Abstract
The nasal type of extranodal natural killer/T‐cell lymphoma is a rare aggressive lymphoma with poor prognosis. To discover a successful treatment, we investigated the efficacy and safety of chemotherapy with methotrexate, etoposide, dexamethasone, and polyethylene glycol‐asparaginase (MESA). Three cycles of MESA were administered to 46 patients with new or relapsed/refractory natural killer/T‐cell lymphoma. Complete response after 3 treatment cycles was 43.5%, the overall response rate was 87%, and 2‐year overall survival was 83.4%. Complete response was significantly better for newly diagnosed patients than for patients with relapsed/refractory disease. Patients with newly diagnosed disease had a significantly better overall response rate after 1, but not after 2 or 3 treatment cycles. Overall survival and progression‐free survival did not differ over 2 years. Grade 1/2 toxicities were frequent, but MESA was associated with fewer grade 3/4 events or treatment‐related deaths. These results will require confirmation in larger prospective trials.
Keywords: chemotherapy, clinical trial, extranodal NK/T‐cell lymphoma, lymphoma, nasal type, non‐Hodgkin, refractory, relapse
1. INTRODUCTION
Extranodal natural killer/T‐cell lymphoma (NKTCL), nasal type, is a distinct and heterogeneous histopathologic subtype of non‐Hodgkin lymphoma (NHL), accounting for 5% to 10% of cases.1, 2, 3 The frequency of NKTCL among patients with NHL is significantly higher in Asia than in Europe and North America.4, 5, 6, 7 A recent analysis by the International T‐cell lymphoma Project revealed that NKTCL was among the most common lymphomas in Asia, with an incidence of 22.4%, and excluding Japan, just under half of the cases of peripheral T‐cell lymphoma among Asian patients were NKTCL.8, 9
The prognosis for NKTCL patients is poor, with 5‐year progression‐free survival (PFS) and overall survival (OS) rates of approximately 30% and 40%, respectively.9, 10 Historically, the poor outcome and high incidence of resistance to conventional therapy may be associated with overexpression of P‐glycoprotein, a transmembrane pump that transports anthracycline‐based chemotherapeutics and results in multidrug resistance.11, 12, 13, 14, 15 Given this complication, optimal treatment strategies for patients with NKTCL have not been determined, and studies have focused on improving the efficacy of chemotherapy and reducing the risk of disease recurrence.16, 17, 18
Recently, novel regimens have been tested, with promising results. One of the new first‐line therapies for NKTCL is dexamethasone, methotrexate, ifosfamide, etoposide, and l‐asparaginase (SMILE) chemotherapy.19 The SMILE therapy is associated with overall response rates (ORRs) ranging from 60% to 80%.19, 20, 21, 22 However, SMILE has resulted in a high level of hematologic toxicities, such as grade 3/4 neutropenia, thrombocytopenia, and nephrotoxicity.23 Asian patients may be at higher risk of hematologic toxicity as 1 study24 reported 92% of patients had grade 4 neutropenia. Asian and non‐Asian patients also experienced other non‐hematologic events such as hyperbilirubinemia and fibrinogenemia.19, 21, 22, 24, 25 These adverse events are frequently accompanied by hypersensitivity to l‐asparaginase.21, 22, 26, 27 This enzyme is a key component of chemotherapy because of the selective inability of transformed lymphocytes to synthesize asparagine.28 A recent review of the literature indicates that an allergic reaction to l‐asparaginase occurs in up to half of patients who receive this compound.27
Thus, to explore the possibility of more effective and less toxic chemotherapy for NKTCL, we have formulated methotrexate, etoposide, dexamethasone, and polyethylene glycol‐asparaginase (MESA), a novel chemotherapeutic regimen containing methotrexate, etoposide, dexamethasone, and polyethylene glycol‐asparaginase (pegaspargase). These agents are not affected by multidrug resistance and may be key drugs for first‐ and second‐line treatments for NKTCL. We omitted ifosfamide, which is included in the SMILE protocol, as NKTCL may develop resistance to the drug. Polyethylene glycol‐asparaginase is a monoethoxypolyethylene glycol succinimidyl conjugate of l‐asparaginase produced in Escherichia coli. This form of asparaginase has advantages over other sources because it is more stable and less likely to trigger an immune reaction.29, 30 It has been reported that the incidence of allergic reaction is as high as 30% after l‐asparaginase treatment.31 Studies have found that pegaspargase has similar efficacy as l‐asparaginase in treating acute lymphoblastic leukemia in children32, 33, 34, 35 and has about a 5 times longer half‐life with therapeutic effects being maintained for about 2 weeks.23, 36, 37 Polyethylene glycol‐asparaginase also requires lower and less frequent dosing.36 Therefore, the use of pegaspargase may reduce the pain due to continuously intravenous infusion, decrease hospital stay, and increase therapeutic compliance. Other drugs included in MESA, etoposide and steroid hormones, are effective treatments for hemophagocytic syndrome, which can be a concomitant disease in patients with NKTCL. Here, we report the findings of a multicenter study to evaluate the initial outcomes of a MESA regimen and involved‐field radiotherapy (RT). We assessed its effectiveness, toxicity, and feasibility in Asian patients with newly diagnosed, relapsed, or refractory NKTCL.
2. MATERIALS AND METHODS
2.1. Patients and staging of disease
This study (ClinicalTrial.gov: NCT01933282) was a retrospective analysis that enrolled 46 consecutive patients who were recruited at the Departments of Hematology of Xijing Hospital of Fourth Military Medical University, Guiyang Medical College, Kunming Medical University, and Xinan Hospital of Third Military Medical University in China between December 2012 and January 2015. All of the cases were histologically confirmed as NKTCL, nasal type, in accordance with the criteria of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues.38 Aggressive NKTCL was excluded. Patient demographics, Eastern Cooperative Oncology Group performance status (ECOG PS), and the primary sites of involvement were analyzed.
Patients underwent a pretreatment staging evaluation including a physical examination; complete blood count; determination of serum lactate dehydrogenase, ferroprotein, β‐microglobulin, and serum albumin levels; bone marrow aspiration and/or biopsy; computed tomography scan or magnetic resonance imaging scan of the involved region; and type B ultrasound. Ultrasonography was performed at superficial areas (posterior and anterior ear regions, submandibular region, neck, supraclavicular region, armpits, and groin) and the abdomen (liver, spleen, gallbladder, pancreas, and abdominal lymph nodes). Computed tomography was performed at the nasopharynx and chest. The tumors of all patients were staged in accordance with the Ann Arbor Staging system. The stage of IE could include local IE (site confined to the nasal cavity) and invasive IE (lesions extending outside of the nasal cavity and invading the adjacent organs and structures). B symptoms were defined as unexplained fever with a temperature higher than 38°C for 3 consecutive days, drenching night sweats, and/or unexplained weight loss exceeding 10% of the patient's baseline value. The international prognostic index (IPI) score was calculated on the basis of age, performance score, stage of disease, lactate dehydrogenase level, and extranodal site. Patients were divided into low/low to middle risk (IPI = 0‐2) and high risk (IPI = 3‐5). The observation time was 12 to 24 months. Informed consent was obtained from all of the patients, and the study was approved by the Medical Ethics Committee of Clinical Trials of Xijing hospital.
2.2. Diagnosis of pathology
Morphological analysis of the tissue samples was performed on formalin‐fixed, paraffin‐embedded tissue sections. A panel of primary antibodies specific to leukocyte common antigen, cluster of differentiation (CD)3, CD20, CD79a, CD56, cytotoxic granule‐associated RNA binding protein (TIA1), granzyme B, and Ki‐67 (DAKO, Glostrup, Denmark) was used. Immunohistochemical staining was performed with the EnVision System and the DAB Detection System (DAKO). The primary antibody was substituted with phosphate‐buffered saline (PBS) as a negative control.
DNA from paraffin‐embedded tissue was prepared with a tissue DNA extraction and purification kit (Dneasy Tissue Kit, Qiagen, GmbH, Hilden, Germany). Two sets of primers were used to amplify the rearranged T‐cell receptor gene in accordance with the BIOMED‐2 system as previously described.39 The positive control was a sample from a case of T‐cell lymphoma with a known monoclonal rearrangement; the negative control, run simultaneously, was a reaction without template DNA.
The fluorescent in situ hybridization probe for Epstein‐Barr virus (EBV)‐encoded RNA (EBER) and the fluorescent in situ hybridization kit were purchased from DAKO. The probe was a single‐stranded DNA probe that binds to EBER1 and EBER2. The kit provides a known positive control. A known negative sample from a reactive hyperplasia of lymph node tissue served as a negative control, and the blank control was phosphate‐buffered saline substituted for hybridization buffer. A positive cell had brown product in the nucleus, but cytoplasmic and membrane staining was regarded as negative.
2.3. Quantitative polymerase chain reaction of plasma EBV DNA
For quantitative polymerase chain reaction (qPCR), cell‐free plasma was separated immediately after venipuncture by centrifugation at 1200g for 15 minutes and frozen at −20°C until the assay was performed. DNA was extracted from 500‐μL plasma by using a QIAamp DNA mini blood kit (Qiagen). For each reaction, 5‐μL DNA eluate (or 25‐μL plasma volume equivalent) was used. qPCR was performed by using a real‐time polymerase chain reaction (PCR) assay and the ABI Prism 7700 Sequence Detector (PE Biosystems, Foster City, California). Sequences for the PCR primers, which were designed with the Primer Express software (PE Biosystems), were as follows: Epstein‐Barr nuclear antigen 1 forward primer (Ef), 5′‐TCA TCA TCA TCC GGG TCTCC‐3′, and reverse primer (Er), 5′‐CCT ACA GGG TGG AAA AATGGC‐3′. The sequence of the TaqMan probe (Ep) was 5′‐CGC AGG CCC CCT CCA GGT AGA A‐3′. The qPCR was performed in a volume of 50 μL with the TaqMan Universal PCR Master Mix (PE Biosystems). The reactions contained 5‐μL purified DNA, 300nM Ef/Er, and 200nM Ep. Thermal cycling was initiated with a 2‐minute incubation at 50°C, followed by a first denaturation step of 10 minutes at 95°C, and then 40 cycles of 95°C for 15 seconds (denaturation) and 60°C for 1 minute (reannealing and extension). A positive quantitative standard was the EBV DNA BamHI‐W fragment. In our hospital, the normal reference range of EBV DNA is 5000 copies/mL.
2.4. Chemotherapeutic regimen and eligibility criteria
All patients included have newly diagnosed, relapsed, or refractory NKTCL. The inclusion criteria were as follows: (1) a pathological diagnosis of NKTCL; (2) at least 1 objective evaluation (a measurable lesion); (3) age 15 to 60 years, male or female; (4) ECOG PS 0 to 3 and expected to survival of more than 3 months; (5) heart and kidney function in the normal range; (6) liver function (transaminase less than 2 times the normal value); and (7) a negative pregnancy test for women of childbearing age; all men and women had to agree to use effective contraception during the treatment and for 1 year after. The exclusion criteria were as follows: (1) previous use of methotrexate and/or l‐asparaginase; (2) patients with a malignant tumor who were also pregnant, breast‐feeding, or having mental diseases; (3) concurrent use of another trial drug or a contraindication for use of the drugs being tested in the study; (4) a serious infection (such as pneumonia or other fever‐accompanied infections) or metabolic (such as diabetes or diabetic ketoacidosis) disease; (5) liver dysfunction (serum direct bilirubin, indirect bilirubin, and transaminase at least 2 times higher than normal; a level of serum total protein or albumin below normal); (6) renal insufficiency, as indicated by a creatinine clearance at least 2 times higher than normal or less than 30 mL/min; (7) at the time of enrollment, a white blood cell count <3 × 109/L, absolute neutrophil count <1.5 × 109/L, platelet count <100 × 109/L (with no bone marrow invasion), platelet count <75 × 109/L (without bone marrow invasion), and hemoglobin <100 g/L; (8) in the 6 months prior to enrollment, a history of diabetes, phlebitis, or uncontrolled or serious cardiovascular disease including myocardial infarction, class III or IV heart failure, uncontrolled angina, or clinically significant pericardial disease; (9) a positive test for human immunodeficiency virus (HIV) antibody or for hepatitis B surface antibody or a DNA titer of greater than 1 × 104 copies/mL for HIV antibody or hepatitis B surface antibody after antiviral therapy for hepatitis B virus; and (10) a history of coagulation abnormalities.
Methotrexate‐etoposide‐dexamethasone‐pegaspargase chemotherapy was administrated as summarized in Table 1. The use of a pegaspargase schedule of 2500 IU/m2 intramuscularly every 2 weeks is based on the manufacturer's instructions and what is approved for use by the Chinese State Food and Drug Administration for therapy of acute lymphoblastic leukemia.40 The dose of the drug used was calculated according to the child's surface area. Each course of treatment was a 21‐day cycle. In the intervals between chemotherapy, patients with hematologic toxicities who had a peripheral white blood cell count less than 2.0 × 109 cells/L received 5‐μg/kg granulocyte colony‐stimulating factor daily until neutrophil recovery. A patient received an additional course of chemotherapy only if tests conducted before each cycle revealed an absolute neutrophil count of equal to or greater than 1.5 × 109/L and a platelet count of equal to or greater than 75 × 109/L. If a patient developed a grade 4 hematologic toxicity, then the doses of all chemotherapy drugs were reduced by 20% in all subsequent cycles. After at least 3 cycles of MESA chemotherapy, patients were referred for RT with a dose of 45‐50 Gy for 25 times. An additional 2 to 4 cycles of MESA chemotherapy were started within 1 week after the completion of RT, resulting in a maximum total of 7 cycles of MESA chemotherapy. If there was no recovery by 4 weeks after the day of the scheduled second course of chemotherapy, then the treatment protocol was terminated. The treatment protocol involved at least 3 courses of MESA chemotherapy with or without RT and then MESA chemotherapy. If the planned courses were completed, patients could choose to receive autologous hematopoietic stem cell transplant (HSCT). For HSCT, chemotherapy was performed with the etoposide‐methylprednisolone‐cytarabine‐cisplatin protocol, and the stem cells were mobilized by granulocyte colony‐stimulating factor treatment and subsequently collected and frozen. The standard carmustine‐etoposide‐cytarabine‐melphalan protocol was used to treat stem cells, which were then transfused back into the patients. The decision to receive HSCT was made at the discretion of the treating physician and the patient on the basis of the patient's age and condition.
Table 1.
Treatment protocol for MESA chemotherapy
| Agent | Dose Per Day | Route | Day of Administrationa |
|---|---|---|---|
| Methotrexate | 2 g/m2 | IV | 1 |
| Etoposide | 100 mg/m2 | VD | 2, 3, 4, |
| Dexamethasone | 20 mg/m2 | VD | 2, 3, 4, 5 |
| Polyethylene glycol‐asparaginase | 2500 IU/m2 | IM | 5 |
Cycles were repeated every 21 days. Three courses were planned as the protocol treatment.
Abbreviations: IM, intramuscular; IU, international units; IV, intravenous; MESA, methotrexate, etoposide, dexamethasone, polyethylene glycol‐asparaginase; VD, intravenous drip.
2.5. Response and toxicity criteria
An examination to restage the patients' cancer was conducted after every course of MESA. The tumor response was classified as complete response (CR), partial response (PR), or stable disease in accordance with the criteria of the International Workshop to Standardize the Response Criteria for NHL was used.41 The ORR was defined as the proportion of all patients who were able to be evaluated for response who experienced CR or PR, as defined by the National Comprehensive Cancer Network guideline.
Adverse reactions and toxicities were evaluated in accordance with the National Institute Common Toxicity Criteria version 3.0. If a patient experienced grade 4 thrombocytopenia, then the doses of methotrexate and etoposide were reduced to two‐thirds of the previous dose. Polyethylene glycol‐asparaginase was discontinued if it induced a grade 3 or 4 allergic reaction/hypersensitivity, pancreatitis, or hypotension or reduced by half if it induced a grade 1 or 2 allergic reaction/hypersensitivity. Patients who experienced a grade 1 or 2 allergic reaction/hypersensitivity could be administered prednisone at a dose of 1 mg/kg/d. If a patient experienced grade 4 thrombocytopenia or grade 3 non‐hematologic toxicity, pegaspargase was discontinued, but if the adverse event occurred during the first course of MESA and the patient recovered, then pegaspargase was readministered. During the first course, if the concentration of methotrexate exceeded 1 × 10−7 mol/L by 72 hours after the drug was administered, then the dose in the second course was reduced to two‐thirds that of the first course.
2.6. Statistical analysis
The primary end points were CR, PR, and ORR (ORR = CR + PR) after 3 treatment cycles. The secondary end points were OS, PFS, and toxicity. The duration of OS was defined as the time interval between enrollment and the death of the patient or, for patients who were censored, the date of the last follow‐up. PFS was calculated from the time of enrollment to the day that progression was first identified, the time of death, or the date of the last follow‐up.
Overall survival and PFS were estimated by using the Kaplan‐Meier method. For effectors of OS and PFS, the hazard ratio and the 95% confidence interval were estimated by using a Cox proportional hazard model. The log‐rank test or the Breslow test was implemented to compare differences in OS and PFS. All analyses were performed by using SPSS 22 software (IBM Corporation, Armonk, New York).
3. RESULTS
3.1. Patient characteristics
This study enrolled 46 patients, whose clinical characteristics are summarized in Table 2. The average age of the patients was 38.6 years (standard deviation = 10.7 years, range: 16‐54 years), and approximately 80% were men. The majority of patients (76.1%) were newly diagnosed with NKTCL, with the upper respiratory tract being the most common site (76.1%) of tumor localization. Among all patients, III or IV was the most prevalent stage of cancer (43.5%), followed by stage II (32.6%) and then stage I (23.9%). B symptoms and elevated ferroprotein were reported by almost two‐thirds of patients; approximately half had elevated low‐density lipoprotein cholesterol, elevated β‐microglobulin, an ECOG PS ≥1, and >60% Ki‐67‐positive tumor cells. Approximately one‐third of patients had an IPI ≥2. Among the patients, 15 received RT and 2 received HSCT. Not all patients received RT, which was administered depending on their disease condition (intensive therapy of the primary site or therapy of stage I/II lymphoma).
Table 2.
Baseline characteristics
| Variable | No. (%), N = 46 |
|---|---|
| Age, y (mean ± standard deviation) | 38.6 ± 10.7 |
| ≤40 | 23 (50) |
| >40 | 23 (50) |
| Disease state | |
| New diagnosis | 35 (76.1) |
| Refractory or relapsed | 11 (23.9) |
| Gender | |
| Female | 10 (21.7) |
| Male | 36 (78.3) |
| Primary site at diagnosis | |
| Nonupper respiratory tract | 11 (23.9) |
| Upper respiratory tract | 35 (76.1) |
| Stage | |
| I | 11 (23.9) |
| II | 15 (32.6) |
| III or IV | 20 (43.5) |
| B symptoms | 32 (69.6) |
| Elevated LDL cholesterol | 25 (54.3) |
| Elevated ferroprotein | 32 (69.6) |
| Elevated β‐microglobulin | 22 (47.8) |
| Cells positive for Ki‐67 expression, %a | |
| ≤60 | 22 (52.4) |
| >60 | 20 (47.6) |
| ECOG performance status | |
| 0 | 21 (45.6) |
| ≥1 | 25 (54.4) |
| International prognostic index | |
| 0 | 11 (23.9) |
| 1 | 21 (45.7) |
| ≥2 | 14 (30.4) |
| Prior treatment | |
| None | 38 (82.6) |
| Chemotherapyb | 8 (17.4) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDL, low‐density lipoprotein.
Four patients had no data on the percentage of cells positive for Ki‐67 expression.
Prior treatment included cyclophosphamide‐hydroxydaunorubicin‐vincristine‐prednisone (CHOP) in 2 cases (1 patient received both CHOP and CHOP‐etoposide), CHOP‐etoposide in 2 cases, and CHOP‐L‐asparaginase in 5 cases. No patients received radiotherapy in prior treatment.
3.2. Response
After 3 treatment cycles, 20 of 46 patients (43.5%) had CR, 20 (43.5%) had PR, and 6 (13%) had no response. The ORR was 87%. As compared with patients with refractory or relapsed disease, those with newly diagnosed NKTCL had a significantly higher rate of CR (54.3% vs 9.1%, P = .013) and lower rate of PR (34.3% vs 72.7%) (Table 3). Despite these differences, the ORRs were similar for the 2 treatment groups of patients. The responses of patients by stage of disease, IPI, and Ki‐67 expression are reported in Table 4. Although patients with stage I disease tended to have a higher CR and lower PR rates than did those with stage II or above, the differences did not reach statistical significance. Likewise, no significant differences were found in the rates of CR or PR between patients with different IPI scores or those whose tumors had ≤60% or >60% cells that were positive for Ki‐67.
Table 3.
Patient response after the first 3 treatment cycles by disease state
| Disease State | Response | Cycle 1 (n = 46) | Cycle 2 (n = 46) | Cycle 3 (n = 46) | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| New diagnosis | CR | 15 | 42.9a | 17 | 48.6a | 19 | 54.3a |
| PR | 14 | 40.0 | 13 | 37.1 | 12 | 34.3a | |
| ORR | 29 | 82.9a | 30 | 85.7 | 31 | 88.6 | |
| SD | 3 | 8.6 | 2 | 5.7 | 0 | 0.0 | |
| NR | 3 | 8.6 | 3 | 8.6 | 4 | 11.4 | |
| Refractory or relapsed | CR | 0 | 0.0 | 1 | 9.1 | 1 | 9.1 |
| PR | 5 | 45.5 | 7 | 63.6 | 8 | 72.7 | |
| ORR | 5 | 45.5 | 8 | 72.7 | 9 | 81.8 | |
| SD | 1 | 9.1 | 0 | 0.0 | 0 | 0.0 | |
| NR | 5 | 45.5 | 3 | 27.3 | 2 | 18.2 | |
Abbreviations: CR, complete response; ORR, overall response rate; PR, partial response; SD, stable disease; No., number; NR, no response.
Significant difference between newly diagnosed patients and those with refractory or relapsed disease, P < .05.
Table 4.
Response after 3 treatment cycles by stage of disease, IPI score, and Ki‐67 expression
| Response | Disease Stage | IPI Score | Ki‐67‐Positive Cells | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I (n = 11) | II (n = 15) | III or IV (n = 20) | 0 (n = 11) | 1 (n = 21) | ≥2 (n = 14) | ≤60% (n = 22) | >60% (n = 20) | |||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| CR | 6 | 54.5 | 6 | 40.0 | 8 | 40.0 | 8 | 72.7 | 8 | 38.1 | 4 | 28.6 | 11 | 50.0 | 8 | 40.0 |
| PR | 3 | 27.3 | 7 | 46.7 | 10 | 50.0 | 2 | 18.2 | 9 | 42.9 | 9 | 64.3 | 7 | 31.8 | 10 | 50.0 |
| ORR | 9 | 81.8 | 13 | 86.7 | 18 | 90.0 | 10 | 90.9 | 17 | 81.0 | 13 | 92.9 | 18 | 81.8 | 18 | 90.0 |
| NR | 2 | 18.2 | 2 | 13.3 | 2 | 10.0 | 1 | 9.1 | 4 | 19.0 | 1 | 7.1 | 4 | 18.2 | 2 | 10.0 |
Abbreviations: CR, complete response; PR, partial response; IPI, international prognostic index; No., number; NR, no response; ORR, overall response rate.
3.3. Prognostic factors and survival
Four patients died (8.7%) from hemophagocytic syndrome. Across the patients, there were 13 events of disease progression. For both OS and PFS, no events were reported after 1 year of follow‐up. Five patients were lost to follow‐up within 1 year because of lack of efficacy or financial difficulty. In addition, 5 to 7 patients discontinued; they only finished 1 course of therapy and therefore did not meet the requirement of completing at least 3 courses of therapy. The mean duration of follow‐up was 12.3 ± 7.5 months (range: 2‐24 months) for OS and 10.7 ± 7.2 months (range: 0‐24 months) for PFS.
The results of univariate analyses of OS and PFS are shown in Table 5. None of the factors that were examined were significantly associated with OS or PFS. Because of the limited number of patients and the lack of significance of effectors of OS and PFS in the univariate analysis, multivariate analysis was not performed.
Table 5.
Univariate analysis of survival
| Variable | OS | PFS | ||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age, y | 1.02 (0.94‐1.09) | 670 | 1.02 (0.96‐1.07) | 571 |
| Refractory or relapsed (Ref: newly diagnosed) | 1.25 (0.24‐6.45) | 792 | 2.25 (0.73‐6.91) | 157 |
| Male gender (Ref: female) | 0.63 (0.12‐3.27) | 585 | 0.78 (0.21‐2.83) | 703 |
| Primary site is URT (Ref: non‐URT) | 0.75 (0.15‐3.88) | 754 | 0.73 (0.22‐2.36) | 596 |
| Disease stage (Ref: stage I) | ||||
| II | 0.79 (0.11‐5.64) | 816 | 1.35 (0.32‐5.66) | 679 |
| III or IV | 0.81 (0.14‐4.87) | 820 | 0.85 (0.20‐3.54) | 818 |
| ECOG performance status ≥ 1 (Ref: 0) | 0.87 (0.39‐1.97) | 744 | 0.95 (0.32‐2.82) | 921 |
| IPI score (Ref: IPI = 0) | ||||
| 1 | 1.47 (0.15‐14.14) | 739 | 3.29 (0.40‐27.37) | 270 |
| ≥2 | 2.17 (0.22‐20.9) | 504 | 4.37 (0.52‐36.39) | 173 |
| Elevated LDL cholesterol (Ref: normal) | 0.56 (0.13‐2.52) | 451 | 0.67 (0.23‐2.01) | 480 |
| Elevated ferroprotein (Ref: normal) | 1.08 (0.21‐5.58) | 926 | 1.48 (0.41‐5.39) | 550 |
| Elevated β‐microglobulin (Ref: normal) | 6.1 (0.73‐50.75) | 094 | 1.28 (0.43‐3.80) | 662 |
| Ki‐67‐positive cells > 60% (Ref: ≤60%) | 0.43 (0.08‐2.23) | 316 | 0.87 (0.29‐2.60) | 808 |
| CD56 (+) (Ref: negative) | 0.69 (0.08‐5.71) | 728 | 1.75 (0.23‐13.43) | 593 |
| CD3 (+) (Ref: negative) | 0.99 (0.12‐8.22) | 992 | 2.63 (0.34‐20.26) | 354 |
| TCR (+) (Ref: negative) | 1.76 (0.21‐14.67) | 600 | 1.98 (0.44‐8.94) | 376 |
| B symptoms (Ref: none) | 1.09 (0.21‐5.64) | 922 | 0.82 (0.25‐2.71) | 748 |
Abbreviations: CD, cluster of differentiation; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; IPI, international prognostic index; LDL, low‐density lipoprotein; NA, data not available owing to no events in the referent group or in the reference group; OR, odds ratio; OS, overall survival; PFS, progression‐free survival; Ref, referent group; TCR, T‐cell receptor; URT, upper respiratory tract.
The rates of OS and PFS for the study population are illustrated in Figures 1A and 2A, respectively. The 2‐year OS and PFS rates were 83.4% and 66%, respectively. Although we found no significant differences between groups, we determined that patients with refractory or relapsed disease had a lower 2‐year OS rate (Figure 1B; 81.8% vs 84.3%, P = .814) and lower 2‐year PFS rate (Figure 2B; 47.7% vs 71.7%, P = .133) than did patients with newly diagnosed disease. The rates of OS (Figure 3) and PFS (Figure 4) were not significantly different between patients with different stages of disease, IPI score, or Ki‐67 expression. The 2‐year OS rate was 81.8%, 86.2%, and 82.8% for patients with stage I, II, or III/IV disease, respectively (P = .921), and the 2‐year PFS was 70.7%, 53.3%, and 70.6% (P = .656). For patients with an IPI score of 0, 1, or, ≥ 2, the 2‐year OS was 90.9%, 85.7%, and 76.6%, respectively (P = .861), while the 2‐year PFS was 80%, 71.4%, and 51.9% (P = 0.160). Among patients whose tumors had ≤60% and >60% Ki‐67‐positive cells, the 2‐year survival was 75.0% and 89.7%, respectively (P = .338) and the 2‐year PFS was 61% and 65.2% (P = .825).
Figure 1.
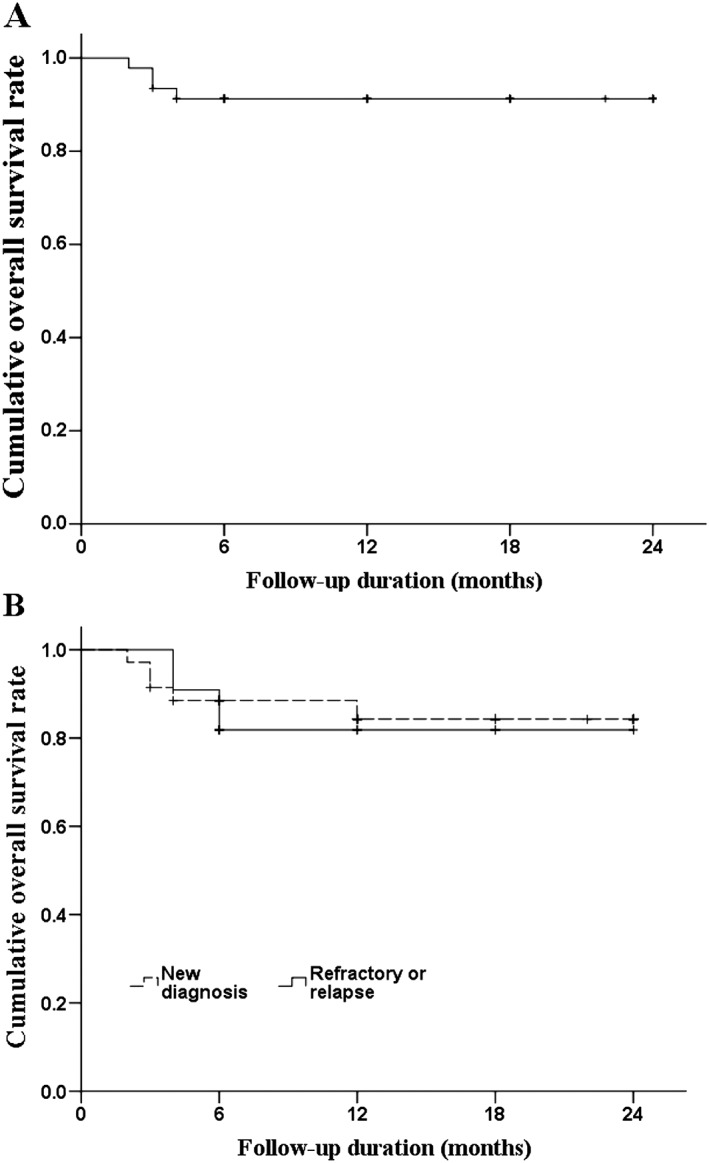
Kaplan‐Meier curves of OS. A, OS of all patients. B, OS of patients by disease state. The log‐rank test found no difference between newly diagnosed patients and those who relapsed (P = .249). Abbreviations: OS, overall survival
Figure 2.
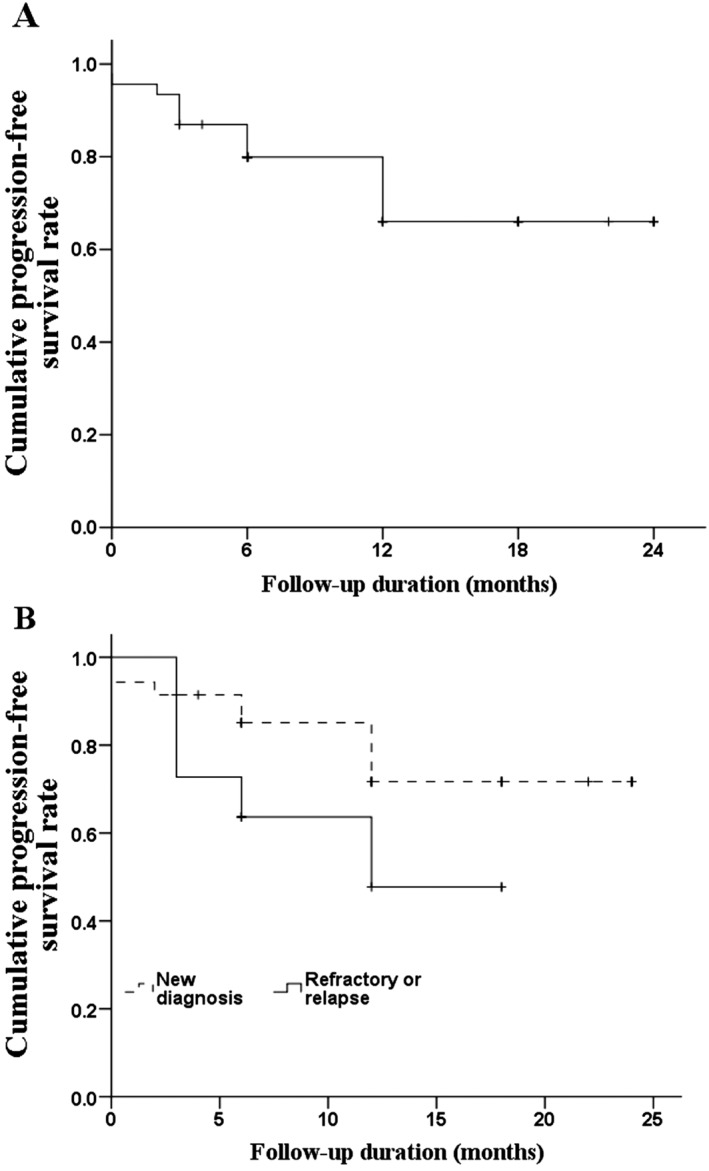
Kaplan‐Meier curves of PFS. A, PFS of all patients. B, PFS of patients by disease state. The Breslow test found no difference between newly diagnosed patients and those who relapsed (P = .814). Abbreviations: PFS, progression‐free survival
Figure 3.
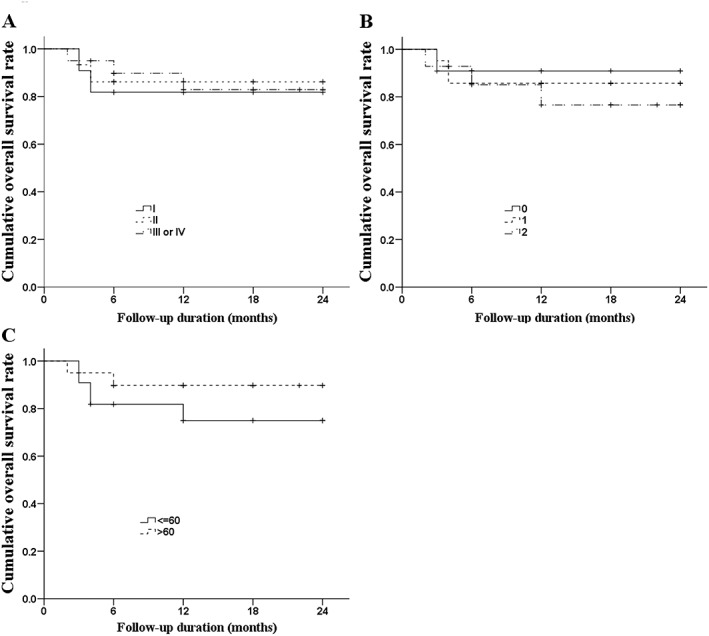
Kaplan‐Meier curves of OS. A, OS by stage of disease; B, OS by IPI; C, OS by Ki‐67 expression. The Breslow test found no differences among the groups (P = .921 for stage, P = .861 for IPI, and P = .338 for Ki‐67 expression). Abbreviations: IPI, international prognostic index; OS, overall survival
Figure 4.
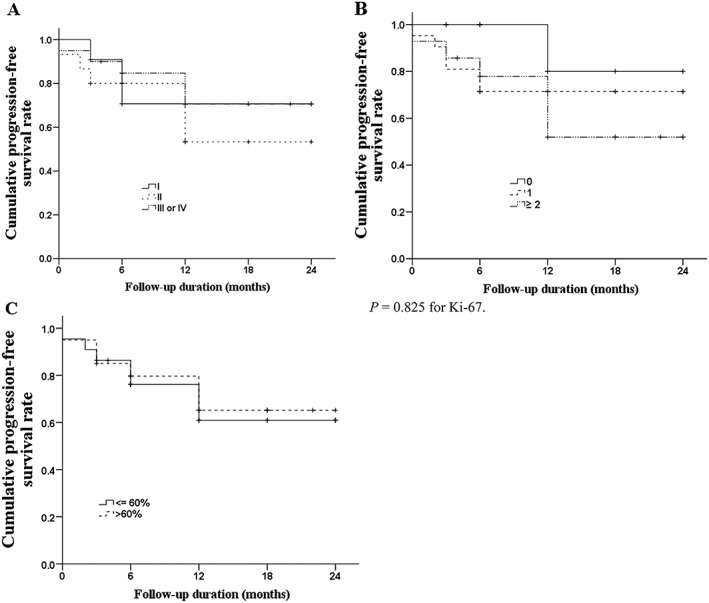
Kaplan‐Meier curves of PFS. A, PFS by stage of disease; B, PFS by IPI; C, PFS by Ki‐67 expression. The Breslow test found no differences among the groups (P = .656 for stage, P = .160 for IPI, and P = .825 for Ki‐67 expression). Abbreviations: IPI, international prognostic index; PFS, progression‐free survival
3.4. Toxicity
Grade 1 and 2 toxicities such as leukopenia, anemia, thrombocytopenia, nausea, vomiting, and liver dysfunction were frequent during treatment with MESA. Two patients (4.3%) developed grade 1 hypofibrinogenemia (a decrease in fibrinogen), which was treated with fresh‐frozen plasma and cryoprecipitate. Two patients (4.3%) experienced grade 3/4 thrombocytopenia, and 2 (4.3%) suffered from severe infection. Grade 1/2 abnormal liver function was found in 16 patients and grade 3 dysfunction in 1 patient, none of which required a delay in chemotherapy. Ten grade 3 or above adverse effects were reported, with 1 grade 3 and 1 grade 4 thrombocytopenia, 3 grade 3 and 2 grade 4 agranulocytosis, and 2 agranulocytotic fever. There were no treatment‐related deaths (Table 6).
Table 6.
Adverse events by severity
| Adverse Events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Leukopenia | 7 (15.2) | 0 (0) | 0 (0) | 0 (0) |
| Thrombocytopenia | 2 (4.3) | 2 (4.3) | 1 (2.2) | 1 (2.2) |
| Agranulocytosis | 0 (0) | 0 (0) | 3 (6.5) | 2 (4.3) |
| Agranulocytotic fever | 0 (0) | 0 (0) | 1 (2.2) | 1 (2.2) |
| Hyperbilirubinemia | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
| Hypofibrinogenemia | 2 (4.3) | 0 (0) | 0 (0) | 0 (0) |
| Liver dysfunction | 11 (23.9) | 5 (10.9) | 1 (2.2) | 0 (0) |
| Renal dysfunction | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) |
| Nausea | 11 (23.9) | 0 (0) | 0 (0) | 0 (0) |
| Vomiting | 10 (21.7) | 0 (0) | 0 (0) | 0 (0) |
4. DISCUSSION
Although the addition of l‐asparaginase to standard chemotherapy regimens for NKTCL has improved survival, hypersensitivity remains a problem for many patients. In this report, we present the results of a phase 2 trial that assessed the efficacy and safety of MESA chemotherapy with or without RT or HSCT for treating NKTCL. We found MESA showed efficacy in treating NKTCL and was associated and was well tolerated. Therefore, this regimen has potential as a new first‐ or second‐line treatment for NKTCL and supports its further evaluation in treating NKTCL.
Our findings for first‐line treatment for NKTCL compares favorably with those of other clinical trials that assessed different treatment regimens in stage I/II newly diagnosed NKTCL. Three studies using simultaneous chemoradiotherapy42, 43, 44, 45 and 3 assessing chemotherapy followed by RT46, 47, 48 had ORRs of 78% to 96%, 2‐year OS rates of 78% to 88%, and 3‐year OS rates of 54% to 86%. The newly diagnosed patients in our study had an ORR of 88% and a 2‐year OS rate of 84%. In addition, there were no treatment‐related deaths in our study.
In our study, not all patients received RT. A recent meta‐analysis has suggested that chemoradiotherapy results in better 3‐year survival than chemotherapy alone.6 Methotrexate‐etoposide‐dexamethasone‐pegaspargase also performed at least as well as other first‐line treatments that also did not include RT. Studies evaluating SMILE, ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) and gemcitabine, pegaspargase, cisplatin, and dexamethasone chemotherapies yielded ORRs ranging from 55% to 100%.20, 21, 49, 50, 51
When assessing second‐line treatments, MESA therapy showed an efficacy similar or better to prior studies. A phase 2 study that evaluated the efficacy and safety of SMILE therapy showed an excellent antitumor activity for patients with relapsed or refractory NKTCL, with an ORR of 77% and a 1‐year OS of 79% or 25%, respectively, and with a 3‐year OS of 50%.22, 52 Phase 2 studies of l‐asparaginase, methotrexate, and dexamethasone, IMEP, and gemcitabine‐pegaspargase‐cisplatin‐dexamethasone also reported ORRs of 77%, 43%, and 88%, respectively.26, 53, 54
Ding et al recently published the results of another study that evaluated MESA therapy (referred to as MEDA in their report).55 Patients in this trial achieved an ORR of 76% and a 1‐year OS of 69%. The treatment protocols for the MESA and MEDA trials differed in 2 key aspects: first, the doses of methotrexate and dexamethasone were not equivalent; second, the treatment was distributed over 5 days in our trial and 4 days in the Ding et al study. In our trial, the 11 patients with relapsed or refractory disease had an ORR of 81% and a 1‐year OS of 81.8%.
The adverse events experienced by patients in this trial were generally less severe than those of other studies and supports the use of MESA chemotherapy as a new first‐ or second‐line therapy for NKTCL. Although grade 1 and 2 toxicities were frequent, grade 3 events were few and there were no grade 4 events or treatment‐related deaths. Moreover, pegaspargase was well tolerated by all patients.
The number of grade 3 or 4 toxicities of this trial was less than that of the MEDA trial.55 As the treatment protocols of our trial and the MEDA trial were not the same (see above), the differences in adverse events highlight the importance of determining an optimal treatment regimen either through retrospective analysis of completed trials or prospective analysis of additional patients in randomized controlled trials. Future studies should also include subtype analysis of distinct patient populations, as our trial enrolled newly diagnosed patients with stage I or II disease and the MEDA trial did not. However, in the study by Kwong et al, adverse events were not more frequent in those with relapsed/refractory disease.21
Among the trials of SMILE chemotherapy, the rates of adverse events and treatment‐related deaths were much higher than those of the MEDA and MESA trials, even reaching 100% for some types of toxicities.19, 21, 22 In a study of IMEP, the rates of hematologic toxicities were also higher.47 SMILE, MESA, and IMEP are similar treatments, varying only in the use of ifosfamide, and prednisolone. Given this similarity, randomized controlled trials of these 3 regimens should be undertaken to confirm the superior safety outcomes of MESA chemotherapy. Kurtzberg and colleagues have found that, in patients with acute lymphoblastic leukemia, inclusion of either conjugated or native asparaginase showed no differences in adverse events56; therefore, the divergent safety profiles of MESA, SMILE, and IMEP may be related to ifosfamide. It is possible that any differences in clinical response and safety profile between the MESA, SMILE, and IMEP protocols may also be due to differences in the dose of chemotherapy administered, which was not evaluated in our study.
Our results highlight the need to characterize prognostic factors for patients with nasal‐type NKTCL treated with new and emerging chemotherapy regimens. Although patients whose tumors had ≥60% cells positive for Ki‐67 had a lower 3‐cycle rate of CR and lower 1‐year OS and PFS than did those whose tumors had <60% positive cells, the results were not statistically significant, and univariate analysis determined that a high Ki‐67 expression was not associated with a poor survival. This finding differs from those of previous studies.57, 58, 59, 60, 61, 62 Similarly, IPI score, disease stage, and expression of β2‐microglobulin and CD56, which have all been shown to be associated with survival for patients with nasal or extranasal NKTCL,59, 63, 64, 65, 66 were not associated with survival in our study. Additional research will be needed to determine which clinicopathological factors best predict the success of MESA chemotherapy.
This study had several limitations. First, the clinical trial is still ongoing, and this report is the initial summarization of the clinical findings. Second, the short follow‐up and the retrospective design of the study may have influenced our results. The fact the study was retrospective limits the type and quality of the data available for analysis. Third, the patient population is heterogeneous as we recruited patients receiving initial treatment and those receiving treatment due to recurrence, with localized disease patients as well as stage III/IV disease patients. Prospective studies with a larger sample size and long‐term follow‐up will be needed to confirm the therapeutic efficacy in these patients.
In conclusion, we have conducted a phase 2 trial of a new chemotherapy regimen, MESA, in hopes of identifying a treatment that achieves the superior outcomes of SMILE therapy without the accompanying toxicities. Our results demonstrate that MESA is a highly effective and safe chemotherapy regimen for patients with newly diagnosed, relapsed, or refractory NKTCL. Careful patient monitoring is needed, because the MESA regimen, like all chemotherapies, is potentially toxic. Additional studies in an expanded patient population should be conducted to define an optimal treatment protocol and to determine if the risk/benefit profile is similar for non‐Asian patients.
CONFLICT OF INTERESTS
The authors have no competing interest.
Liang R, Gao G‐x, Chen J‐p, et al. A phase 2 study of methotrexate, etoposide, dexamethasone, and pegaspargase chemotherapy for newly diagnosed, relapsed, or refractory extranodal natural killer/T‐cell lymphoma, nasal type: a multicenter trial in Northwest China. Hematological Oncology. 2017;35:619–629. https://doi.org/10.1002/hon.2325
REFERENCES
- 1. The Non‐Hodgkin's Lymphoma Classification Project . A clinical evaluation of the International Lymphoma Study Group classification of non‐Hodgkin's lymphoma. Blood. 1997;89(11):3909–3918. [PubMed] [Google Scholar]
- 2. The World Health Organization classification of malignant lymphomas in Japan: incidence of recently recognized entities. Lymphoma Study Group of Japanese Pathologists . Pathol Int 2000; 50(9):696‐702. [DOI] [PubMed] [Google Scholar]
- 3. Au WY, Ma SY, Chim CS, et al. Clinicopathologic features and treatment outcome of mature T‐cell and natural killer‐cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. 2005;16(2):206–214. [DOI] [PubMed] [Google Scholar]
- 4. Wang XM, Bassig BA, Wen JJ, et al. Clinical analysis of 1629 newly diagnosed malignant lymphomas in current residents of Sichuan province, China. Hematol Oncol. 2015;doi: 10.1002/hon.2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yang QP, Zhang WY, Yu JB, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011;6:77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li H, Wang CS, Wang XD. Meta analysis of treatment for stage IE~IIE extranodal natural killer/T cell lymphomas in China. Asian Pac J Cancer Prev. 2014;15(5):2297–2302. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki R. Pathogenesis and treatment of extranodal natural killer/T‐cell lymphoma. Semin Hematol. 2014;51(1):42–51. [DOI] [PubMed] [Google Scholar]
- 8. Vose J, Armitage J, Weisenburger D. International peripheral T‐cell and natural killer/T‐cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130. [DOI] [PubMed] [Google Scholar]
- 9. William BM, Armitage JO. International analysis of the frequency and outcomes of NK/T‐cell lymphomas. Best Pract Res Clin Haematol. 2013;26(1):23–32. [DOI] [PubMed] [Google Scholar]
- 10. Yamaguchi M. Current and future management of NK/T‐cell lymphoma based on clinical trials. Int J Hematol. 2012;96(5):562–571. [DOI] [PubMed] [Google Scholar]
- 11. Egashira M, Kawamata N, Sugimoto K, Kaneko T, Oshimi K. P‐glycoprotein expression on normal and abnormally expanded natural killer cells and inhibition of P‐glycoprotein function by cyclosporin A and its analogue, PSC833. Blood. 1999;93(2):599–606. [PubMed] [Google Scholar]
- 12. Trambas C, Wang Z, Cianfriglia M, Woods G. Evidence that natural killer cells express mini P‐glycoproteins but not classic 170 kDa P‐glycoprotein. Br J Haematol. 2001;114(1):177–184. [DOI] [PubMed] [Google Scholar]
- 13. Tse E, Kwong YL. Practical management of natural killer/T‐cell lymphoma. Curr Opin Oncol. 2012;24(5):480–486. [DOI] [PubMed] [Google Scholar]
- 14. Wang B, Li XQ, Ma X, Hong X, Lu H, Guo Y. Immunohistochemical expression and clinical significance of P‐glycoprotein in previously untreated extranodal NK/T‐cell lymphoma, nasal type. Am J Hematol. 2008;83(10):795–799. [DOI] [PubMed] [Google Scholar]
- 15. Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P‐glycoprotein/MDR1 by nasal T‐cell lymphoma cells. Cancer. 1995;76(11):2351–2356. [DOI] [PubMed] [Google Scholar]
- 16. Dearden CE, Johnson R, Pettengell R, et al. Guidelines for the management of mature T‐cell and NK‐cell neoplasms (excluding cutaneous T‐cell lymphoma). Br J Haematol. 2011;153(4):451–485. [DOI] [PubMed] [Google Scholar]
- 17. Kwong YL, Anderson BO, Advani R, Kim WS, Levine AM, Lim ST. Management of T‐cell and natural‐killer‐cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol. 2009;10(11):1093–1101. [DOI] [PubMed] [Google Scholar]
- 18. Ying Z, Zhu J. Extranodal natural killer/T cell lymphoma: we should and we can do more. Chin Clin Oncol. 2015;4(1):12 [DOI] [PubMed] [Google Scholar]
- 19. Yamaguchi M, Suzuki R, Kwong YL, et al. Phase I study of dexamethasone, methotrexate, ifosfamide, l‐asparaginase, and etoposide (SMILE) chemotherapy for advanced‐stage, relapsed or refractory extranodal natural killer (NK)/T‐cell lymphoma and leukemia. Cancer Sci. 2008;99(5):1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim SJ, Park S, Kang ES, et al. Induction treatment with SMILE and consolidation with autologous stem cell transplantation for newly diagnosed stage IV extranodal natural killer/T‐cell lymphoma patients. Ann Hematol. 2015;94(1):71–78. [DOI] [PubMed] [Google Scholar]
- 21. Kwong YL, Kim WS, Lim ST, et al. SMILE for natural killer/T‐cell lymphoma: analysis of safety and efficacy from the Asia Lymphoma Study Group. Blood. 2012;120(15):2973–2980. [DOI] [PubMed] [Google Scholar]
- 22. Yamaguchi M, Kwong YL, Kim WS, et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T‐cell lymphoma, nasal type: the NK‐Cell Tumor Study Group study. J Clin Oncol. 2011;29(33):4410–4416. [DOI] [PubMed] [Google Scholar]
- 23. Zeidan A, Wang ES, Wetzler M. Pegasparaginase: where do we stand? Expert Opin Biol Ther. 2009;9(1):111–119. [DOI] [PubMed] [Google Scholar]
- 24. Chang WJ, Ko YH, Kim SJ, Kim WS. Dexamethasone, methotrexate, ifosfamide, l‐asparaginase and etoposide (SMILE) chemotherapy for relapsed or refractory adult lymphoblastic lymphoma. Leuk Lymphoma. 2014;55(9):2196–2198. [DOI] [PubMed] [Google Scholar]
- 25. Tse E, Kwong YL. How I treat NK/T‐cell lymphomas. Blood. 2013;121(25):4997–5005. [DOI] [PubMed] [Google Scholar]
- 26. Jaccard A, Gachard N, Marin B, et al. Efficacy of l‐asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T‐cell lymphoma, a phase 2 study. Blood. 2011;117(6):1834–1839. [DOI] [PubMed] [Google Scholar]
- 27. Yong W. Clinical study of l‐asparaginase in the treatment of extranodal NK/T‐cell lymphoma, nasal type. Hematol Oncol. 2015;34(2):61–68. [DOI] [PubMed] [Google Scholar]
- 28. Codegoni AM, Biondi A, Conter V, Masera G, Rambaldi A, D'Incalci M. Human monocytic leukemia expresses low levels of asparagine synthase and is potentially sensitive to l‐asparaginase. Leukemia. 1995;9(2):360–361. [PubMed] [Google Scholar]
- 29. Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. J Clin Oncol. 1993;11(9):1780–1786. [DOI] [PubMed] [Google Scholar]
- 30. Keating MJ, Holmes R, Lerner S, Ho DH. l‐asparaginase and PEG asparaginase—past, present, and future. Leuk Lymphoma. 1993;10(Suppl):153–157. [DOI] [PubMed] [Google Scholar]
- 31. Earl M. Incidence and management of asparaginase‐associated adverse events in patients with acute lymphoblastic leukemia. Clin Adv Hematol Oncol. 2009;7(9):600–606. [PubMed] [Google Scholar]
- 32. Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard‐risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2002;99(6):1986–1994. [DOI] [PubMed] [Google Scholar]
- 33. Wetzler M, Sanford BL, Kurtzberg J, et al. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood. 2007;109(10):4164–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yong W, Zheng W, Zhu J, et al. l‐asparaginase in the treatment of refractory and relapsed extranodal NK/T‐cell lymphoma, nasal type. Ann Hematol. 2009;88(7):647–652. [DOI] [PubMed] [Google Scholar]
- 35. Silverman LB, Supko JG, Stevenson KE, et al. Intravenous PEG‐asparaginase during remission induction in children and adolescents with newly diagnosed acute lymphoblastic leukemia. Blood. 2010;115(7):1351–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asselin BL, Ryan D, Frantz CN, et al. In vitro and in vivo killing of acute lymphoblastic leukemia cells by l‐asparaginase. Cancer Res. 1989;49(15):4363–4368. [PubMed] [Google Scholar]
- 37. Douer D, Yampolsky H, Cohen LJ, et al. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood. 2007;109(7):2744–2750. [DOI] [PubMed] [Google Scholar]
- 38. World Health Organization . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Geneva, Switzerland: International Agency for Research on Cancer;2008:439. [Google Scholar]
- 39. van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T‐cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED‐2 Concerted Action BMH4‐CT98‐3936. Leukemia. 2003;17(12):2257–2317. [DOI] [PubMed] [Google Scholar]
- 40. Cooperative Group of Phase II Pegaspargase Clinical Trials . A randomized, controlled study on the therapy of acute lymphoblastic leukemia with pegaspargase vs l‐asparaginase in children. Chin J Hematol. 2008;29:29–33. [Google Scholar]
- 41. Cheson BD, Horning SJ, Coiffier B, et al. Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI Sponsored International Working Group. J Clin Oncol. 1999;17(4):1244 [DOI] [PubMed] [Google Scholar]
- 42. Kim SJ, Kim K, Kim BS, et al. Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T‐cell lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol. 2009;27(35):6027–6032. [DOI] [PubMed] [Google Scholar]
- 43. Kim SJ, Yang DH, Kim JS, et al. Concurrent chemoradiotherapy followed by l‐asparaginase‐containing chemotherapy, VIDL, for localized nasal extranodal NK/T cell lymphoma: CISL08‐01 phase II study. Ann Hematol. 2014;93(11):1895–1901. [DOI] [PubMed] [Google Scholar]
- 44. Yamaguchi M, Tobinai K, Oguchi M, et al. Concurrent chemoradiotherapy for localized nasal natural killer/T‐cell lymphoma: an updated analysis of the Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2012;30(32):4044–4046. [DOI] [PubMed] [Google Scholar]
- 45. Yamaguchi M, Tobinai K, Oguchi M, et al. Phase I/II study of concurrent chemoradiotherapy for localized nasal natural killer/T‐cell lymphoma: Japan Clinical Oncology Group Study JCOG0211. J Clin Oncol. 2009;27(33):5594–5600. [DOI] [PubMed] [Google Scholar]
- 46. Jiang M, Zhang H, Jiang Y, et al. Phase 2 trial of “sandwich” l‐asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T‐cell lymphoma. Cancer. 2012;118(13):3294–3301. [DOI] [PubMed] [Google Scholar]
- 47. Kim TM, Kim DW, Kang YK, et al. A phase II study of ifosfamide, methotrexate, etoposide, and prednisolone for previously untreated stage I/II extranodal natural killer/T‐cell lymphoma, nasal type: a multicenter trial of the Korean Cancer Study Group. Oncologist. 2014;19(11):1129–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang L, Wang ZH, Chen XQ, et al. First‐line combination of gemcitabine, oxaliplatin, and l‐asparaginase (GELOX) followed by involved‐field radiation therapy for patients with stage IE/IIE extranodal natural killer/T‐cell lymphoma. Cancer. 2013;119(2):348–355. [DOI] [PubMed] [Google Scholar]
- 49. Jaccard A, Suarez F, Delmer A, et al. A prospective phase II trial of an l‐asparaginase containing regimen in extra nodal NK/T‐cell lymphoma. Hematol Oncol. 2013;31(Suppl. S1):129 [abstract 099] [Google Scholar]
- 50. Kim M, Kim TM, Kim KH, et al. Ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) plus l‐asparaginase as a first‐line therapy improves outcomes in stage III/IV NK/T cell‐lymphoma, nasal type (NTCL). Ann Hematol. 2015;94(3):437–444. [DOI] [PubMed] [Google Scholar]
- 51. Li L, Zhang C, Zhang L, et al. Efficacy of a pegaspargase‐based regimen in the treatment of newly‐diagnosed extranodal natural killer/T‐cell lymphoma. Neoplasma. 2014;61(2):225–232. [DOI] [PubMed] [Google Scholar]
- 52. Suzuki R, Kwong Y, Maeda Y, et al. Long‐term follow‐up of the phase II study of SMILE chemotherapy for patients with newly diagnosed stage IV, relapsed or refractory extranodal NK/T‐cell lymphoma, nasal type: the NK‐cell Tumor Study Group study. Hematol Oncol. 2013;31(Suppl. S1):175 [abstract 235] [Google Scholar]
- 53. Kim BS, Kim DW, Im SA, et al. Effective second‐line chemotherapy for extranodal NK/T‐cell lymphoma consisting of etoposide, ifosfamide, methotrexate, and prednisolone. Ann Oncol. 2009;20(1):121–128. [DOI] [PubMed] [Google Scholar]
- 54. Zhou Z, Li X, Chen C, et al. Effectiveness of gemcitabine, pegaspargase, cisplatin, and dexamethasone (DDGP) combination chemotherapy in the treatment of relapsed/refractory extranodal NK/T cell lymphoma: a retrospective study of 17 patients. Ann Hematol. 2014;93(11):1889–1894. [DOI] [PubMed] [Google Scholar]
- 55. Ding H, Chang J, Liu LG, et al. High‐dose methotrexate, etoposide, dexamethasone and pegaspargase (MEDA) combination chemotherapy is effective for advanced and relapsed/refractory extranodal natural killer/T cell lymphoma: a retrospective study. Int J Hematol. 2015;102(2):181–187. [DOI] [PubMed] [Google Scholar]
- 56. Kurtzberg J, Asselin B, Bernstein M, Buchanan GR, Pollock BH, Camitta BM. Polyethylene glycol‐conjugated l‐asparaginase versus native l‐asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: a Children's Oncology Group Study (POG 8866). J Pediatr Hematol Oncol. 2011;33(8):610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. He X, Chen Z, Fu T, et al. Ki‐67 is a valuable prognostic predictor of lymphoma but its utility varies in lymphoma subtypes: evidence from a systematic meta‐analysis. BMC Cancer. 2014;14:153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiang L, Li P, Wang H, et al. Prognostic significance of Ki‐67 antigen expression in extranodal natural killer/T‐cell lymphoma, nasal type. Med Oncol. 2014;31(10):218 [DOI] [PubMed] [Google Scholar]
- 59. Li S, Feng X, Li T, et al. Extranodal NK/T‐cell lymphoma, nasal type: a report of 73 cases at MD Anderson Cancer Center. Am J Surg Pathol. 2013;37(1):14–23. [DOI] [PubMed] [Google Scholar]
- 60. Yasuda H, Sugimoto K, Imai H, et al. Expression levels of apoptosis‐related proteins and Ki‐67 in nasal NK/T‐cell lymphoma. Eur J Haematol. 2009;82(1):39–45. [DOI] [PubMed] [Google Scholar]
- 61. Huang X, Sun Q, Fu H, Zhou X, Guan X, Wang J. Both c‐Myc and Ki‐67 expression are predictive markers in patients with extranodal NK/T‐cell lymphoma, nasal type: a retrospective study in China. Pathol Res Pract. 2014;210(6):351–356. [DOI] [PubMed] [Google Scholar]
- 62. Kim SJ, Kim BS, Choi CW, et al. Ki‐67 expression is predictive of prognosis in patients with stage I/II extranodal NK/T‐cell lymphoma, nasal type. Ann Oncol. 2007;18(8):1382–1387. [DOI] [PubMed] [Google Scholar]
- 63. Li ZM, Zhu YJ, Sun J, et al. Serum beta2‐microglobin is a predictor of prognosis in patients with upper aerodigestive tract NK/T‐cell lymphoma. Ann Hematol. 2012;91(8):1265–1270. [DOI] [PubMed] [Google Scholar]
- 64. Wang L, Wang Z, Xia ZJ, Lu Y, Huang HQ, Zhang YJ. CD56‐negative extranodal NK/T cell lymphoma should be regarded as a distinct subtype with poor prognosis. Tumour Biol. 2015;36(10):771–723. [DOI] [PubMed] [Google Scholar]
- 65. Wu X, Li P, Zhao J, et al. A clinical study of 115 patients with extranodal natural killer/T‐cell lymphoma, nasal type. Clin Oncol (R Coll Radiol). 2008;20(8):619–625. [DOI] [PubMed] [Google Scholar]
- 66. Yoo C, Yoon DH, Jo JC, et al. Prognostic impact of beta‐2 microglobulin in patients with extranodal natural killer/T cell lymphoma. Ann Hematol. 2014;93(6):995–1000. [DOI] [PubMed] [Google Scholar]


