Abstract
Aim
Preterm infants display aberrant gut microbial colonisation. We investigated whether the differences in gut microbiota between late preterm and full‐term infants results from prematurity or external exposures.
Methods
This study comprised 43 late preterm infants (340/7–366/7) and 75 full‐term infants based on faecal samples collected following birth and at two to four weeks and six months of age. We assessed clinically relevant bacteria using quantitative polymerase chain reaction. Logistic regression analyses were performed to determine whether the observed differences in gut microbiota were attributable to prematurity or perinatal exposure.
Results
The prevalence of bifidobacteria differed in the intestinal microbiota of the full‐term and late preterm neonates. Differences in the presence of specific species were detected at the age of six months, although the microbiota alterations were most prominent following delivery. As well as prematurity, the mode of birth, intrapartum and neonatal antibiotic exposure, and the duration of breastfeeding had an additional impact on gut microbiota development.
Conclusion
The gut microbiota composition was significantly different between late preterm and full‐term infants at least six months after birth. Antibiotic exposure was common in late preterm infants and modulated gut colonisation, but preterm birth also affected gut microbiota development independently.
Keywords: Antibiotics, Caesarean section, Gut colonisation, Intestinal microbiota, Late preterm neonate
Abbreviation
- DNA
deoxyribonucleic acid
Key notes.
We investigated whether differences in gut microbiota between 43 late preterm infants and 75 full‐term infants resulted from prematurity or external exposure.
The prevalence of bifidobacteria differed in the intestinal microbiota of the two groups at six months of age, but the microbiota alterations were most prominent following delivery.
As well as prematurity, the birth mode, intrapartum and neonatal antibiotic exposure and the duration of breastfeeding affected gut microbiota development.
Introduction
Preterm birth, accounts for 5%–18% of all deliveries and is a leading cause of infant morbidity and mortality 1. The problem is expected to escalate, as an increasing trend in preterm births has been reported, particularly in industrialised countries. In addition to heightened morbidity in the immediate neonatal period, preterm birth also increases the risk of long‐term health problems. These sequelae may arise from prematurity per se or develop as a complication of neonatal intensive care, suggesting a vicious circle of immature host defences and extensive environmental challenges during a critical period of development.
The gut microbiota development in preterm infants is different from that of term infants 2. It is not known whether the differences in gut colonisation patterns are a direct result of prematurity or mediated by detrimental environmental exposure, including increased rates of Caesarean section delivery and antibiotic use or various components of neonatal intensive care, which often cluster in preterm infants. Early gut colonisation has been recognised to exert a significant impact on health and disease 3 and specific members of the infant gut microbiota, including bifidobacteria, clostridia and Akkermansia muciniphila, have been associated with subsequent development of atopic disease and allergy 4, 5 as well as overweight and obesity 6, 7. Humans and microbes accomplish bidirectional exchanges of endocrine, immune and neural signals with targets in metabolic, immune, humoral and neural pathways. Consequently dysbiosis, perturbations in bacterial colonisation may result in an increased risk of diseases such as atopic diseases, autism spectrum disorders, and metabolic disease including obesity and insulin resistance 3.
The aim of this study was to investigate gut microbiota development during the first six months of life in late preterm infants compared to those born at full term. Specifically, we aimed to establish whether the differences in gut microbiota development between late preterm and full‐term infants were caused by prematurity or resulted from external factors during early life.
Methods
Design and sampling
The study was based on the Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Research Group's randomised controlled trials, which evaluated the impact of prenatal probiotic supplementation in infants at high risk of abnormal early microbial contact. A total of 118 infants 43 born late preterm and 75 born full term from ongoing clinical trials 8, 9 were included in this study. Late preterm birth was defined as delivery between 340/7 and 366/7 weeks of gestation and full term birth as delivery between 390/7 and 406/7 weeks of gestation. The study subjects were selected based on the availability of faecal samples for the desired time point and the final number of subjects included in the study was determined by faecal sample availability. The follow‐up of the studies proceeded with a uniform protocol in the same population base, in the same area and by the same research personnel, blinded to randomisation. The studies were designed to fulfil the Declaration of Helsinki and the European Convention on Human Rights. The regulations on good clinical and laboratory practice and clinical trials were followed and the data were saved accordingly. Faecal samples were collected for microbial analyses immediately after birth, two to four weeks after birth and at six months of age. We analysed the prevalence of clinically relevant bacteria, including: the Bifidobacterium genus and the Bidiobacterium species B. adolescentis, B. bifidum, B. breve, B. catenulatum, B. lactis, B. longum and B. infantis; the Clostridium species C. coccoides, C. leptum, C. difficile and C. perfringens and, Staphylococcus aureus and Akkermansia muciniphila. Bifidobacteria are a major component of the gut microbiota in healthy breastfed infants 10. Previous studies have demonstrated the significance of these gut bacteria in early infancy for the later risk of chronic diseases such as atopic disease 4, allergy 5 and obesity 6, 7 in children. We provided 54% of the full‐term infants and 61% of the late preterm infants with probiotic Lactobacillus rhamnosus GG supplements 8, 9. The other late preterm and full‐term infants in the study received a placebo.
DNA isolation and quantitative PCR assay
Deoxyribonucleic acid (DNA) was isolated from faecal samples that were stored at −80°C until analysed. Samples were pre‐treated and DNA was extracted using an automated KingFisher DNA extraction system (Thermo Fisher Scientific Oy, Vantaa, Finland) and InviMag Stool DNA kit (Stratec Molecular, Berlin, Germany), as previously described 11. The standard DNA for quantitative polymerase chain reaction was also prepared as previously described 11. All DNA samples were stored at −20°C until analysed. Quantitative PCRs were completed as previously described 12, 13. PCR amplification and detection were performed with an ABI PRISM 7300‐PCR sequence detection system (Applied Biosystems, Foster City, CA, USA). The primers used in the analyses are described in Table S1.
Statistical analyses
The baseline characteristics of the preterm and full‐term groups were compared using the t‐test for independent samples. The Mann–Whitney U test was used for non‐normal continuous variables and the chi‐square test was used for categorical variables. The chi‐square test was also used to compare the presence of bacteria between preterm and full‐term groups.
Whether the infant was born late preterm or full term was the primary predictor variable in predicting the presence of different bacteria during the follow‐up. Other potential prognostic factors included the mode of delivery, intrapartum and neonatal antibiotic exposure, duration of exclusive breastfeeding, total duration of breastfeeding and probiotic intervention. The associations between those potential prognostic factors and the presence of specific bacteria at birth, at two to four weeks and at six months were first screened with univariable analysis using the chi‐square test. Subsequently, univariable logistic regression analysis with the generalised estimating equations method was used to include all infants with at least one microbiota measurement.
The final multivariable logistic regression analyses with generalised estimating equations included gestational age and the prognostic factors that resulted in a p‐value of ≤0.10 in the analyses. The results are given as adjusted odds ratios with 95% confidence intervals.
Two‐tailed p‐values <0.05 were considered statistically significant. The analyses were performed using IBM SPSS Statistics for Windows version 23.0 (IBM Corporation, New York, NY, USA).
Results
Clinical characteristics
The clinical characteristics of the mothers and infants in this study are presented in Table 1. Smoking during pregnancy was more prevalent in mothers of preterm infants compared to those delivered full‐term. Mothers of late preterm infants had a considerably higher body mass index before pregnancy in comparison to mothers of full‐term infants. Exposure to intrapartum and neonatal antibiotics as well as elective Caesarean section as the mode of birth were less frequent in full‐term than in late preterm infants.
Table 1.
Clinical characteristics of preterm and full‐term infants
| Full‐term (n = 75) | Preterm (n = 43) | Total (n = 118) | p valuea | |
|---|---|---|---|---|
| Maternal age (years) | 29 (19–44) | 31 (21–41) | 30 (19–44) | 0.003 |
| Maternal smoking during pregnancy | 3/75 (4%) | 9/43 (21%) | 12/118 (10%) | |
| Maternal weight before pregnancy (kg) | 60 (43–105) | 66.1 (49–134) | 63.2 (43–134) | |
| Maternal BMI before pregnancy (kg/m2) | 21.9 (17.7–37.2) | 24.2 (19.0–45.3) | 23.1 (17.7–45.3) | |
| Gestational age (weeks) | 40 (37–42) | 35 (33–36) | 39 (33–42) | <0.001 |
| First child | 54 (72%) | 25 (58%) | 79 (67%) | 0.12 |
| Boys | 48 (64%) | 31 (72%) | 79 (70%) | 0.37 |
| Birthweight (g) | 3693 (2750–4800) | 2565 (1770–3650) | 3282 (1770–4800) | <0.001 |
| Mode of delivery | ||||
| Vaginal | 47 (63%) | 29 (67%) | 76 (64%) | 0.01 |
| Vacuum extraction | 15 (20%) | 3 (7%) | 18 (15%) | |
| Elective section | 4 (5%) | 9 (21%) | 13 (11%) | |
| Non‐elective section | 9 (12%) | 2 (5%) | 11 (9%) | |
| Intrapartum antibiotics | 13 (18%) | 11 (26%) | 24 (21%) | 0.34 |
| Exclusive breastfeeding (months) | 3 (0–6) | 0 (0–8) | 2 (0–8) | 0.04 |
| Total breastfeeding (months) | 6.0 (0.5–15.0) | 4.0 (0.5–12.0) | 5.6 (0.5–15.0) | 0.31 |
| Probiotic intervention | 40 (54%) | 22 (61%) | 62 (48%) | 0.82 |
| Neonatal antibiotic exposure | ||||
| None | 72 (97%) | 20 (47%) | 92 (79%) | <0.001 |
| Less than five days | 2 (3%) | 18 (42%) | 20 (17%) | |
| Five days or longer | 0 (0%) | 5 (12%) | 5 (4%) | |
The data are presented as means (SD range) for continuous variables with normal distribution, as medians (range) for non‐normal continuous variable and as number (%) for categories variable.
t‐test for independent samples was used for continuous variables with normal distributions, Mann–Whitney U‐test for non‐normal continuous variables and chi‐square test for categorical variables.
Compositional development of gut microbiota in full‐term and late preterm infants
The intestinal microbiota in full‐term infants was characterised by a high frequency of bifidobacteria following delivery. At the genus level, bacteria belonging to the genus Bifidobacterium were detected at all time points in all of the full‐term infants (Fig. 1), while B. longum was the most frequent species throughout the study period (Fig. 1). The frequency of the Clostridium species followed an increasing trend over time and C. difficile was only detectable at the age of six months in full‐term infants (Fig. 1).
Figure 1.
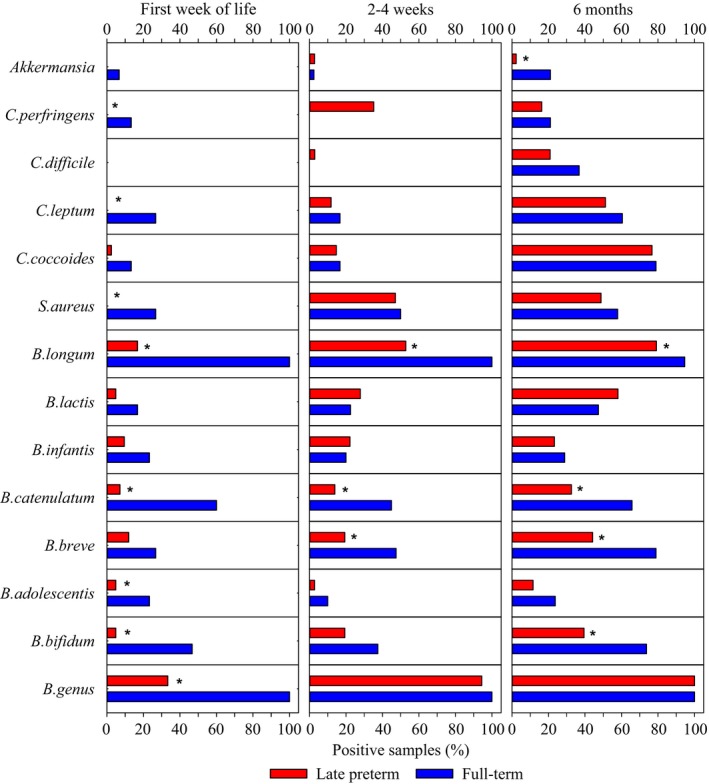
Gut colonisation patterns in full‐term and late preterm infants during the first six months of life. The prevalence of clinically relevant bacteria in full‐term (n = 75) and preterm (n = 43) infant fecal samples collected during first week of life and at two to four weeks and six months of age (*denotes p < 0.05).
Intestinal colonisation in late preterm infants, on the other hand, displayed a delayed progress compared to full‐term infants. The frequency of Bifidobacterium on both genus and species levels was significantly lower in late preterm infants during the first weeks of life when compared to full‐term infants (Fig. 1). The frequency of the Bifidobacterium genus in late preterm infants reached the level of full‐term infants by the age of six months, while differences in the presence of the specific Bifidobacterium species were still detectable at that age.
Effect of prematurity and perinatal confounding factors on gut microbiota composition
We sought to establish whether the observed differences were attributable to prematurity per se or to various perinatal risk factors that might cluster in late preterm infants. In the multivariable model, neonatal antibiotic exposure was associated with reduced prevalence of B. bifidum, B. breve, B. lactis and B. longum (Table 2). Caesarean delivery as the mode of birth was associated with the decreased prevalence of B. catenulatum and B. longum but the increased prevalence of C. perfringens (Table 2). Preterm birth affected gut microbiota development independently of the confounding factors (Table 2). In particular, prematurity delayed intestinal Bifidobacterium colonisation and, interestingly, the effect of preterm birth on bifidobacteria appeared to be species‐specific (Table 2). Use of the specific probiotic Lactobacillus rhamnosus exerted no significant effect on the prevalence of the gut microbes assessed in this study.
Table 2.
Exposure affecting the gut microbiota during the first six months of life
| Bacteria | Factors | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|---|
| B. adolescentis | Late preterm birth | 0.37 | 0.15–0.92 | 0.03 |
| Antibiotics during delivery | 0.31 | 0.07–1.31 | 0.110 | |
| B. bifidum | Late preterm | 0.43 | 0.22–0.85 | 0.02 |
| Vaginal delivery | 1.88 | 0.96–3.67 | 0.07 | |
| Infant's antibiotic treatment | 0.27 | 0.10–0.69 | 0.01 | |
| Antibiotics during delivery | 0.54 | 0.25–1.18 | 0.12 | |
| B. breve | Late preterm | 0.66 | 0.24–1.49 | 0.27 |
| Infant's antibiotic treatment | 0.27 | 0.10–0.72 | 0.01 | |
| Antibiotics during delivery | 0.36 | 0.13–1.03 | 0.06 | |
| Duration of breastfeeding greater than or equal to six months | 0.54 | 0.27–1.11 | 0.09 | |
| B. catenulatum | Late preterm | 11.11 | 3.85–33.33 | <0.001 |
| Vaginal delivery | 2.96 | 1.33–6.59 | 0.01 | |
| Infant's antibiotic treatment | 0.4 | 0.14–1.11 | 0.08 | |
| Duration of exclusive breastfeeding greater than or equal to three months | 2.21 | 1.16–4.22 | 0.02 | |
| B. lactis | Late preterm | 1.67 | 0.83–3.33 | 0.16 |
| Infant's antibiotic treatment | 0.43 | 0.21–0.90 | 0.02 | |
| Duration of breastfeeding greater than or equal to six months | 0.46 | 0.25–0.85 | 0.01 | |
| B. longum | Late preterm | 0.02 | 0.00–0.15 | <0.001 |
| Vaginal delivery | 2.41 | 1.04–5.57 | 0.04 | |
| Infant's antibiotic treatment | 0.14 | 0.07–0.30 | <0.001 | |
| Antibiotics during delivery | 0.40 | 0.20–0.78 | 0.01 | |
| C. coccoides | Late preterm | 0.65 | 0.36–1.16 | 0.14 |
| Duration of breastfeeding greater than or equal to six months | 0.52 | 0.31–0.89 | 0.02 | |
| C. leptum | Late preterm | 0.35 | 0.16–0.76 | 0.01 |
| Infant's antibiotic treatment | 0.69 | 0.33–1.45 | 0.331 | |
| Duration of breastfeeding greater than or equal to six months | 0.31 | 0.15–0.65 | 0.002 | |
| C. difficile | Term | 0.25 | 0.10–0.62 | 0.003 |
| Duration of exclusive breastfeeding greater than or equal to three months | 0.14 | 0.04–0.14 | 0.001 | |
| C. perfringens | Late preterm | 0.62 | 0.26–1.49 | 0.29 |
| Vaginal delivery | 0.47 | 0.25–0.86 | 0.02 | |
| Time of breastfeeding six months | 0.09 | 0.03–0.26 | <0.001 | |
| Akkermansia | Late preterm | 0.19 | 0.04–0.90 | 0.04 |
| Infant's antibiotic treatment | 0.51 | 0.06–4.27 | 0.53 |
Preterm birth and other factors associated with the presence of bacterial species in faecal samples of infants throughout the study period at birth, two to four weeks and six months. Results are based on multivariable logistic regression analyses and generalised estimating equations (GEE).
Discussion
Our study demonstrated significant differences in gut colonisation patterns between late preterm and full‐term infants during the first six months of life. In particular, delayed colonisation with bifidobacteria was evident in late preterm infants. Preterm infants are exposed to numerous microbiota‐modifying practices, such as Caesarean section delivery, shorter or no contact with the mother's microbes at delivery, early antibiotic exposure and longer hospitalisation influencing the stepwise intestinal colonisation process. Consistently with this, delivery by Caesarean section and neonatal antibiotic exposure were more common in late preterm than in full‐term infants in this study and the same factors were shown to modulate gut colonisation. Nonetheless, our present data indicated that the differences in intestinal microbial colonisation observed between late preterm and full‐term infants were also directly attributable to preterm birth and not a simple reflection of the distinctive environmental exposures associated with the care of these children. It is of note that the differences in early gut microbiota composition between late preterm and full‐term infants were still detectable six months after birth.
Previous studies have mostly provided data on gut microbiota composition in very low birthweight infants during their stay in the neonatal intensive care unit. The results from studies comparing very low birthweight and full‐term infants are liable to be confounded by interacting exposures related to neonatal morbidity and treatment modalities. In contrast, we investigated gut colonisation in late preterm infants who were not subjected to intensive care or prolonged stay in the neonatal intensive care unit 2. Therefore, the postnatal exposure of late preterm infants resemble more closely those of full‐term infants. This facilitates direct assessment of our study objectives when early confounding factors such as mode of delivery, intrapartum and neonatal antibiotic exposure, duration of exclusive breastfeeding, total duration of breastfeeding and probiotic intervention were considered in the analyses. Our data confirmed the previous findings of aberrant gut colonisation in preterm infants 14 and extended these observations to late preterm infants. Moreover, we provided direct corroboration for the notion that prematurity per se affects gut colonisation patterns. The differences in gut microbiota activity and their functional correlates in the host in term and late preterm infants remain to be established in future studies.
The mechanism underlying deviant gut colonisation resulting from prematurity irrespective of environmental exposures remains elusive. Preterm neonates are characterised by immaturity of immune regulation and gut barrier function, which may selectively modulate microbial adhesion and colonisation. On the other hand, maternal gut microbiota composition is known to undergo significant changes during pregnancy 15. It is therefore likely that the initial colonising inoculum received from the mother varies as a function of duration of pregnancy. Finally, accumulating evidence suggests that preterm birth may be causally related not only to maternal infection but also more subtle microbial disturbances in the birth canal or even poor dental health 16. These data raise the question about whether the dysbiosis detected in preterm neonates reflects the cause and not the consequence of premature birth.
Most previous studies have focused on gut microbiota composition in preterm infants during the first days or weeks of life 17. Arboleya et al. 18 reported that the quantitative levels of most microbial groups generally tended to increase over time in preterm and full‐term infants. In particular, preterm infants had a lower level of Bifidobacterium, Bacteroides and Atopobium group bacteria, but higher levels of Enterococcaceae and Lactobacillus groups compared to full‐term infants during a 90‐day study period 18. Our present data indicated that the aberrant gut colonisation patterns detected in late preterm infants extended until at least the age of six months. This long‐term gut microbiota perturbation may have a considerable impact on later health. Given the recently discovered role of gut microbiota in the development of obesity and metabolic disease 3, it is conceivable that the increase in cardiometabolic risk factors observed in young adults born preterm and late preterm 19 may be, at least partly, mediated by the intestinal microbiota.
While no effective means of preventing preterm birth are currently available, there are measures that might attenuate the detrimental consequences of preterm birth on the gut microbiota. According to current scientific evidence, the perinatal period constitutes not only the most critical stage but also an optimal target for interventions that aim to reduce the risk of non‐communicable disease. An optimal objective would be to support healthy gut microbiota composition. Prudence in the use of antibiotics is clearly called for in the case of these fragile infants. Antibiotics can be life‐saving treatment for infants, but they may also have untoward long‐term effects on later health. Breastmilk is beneficial for the balanced development of the gut microbiota and mothers should be encouraged to breastfeed their infants. The potentially detrimental effects of aberrant early gut colonisation in late preterm infants may be amenable to intervention by prebiotics or probiotics. The potential of such interventions need to be assessed in well‐powered clinical studies with clinically significant endpoints.
Conclusion
Our study is the first to demonstrate that, in addition to known exposures including Caesarean section delivery, antibiotic use and lack of breastfeeding, late preterm birth independently affected gut microbiota development during the first six months of life. Neonates exposed to these potentially detrimental environmental factors may be at increased risk of long‐term adverse health effects and warrant further investigation.
Funding
This study was funded by the Emil Aaltonen Foundation. The study sponsor had no role in any aspect of the study.
Conflicts of interest
The authors have no potential conflict of interest, real or perceived.
Supporting information
Table S1 The primers used in the qPCR analyses.
Acknowledgements
We would like to thank Tuija Poussa for statistical consulting and analyses.
References
- 1. Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014; 15: 760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stark PL, Lee A. The bacterial colonization of the large bowel of pre‐term low birth weight neonates. J Hyg (Lond) 1982; 89: 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rautava S, Luoto R, Salminen S, Isolauri E. Microbial contact during pregnancy, intestinal colonization and human disease. Nat Rev Gastroenterol Hepatol 2012; 9: 565–76. [DOI] [PubMed] [Google Scholar]
- 4. Kalliomäki M, Kirjavainen P, Eerola E, Kero P, Salminen S, Isolauri E. Distinct patterns of neonatal gut microflora in infants in whom allergy was and was not developing. J Allergy Clin Immunol 2001; 107: 129–34. [DOI] [PubMed] [Google Scholar]
- 5. Björkstén B, Sepp E, Julge K, Voor T, Mikelsaar M. Allergy development and the intestinal microflora during the first year of life. J Allergy Clin Immunol 2001; 108: 516–20. [DOI] [PubMed] [Google Scholar]
- 6. Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr 2008; 87: 534–8. [DOI] [PubMed] [Google Scholar]
- 7. Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren‐Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 2012; 20: 2257–61. [DOI] [PubMed] [Google Scholar]
- 8. Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease. A randomised placebo‐controlled trial. Lancet 2001; 357: 1076–9. [DOI] [PubMed] [Google Scholar]
- 9. Luoto R, Ruuskanen O, Waris M, Kalliomäki M, Salminen S, Isolauri E. Prebiotic and probiotic supplementation prevents rhinovirus infections in preterm infants. A randomized, placebo‐controlled trial. J Allergy Clin Immunol 2014; 133: 405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harmsen HJ, Wildeboer‐Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast‐fed and formula‐fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr 2000; 30: 61–7. [DOI] [PubMed] [Google Scholar]
- 11. Nylund L, Heilig HG, Salminen S, de Vos WM, Satokari R. Semiautomated extraction of microbial DNA from feces for qPCR and phylogenetic microarray analysis. J Microbiol Methods 2010; 83: 231–5. [DOI] [PubMed] [Google Scholar]
- 12. Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal‐weight women. Am J Clin Nutr 2008; 88: 894–9. [DOI] [PubMed] [Google Scholar]
- 13. Scalabrin DM, Mitmesser SH, Welling GW, Harris CL, Marunycz JD, Walker DC, et al. New prebiotic blend of polydextrose and galacto‐oligosaccharides has bifidogenic effect in young infants. J Pediatr Gastroenterol Nutr 2012; 54: 343–52. [DOI] [PubMed] [Google Scholar]
- 14. Arboleya S, Sánchez B, Solís G, Fernández N, Suárez M, Hernández‐Barranco AM, et al. Impact of prematurity and perinatal antibiotics on the developing intestinal microbiota: a functional inference study. Int J Mol Sci 2016; 17: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012; 3: 470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dörtbudak O, Eberhardt R, Ulm M, Persson GR. Periodontitis, a marker of risk in pregnancy for preterm birth. J Clin Periodontol 2005; 32: 45–52. [DOI] [PubMed] [Google Scholar]
- 17. Rougé C, Goldenberg O, Ferraris L, Berger B, Rochat F, Legrand A, et al. Investigation of the intestinal microbiota in preterm infants using different methods. Anaerobe 2010; 16: 362–70. [DOI] [PubMed] [Google Scholar]
- 18. Arboleya S, Binetti A, Salazar N, Fernández N, Solís G, Hernández‐Barranco A, et al. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol Ecol 2012; 79: 763–72. [DOI] [PubMed] [Google Scholar]
- 19. Sipola‐Leppänen M, Vääräsmäki M, Tikanmäki M, Matinolli HM, Miettola S, Hovi P, et al. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol 2015; 181: 861–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 The primers used in the qPCR analyses.


