Abstract
Intragastric Balloons are a temporary, reversible and safer option compared to bariatric surgery to promote significant weight loss, leading to improved metabolic outcomes. However, due to subsequent weight regain, alternative procedures are now preferred in adults. In adolescents, more amenable to lifestyle change, balloons may be an alternative to less reversible procedures. Our aim was to assess the tolerability and efficacy of the intragastric balloon in severely obese adolescents and the impact of associated weight loss on biomedical outcomes (glucose metabolism, blood pressure, lipid profiles) and bone density. A 2-year cohort study of 12 adolescents (BMI >3.5 s.d., Tanner stage >4) following 6 months intragastric balloon placement was carried out. Subjects underwent anthropometry, oral glucose tolerance test, and DEXA scans at 0, 6 and 24 months. The results showed clinically relevant improvements in blood pressure, insulin: glucose metabolism, liver function and sleep apnoea at 6 months. Changes were not sustained at 2 years though some parameters (Diastolic BP, HBA1c, insulin AUC) demonstrated longer-term improvement despite weight regain. Despite weight loss, bone mass accrual showed age appropriate increases. In conclusion, the intragastric balloon was safe, well tolerated and effective in supporting short-term weight loss and clinically relevant improvement in obesity-related complications, which resolved in some individuals. Benefits were not sustained in the majority at 2 years.
Introduction
A small proportion of children and adolescents have very severe obesity and develop obesity-related complications in adolescence.1, 2
Such individuals tend to become obese adults with a consequent decrease in life expectancy of between 5 and 20 years.3
Bariatric surgery is effective in adolescents though as procedures have only relatively recently been undertaken in such populations data on longer-term outcomes is lacking4 In a recent UK single-centre series of young adolescents, the average weight loss was 54 kg with a reduction in BMI of 16.2 kg m−2.5
Whilst NICE guidance makes provision for adolescent bariatric surgery in ‘exceptional circumstances’,6 there remains an understandable reluctance amongst pediatricians and commissioners to consider this.
We undertook a pilot study to evaluate the effectiveness of intragastric balloons supported by a behavioural management programme in very severe adolescent obesity.
Our premise was that adolescents are more amenable to lifestyle changes than adults and that the balloon supported by lifestyle intervention would ‘kickstart’ weight loss and facilitate longer-term changes.
Intragastric balloons (IGBs) have been used as an adjunct to weight loss for 30 years.7 A review of adult studies reported a mean weight loss of 17.8 kg (range 4.9–28.5 kg, BMI change 4.0–9.0 kg m−2), (30 studies, 4877 patients8). The mortality rate was 0.07% (2 deaths in patients with previous gastric surgery).
Minor side-effects were common with 8.6% of patients experiencing nausea and vomiting and 5% reporting abdominal discomfort.9 Deflation or displacement of the balloon occurred in 2.5%, and obstruction in 0.8% of patients.
Only two studies examined weight loss a year after balloon removal. In one RCT, which included a sham treatment arm, weight loss after a year of balloon therapy was 21.3 kg (17.1% of total body weight). A year post balloon mean weight loss was 12.6 kg.10
In adults IGBs are now used primarily in high anaesthetic risk patients prior to definitive bariatric procedures.11 Evidence that weight loss improves obesity-related co-morbidities is strong.12 In a retrospective study of 2500 IGB patients (mean BMI 44 kg m−2) rates of hypertension, diabetes and obstructive sleep apnoea (OSA) improved.13
There is a concern that weight loss in childhood may reduce the accrual of bone mass that continues until 25 years. However, obese children have reduced bone density and an increased fracture risk.14 Adult studies demonstrate decreased bone mass with both diet-induced weight loss15 and surgery.16 What happens following significant weight loss at time of peak bone mass accrual is unclear.
The aim of our study was to examine longitudinally at 0, 6 and 24 months the impact of weight loss associated with IGB on
Co-morbidities such as glucose metabolism, blood pressure, lipids and liver enzymes.
Developing skeleton by comparing change in fat mass and bone density.
Psychosocial outcomes and physical activity. 17
A cohort of 12 severely obese adolescents (BMI SDS >3.5, Tanner stage ⩾4) were recruited to an open, non-randomised, feasibility study. Informed consent was taken.
A detailed pre-balloon assessment included psychology, anthropometry, oral glucose tolerance test (OGTT) and DXA (lunar iDEXA) scans.
Balloon insertion
Balloons were sited endoscopically under general anaesthetic and inflated with 500 ml saline stained with methylene blue to alert patients to balloon deflation. An antiemetic regimen of dexamethasone (single dose), cyclizine, ondansetron and buscapan was given intravenously initially and orally when tolerating fluids. All patients were discharged the following day on anti-emetics and antispasmodics. Lansoprazole was prescribed while the balloon was in situ to prevent gastric erosion/ulceration and to protect the balloon.
Patients were advised a fully liquid diet in the first week post insertion with semi solids in the second week and normal, healthy diet by week 3. Calories were not restricted to a set number.
Statistical considerations
A sample of 12 patients was selected as the optimal size for a feasibility study.18
Results are expressed as means and s.d. with 95% confidence interval (95% CI). Effect sizes for relationships were calculated using Pearson’s correlation co-efficient.
Co-morbidities
Hypertension was defined as blood pressure >95th centile for age, sex and height. OSA was considered present if symptoms of sleep disordered breathing were reported or sleep study was abnormal. Mobility issues were as reported by participants. Insulin resistance was defined as a Homeostatic Metabolic Assessment score (HOMA) >4.4 or fasting hyperinsulinemia >120 pmol l−1 as per the OSCA (Obesity Services for Children and Adolescents) guidelines.19 Psychosocial issues were defined as ongoing child and adolescent mental health services (CAMHS) involvement. Liver function was considered abnormal if alanine transaminase (ALT) levels were twice upper limit of normal.
Dyslipidemia was defined as in the OSCA guideline. (Cholesterol>5.2, TGL>1.47, HDL<1.09).
Patient demographics
Twelve patients were recruited (5 males).
Mean weight at baseline was 138.5 kg (s.d.±23.9), BMI 46.4 kg/m2 (s.d.±5.6) and BMI SDS +4.0 (s.d.±0.3).
Patients had gained a mean of 11.1(±9.5) kg in the year prior to entering the study and a mean of 20.8 (±12.9) in the 2 years prior to entering the study.
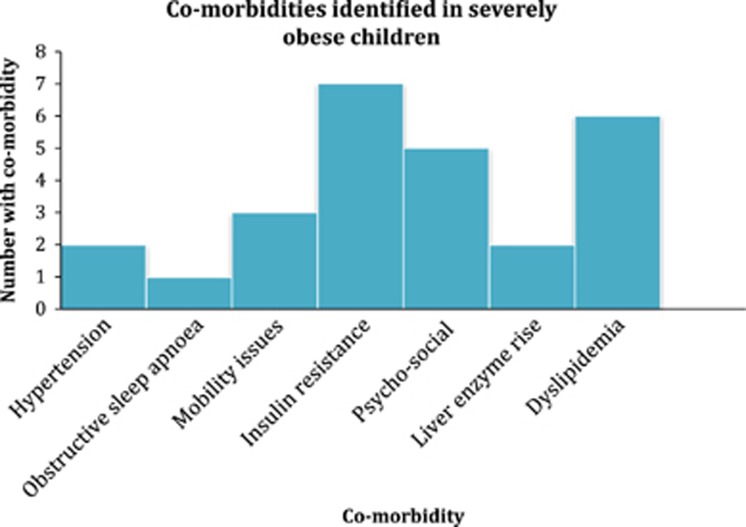
(The co-morbidities within the cohort are detailed in Figure 1). One patient was on continuous positive airway pressure (CPAP) at night.
Figure 1.
Title Co-morbidities identified in severely obese children. X axis co-morbidity. Y axis number with co-morbidity.
Tolerability and complications
The balloon was well tolerated. All patients (except one) experienced nausea, vomiting and abdominal discomfort in the first week. One patient developed non-infective diarrhea 3 weeks after IGB insertion, which resolved spontaneously. One individual developed a subconjunctival haemorrhage (following vomiting). One patient was lost to follow-up after balloon removal due to mental health problems (unrelated to the balloon). Another patient dropped out at 24 months. No serious complications (balloon deflation, intestinal obstruction) were seen and there were no early balloon removals.
Weight loss
Average weight loss at 6 months at balloon removal was 7.0 kg (P=0.005) with reduction in BMI of −2.53 kg m−2 (BMI SDS −0.2 SD (P=0.002). This represented a mean loss of 5% of initial body weight. However, weight loss was sustained in only 2 participants at 24 months.
(Table 1 outlines the changes in the anthropometric, biomedical and bone data during the study).
Table 1. Table outlines the change in weight, BMI, blood pressure, insulin glucose metabolism, lipids, TBLH fat mass %, TBLH bone mineral density, TBLH bone area and bone mineral content, Lumbar bone mineral density, Bone area and bone mineral content between baseline, balloon removal at 6 months and 2 years (18 months after balloon removal).
| Before Balloon insertion N=12 Mean (±s.d.) | After Balloon removal N=12 Mean (±s.d.) | At 2 years N=10 Mean (±s.d.) | Mean difference between baseline and balloon removal at 6 months (95 CI) (P-value) | Mean difference between baseline and 24 months (95 CI) (P-value) | |
|---|---|---|---|---|---|
| Body weight (kg) | 138.5 (23.9) | 131.4 (23.1) | 148.4 (25.2) | −7.1 (−27, 12.8) (P=0.005) | +9.9 (−11.8, 31.8) (P=0.4) |
| BMI (kg m−2) | 46.4 (5.6) | 43.9 (5.5) | 49.3 (8.1) | −2.5 (−7.2, 2.2) (P=0.004) | +2.9 (−3,8.8) (P=0.5) |
| BMI SDS | 4 (0.3) | 3.8 (0.3) | 4.2 (0.5) | −0.2 (−0.37, −0.03) (P=0.002) | +0.2 (−0.1, 0.5) (P=0.5) |
| Waist circumference (cm) | 128.3 (19.1) | 115.9 (15.6) | 138.7 (21.2) | −12.4 (−27.2, 2.4) (P=0.016) | +10.4 (−6.7, 27.5) (P=0.3) |
| Systolic BP (mm of Hg) | 127.8 (11.9) | 122 (16) | 132.2 (18.3) | −5.8 (−17.7, 6.1) (P=0.3) | +4.4 (−9.1, 17.1) (P=0.5) |
| Diastolic BP (mm of Hg) | 74.8 (5.4) | 72.8 (9.5) | 72 (7.5) | −2.0 (−8.5, 4.5) (P=0.6) | −2.8 (−8.3, 2.3) (P=0.2) |
| Fasting glucose (mmol) | 4.3 (0.3) | 4.3 (0.3) | 4.7 (0.4) | −0.03 (−0.25, 0.25) (P=0.5) | +0.4 (0.1, 0.7) (P=0.1) |
| Fasting Hyperinsulinemia (pmol/l) | 209.6 (141.6) | 189 (116.4) | 213.6 (106.4) | −20.6 (−130.3, 89) (P=0.3) | +4 (109.4, 117) (P=0.8) |
| HOMA IR | 6.6 (4.7) | 5.2 (3.2) | 7.4 (3.8) | −1.4 (−4.8, 2) (P=0.5) | +0.8 (−3.1, 4.7) (P=0.6) |
| Insulin (AUC; pmol l−1) | 3387 (2417) | 2173 (1845) | 2780 (2588) | −1214 (3034, 606) (P=0.05) | −607 (−2835, 1621) (P=0.4) |
| ALT | 47.3 (25.6) | 43 (23.8) | 48.8 (28.7) | −4.3 (−25.2, 16.6) (P=0.4) | +1.5 (−22.7, 25.7) (P=0.7) |
| Gamma GT | 32.9 (13.1) | 25.8 (9.5) | 29.4 (12.5) | −7.1 (−16.8, 2.6) (P=0.03) | −3.5 (−15, 8) (P=0.3) |
| Cholesterol | 4.4 (0.7) | 4.1 (0.6) | 4.3 (0.8) | −0.3 (−0.85, 0.25) (P=0.2) | −0.1 (−0.8, 0.6) (P=0.4) |
| Triglycerides | 1.5 (0.7) | 1.9 (0.8) | 1.3 (0.6) | +0.4 (−0.24, 1.04) (P=0.1) | −0.2 (−0.8, 0.4) (P=0.4) |
| HBA1c (mmol mol−1) | 36.2 (2.5) | 35.1 (1.8) | 34.1 (2) | −1.1 (−2.9, 0.7) (P=0.08) | −2.1 (−4.1, −0.1) (P=0.005) |
| TBLH fat mass % | 54.9 (3.5) | 52.8 (5.2) | 54.9 (7.5) | −2.1 (−5.9, 1.7) (P<0.05) | 0.0 (−5.0, 5.0) (P=0.5) |
| TBLH BMD (g cm−2) | 1.15 (0.07) | 1.15 (0.06) | 1.19 (0.08) | 0.002 (−0.03, 0.03) (P=0.9) | 0.04 (0.01, 0.06) (P=0.01) |
| TBLH BMD Z score | 1.7 (1) | 1.64 (0.9) | 1.97 (1.1) | −0.06 (−0.9, 0.7) (P=0.3) | 0.27 (−0.65, 1.19) (P=0.3) |
| TBLH BA (cm2) | 2010.2 (240.7) | 2056.5 (205.3) | 1958.3 (252.8) | 46.4 (−15.8, 108) (P=0.13) | −22.5 (−97.5, 52.5) (P=0.52) |
| TBLH BMC (gm) | 2307.9 (287.6) | 2368.6 (254.1) | 2343.9 (334.5) | 60.7 (5.5, 115.9) (P=0.03) | 43.9 (−20.6, 108.4) (P=0.16) |
| L1-L4 BMD(g cm−2) | 1.23 (0.2) | 1.26 (0.2) | 1.28 (0.2) | 0.03 (0.01, 0.04) (P=0.01) | 0.04 (−0.02, 0.10) (P=0.14) |
| L1-L4 BMD z score | 0.9 (1.2) | 0.87 (1.2) | 0.76 (1.3) | −0.03 (−1.09, 1.03) (P=0.6) | −0.14 (−1.26, 0.98) (P=0.6) |
| L1-L4 BA (cm2) | 57.4 (7.6) | 58.1 (7.5) | 58.8 (7.1) | 0.8 (0.4, 1.2) (P=0.002) | 2.0 (0.9, 3.0) (P=0.003) |
| L1-L4 BMC (gm) | 70.5 (13.0) | 73.0 (13.3) | 75.6 (14.0) | 2.5 (1.4, 3.6) (P=0.001) | 5.3 (1.0, 9.5) (P=0.02) |
Abbreviations:
ALT, alanine transaminase; BA, bone area; BMC, bone
mineral content; BMD, bone mineral density; TBLH, total body less head.
Reference population used to calculate the BMI Z score was based on the UK90 growth charts.
Blood pressure
Systolic and diastolic blood pressure (BP) fell by 5.8 mm Hg (s.d.16.8, P=0.3) and 2.0 mm Hg (s.d. 10.9, P=0.6), respectively. Of the two patients with established hypertension, blood pressure normalised in one at 6 months. As patients regained weight after balloon removal, systolic and mean blood pressures subsequently rose and were above baseline levels at 24 months though diastolic BP remained below that at baseline (Table 1).
Glucose and insulin metabolism
Insulin area under the curve (AUC) following OGTT improved at 6 months (P=0.05) though was not sustained at 2 years. HOMA scores also declined at 6 months. Two of the seven individuals with raised HOMA at balloon insertion had normal markers at balloon removal. Insulin AUC remained below pre intervention levels despite subsequent weight regain. There was also a fall in HBA1c at 6 months that was maintained despite weight regain (P=0.005).
There was an association between initial weight loss and improved insulin glucose metabolism (AUC insulin r=0.3, HBA1c r=0.4). This association was stronger at 24 months (AUC insulin r=0.66 and HBA1c r=0.64).
Lipid profiles
Dyslipidemia did not resolve in the 6 individuals with baseline anomalies.
Liver enzymes
Of two with raised ALT at study inception, one improved but the other persisted. Gamma glutamyl transferase levels fell at 6 months (P=0.03) and remained below levels at baseline at 24 months.
Obstructive sleep apnoea
The patient on CPAP for sleep apnoea continued to lose weight at 24 months and was weaned off this.
Bone
Total percentage fat mass decreased −2.1% (CI −5.9, 1.7, P<0.05) as did truncal fat mass percentage −2.1% (CI: −4, −0.1, P<0.04) in the 6 months the balloon was in situ. However, there was no evidence that weight loss had a deleterious impact on bone with improved bone mineral density (BMD), and lumbar area and bone mineral content at 2 years. TBLH BMD Z score increased by 0.27 (CI: −0.65, 1.19, P=0.3) over the 2 years (Table 1).
This study demonstrated that the IGB was safe and well tolerated in young people. There were no early balloon removals. There was some initial nausea, vomiting and abdominal discomfort, but none of the more serious complications described in adult studies such as perforation, or obstruction were reported.9 At 15.6 years, participants were much younger than in adolescent cohorts reported previously.
The magnitude of weight loss, (5%) while likely to be clinically significant, was lower than described in adult cohorts and in the minimal data available on younger patients (BMI reduction of 5 kg m−2 in Brazilian study versus 2.5 kg m−2 in our cohort).20 However, stringent entry criteria in our study meant that all patients had to have undergone at least a 3-month lifestyle intervention (Most had done substantially more than this) and pharmacotherapy (orlistat and/or sibutramine, which was still licensed at that time. The study perhaps had a positive effect even for individuals with no/minimal weight loss when one considers that the cohort had gained an average of 20 kg in the 2-years prior to the study.
This was a feasibility study with limited numbers and therefore not powered to show statistical significance. There was considerable public patient involvement in study design who felt that recruitment to a ‘standard’ care group for a randomised trial would have been difficult, as the young people felt ‘there was nothing in it for them.’ However, one of the studies weaknesses is the lack of a control group, which may have shown more clearly the weight trajectories in a non-intervention group. Given the PPI concerns, a waiting list control cross over design in the setting of a larger study could be the next step in evaluating the efficacy of the intragastric balloons in obese adolescents.
One key objective of the study was to ascertain whether rapid weight loss would adversely affect the developing skeleton as seen in adults.16 We demonstrated that adolescents continued to demonstrate normal accrual of bone mass despite significant weight loss.
At balloon removal, there were clinically relevant improvements in blood pressure, liver function and insulin glucose metabolism with successful resolution of co-morbidities in some subjects. Unfortunately, 8 out of 10 individual’s subsequently regained weight but a criterion for entry into the pilot was that individuals had been unsuccessful losing weight with previous lifestyle interventions. However there appeared to be a persisting benefit on diastolic BP, insulin AUC production and HBA1c.
In conclusion, the intragastric balloon is safe, well tolerated and effective supporting modest short-term weight loss. There was clinically important improvement in co-morbidities, albeit short-term in most instances. There was no detrimental effect on bone of rapid weight loss in adolescents. While the technique was safe and effective, a larger RCT would be needed to fully evaluate clinical benefit and cost effectiveness.
Acknowledgments
We express our gratitude to all families who participated in this research and to Lizzy DeAngelis for providing the dietetic support in this study. CLAHRC SY would also like to acknowledge the participation and resources of our partner organisations. Further details can be found at www.clahrc-sy.nihr.ac.uk. The funding for this work was also received from the British Society of Paediatric Endocrinology and Diabetes (BSPED).
Disclaimer
The paper presents independent research as part of the Obesity Theme within the National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care for South Yorkshire (NIHR CLAHRC SY). The views and opinions expressed are those of the authors, and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
The authors declare no conflict of interest.
References
- Baker JL, Olsen LW, Sorensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med 2007; 357: 2329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med 2002; 346: 802–810. [DOI] [PubMed] [Google Scholar]
- Prospective Studies CollaborationProspective Studies CollaborationWhitlock G Prospective Studies CollaborationLewington S Prospective Studies CollaborationSherliker P Prospective Studies CollaborationClarke R Prospective Studies CollaborationEmberson J. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. The Lancet 2009; 373: 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadwell JR, Sun F, Schoelles K. Systematic review and meta-analysis of bariatric surgery for pediatric obesity. Ann Surg 2008; 248: 763–776. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Makaya T, Marven S, Ackroyd R, Wales J, Wright N. Bariatric surgery in severely obese adolescents:a single centre experience. Arch Dis Child 2014; 99: 894–898. [DOI] [PubMed] [Google Scholar]
- (UK). NIfHaCE2014. Identification, assessment and management of overweight and obesity in children, young people and adults: partial update of CG43.NICE Clinical Guidelines No.189 https://www.nice.org.uk/guidance/cg43. [PubMed]
- Nieben OG, Harboe H. Intragastric balloon as an artificial bezoar for treatment of obesity. Lancet 1982; 1: 198–199. [DOI] [PubMed] [Google Scholar]
- Dumonceau JM. Evidence-based review of the Bioenterics intragastric balloon for weight loss. Obes Surg 2008; 18: 1611–1617. [DOI] [PubMed] [Google Scholar]
- Imaz I, Martínez-Cervell C, García-Álvarez EE, Sendra-Gutiérrez JM, González-Enríquez J. Safety and effectiveness of the intragastric balloon for obesity. A meta-analysis. Obes Surg 2008; 18: 841–846. [DOI] [PubMed] [Google Scholar]
- Mathus-Vliegen EMH, Tytgat GNJ. Intragastric balloon for treatment-resistant obesity: safety, tolerance, and efficacy of 1-year balloon treatment followed by a 1-year balloon-free follow-up. Gastrointest Endosc 2005; 61: 19–27. [DOI] [PubMed] [Google Scholar]
- Gottig S, Daskalakis M, Weiner S, Weiner RA. Analysis of safety and efficacy of intragastric balloon in extremely obese patients. Obes Surg 2009; 19: 677–683. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Related Metab Disord 1992; 16: 397. [PubMed] [Google Scholar]
- Genco A, Bruni T, Doldi SB, Forestieri P, Marino M, Busetto L et al. BioEnterics intragastric balloon: the Italian experience with 2515 patients. Obes Surg 2005; 15: 1161–1164. [DOI] [PubMed] [Google Scholar]
- Dimitri P, Wales JK, Bishop N. Fat and bone in children: differential effects of obesity on bone size and mass according to fracture history. J Bone Miner Res 2010; 25: 527–536. [DOI] [PubMed] [Google Scholar]
- Jenson L, Quaade F, Sorenson O. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res 1994; 9: 459–463. [DOI] [PubMed] [Google Scholar]
- Mahdy T, Atia S, Farid M, Adulatif A. Effect of Roux-en Y gastric bypass on bone metabolism in patients with morbid obesity: Mansoura experiences. Obes Surg 2008; 18: 1526–1531. [DOI] [PubMed] [Google Scholar]
- Reece LJ, Sachdev P, Copeland RJ, Thomson M, Wales JK, Wright NP. Intra-gastric balloon as an adjunct to lifestyle support in severely obese adolescents; impact on weight, physical activity, cardiorespiratory fitness and psychosocial well-being. Int J Obes 41: 591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharma Stat 2005; 4: 287–291. [Google Scholar]
- Viner RM, White B, Barrett T, Candy DCA, Gibson P, Gregory JW et al. Assessment of childhood obesity in secondary care: OSCA consensus statement. Arch Dis Child Educ Pract Ed 2012; 97: 98–105. [DOI] [PubMed] [Google Scholar]
- Sallet J, Marchesini J, Paiva D. Brazilian multicenter study of the intragastric balloon. Obes Surg 2004; 14: 991–998. [DOI] [PubMed] [Google Scholar]