ABSTRACT
Background
The treatment of levodopa‐induced dyskinesia in Parkinson's disease (PD) is an unmet need with no approved drug therapy.
Objective
The purpose of this study was to investigate the efficacy and safety of 274 mg ADS‐5102 (amantadine) extended‐release capsules (equivalent to 340‐mg amantadine HCl) for levodopa‐induced dyskinesia in a randomized controlled trial.
Methods
PD patients with ≥1 hour of troublesome dyskinesia and at least mild functional impact were randomized to placebo or ADS‐5102 once daily at bedtime for 13 weeks. The primary efficacy analysis was based on change from baseline to week 12 on the Unified Dyskinesia Rating Scale total score in the modified intent‐to‐treat population. OFF time was a key secondary measure.
Results
At week 12, least‐squares mean change in the Unified Dyskinesia Rating Scale was −20.7 (standard error 2.2) for ADS‐5102 (n = 37) and –6.3 (standard error 2.1) for placebo (n = 38; treatment difference −14.4, 95% confidence interval −20.4 to −8.3, P < .0001), indicating improvement in levodopa‐induced dyskinesia. OFF time decreased 0.5 hours (standard error 0.3) for ADS‐5102 from a baseline mean of 2.6 hours and increased 0.6 hours (standard error 0.3) for placebo from a baseline mean of 2.0 hours (treatment difference −1.1 hours, 95% confidence interval −2.0 to −0.2, P = .0199). The most common adverse events (ADS‐5102 versus placebo) included dry mouth (13.5% versus 2.6%), nausea (13.5% versus 2.6%), decreased appetite (10.8% versus 0%), insomnia (10.8% versus 0%), orthostatic hypotension (10.8% versus 0%), constipation (8.1% versus 0%), falls (8.1% versus 5.3%), and visual hallucinations (8.1% versus 5.3%). Adverse events led to treatment discontinuation in 19% versus 8%, respectively.
Conclusion
ADS‐5102 274 mg is an oral pharmacotherapy demonstrating a significant decrease in levodopa‐induced dyskinesia and improving OFF time. © 2017 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease, amantadine, Unified Dyskinesia Rating Scale, levodopa‐induced dyskinesia, randomized controlled trial
Levodopa‐induced dyskinesia (LID) is a significant treatment‐limiting adverse effect of levodopa therapy that results in the suboptimal management of Parkinson's disease (PD). As PD progresses, patients experience diminished duration of benefit from each levodopa dose, termed wearing‐off, and motor fluctuations with OFF time between doses. In addition, chronic levodopa treatment often leads to the development of dyskinesia. In patients treated with levodopa, dyskinesia can occur in approximately 50% of patients by 5 years and nearly 90% of patients by approximately 10 years of treatment.1 LID is associated with impaired activities of daily living, decreased health‐related quality of life, increased risk of falls, increased health care utilization, and increased caregiver burden.2, 3, 4, 5
Many patients on levodopa experience both wearing‐off and dyskinesia. LID may be managed by modifying the patient's levodopa dose by either decreasing each dose or fractionating the total daily dose.6 A monoamine oxidase type B inhibitor, dopamine agonist, or catechol‐O‐methyltransferase inhibitor can be used to treat the underlying PD but may exacerbate dyskinesia.7 A critical objective in reducing OFF time is to avoid a simultaneous increase in dyskinesia. As there is no approved drug therapy for LID, physicians and patients are faced with a trade‐off between ON time with dyskinesia, or greater OFF time without dyskinesia. Patient research shows a strong preference for avoiding the uncomfortable or painful OFF state.3 If a drug could reduce both OFF time and dyskinesia, it would be a major advance in therapeutics. So far, no drug has been proven to accomplish this goal.
Amantadine immediate release (amantadine IR) was approved in the United States as a prophylactic agent against Asian influenza in 1966.8 In 1969, Schwab and colleagues9 discovered by serendipity the beneficial effect of amantadine hydrochloride (HCl) on the motor symptoms of PD, but it took more than 30 years to discover its antidyskinetic effect. Relatively short studies have shown an antidyskinetic effect with amantadine IR; however, this effect has not been extensively studied in well‐controlled, randomized, long‐term clinical trials.9, 10, 11, 12 Despite the same body of trial evidence, there are differing guideline recommendations regarding the use of amantadine in the treatment of levodopa‐induced dyskinesia.13, 14 A Cochrane review concluded that there was insufficient evidence to conclude whether amantadine is an effective treatment for LID in patients with PD.15 American Academy of Neurology guidelines concluded amantadine to be “possibly effective,”13 whereas a Movement Disorders Society evidence‐based review reported that amantadine IR was “efficacious” in the treatment of dyskinesia.14
The mechanism of action of amantadine in decreasing LID is not known. Amantadine is an uncompetitive (open‐channel) antagonist of the N‐methyl‐d‐aspartate (NMDA) receptor (Ki = 10 μM), a type of glutamatergic receptor, and has direct and indirect effects on glutamatergic and dopaminergic signaling.16, 17, 18, 19 Amantadine exhibits predictable anticholinergic (eg, dry mouth, urinary retention, and constipation) and NMDA receptor antagonist activity (eg, hallucinations).10, 20, 21, 22
The safety profile of amantadine has been well characterized.23, 24 Although most patients with PD can tolerate 200 mg daily of amantadine IR (amantadine HCl, equivalent to 162 mg amantadine),23 the increased frequency of adverse events (AEs) at higher doses,25 in particular central nervous system events (including sleep disturbances), limits the routine use of amantadine IR at doses of 300 mg/day (equivalent to 243 mg amantadine) or higher. Dosing instructions for amantadine IR in Germany state that patients' last dose of the day should be taken no later than 4:00 in the afternoon.24
ADS‐5102 (amantadine) extended‐release capsules is being developed for the treatment of LID in patients with PD. The pharmacokinetic profile of ADS‐5102 has been designed to exhibit an initially slow rate of rise in amantadine levels during sleep and high levels in the morning that are sustained throughout waking hours when patients most need relief from their dyskinesia. This profile enables higher dosing of ADS‐5102, which is not interchangeable with amantadine IR on a mg‐per‐mg basis. The target dose of 274 mg ADS‐5102 daily (equivalent to 340 mg amantadine HCl) was selected based on the results of an earlier phase 2/3 dose‐ranging study (EASED study).26 The present study was the second pivotal phase 3 study (prior study, EASE LID study27) designed to confirm the efficacy and safety of ADS‐5102 274 mg dosed once daily at bedtime for the treatment of LID in patients with PD.
Methods
Study Design and Participants
A phase 3, randomized, double‐blind, placebo‐controlled study (EASE LID 3 Study, Adamas Pharmaceuticals, Inc., ADS‐AMT‐PD304) was conducted at 39 sites in the United States and Western Europe (Germany, France, Spain, and Austria). The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Prior to initiating the study, all participating sites received approval from an institutional review board, research ethics board, or independent ethics committee. Written informed consent was obtained from all study participants before any study‐related procedures were performed.
Key inclusion criteria included age between 30 and 85 years, inclusive; diagnosis of PD based on the United Kingdom Parkinson's Disease Society Brain Bank Clinical Diagnostic Criteria28; score of at least 2 on part IV, item 4.2 (functional impact of dyskinesia) of the Movement Disorder Society–Unified Parkinson's Disease Rating Scale (MDS‐UPDRS) at screening and at day 1 (baseline); and at least 2 half‐hour time periods between 9:00 am and 4:00 pm documented as ON time with troublesome dyskinesia on a 24‐hour PD patient diary29 on each of 2 consecutive days just prior to day 1. All antiparkinson medications, including levodopa preparations, were to be unchanged for at least 30 days prior to screening and during study participation. Levodopa preparations had to be administered at least 3 times daily.
Key exclusion criteria included history of dyskinesia that was not caused by dopaminergic stimulation in PD; neurosurgical intervention related to PD; atypical parkinsonism; levodopa‐ or dopamine‐agonist–induced psychosis; cognitive impairment as evidenced by a Mini‐Mental State Examination score of less than 24 during screening; estimated glomerular filtration rate <50 mL/min/1.73 m2; use of amantadine within 30 days prior to screening; documented inability to tolerate or lack of dyskinesia response to prior amantadine treatment; current treatment with apomorphine or dopamine receptor blocking agents; current treatment with medications that prolong the QT interval and have a known risk of torsades de pointes; clinically significant electrocardiographic abnormalities; use of rimantadine; or history of hypersensitivity or allergic reaction to amantadine, rimantadine, or memantine.
Randomization and Blinding
Eligible patients were randomized on day 1 in a 1:1 ratio to receive placebo or ADS‐5102. ADS‐5102 and placebo capsules and packaging were identical in appearance. The randomization list was generated and validated by Pharmaceutical Product Development, LLC (Wilmington, North Carolina). Randomization was accomplished through an interactive web‐based response system managed by Endpoint Clinical (San Francisco, California), which also allowed for unblinding of treatment assignment if necessary for patient safety. All patients, study‐site personnel, raters, sponsor, and contract research organization staff were blinded to treatment assignment. Data analyzers were blinded until after the database was locked.
Procedures
Prequalified raters completed training and were certified to administer efficacy scales. Rater training utilized the MDS teaching modules for the Unified Dyskinesia Rating Scale (UDysRS) and MDS‐UPDRS. If possible, the same rater conducted efficacy assessments for a patient at least 30 minutes following the patient's regularly scheduled levodopa dose, and when the patient was ON (PD medications providing good effect on motor symptoms) and experiencing typical dyskinesia. There were up to 9 visits (Screening, Baseline, weeks 1, 2, 4, 8, 12, 13, and safety follow‐up visits) in this study. Study visits and assessments were scheduled between 9:00 am and 4:00 pm, and all study visits for an individual participant were to be scheduled at approximately the same time of day. Doses and regimens of antiparkinson medications were maintained without changes during study participation.
During screening (up to 3 weeks prior to baseline), written informed consent was obtained, training and concordance testing for the PD home diary was completed, and study eligibility criteria were assessed. A set of two 24‐hour PD home diaries was distributed for completion just prior to the scheduled baseline visit. During the baseline visit (week 0, day 1), study eligibility was confirmed and patients were randomized to ADS‐5102 or placebo.
In addition, the following efficacy assessments were completed: UDysRS, review of completed PD home diaries, MDS‐UPDRS, and baseline notes to allow future assessment of the Clinician's Global Impression of Change (CGI‐C).
During the first week of dosing, patients randomized to ADS‐5102 received a daily ADS‐5102 dose of 137 mg (one ADS‐5102–containing capsule and one placebo capsule, identical in appearance). During weeks 2 through 12, the daily ADS‐5102 dose was 274 mg, administered as two 137‐mg capsules. During the last week of dosing, the dose was reduced to 137 mg daily. Patients randomized to placebo received 2 placebo capsules for 13 weeks. Individuals who discontinued the study drug were encouraged to continue study participation and complete all study visits.
The UDysRS, MDS‐UPDRS, CGI‐C, and standard safety assessments were performed at weeks 2, 4, 8, and 12. The PD home diaries were completed prior to each of these visits. A final safety follow‐up visit occurred approximately 7 days following treatment completion unless a patient elected to enroll directly into a companion open‐label safety study (NCT02202551).30
Outcomes
The primary outcome measure was the change from baseline to week 12 in the UDysRS total score. Key secondary outcome measures included the change from baseline to week 12 in ON time without troublesome dyskinesia (ON time without dyskinesia plus ON time with non‐troublesome dyskinesia) and OFF time. Other secondary measures included change from baseline to week 12 in MDS‐UPDRS, ON time with troublesome dyskinesia, total ON time with dyskinesia (non‐troublesome plus troublesome), and the CGI‐C. Safety assessments included AEs, reasons for discontinuation, physical examinations, vital signs, and clinical laboratory testing.
Statistical Analyses
The sample size was estimated based on the previous phase 2/3 study,26 which suggested that 36 patients per group would provide 90% power to detect a treatment difference between the ADS‐5102 and placebo groups of 11.5 units (standard deviation 14) with an α level of 0.05 and a potential dropout rate of up to 10%.
The primary efficacy analysis compared the active (274 mg ADS‐5102) group with the placebo group at week 12 using a linear mixed model with repeated measures with the changes from baseline in the UDysRS total score at weeks 2, 4, 8, and 12 as the dependent variable. The model included categorical effects for treatment group, visit (4 levels corresponding to weeks 2, 4, 8, and 12), and the interaction between treatment group and visit. The baseline UDysRS total score was included as a covariate. Estimates for the least squares mean change from baseline at week 12 in each treatment arm along with the least squares mean treatment difference were provided with 95% confidence intervals (CIs) using an appropriate contrast from the model.
The following key secondary analyses were conducted using a fixed‐sequence hierarchical procedure to control the overall level of significance, in the order shown below:
274 mg ADS‐5102 versus placebo for ON time without troublesome dyskinesia at week 12
274 mg ADS‐5102 versus placebo for OFF time at week 12.
The week 12 secondary analyses in this list were performed using linear mixed model with repeated measures models.
The hypotheses were tested using 2‐sided tests at the 5% level of significance, but a specified comparison was considered confirmatory only if the primary efficacy analysis and all previously conducted key secondary analyses were statistically significant (P < .05).
The modified intent‐to‐treat population (mITT) was the prespecified efficacy analysis population and included all randomized patients who were dosed and who provided at least 1 post‐baseline assessment of the UDysRS. The safety population included all randomized patients who received at least 1 dose of study drug. Prespecified directions for the handling of individual missing data values related to each efficacy outcome measure were included in the Statistical Analysis Plan. The software package SAS (version 9.4; SAS Institute, Cary, North Carolina) was used for analysis. A data monitoring committee was not used for this trial.
This study is registered with ClinicalTrials.gov, number NCT02274766.
Results
Between October 16, 2014, and December 9, 2015, 114 patients were screened and 77 patients were randomized (Fig. 1). The most common reason for screen failure was that the patient did not report at least 2 half‐hour time periods of ON time with troublesome dyskinesia. The mITT and safety populations included 75 patients. Two patients were inadvertently randomized but never dosed. A week 12 visit (primary efficacy endpoint) was completed by 83% of randomized patients. The most common reason for study drug discontinuation was AEs. No unblinding of the treatment assignment was necessary during the study. Demographics and baseline characteristics of the mITT population are shown in Table 1. The treatment groups were balanced at study entry for patient demographics.
Figure 1.
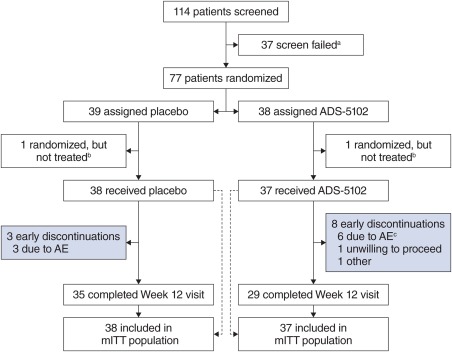
Trial profile. aThe most common reason for screen failure was that the patient did not report at least two half‐hour time periods of ON with troublesome dyskinesia at baseline. bTwo participants, randomized to ADS‐5102 and placebo, respectively, were randomized in error and did not receive study drug. cOne additional patient discontinued study drug as a result of AEs but continued the study. AE, adverse event; mITT, modified intent‐to‐treat. [Color figure can be viewed at wileyonlinelibrary.com]
Table 1.
Baseline demographics and PD characteristics (modified intent‐to‐treat population)
| Placebo, n = 38 | ADS‐5102, n = 37 | |
|---|---|---|
| Age, y | 64.9 (9.1) | 64.7 (9.7) |
| Sex, male | 20 (52.6) | 19 (51.4) |
| Race, white | 38 (100.0) | 36 (97.3) |
| Baseline levodopa (any preparation), dose (mg) | 635.8 (446.7) | 671.9 (465.7) |
| Duration of LID, y | 4.0 (2.6) | 3.8 (3.2) |
| Years since PD diagnosis | 10.7 (4.3) | 10.4 (5.1) |
| Mini‐Mental State Examination | 28.7 (1.4) | 29.1 (1.6) |
| Hoehn and Yahr (while ON) | 2.4 (0.6) | 2.1 (0.6) |
| UDysRS, total (max possible score: 104) | 41.2 (10.3) | 40.2 (13.1) |
| PD home diary | ||
| ON time with troublesome dyskinesia, h | 6.0 (3.4) | 4.7 (2.5) |
| ON time without troublesome dyskinesia, h | 7.8 (3.2) | 8.8 (2.5) |
| OFF time, h | 2.0 (1.7) | 2.6 (2.0) |
| Participants with OFF time at baseline | 35 (84.2) | 29 (86.5) |
| MDS‐UPDRS (while ON) | ||
| Part I (max possible score: 52) | 9.9 (4.9) | 11.3 (59) |
| Part II (max possible score: 52) | 14.8 (6.1) | 14.1 (6.2) |
| Part III (max possible score: 132) | 21.4 (10.2) | 21.2 (9.2) |
| Combined score (Parts I–III) (max possible score: 236) | 46.1 (17.0) | 46.6 (14.8) |
| Part IV (max possible score: 24) | 11.1 (2.4) | 9.8 (2.8) |
| Part IV, item 4.1, time spent with dyskinesia | 2.8 (0.9) | 2.2 (0.8) |
| Part IV, item 4.2, functional impact of dyskinesia | 2.5 (0.5) | 2.5 (0.6) |
| Concomitant medication use at baseline | ||
| Dopamine agonist | 25 (65.8) | 21 (56.8) |
| MAO inhibitor | 20 (52.6) | 17 (45.9) |
| COMT inhibitor | 1 (2.6) | 3 (8.1) |
| Anticholinergic | 2 (5.3) | 0 (0.0) |
Data are mean (standard deviation) or n (%). COMT, catechol‐O‐methyltransferase; LID, levodopa‐induced dyskinesia; MAO, monoamine oxidase; MDS‐UPDRS, Movement Disorder Society–Unified Parkinson's Disease Rating Scale; PD, Parkinson's disease; UDysRS, Unified Dyskinesia Rating Scale.
The primary efficacy analysis demonstrated a significantly greater reduction in UDysRS total score (improvement in dyskinesia) in the ADS‐5102 group when compared with placebo at week 12 (least squares mean treatment difference −14.4 [95% CI −20.4 to −8.3], P < .0001; Table 2). Changes in UDysRS total scores at all study visits for ADS‐5102 and placebo groups are shown in Figure 2A. The treatment effect of ADS‐5102 on UDysRS total score was consistent across the following subgroups: sex, age, baseline renal function, body mass index, dyskinesia severity, baseline OFF time, and baseline levodopa dose (Supplemental Fig. 1).
Table 2.
Efficacy results (modified intent‐to‐treat population)
| LS mean change from baseline to week 12 (SE) | Treatment difference, 95% CI | P value | ||
|---|---|---|---|---|
| Placebo, n = 38 | ADS‐5102, n = 37 | |||
| Primary endpoint | ||||
| UDysRS total score | −6.3 (2.1) | −20.7 (2.2) | −14.4 (−20.4 to −8.3) | <.0001 |
| Key secondary endpoints | ||||
| ON time without troublesome dyskinesia | 2.1 (0.5) | 4.0 (0.6) | 1.9 (0.4 to 3.5) | .0168 |
| OFF time | 0.6 (0.3) | −0.5 (0.3) | −1.1 (−2.0 to −0.2) | .0199 |
| Other secondary endpoints | ||||
| ON time with troublesome dyskinesia | −2.5 (0.4) | −3.6 (0.5) | −1.1 (−2.4 to 0.2) | .0853 |
| Total time with dyskinesia | −2.7 (0.7) | −4.2 (0.7) | −1.6 (−3.59 to 0.45) | .1254 |
| ASLEEP time | 8.1 (1.6) | 8.1 (1.6) | n/a | n/a |
| UDysRS historical score, parts I & II | −4.0 (1.4) | −12.1 (1.5) | −8.1 (−12.1 to −4.1) | .0001 |
| UDysRS objective score, parts III & IV | −2.2 (1.2) | −8.7 (1.3) | −6.5 (−10.1 to −3.0) | .0004 |
| MDS‐UPDRS, part IV, motor complications | −1.3 (0.5) | −4.3 (0.5) | −3.0 (−4.5 to −1.6) | <.0001 |
| MDS‐UPDRS, part IV, item 4.1, time spent with dyskinesia | −0.6 (0.2) | −0.9 (0.2) | −0.3 (−0.8 to 0.2) | .1929 |
| MDS‐UPDRS, Part IV, item 4.2, functional impact of dyskinesia | −0.6 (0.1) | −1.6 (0.2) | −0.9 (−1.4 to −0.5) | <.0001 |
| MDS‐UPDRS, combined score, parts I‐III | −2.0 (2.1) | −8.4 (2.2) | −6.5 (−12.7 to −0.3) | .0398 |
| Daily levodopa dose (any preparation), mg, mean change from baseline (SD) | 0.0 (0.0) | −13.5 (67.3) | n/a | n/a |
CI, confidence interval; LS, least squares; MDS‐UPDRS, Movement Disorder Society–Unified Parkinson's Disease Rating Scale; n/a, not applicable; SD, standard deviation; SE, standard error; UDysRS, Unified Dyskinesia Rating Scale.
Figure 2.

(A) Change in UDysRS over time (mITT population). Range in parentheses indicates 95% confidence interval. (B) Change in PD home diary data over time (mITT population). CI, confidence interval; LS, least squares; mITT, modified intent‐to‐treat; PD, Parkinson's disease; UDysRS, Unified Dyskinesia Rating Scale; SE, standard error.
The historical (patient‐reported duration and impact) and objective (rater assessment of impairment and disability) UDysRS scores showed a significantly greater improvement in the ADS‐5102 group when compared with placebo at 12 weeks (Table 2). The key secondary PD diary endpoints (ON time without troublesome dyskinesia and OFF time) also showed significant improvements in the ADS‐5102 group (Table 2). Changes in PD diary endpoints at all study visits for ADS‐5102 and placebo groups are shown in Figure 2B.
To further characterize the effect of ADS‐5102 on patient‐reported diary states throughout waking hours, a summary of diary states across waking hours for the study population was generated (Supplemental Fig. 2). The results showed a greater increase in ON time without troublesome dyskinesia (as a result of decreases in both OFF time and ON time with troublesome dyskinesia) throughout waking hours for the ADS‐5102–treated patients when compared with placebo‐treated patients.
At week 12, changes in MDS‐UPDRS showed a statistically significant treatment effect for ADS‐5102 at week 12 for the part II score (P = .0076), the combined scores for parts I, II, and III (P = .0398), and for the part IV score (P < .0001; Table 2). The CGI‐C results showed that 19 patients (51%) in the ADS‐5102 group and 4 patients (11%) in the placebo group were assessed as moderately or markedly improved in overall PD symptoms, including dyskinesia, at week 12 (P = .0009 for overall distribution; Supplemental Table 1).
Overall, AEs were reported for 84% of ADS‐5102 patients and 50% of placebo patients. Most patients reported AEs that were mild to moderate in intensity (26 [70%] in ADS‐5102 and 17 [45%] in placebo). The most common AEs (≥5% in the ADS‐5102 group) were dry mouth, nausea, decreased appetite, insomnia, orthostatic hypotension, constipation, falls, and visual hallucinations (Table 3). Two study drug–related serious AEs (constipation and urinary retention) were reported for 1 patient during the study. One patient treated with ADS‐5102 experienced suicidal ideation (assessed by the investigator as related to the study drug), and a second patient attempted suicide (assessed by the investigator as not related to the study drug). Both patients had a history of depression and discontinued the study drug. The patient who attempted suicide had stopped the study drug 4 days prior to the attempt. There were no deaths. In general, vital signs and laboratory results remained consistent with baseline values throughout the study.
Table 3.
Adverse events (AEs) overview (safety population)
| Placebo, n = 38 | ADS‐5102, n = 37 | |
|---|---|---|
| Number (%) of participants with any: | ||
| AEs | 19 (50.0) | 31 (83.8) |
| Study drug‐related AEs | 10 (26.3) | 21 (56.8) |
| Serious AEs | 0 | 4 (10.8) |
| Study drug‐related serious AEs | 0 | 1 (2.7) |
| Number (%) of participants who permanently discontinued treatment as a result of any: | ||
| AEs | 3 (7.9) | 7 (18.9) |
| Study drug‐related AEs | 2 (5.3) | 6 (16.2) |
| Most common AEs (at least 5% in active arm) | ||
| Dry mouth | 1 (2.6) | 5 (13.5) |
| Nausea | 1 (2.6) | 5 (13.5) |
| Decreased appetite | 0 | 4 (10.8) |
| Insomnia | 0 | 4 (10.8) |
| Orthostatic hypotension | 0 | 4 (10.8) |
| Constipation | 0 | 3 (8.1) |
| Fall | 2 (5.3) | 3 (8.1) |
| Hallucination, any type | 2 (5.3) | 3 (8.1) |
| Hallucination, visual | 2 (5.3) | 3 (8.1) |
| Hallucination, auditory | 0 | 1 (2.7) |
Of the 37 ADS‐5102 patients, 7 (19%) and 3 (8%) of the 38 placebo patients discontinued the study drug because of AEs. In the ADS‐5102 group, 5 of these 7 patients discontinued treatment during the first month (16% of ADS‐5102 group). The most common AE leading to treatment discontinuation in the ADS‐5102 group was visual hallucinations (2 [5.4%]).
Discussion
ADS‐5102 is a high‐dose, extended‐release amantadine administered once daily at bedtime with a slow initial rate of rise in amantadine concentrations and a prolonged Tmax without exacerbating adverse events. This pharmacokinetic profile provides a peak concentration in the morning with continuous coverage throughout the day to alleviate LID, with high plasma concentrations (approximately 1500 ng/mL) that cannot be achieved with conventional dosing (100 mg bid/tid) with amantadine IR.
This is the second pivotal phase 3 study confirming that ADS‐5102 significantly improves dyskinesia in patients with PD.27 Efficacy of ADS‐5102 was demonstrated across multiple outcome measures assessing several aspects of dyskinesia. Bedtime administration of ADS‐5102 274 mg (equivalent to 340 mg amantadine HCl) was associated with a clinically significant reduction in the UDysRS total score when compared with placebo. ADS‐5102 was also associated with a significant increase in ON time without troublesome dyskinesia. These diary results, along with the marked improvement in CGI‐C, support the clinical relevance of the decrease in the UDysRS total score and the reduction in LID. In addition, the antidyskinetic benefit of ADS‐5102 was achieved with a significant reduction in OFF time as well as an improvement in MDS‐UPDRS scores.31
Statistical significance in reducing ON time with troublesome dyskinesia was not met largely because of the placebo response (change from baseline of −2.5 hours). In comparison, the placebo response in the companion EASE LID study27 was −1.6 hours at week 12. In both studies, the ADS‐5102 group had a similar decrease in ON time with troublesome dyskinesia at week 12 (EASE LID: ‐3.2 hours, EASE LID 3: −3.6 hours).
The most common AEs with ADS‐5102 treatment are largely predicated by its NMDA receptor antagonist activity (eg, hallucinations) and anticholinergic activity (eg, dry mouth and constipation). Most AEs were of mild to moderate intensity, transient, and did not result in treatment discontinuation. The most common reason for discontinuation of treatment was visual hallucinations (n = 2). Participants with a major psychiatric disorder, including suicidal ideation, were excluded from participation in the study. Of the participants, 2 (both in the ADS‐5102 group) had AEs related to suicidal attempt or ideation (1 each); both participants had a prestudy history of depression, and 1 participant had an undisclosed history of suicidal ideation. Patients should be monitored during treatment for the development of depressed mood, depression, and changes in behavior or thinking that are not typical for the patient and for suicidal ideation or behavior. Orthostatic hypotension may manifest as dizziness or result in falls. Risk factors for falls in patients with PD include prior history of falls, disease severity, gait abnormalities, and cognitive impairment.32 Precautions should be taken to reduce the risk of falls. Although incidences of AEs such as hallucinations, orthostatic hypotension, and falls were increased in the ADS‐5102 group, these events represented mostly reversible morbidity, were generally mild to moderate in nature, and most did not result in study drug discontinuation. ADS‐5102 has a manageable safety profile.
In the study, the rate of visual hallucination in the ADS‐5102 group was lower than that reported in the previous phase 3 study27 (24% vs 8%). This does not appear to be explained by baseline PD duration, LID duration or severity, or age, as both studies had similar baseline demographics. However, participants randomized to ADS‐5102 in the current study reported a lower mean baseline levodopa dose (672 mg vs 906 mg) and a greater prevalence of dopamine agonists (57% vs 46%) and monoamine oxidase inhibitor use (46% vs 41%) compared with patients who received ADS‐5102 in the previous phase 3 study. Use of anticholinergics was low in both study populations (<5% of total participants).
Goetz and colleagues33 reported a placebo‐corrected reduction in the UDysRS total score of −6.6 points after 8 weeks of treatment with amantadine IR (mean dose: 229 mg of amantadine per day). In the EASE LID 3 study, which did not include a direct comparison with amantadine IR, ADS‐5102 treatment at 274 mg resulted in a placebo‐corrected −14.4‐point decrease in UDysRS total score after 12 weeks.33 However, any conclusions regarding the relative efficacy and safety of ADS‐5102 and amantadine IR would require a direct comparison of these agents in a randomized trial, and no such direct comparison exists. Based on results from this pivotal phase 3 study, and the first pivotal phase 3 study (EASE LID),27 there is substantial evidence that 274 mg of ADS‐5102 administered at bedtime is an effective dosage for the treatment of dyskinesia. Long‐term safety of this ADS‐5102 regimen is currently being evaluated in an open‐label safety study (ClinicalTrials.gov identifier: NCT02202551).30 Future research efforts should establish the minimal clinically important difference for the UDysRS. In addition, studies that address converting treatment from amantadine IR to ADS‐5102 may be useful.
There is no approved drug therapy for LID. DBS (Activa, Medtronic, Minneapolis, Minnesota; Brio, St. Jude Medical, St. Paul, Minnesota) is currently the only approved treatment for reducing symptoms of advanced levodopa‐responsive PD that are not adequately controlled. There remains an unmet need for an approved pharmacotherapy that treats dyskinesia without compromising underlying PD control and has a manageable safety profile.27 Of note, 60 patients who continue to experience dyskinesia despite undergoing DBS have been enrolled in an ongoing ADS‐5102 open‐label safety study.30
The effect of ADS‐5102 reducing both dyskinesia and OFF time has now been confirmed in 2 phase 3 controlled studies. These data support ADS‐5102 as an adjunctive therapy to levodopa for the treatment of dyskinesia and OFF in PD patients with LID. In addition, 95% of patients who completed this study continued into a 2‐year extension study that will allow further evaluation of the long‐term efficacy and safety of ADS‐5102 in this patient population.
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of first draft, B. Review and critique of the manuscript.
W.O.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
K.E.: 1B, 1C, 2C, 3B
R.P.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
C.M.T.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
R.A.H.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
C.T.: 1B, 1C, 2C, 3B
R.E.: 1B, 1C, 2C, 3B
J.P.A.: 1B, 1C, 2C, 3B
S.I.: 1B, 1C, 2C, 3B
L.F.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
M.J.S.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
Full financial disclosure for the previous 12 months
W.O. has served as a consultant for Adamas and Novartis; on advisory boards for Mundipharma, Novartis, Roche, UCB, Adamas, GE Healthcare, Bristol‐Myers Squibb, and Eisai; and received honoraria from Abbvie, Desitin, Mundipharma, Novartis, Prothena, and UCB. He has received scientific grants from the German Ministry of Education and Research, the German Research Foundation, the Charitable Hertie Foundation, the Internationaal ParkinsonFonds, ParkinsonFonds Deutschland, the Michael J. Fox Foundation, and Novartis Pharma Germany, and holds shares in Biogen Idec, Merck Darmstadt, Medigene, and Roche. K.E. is an employee of Philipps University, Marburg, Germany. She has received consulting fees from UCB, Novartis, Mundipharma, and Zambon, and honoraria from UCB, Solvay Pharmaceuticals, Desitin, Novartis, GlaxoSmithKline, Teva Pharma, Mundipharma, Zambo, and Grünenthal. She has served on the advisory boards of Mundipharma, Zambon, Abbvie, and Grünenthal, and has received grants from the Michael J. Fox Foundation. R.P. is receiving or has received honoraria or payments for consulting from AbbVie, Acadia, Acorda, Adamas, Cynapsus, Impax, Lundbeck, Medtronic, Neurocrine, Sage, St Jude Medical, Teva Neuroscience, Medtronic, UCB, and US WorldMeds. He has received research grants from Acadia, Acorda, Adamas, Avid, Biotie, Boston Scientific, Civitas, Cynapsus, Kyowa, National Institutes of Health/National Institute of Neurological Disorders and Stroke, NPF, Pfizer, and Parkinson Study Group/University of Rochester. He has also served on the data monitoring committee for Ceregene. He has received personal compensation as the Co‐Editor‐in‐Chief of the International Journal of Neuroscience. C.M.T. is an employee of the University of California‐San Francisco and the San Francisco Veterans Affairs Medical Center and an intermittent employee of the Parkinson's Institute. She serves on the Scientific Advisory Boards of the Michael J. Fox Foundation and the National Spasmodic Dysphonia Association as a voluntary consultant and has provided paid consulting services to Neurocrine Biosciences, Sage Biometrics, and Adamas Pharmaceuticals. She has received compensation for serving on Data Monitoring Committees from Biotie Therapeutics, Voyager Therapeutics, and Intec Pharma. She receives grant support from the Michael J. Fox Foundation, the Parkinson's Disease Foundation, the Department of Defense, and the National Institutes of Health. R.A.H. is supported in part by a center grant from the National Parkinson's Disease Foundation. He received payment from Adamas for participating as a Steering Committee member and reports consulting fees from Teva Pharmaceuticals, USB Biosciences, AbbVie, Novartis, Biotie Therapies, Lundbeck, Pfizer, Allergan Neuroscience, Neurocrine Biosciences, Chelsea Therapeutics, Auspex, Acadia Pharmaceuticals, Michael J. Fox Foundation, GLG, AstraZeneca, Acorda Therapeutics, Impax Pharmaceuticals, Cynapsus Therapeutics, US WorldMeds, Neurospore, and Prexton; salary support grants from National Parkinson Foundation; and salary from University of South Florida. C.T. reports personal fees from Mundipharma Research GmbH & Co; payment for advisory board from Mundipharma Germany GmbH & Co., UCB, Vifor, Britannia, Novartis; payment for lectures from UCB, Desitin, Britannia. R.E. has served on advisory boards for Bial, Desitin, Global Kinetics, Gruenenthal, Mundipharma Germany GmbH & Co, Novartis, Roche, Sanofi, Teva, and UCB Pharma. He has received honoria for presenting lectures from Abbvie, Desitin, GE Healthcare, Medtronics, Mundipharma Germany GmbH & Co, Novartis, Zambon. J.P.A. has served on advisory boards for Teva, UCB Pharma, AbbVie, and Zambon. He has received honoraria for presenting lectures from AbbVie, Medtronics, Novartis, Zambon, and UCB. S.I. has received honoraria for continuing medical education, consultant, research grants, and/or promotional speaker on behalf of AbbVie, Acadia, Acorda, Adamas, Addex, Allergan, Amarantus, Auspex, Avid, Axovant, AZ Therapies, Biogen, Biotie, Britannia, Cynapsus, Eisai, Eli Lilly, GE Healthcare, Impax, Intec Pharma, Ipsen, Kyowa, Lundbeck, Medtronics, Merz, Michael J. Fox Foundation, Neurocrine, Neuroderm, National Institutes of Health/National Institute of Neurological Disorders and Stroke, Parkinson Study Group, Pfizer, Pharma2B, Prothena, Roche, Sanofi, Shire, Sunovion, Teva, UCB, US World Meds, and XenoPort. L.F. is an employee of and has received compensation and stock options from Adamas. M.J.S. is a consultant to and has received compensation and stock options from Adamas. She has also received consultancy payments from Dermira, Inc.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Supplementary Information 1
Acknowledgments
We acknowledge and thank the study participants; the EASE LID 3 study investigators, including the Steering Committee and their staff; and Charles Davis, PhD, CSD Biostatistics, who provided statistical and analysis support. Under the direction of the authors, editorial assistance was provided by Cory Hussar, PhD, of The Curry Rockefeller Group, LLC, which was funded by Adamas Pharmaceuticals, Inc. EASE LID 3 principal investigators/sites that screened study participants included Pinky Agarwal, Evergreen Health, Kirkland, Washington; Jean‐Philippe Azulay, Hôpital de la Timone, Marseille, France; Danny Bega, Northwestern University, Chicago, Illinois; John Burch, Blue Ridge Research Center, Roanoke, Virginia; Andres Ceballos‐Baumann, Schön Klinik München Schwabing, Munich, Germany; Joseph Classen, Universitätsklinikum Leipzig, Leipzig, Germany; Luc Defebvre, Hôpital Roger Salengro, Lille, France; Rohit Dhall, Parkinson's Institute and Clinical Center, Sunnyvale, California; Atbin Djamshidian‐Tehrani, Universitätsklinikum Innsbruck, Innsbruck, Austria; Franck Durif, Hôpital Gabriel Montpied, Clermont‐Ferrand, France; Georg Ebersbach, Neurologisches Fachkrankenhaus für Bewegungsstörungen / Parkinson, Brandenburg, Germany; Karla Eggert, Universitätsklinikum Gießen und Marburg GmbH, Marburg, Germany; Reinhard Ehret, Gemeinschaftspraxis Neurologie Berlin, Berlin, Germany; Christian Geny, CHU Gui De Chauliac, Montpellier, France; Ramon Gil, Parkinson's Disease Treatment Center of Southwest Florida, Port Charlotte, Florida; Jorge Hernandez‐Vara, Hospital Universitario Vall d'Hebron, Barcelona, Spain; Jean‐Luc Houeto, CHRU de Poitiers La Miletrie, Poitiers, France; Keith Hull, Raleigh Neurology Associates PA, Raleigh, North Carolina; Stuart Isaacson, Parkinson's Disease and Movement Disorders Center of Boca Raton Inc, Boca Raton, Florida; Kevin Klos, Movement Disorder Clinic of Oklahoma PLLC, Tulsa, Oklahoma; Andrea Kuhn, Charité ‐ Universitätsmedizin Berlin, Berlin, Germany; Jaime Kulisevsky, Hospital de La Santa Creu i Sant Pau, Barcelona, Spain; David Maltete, Hôpital Charles Nicolle, Rouen, France; Maria Jose Marti Domenech, Hospital Clinic de Barcelona, Barcelona, Spain; Wassilios Meissner, Centre Hospitalier Universitaire de Bordeaux, Hôpital Pellegrin, Bordeaux, France; Kelly Mills, Johns Hopkins Hospital, Baltimore, Maryland; Paul Nausieda, Wisconsin Institute for Neurologic and Sleep Disorders, SC, Milwaukee, Wisconsin; Christian Oehlwein, Neurologische Praxis, Gera, Germany; Berta Pascual, Hospital Universitari Quirón Dexeus Barcelona, Barcelona, Spain; Udo Polzer, Asklepios Fachklinikum Stadtroda, Stadtroda, Germany; Isabelle Roullet‐Solignac, Hôpital Pierre Wertheimer ‐ Hôpital Neurologique, Bron, France; Christos Sidiropoulos, Henry Ford Medical Center West Bloomfield, West Bloomfield, Michigan; Carlos Singer, University of Miami, Miami, Florida; Richard Singer, Infinity Clinical Research, Sunrise, Florida; Martin Sommer, Klinische Neurophysiologie, Gottingen, Germany; Claudia Trenkwalder, Paracelsus‐Elena‐Klinik Kassel, Kassel, Germany; Daniel Truong, Parkinson's and Movement Disorder Institute, Fountain Valley, California; Dieter Volc, Confraternität Privatklinik Josefstadt, Vienna, Austria; Theresa Zesiewicz, Carol and Frank Morsani Center for Advanced Health Care, Tampa, Florida.
EASE LID 3 principal investigators are listed in the Acknowledgments.
Funding agencies: Adamas Pharmaceuticals, Inc.
Relevant conflicts of interests/financial disclosures: W.O., C.M.T., and R.A.H. are on the Adamas LID Steering Committee and received compensation for this service. R.P. is on the Adamas LID Steering Committee and received compensation for this service, and he is receiving or has received honoraria or payments for consulting from Adamas. C.M.T. has provided paid consulting services to Adamas. K.E., C.T., R.E., J.P.A., and S.I. are EASE LID 3 study investigators and have not received any compensation from Adamas. L.F., an employee of Adamas, and M.J.S., a consultant to Adamas, have received compensation and stock options from Adamas. S.I. has received research grants, honoraria for continuing medical education, and payment as a consultant to and/or promotional speaker on behalf of Adamas.
References
- 1. Ahlskog JE, Muenter MD. Frequency of levodopa‐related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 2001;16(3):448‐458. [DOI] [PubMed] [Google Scholar]
- 2. Rascol O, Perez‐Lloret S, Damier P, et al. Falls in ambulatory non‐demented patients with Parkinson's disease. J Neural Transm (Vienna) 2015;122(10):1447‐1455. [DOI] [PubMed] [Google Scholar]
- 3. Khlebtovsky A, Rigbi A, Melamed E, et al. Patient and caregiver perceptions of the social impact of advanced Parkinson's disease and dyskinesias. J Neural Transm (Vienna) 2012;119(11):1367‐1371. [DOI] [PubMed] [Google Scholar]
- 4. Hechtner MC, Vogt T, Zollner Y, et al. Quality of life in Parkinson's disease patients with motor fluctuations and dyskinesias in five European countries. Parkinsonism Relat Disord 2014;20(9):969‐974. [DOI] [PubMed] [Google Scholar]
- 5. Dodel RC, Berger K, Oertel WH. Health‐related quality of life and healthcare utilisation in patients with Parkinson's disease: impact of motor fluctuations and dyskinesias. Pharmacoeconomics 2001;19(10):1013‐1038. [DOI] [PubMed] [Google Scholar]
- 6. Muller T, Woitalla D, Russ H, Hock K, Haeger DA. Prevalence and treatment strategies of dyskinesia in patients with Parkinson's disease. J Neural Transm (Vienna) 2007;114(8):1023‐1026. [DOI] [PubMed] [Google Scholar]
- 7. Schaeffer E, Pilotto A, Berg D. Pharmacological strategies for the management of levodopa‐induced dyskinesia in patients with Parkinson's disease. CNS Drugs 2014;28(12):1155‐1184. [DOI] [PubMed] [Google Scholar]
- 8. Hubsher G, Haider M, Okun MS. Amantadine: the journey from fighting flu to treating Parkinson disease. Neurology 2012;78(14):1096‐1099. [DOI] [PubMed] [Google Scholar]
- 9. Schwab RS, England AC Jr, Poskanzer DC, Young RR. Amantadine in the treatment of Parkinson's disease. JAMA 1969;208(7):1168‐1170. [PubMed] [Google Scholar]
- 10. Silver DE, Sahs AL. Double blind study using amantadine hydrochloride in the therapy of Parkinson's disease. Trans Am Neurol Assoc 1971;96:307‐308. [PubMed] [Google Scholar]
- 11. Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M. Duration of amantadine benefit on dyskinesia of severe Parkinson's disease. J Neurol Neurosurg Psychiatry 2004;75(1):141‐143. [PMC free article] [PubMed] [Google Scholar]
- 12. Ory‐Magne F, Corvol JC, Azulay JP, et al. Withdrawing amantadine in dyskinetic patients with Parkinson disease: the AMANDYSK trial. Neurology 2014;82(4):300‐307. [DOI] [PubMed] [Google Scholar]
- 13. Pahwa R, Factor SA, Lyons KE, et al. Practice Parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence‐based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66(7):983‐995. [DOI] [PubMed] [Google Scholar]
- 14. Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society evidence‐based medicine review update: treatments for the motor symptoms of Parkinson's disease. Mov Disord 2011;26(suppl 3):S2‐S41. [DOI] [PubMed] [Google Scholar]
- 15. Crosby NJ, Deane KH, Clarke CE. Amantadine for dyskinesia in Parkinson's disease. Cochrane Database Syst Rev 2003(2):CD003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farnebo LO, Fuxe K, Goldstein M, Hamberger B, Ungerstedt U. Dopamine and noradrenaline releasing action of amantadine in the central and peripheral nervous system: a possible mode of action in Parkinson's disease. Eur J Pharmacol 1971;16(1):27‐38. [DOI] [PubMed] [Google Scholar]
- 17. Kornhuber J, Bormann J, Hubers M, Rusche K, Riederer P. Effects of the 1‐amino‐adamantanes at the MK‐801‐binding site of the NMDA‐receptor‐gated ion channel: a human postmortem brain study. Eur J Pharmacol 1991;206(4):297‐300. [DOI] [PubMed] [Google Scholar]
- 18. Von Voigtlander PF, Moore KE. Dopamine: release from the brain in vivo by amantadine. Science 1971;174(4007):408‐410. [DOI] [PubMed] [Google Scholar]
- 19. Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents‐‐preclinical studies. Neurosci Biobehav Rev 1997;21(4):455‐468. [DOI] [PubMed] [Google Scholar]
- 20. Metman LV, Del Dotto P, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa‐induced dyskinesias: a 1‐year follow‐up study. Arch Neurol 1999;56(11):1383‐1386. [DOI] [PubMed] [Google Scholar]
- 21. Wolf E, Seppi K, Katzenschlager R, et al. Long‐term antidyskinetic efficacy of amantadine in Parkinson's disease. Mov Disord 2010;25(10):1357‐1363. [DOI] [PubMed] [Google Scholar]
- 22. Hayden FG, Gwaltney JM Jr, Van de Castle RL, Adams KF, Giordani B. Comparative toxicity of amantadine hydrochloride and rimantadine hydrochloride in healthy adults. Antimicrob Agents Chemother 1981;19(2):226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Symmetrel (amantadine hydrochloride, USP) [prescribing information]. Chadds Ford, PA: Endo Pharmaceuticals Inc; January 2009. [Google Scholar]
- 24. Amantadin STADA® . (Amantadinhydrochlorid) [Information for User‐In German]. Bad Vilbel, Germany: STADApharm GmbH; November 2006. [Google Scholar]
- 25. Parkes JD, Zilkha KJ, Marsden P, Baxter RC, Knill‐Jones RP. Amantadine dosage in treatment of Parkinson's disease. Lancet 1970;1(7657):1130‐1133. [DOI] [PubMed] [Google Scholar]
- 26. Pahwa R, Tanner CM, Hauser RA, et al. Amantadine extended release for levodopa‐induced dyskinesia in Parkinson's disease (EASED Study). Mov Disord 2015;30(6):788‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pahwa R, Tanner CM, Hauser RA, et al. ADS‐5102 (amantadine) extended‐release capsules for levodopa‐induced dyskinesia in Parkinson disease (EASE LID study): a randomized clinical trial. JAMA Neurol 2017. Jun 12 [Epub ahead of print]. doi:10.1001/jamaneurol.2017.0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico‐pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55(3):181‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hauser RA, Friedlander J, Zesiewicz TA, et al. A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 2000;23(2):75‐81. [DOI] [PubMed] [Google Scholar]
- 30. Hauser RA, Pahwa R, Tanner CM, et al. ADS‐5102 (amantadine) extended‐release capsules for levodopa‐induced dyskinesia in Parkinson's disease (EASE LID 2 study): interim results of an open‐label safety study. J Parkinsons Dis 2017. Aug 4 [Epub ahead of print]. doi:10.3233/JPD-171134. [DOI] [PMC free article] [PubMed]
- 31. Shulman LM, Gruber‐Baldini AL, Anderson KE, Fishman PS, Reich SG, Weiner WJ. The clinically important difference on the unified Parkinson's disease rating scale. Arch Neurol 2010;67(1):64‐70. [DOI] [PubMed] [Google Scholar]
- 32. van der Marck MA, Klok MP, Okun MS, et al. Consensus‐based clinical practice recommendations for the examination and management of falls in patients with Parkinson's disease. Parkinsonism Relat Disord 2014;20(4):360‐369. [DOI] [PubMed] [Google Scholar]
- 33. Goetz CG, Stebbins GT, Chung KA, et al. Which dyskinesia scale best detects treatment response? Mov Disord 2013;28(3):341‐346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article at the publisher's website.
Supplementary Information 1


