Abstract
Mirogabalin (DS‐5565) is a novel preferentially selective α2δ‐1 ligand being developed for the treatment of diabetic peripheral neuropathic pain and postherpetic neuralgia. The current multicenter open‐label study determined the effect of varying degrees of renal impairment on the pharmacokinetics and safety of a single dose of mirogabalin 5 mg in Japanese subjects. A total of 30 subjects (6 subjects per renal function category [normal, mild, moderate, or severe impairment; and end‐stage renal disease (ESRD)]) were enrolled and completed the study. The AUClast increased with severity of renal impairment; the geometric least‐squares mean ratios of AUClast compared with subjects with normal renal function were 1.3, 1.9, 3.6, and 5.3 for patients with mild, moderate, and severe impairment and ESRD, respectively. In accordance with this AUClast increase, apparent total body clearance (CL/F), renal clearance (CLr), and the cumulative percentage of mirogabalin dose excreted into urine all decreased with severity of renal impairment. There were no deaths and no severe treatment‐related adverse events (TEAEs), serious TEAEs, or TEAEs resulting in study discontinuation. Mirogabalin was well tolerated in Japanese subjects with normal renal function and those with mild to severe renal impairment. It was also tolerated in subjects with ESRD but with a higher incidence of TEAEs. The most frequently reported TEAEs were dizziness (ESRD, n = 3), somnolence (ESRD, n = 2), and vomiting (ESRD, n = 2). Based on these data, a mirogabalin dose adjustment will be considered in Japanese subjects with moderate to severe renal impairment and those with ESRD.
Keywords: mirogabalin, pharmacokinetics, renal impairment, Japanese
Mirogabalin (DS‐5565; Daiichi Sankyo Co., Ltd., Tokyo, Japan), a novel preferentially selective α2δ‐1 ligand, is in phase 3 development in the United States and Europe for the treatment of pain associated with fibromyalgia and in Asia for the treatment of various neuropathic pain conditions including diabetic peripheral neuropathic pain and postherpetic neuralgia.1
Upregulation of the α2δ‐1 subunit of voltage‐gated calcium channels has been reported to occur in neuropathic pain states.2 Ligands of the α2δ‐1 subunit are able to reduce calcium influx into central nervous system (CNS) neurons and exert analgesic effects.3 Therefore, the α2δ‐1 subunit is a therapeutic target in the treatment of neuropathic pain.4, 5
Mirogabalin has demonstrated sustained analgesic effects in preclinical pain models.1 In rats, mirogabalin demonstrates superior analgesic effects with a wider CNS safety margin than pregabalin.1 The safety and tolerability of mirogabalin at doses of up to 15 mg twice daily for 7 days in healthy Japanese subjects were confirmed in a single‐ and multiple‐ascending‐dose study.6
There is a well‐established association between diabetic peripheral neuropathy and renal impairment.7, 8, 9, 10 Prospective data indicate that peripheral neuropathy and chronic kidney disease (CKD) frequently co‐occur in patients with diabetes10 and that there is a significant and independent association between diabetic neuropathy and microalbuminuria.8 Because individuals with diabetic peripheral neuropathic pain often have reduced renal function and mirogabalin is primarily excreted unchanged in urine, it is imperative to determine the effect of renal impairment on mirogabalin pharmacokinetics (PK). Increased mirogabalin exposure concomitant with worsening renal function was observed in white subjects, leading to a dose adjustment in subjects with moderate or severe renal impairment.11
The current study aimed to determine the effect of varying degrees of renal impairment on the PK and safety of a single dose of mirogabalin 5 mg in Japanese subjects, which will guide dose recommendations in this ethnic group.
Methods
Study Design
The study was reviewed and approved by each institutional review board (Medical Corporation Kyosokai AMC Nishi‐umeda Clinic, P‐One Clinic, Kasaoka Daiichi Hospital, and Medical Corporation Applied Bio‐Pharmatech Kurume Clinical Pharmacology Clinic). All subjects provided written informed consent before screening examinations. This study was conducted in accordance with the ethical principles set in the Declaration of Helsinki and is consistent with Good Clinical Practices and applicable regulatory requirements. This was a multicenter open‐label, single‐dose study (JapicCTI 132175). The planned sample size was 30 subjects, equating to 6 subjects per renal function category. This sample size was determined by practical considerations.
Subjects
Eligible Japanese subjects were men and women ≥ 20 years of age with a body mass index (BMI) between 18.5 and 30.0 kg/m2, inclusive. Prior to administration of mirogabalin, subjects were classified into 1 of 5 categories by renal function (based on the Cockcroft‐Gault formula)12: normal, estimated creatinine clearance (CrCl) > 80 mL/min/1.73 m2; mild impairment, 50 mL/min/1.73 m2 ≤ CrCl ≤ 80 mL/min/1.73 m2; moderate impairment, 30 mL/min/1.73 m2 ≤ CrCl <50 mL/min/1.73 m2; severe impairment, CrCl < 30 mL/min/1.73 m2, and end‐stage renal disease (ESRD, patients undergoing hemodialysis, regardless of CrCl.
Subjects were excluded at screening if they had any clinically significant disease; a history of drug allergies or idiosyncratic drug response; a history of drug or alcohol abuse; use of prohibited concomitant drugs including any prescribed or over‐the‐counter drugs for patients with normal renal function or drugs that affect serum creatinine elimination (including trimethoprim and cimetidine) or renal tubular secretion (including probenecid) for patients with impaired renal function, within 60 days prior to screening; a positive test for hepatitis B, hepatitis C, HIV, or syphilis; had participated in another clinical study involving an investigational drug within the past 120 days or any previous clinical study of mirogabalin; or were pregnant, breastfeeding, or unwilling to use contraception throughout the study.
Study Drug
A single 5‐mg mirogabalin tablet was administered 2 hours after a standard low‐calorie meal to subjects who had fasted for ≥8 hours. This dose was selected based on previous clinical pharmacology studies of mirogabalin.11 In subjects with ESRD, mirogabalin was administered 20 to 56 hours after the completion of hemodialysis so that the next hemodialysis could be conducted 24 hours after study drug administration to minimize the effect of hemodialysis. The maximum plasma concentration (Cmax) for a single 5‐mg dose of mirogabalin in subjects with ESRD was not expected to exceed the Cmax for administration of a 20‐mg dose in healthy Japanese adults. The area under the plasma concentration–time curve (AUC) for a 5‐mg dose in subjects with ESRD was not expected to exceed the AUC for administration of mirogabalin 15 mg twice daily; therefore, the risk was considered acceptable for a single 5‐mg dose in subjects with ESRD.
Pharmacokinetic Assessments
Mirogabalin is the besylate salt of A200‐0700. To investigate the PK of mirogabalin, A200‐0700 was targeted for assessment. The analyte is the total plasma A200‐0700, both bound and unbound to protein. The A200‐0700 concentration was measured by liquid chromatography–tandem mass spectrometry (lower limit of quantitation; plasma, 1.00 ng/mL; urine, 0.100 μg/mL; dialysate, 0.100 ng/mL).
Mirogabalin and its internal standard were extracted from plasma samples (0.050 mL) using a 96‐well solid‐phase extraction plate conditioned with 0.5 mL of methanol and 0.5 mL of water. After washing with 0.5 mL of water and 0.75 mL of 5% methanol in water, the plate was eluted with 0.2 mL of an 80:20 mixture of acetonitrile and 20 nM ammonium formate in water with a pH of 2.5. Chromatographic separation performed using a Zorbax 300‐SCX (Agilent Technologies, Santa Clara, California) column (50 × 3.0 mm, 5 mm). Detection was performed by a Sciex API 4000 (Sciex, Framingham, Massachusetts) tandem mass spectrometer with TurboIonSpray source in the positive ion mode and multiple‐reaction monitoring for mirogabalin and the internal standard. The within‐study assay precision was 5.8%, 2.8%, 2.0%, and 2.9% for quality control samples of mirogabalin at 3, 75, 400, and 750 ng/mL, respectively. Assay accuracy was in the range of ‐1.7% to 1.9% (%bias), and the lower limit of quantification was 1 ng/mL.
Mirogabalin and its internal standard were extracted from urine samples (0.025 mL) using a 96‐well solid‐phase extraction plate conditioned with 0.5 mL of methanol and 0.5 mL of water. After washing with 0.5 mL of ultrapure water and 0.75 mL of 5% methanol in water, the plate was eluted with 0.3 mL of an 80:20 mixture of acetonitrile and 20 nM ammonium formate in water with a pH of 2.5; 0.5 mL of 0.1% formic acid in water was added to the wells; and 0.8 mL of 0.1% formic acid in water was added to all urine samples. Chromatographic separation was performed using an Agilent Zorbax 300‐SCX column (50 × 3.0 mm, 5 mm). Detection was performed by a Sciex API 4000 tandem mass spectrometer with TurboIonSpray source in the positive ion mode and multiple‐reaction monitoring for mirogabalin and the internal standard. The within‐study assay precision was 4.7%, 2.7%, 4.5%, and 3.0% for quality control samples of mirogabalin at 0.300, 7.550, 40.0, and 75.0 μg/mL, respectively. Assay accuracy was in the range of ‐2.3% to 2.5% (%bias), and the lower limit of quantification was 0.100 μg/mL.
Blood samples (3 mL) for plasma A200‐0700 were collected before mirogabalin administration and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 9, 12, 24, 36, and 48 hours after administration. Urine samples (20 mL) for urine A200‐0700 concentration measurement were collected before mirogabalin administration and accumulated during the following periods: from 0 to 6, 6 to 12, 12 to 24, and 24 to 48 hours after administration.
Subjects with ESRD underwent 4‐hour hemodialysis 24 hours after drug administration. In these subjects, 3 mL of blood was collected at entry to the hemodialysis device and 3 mL at exit from the device; 0.5, 1, 2, 3, and 4 hours after the initiation of hemodialysis; and 8 mL of dialysate was also collected at 0.5, 1.5. 2.5, and 3.5 hours.
The PK parameters were calculated from A200‐0700 plasma and urine concentrations. Analyses were conducted using WinNonlin Phoenix version 6.0 or higher. PK parameters, evaluated using a noncompartmental model analysis, included time to reach maximum plasma concentration (Tmax), Cmax, area under the plasma concentration–time curve (AUC) up to the last quantifiable time (AUClast), AUC up to infinity (AUCinf), apparent total body clearance (CL/F), renal clearance (CLr), terminal elimination half‐life (t1/2), apparent volume of distribution based on the terminal phase (Vz/F), and cumulative percentage of dose excreted into urine up to 48 hours (Fe0–48h). For the noncompartmental analysis, a regression line was created from the logarithmic conversion of at least 3 quantifiable plasma concentrations at and before the final quantifiable point, with the exception of times prior to Tmax. A time point range that provided that the maximum R 2 (adjusted) value was selected. In subjects with ESRD, 3 time points that occurred after completion of hemodialysis were selected. One subject with ESRD did not have 3 PK sampling points for calculation, and the values were reported as not calculated. AUC during hemodialysis, from the start of hemodialysis to the end of hemodialysis (AUCHD), and dialysis clearance (CLHD) were also assessed at hemodialysis. AUCHD was calculated by the trapezoidal method (linear trapezoidal rule; linear interpolation), using plasma concentrations from the start of hemodialysis (24 hours after administration) to the completion of hemodialysis (4 hours after hemodialysis start). This parameter was calculated as partial AUC when WinNonlin was used for that calculation. CLHD was calculated using the formula amount of the drug removed by the hemodialysis session/AUCHD.
Safety
Safety was assessed using treatment‐emergent adverse event (TEAE) reports and laboratory values and physical evaluations. System organ classes and preferred terms were coded using the Medical Dictionary for Regulatory Activities version 16.1. A follow‐up safety assessment was conducted 6 to 8 days after drug administration.
Statistical Analysis
The planned sample size was 30 subjects, equating to 6 subjects per renal function category. The PK analysis set included all subjects who received a single dose of mirogabalin and had usable plasma or urine concentration data. The safety analysis set included all subjects who received a single dose of mirogabalin.
Demographic and baseline characteristics were summarized according to each renal function category. For AUC0–inf, AUClast, Cmax, Tmax, CL/F, CLr, and Fe0–48h (CLr and Fe0–48h omitted in subjects with ESRD) of A200‐0700, an analysis of variance (ANOVA) was performed using these values after natural logarithmic transformation (untransformed values for Tmax) as response variables. This analysis was performed using a linear model in which the renal function category was considered a fixed effect (using the category with normal renal function as the standard). For the ANOVA of PK parameters, the 2‐sided confidence intervals and P values were calculated along with the geometric least‐squares (LS) mean between each impaired renal function category and the normal renal function category for each PK parameter. Statistical tests were conducted for AUC0–inf, AUClast, Cmax, and CL/F. Analyses were conducted using SAS version 9.2 or higher.
Results
Subject Disposition and Demographics
A total of 30 subjects (16 men and 14 women; 6 subjects per renal function category) were enrolled, received the study drug, and completed the study. All 30 subjects were available for the PK and safety evaluations. For all subjects, the mean age ranged from 61.7 to 70.5 years, and mean body weight ranged from 59.5 to 69.3 kg. There were no notable differences in terms of baseline demographics such as age, body weight, and BMI among renal function groups (Table 1).
Table 1.
Baseline Demographic Data
| Renal Classification According to CrCla | |||||
|---|---|---|---|---|---|
| Normal (n = 6) | Mild (n = 6) | Moderate (n = 6) | Severe (n = 6) | ESRD (n = 6) | |
| Age, mean (SD), years | 64.2 (5.2) | 61.7 (6.5) | 62.3 (14.9) | 70.5 (8.5) | 65.5 (8.7) |
| Sex, M:F | 2:4 | 2:4 | 4:2 | 4:2 | 4:2 |
| Weight, mean (SD), kg | 65.4 (10.7) | 62.5 (8.4) | 69.3 (14.7) | 59.5 (11.7) | 60.6 (9.1) |
| BMI, median (range), kg/m2 | 25.9 (22.4–27.7) | 24.6 (20.7–26.8) | 25.1 (19.4–28.8) | 24.1 (19.9–28.7) | 24.4 (20.4–26.5) |
| Estimated CrCl, mean (SD), mL/min/1.73 m2 | 100.3 (11.4) | 69.8 (8.0) | 40.9 (6.2) | 23.4 (5.8) | 9.7 (3.2) |
BMI, body mass index; CrCl, creatinine clearance;12 ESRD, end‐stage renal disease; F, female; M, male; SD, standard deviation.
Renal function groups, based on the Cockcroft‐Gault formula,12 were normal (estimated CrCl > 80 mL/min/1.73 m2), mild impairment (50 mL/min/1.73 m2 ≤ CrCl ≤ 80 mL/min/1.73 m2), moderate impairment (30 mL/min/1.73 m2 ≤ CrCl < 50 mL/min/1.73 m2), severe impairment (CrCl < 30 mL/min/1.73 m2), and ESRD (patients undergoing hemodialysis, regardless of CrCl).
Pharmacokinetics
In subjects with severe renal impairment and ESRD, Cmax values were approximately 50% and approximately 30% higher, respectively, than those of subjects with normal renal function, based on the geometric LS mean ratio. Cmax was not notably different in the other categories compared with subjects with normal renal function. Median Tmax was 1.3 hours for subjects with normal renal function compared with 2.0, 1.7, 2.0, and 4.0 hours for subjects with mild, moderate, and severe impairment and ESRD, respectively (Figure 1, Table 2).
Figure 1.
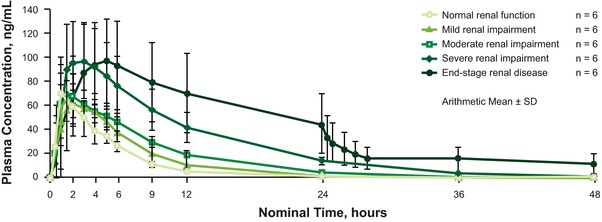
Mean plasma concentrations of mirogabalin in subjects with varying degrees of renal impairment after a single oral 5‐mg dose. ESRD, end‐stage renal disease.
Table 2.
Mirogabalin Plasma and Urinary Pharmacokinetic Parameters
| Renal Classification According to CrCla | |||||
|---|---|---|---|---|---|
| Normal (n = 6) | Mild (n = 6) | Moderate (n = 6) | Severe (n = 6) | ESRD (n = 6) | |
| Plasma parametersb | |||||
| AUClast, ng·h/mL | 341.9 (16.5) | 452.9 (32.6) | 650.6 (10.9) | 1244 (24.4) | 1796 (55.5) |
| AUC0–inf, ng·h/mL | 358.6 (16.1) | 474.7 (30.0) | 671.6 (11.3) | 1266 (23.6) | 2989 (51.8)c |
| Cmax, ng/mL | 73.7 (35.9) | 73.4 (36.8) | 74.8 (20.0) | 109.3 (24.5) | 96.8 (31.9) |
| Tmax, h | 1.3 (1.0, 2.0) | 2.0 (1.0, 4.0) | 1.7 (1.0, 5.0) | 2.0 (1.5, 5.0) | 4.0 (1.9, 5.0) |
| t1/2, h | 2.8 (36.2) | 3.4 (24.4) | 5.6 (9.5) | 7.5 (14.5) | 39.8 (62.9)c |
| CL/F, L/h | 13.9 (16.1) | 10.5 (30.0) | 7.4 (11.3) | 3.9 (23.6) | 1.7 (51.8)c |
| Vz/F, L | 56.0 (47.8) | 52.3 (18.1) | 59.8 (19.2) | 42.7 (32.4) | 96.2 (29.1)c |
| Urinary parametersb | |||||
| Fe0–48 h, % | 75.2 (6.6) | 64.3 (6.8) | 57.0 (10.3) | 46.1 (31.4) | ─ |
| CLr, L/h | 10.2 (15.1) | 6.7 (30.0) | 4.3 (8.7) | 1.8 (20.6) | ─ |
AUC0–inf, area under the plasma concentration–time curve up to infinity; AUClast, area under the plasma concentration–time curve up to the last quantifiable time; CL/F, apparent total body clearance; Cmax, maximum plasma concentration; CLr, renal clearance; CrCl, creatinine clearance; CV, coefficient of variance; ESRD, end‐stage renal disease (4‐hour hemodialysis 24 hours after drug administration); Fe0–48 h, cumulative percentage of dose excreted into urine up to 48 hours; Tmax, time to reach maximum plasma concentration; t1/2, terminal elimination half‐life; Vz/F, apparent volume of distribution based on the terminal phase.
Renal function groups, based on the Cockcroft‐Gault formula,12 were normal (estimated creatinine clearance [CrCl] > 80 mL/min/1.73 m2), mild impairment (50 mL/min/1.73 m2 ≤ CrCl ≤ 80 mL/min/1.73 m2), moderate impairment (30 mL/min/1.73 m2 ≤ CrCl < 50 mL/min/1.73 m2), severe impairment (CrCl < 30 mL/min/1.73 m2), and ESRD (patients undergoing hemodialysis, regardless of CrCl).
Geometric mean (geometric CV%) values are presented for parameters excluding Tmax. Median (minimum, maximum) values are presented for Tmax.
n = 5. One subject had 3 unusable PK sampling points.
AUClast increased with severity of renal impairment. Compared with subjects with normal renal function, geometric LS mean ratios of AUClast were 1.3, 1.9, and 3.6 for patients with mild, moderate, and severe impairment, respectively. In patients with ESRD who undertook 4‐hour hemodialysis 24 hours after drug administration, the geometric LS mean ratio of AUClast compared with subjects with normal renal function was 5.3 (Table 3). CL/F, CLr, and the cumulative percentage of mirogabalin dose excreted into urine were all found to decrease as severity of renal impairment increased (Figure 2), in accordance with the increase in AUClast. In the subjects with ESRD, the geometric mean coefficient of variation (CV%) for AUCHD was 85.4 ng·h/mL, and the geometric mean CV% CLHD was 7.5 L/h. The t½ was substantially increased in patients with ESRD (39.8 hours) compared with subjects with normal renal function (2.8 hours) and those with mild, moderate, or severe renal impairment (range, 3.4–7.5 hours).
Table 3.
Ratio of Geometric LS Means and Corresponding 95%CIs for Mirogabalin PK Parameters Between Subjects With Varying Degrees of Renal Impairment and Those With Normal Renal Function
| Renal Classification According to CrCla | ||||
|---|---|---|---|---|
| Mild vs Normal (n = 6) | Moderate vs Normal (n = 6) | Severe vs Normal (n = 6) | ESRDb vs Normal (n = 6) | |
| AUClast,c ng·h/mL | 1.325 (0.921–1.905) | 1.903 (1.324–2.736) | 3.637 (2.530–5.230) | 5.254 (3.654–7.553) |
| P = .123 | P = .001 | P < .001 | P < .001 | |
| AUC0‐inf,c ng·h/mL | 1.324 (0.951–1.842) | 1.873 (1.346–2.606) | 3.531 (2.538–4.914) | 8.335 (5.894–11.786) |
| P = .093 | P < .001 | P < .001 | P < .001 | |
| Cmax,c ng/mL | 0.997 (0.700–1.420) | 1.015 (0.712–1.445) | 1.483 (1.041–2.113) | 1.314 (0.923–1.873) |
| P = .984 | P = .933 | P = .030 | P = .124 | |
| CL/F,c L/h | 0.755 (0.543–1.051) | 0.534 (0.384–0.743) | 0.283 (0.204–0.394) | 0.120 (0.085–0.170) |
| P = .093 | P < .001 | P < .001 | P < .001 | |
AUC0–inf, area under the plasma concentration–time curve to infinity; AUClast, area under the plasma concentration–time curve to the last quantifiable time; CI, confidence interval; CL/F, apparent total body clearance; Cmax, maximum plasma concentration; CLr, renal clearance12; CrCl, creatinine clearance; ESRD, end‐stage renal disease.
Renal function groups, based on the Cockcroft‐Gault formula,12 were: normal (estimated creatinine clearance [CrCl] > 80 mL/min/1.73 m2); mild impairment (50 mL/min/1.73 m2 ≤ CrCl ≤ 80 mL/min/1.73 m2); moderate impairment (30 mL/min/1.73 m2 ≤ CrCl < 50 mL/min/1.73 m2); severe impairment (CrCl < 30 mL/min/1.73 m2), and ESRD (patients undergoing hemodialysis, regardless of CrCl).
4‐Hour hemodialysis 24 hours after drug administration.
Values are presented as geometric LS mean ratios and 95%CIs.
Figure 2.
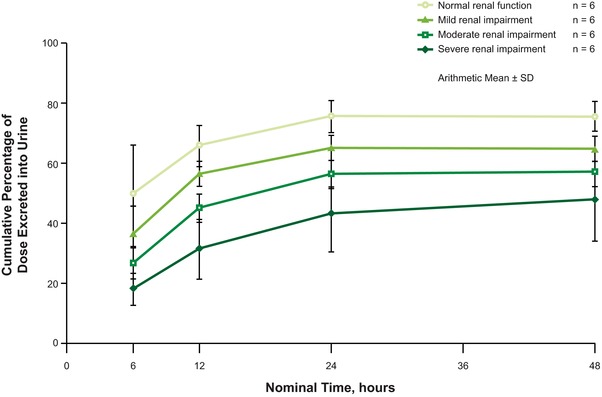
Cumulative percentage of a single oral 5‐mg dose of mirogabalin excreted into urine after administration to subjects with varying degrees of renal impairment.
Safety
No deaths and no severe TEAEs, serious TEAEs, or TEAEs that led to study discontinuation were reported. TEAEs were reported in 2 of 6 subjects (33.3%) with normal renal function and 4 of 6 subjects with ESRD (66.6%); see Table 4. No TEAEs were reported in subjects with mild, moderate, or severe renal impairment. The most frequently reported TEAEs were dizziness (ESRD, n = 3), somnolence (ESRD, n = 2), and vomiting (ESRD, n = 2). Moderate dizziness and vomiting were reported in 1 subject with ESRD; the other TEAEs were mild. There were no notable changes in laboratory parameters or physical evaluations.
Table 4.
Treatment‐Emergent Adverse Events
| Renal Classification According to CrCl | ||||||
|---|---|---|---|---|---|---|
| Normal (n = 6) | Mild (n = 6) | Moderate (n = 6) | Severe (n = 6) | ESRDa (n = 6) | Total (n = 6) | |
| Subjects with any TEAE, n (%) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (66.7) | 6 (20) |
| Decreased appetite | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) |
| Hyperkalemia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (3.3) |
| Dizziness | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (50.5) | 3 (10.0) |
| Somnolence | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 2 (6.7) |
| Headache | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) |
| Vomiting | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 2 (6.7) |
| Constipation | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (3.3) |
CrCl, creatinine clearance; ESRD, end‐stage renal disease; TEAE, treatment‐emergent adverse event.
4‐hour hemodialysis 24 hours after drug administration.
Discussion
Diabetic peripheral neuropathic pain is independently associated with microvascular complications such as microalbuminuria.8, 9 A population‐based study in more than 5000 Asians indicated that the prevalence of CKD and peripheral neuropathy is approximately 5% even at the lowest fasting plasma glucose levels.10 In patients with diabetes, the prevalence of peripheral neuropathy and renal impairment (estimated glomerular filtration rate < 60 mL/min/1.73 m2 and albuminuria) increased to approximately 17% and 9%, respectively.10
Given that mirogabalin is primarily eliminated in the urine, renal function can be expected to play a significant role in its elimination and clearance.11 A 2‐compartment population PK model — designed to describe the concentration–time profiles of mirogabalin 15 mg once or twice daily in European subjects with varying degrees of renal function — demonstrated that in white subjects, mirogabalin exposure increased with increasing degrees of renal impairment.11 Therefore, the current study evaluated the PK profile of mirogabalin in a cohort of Japanese subjects with varying degrees of renal impairment. The results of this study in 30 Japanese subjects confirm that mirogabalin serum plasma concentration increased with worsening renal function.
The results of the current study are also supported by those derived from the population PK model, which demonstrated that total mirogabalin clearance was reduced by 25%, 54%, and 76% in white subjects with mild, moderate, and severe renal impairment, respectively, relative to normal controls.11 Similar results were observed in the Japanese subjects enrolled in the current study; the cumulative percentage of dose excreted into urine decreased with increasing severity of renal impairment. Therefore, based on this simulation, a dose reduction of 50% to 75% was deemed necessary in subjects with moderate or severe renal impairment. No dose adjustment was required in subjects with mild impairment. Consequently, an ongoing phase 3 study in patients with CKD used renally adjusted dosing for mirogabalin; subjects with CrCl 15–29 mL/min received mirogabalin 7.5 mg once daily, and those with CrCl 30–59 mL/min received mirogabalin 7.5 mg twice daily (ClinicalTrials.gov Identifier: NCT02496884).
Because of the decreased renal function in subjects with ESRD, there was a notable increase in AUClast, a prolongation of t1/2, and a decrease in CL/F compared with subjects with normal renal function, although it was considered that the times for blood collection were not sufficient during the elimination phase after hemodialysis. Furthermore, relative to subjects with normal renal function, there was an unexpected delay in Tmax and a 1.3‐fold increase in Cmax in subjects with ESRD.
Administration of a single oral 5‐mg mirogabalin tablet was well tolerated in Japanese subjects with normal renal function and those with mild to severe renal impairment. Although a higher incidence of TEAEs was observed in subjects with ESRD, there were no treatment‐related withdrawals. A single oral 5‐mg mirogabalin tablet was also considered to be tolerated in this patient population. Based on these data, a mirogabalin dose adjustment will be considered in Japanese subjects with moderate to severe renal impairment and those with ESRD.
Declaration of Conflicting Interests
M.K., N.T., T.S., M.S., and H.I. are full‐time employees of Daiichi Sankyo Co., Ltd. K.F. and K.H. have nothing to disclose. Editorial assistance was provided by Anna Battershill, MSc, and Lynn Brown, PhD (ApotheCom, Yardley, Pennsylvania); and by Jennifer Meyering, RN, BSN, MS (AlphaBioCom, LLC, King of Prussia, Pennsylvania) and was funded by Daiichi Sankyo, Inc (Basking Ridge, New Jersey).
Data were presented previously at the 16th World Congress on Pain 2016 Annual Meeting, September 26–30, 2016, Yokohama, Japan.
Funding
This study was funded by Daiichi Sankyo, Inc (Basking Ridge, New Jersey).
References
- 1. Yokoyama T, Arakawa N, Domon Y, et al. Pharmacological, pharmacokinetics and safety profiles of DS‐5565, a novel α2δ ligand (P7.301). Neurology. 2014;82(10 Suppl P7.301). [Google Scholar]
- 2. Bauer CS, Nieto‐Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2δ‐1 to presynaptic terminals in neuropathic pain is inhibited by the α2δ ligand pregabalin. J Neurosci. 2009;29(13):4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tesfaye S, Boulton AJ, Dickenson AH. Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care. 2013;36(9): 2456–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Field MJ, Cox PJ, Stott E, et al. Identification of the α2δ‐1 subunit of voltage‐dependent calcium channels as a molecular target for pain mediating the analgesic actions of pregabalin. Proc Natl Acad Sci U S A. 2006;103(46):17537–17542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the α2δ subunit of a calcium channel. J Biol Chem. 1996;271(10):5768–5776. [DOI] [PubMed] [Google Scholar]
- 6. Jansen M, Warrington S, Dishy V, Ohwada S, Johnson L, Brown K, Ishizuka H. A randomized, placebo‐controlled, double‐blind study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of a single and multiple doses of mirogabalin in healthy Asian Volunteers. 2016. [DOI] [PubMed]
- 7. Alwakeel JS, Al‐Suwaida A, Isnani AC, Al‐Harbi A, Alam A. Concomitant macro and microvascular complications in diabetic nephropathy. Saudi J Kidney Dis Transpl. 2009;20(3):402–409. [PubMed] [Google Scholar]
- 8. Bell DS, Ketchum CH, Robinson CA, Wagenknecht LE, Williams BT. Microalbuminuria associated with diabetic neuropathy. Diabetes Care. 1992;15(4):528–531. [DOI] [PubMed] [Google Scholar]
- 9. Cameron NE, Eaton SE, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia. 2001;44(11):1973–1988. [DOI] [PubMed] [Google Scholar]
- 10. Nang EE, Khoo CM, Tai ES, et al. Is there a clear threshold for fasting plasma glucose that differentiates between those with and without neuropathy and chronic kidney disease? The Singapore Prospective Study Program. Am J Epidemiol. 2009;169(12):1454–1462. [DOI] [PubMed] [Google Scholar]
- 11. Yin OQ, Merante D, Truitt K, Miller R. Population pharmacokinetic modeling and simulation for assessing renal impairment effect on the pharmacokinetics of mirogabalin. J Clin Pharmacol. 2016;56(2):203–212. [DOI] [PubMed] [Google Scholar]
- 12. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. [DOI] [PubMed] [Google Scholar]


