Abstract
In the past 15 years, encouraging clinical results for the detection of small lymph node metastases was obtained by the use of Combidex‐enhanced MRI (CEM, also known as magnetic resonance lymphography). Withdrawal of the European Medicines Agency approval application by the manufacturer made it impossible for patients to benefit from this agent; a loss, especially for men with prostate cancer. Current conventional imaging techniques are not as accurate as CEM is, thus a surgical diagnostic exploration (extended lymph node dissection) is still the preferred technique to evaluate the lymph nodes, resulting in peri‐ and postoperative complications. In 2013, the Radboud University Medical Center (Radboudumc) obtained all licenses and documentation for the production process of Combidex (ferumoxtran‐10), and manufactured the contrast agent under supervision of the Department of Pharmacy. Since 2014, 310 men with prostate cancer have been examined with CEM in the Radboudumc. Within this cohort, seven minor possibly contrast‐related adverse effects were observed after administration of Combidex. As the contrast agent is now back again in the Netherlands, this review highlights the working mechanism, previous results, observed side effects since the reintroduction, and the future perspectives for Combidex. WIREs Nanomed Nanobiotechnol 2018, 10:e1471. doi: 10.1002/wnan.1471
This article is categorized under:
-
1
Diagnostic Tools > In Vivo Nanodiagnostics and Imaging
-
2
Therapeutic Approaches and Drug Discovery > Nanomedicine for Oncologic Disease
INTRODUCTION
Knowledge of the presence of lymph node metastases is important to determine the prognosis and therapy choice in cancer patients. Lymph node status in prostate cancer patients can either be assessed by lymphadenectomy or by imaging.
Because of the significant limitations of available preoperative imaging methods in the detection of (small) lymph node metastases of prostate cancer, an extended pelvic lymph node dissection (e‐PLND) is the gold standard to detect metastatic lymph nodes in intermediate and high‐risk prostate cancer patients (current European guidelines1). However, this procedure is invasive with a considerable complication rate and underestimates lymph node involvement by missing small and/or distant nodes, especially in the pararectal and internal iliac area. Only lymph nodes within the dissection field are harvested, and it is shown in a substantial percentage (60–85%) of proven lymph node‐positive patients that positive lymph nodes were present outside the (e‐)PLND region.2, 3, 4 Moreover, in 30% of the patients positive lymph nodes were found only outside the (e‐)PLND region. This results not only in an underestimation of lymph node involvement but also in a false‐negative diagnosis for the patient.2 A follow‐up study suggested a clinical benefit of removing as many metastatic lymph nodes as possible in comparison with subtotal removal, as was shown in a better 5‐year survival of 80% versus 35%, respectively.5 In a recent systematic review, however, a direct therapeutic effect was not evident, and more e‐PLNDs were associated with worse intraoperative and perioperative outcomes.6 They concluded that (e‐)PLND was only useful for staging. In addition, for lymph node‐negative patients, removal of lymph nodes is of no benefit to the patient, even though it puts them at risk for the surgical complications. An accurate noninvasive imaging technique for the detection of metastatic lymph nodes could solve these problems.
Imaging techniques for lymph node assessment can be divided into two groups: conventional (anatomical) and functional. Both are noninvasive. Conventional imaging techniques, i.e., computed tomography (CT) and anatomical magnetic resonance imaging (MRI), are widely available. These techniques rely on size criteria to determine lymph node status. As metastatic nodes in prostate cancer usually are small,7, 8, 9 the sensitivity of these techniques is low (30%).10 Not only in prostate cancer, but also in other cancers like gynecological, breast, and rectal cancer the value of size as a criterion for positive nodes is limited.11, 12, 13, 14
Newer, functional imaging techniques rely on hallmarks and behavior of metastatic cells in lymph nodes or on the lymphatic drainage from the primary tumor to surrounding lymph nodes.15 Different functional techniques have been investigated for prostate cancer. Choline positron emission tomography–CT (PET‐CT) appeared to be suitable for the detection of larger lymph node metastases but is unsatisfactory for nodes smaller than 7 mm.7 In recurrent prostate cancer, choline, PET‐CT may have some added value, but in primary intermediate‐ to high‐risk prostate cancer its sensitivity is poor (49%).16, 17
Recently, promising results have been shown for 68Ga‐labeled prostate‐specific membrane antigen (PSMA) ligand PET‐CT. This technique appears superior to choline PET‐CT for small lymph nodes18 and lower Prostate‐specific antigen (PSA) levels.19 A recent meta‐analysis for 68Ga‐PSMA PET included 16 articles involving 1309 patients. Summary sensitivity and specificity on a per‐patient basis were both 86%. On a per‐lesion analysis, summary sensitivity and specificity were 80% and 97%, respectively.20 The overall percentage of positive scans was higher for patients with biochemical recurrence than for patients with primary disease (76% vs 40%), and 68Ga‐PSMA PET‐CT positive patients had higher prescan PSA levels. But even with improved tumor specificity these new tracers appear not to be capable of reliably detecting metastatic lymph nodes below 5 mm in size,21, 22 as the median size of missed nodal metastases with 68Ga‐PSMA PET/CT was 4.3 mm.23 A disadvantage of PSMA ligands is uptake in other organs such as the kidney, small bowel loops, salivary glands, ureters, and the urinary bladder.24 Furthermore, reports are accumulating on uptake of the tracer in other cancers or benign conditions, like sarcoidosis and inflammation.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36
Since the beginning of the 1990s, encouraging results have been shown for Combidex‐enhanced MRI (CEM, also known as magnetic resonance lymphography). Combidex is a solution of iron oxide particles coated with dextran, which is slowly intravenously administered in patients 24–36 h prior to MRI. The ultra‐small superparamagnetic iron oxide (USPIO) particles are internalized by macrophages, which accumulate in normal lymph nodes. On iron‐sensitive MR‐images, these normal nodes lose MR signal and are black, whereas metastatic lymph nodes remain white.8 Moreover, MRI provides anatomical soft‐tissue contrast with a high spatial resolution. In comparison with lymphadenectomy, MRI can visualize nodes in the entire pelvis, upper abdomen, and the rest of the body, and can therefore identify positive lymph nodes outside the routine surgical field.2
Unfortunately, in 2009, Combidex was withdrawn from the registration process in Europe by the manufacturer. An alternative agent with iron oxide nanoparticles, Feraheme (ferumoxytol; AMAG Pharma, Waltham, MA, USA), was approved for therapeutic use as intravenous iron replacement therapy in anemia, and was used off‐label as an alternative for Combidex.37 However, in March 2015, the Food and Drug Administration (FDA) issued a ‘Boxed Warning’ regarding serious risks for Feraheme because of association with fatal anaphylaxis.38, 39 In addition, the ‘darkening’ of normal nodes with Feraheme did not appear as prominent as with Combidex, even with triple doses of iron oxide,40 resulting in inferior diagnostic quality compared with Combidex.
In 2013, the Radboud University Medical Center (Radboudumc) obtained all rights and documents regarding Combidex, and was able to manufacture the contrast agent again, exactly according to original specifications and under supervision of an accredited pharmacy. In 2015, all the rights were transferred to SPL Medical B.V. in Nijmegen, The Netherlands, who is currently the only rights owner and manufacturer of Combidex. With the availability of the agent in clinical practice in the Netherlands, the time is right to review its working mechanism and results from earlier studies. Furthermore, the reintroduction of Combidex, its clinical use, and side effects because of the reintroduction are described, together with the future perspectives for the contrast agent.
USPIO‐ENHANCED MRI: WORKING MECHANISM OF COMBIDEX
Combidex is a solution of dextran‐coated iron oxide nanoparticles (size 20–50 nm) intravenously administered with a slow‐drip infusion (30 min) to the patient 24–36 h prior to MRI imaging. These nanoparticles are ingested by macrophages, which accumulate in normal lymph nodes throughout the body. Metastatic lymph nodes will not pick up the iron nanoparticles (Figure 1(a) and (b)). Benign and metastatic lymph nodes can therefore be differentiated on an iron‐sensitive (T2*‐weighted) MRI sequence 24–36 h after administration of Combidex. Normal lymph nodes are black on these MR‐images, whereas nodes with metastases are white, retaining MR signal.41 This is an indirect functional imaging technique: it does not rely on minimal uptake in viable tumor tissue, and there is no issue with background uptake in organs. In a T1‐weighted MRI image series after the infusion of Combidex, the anatomy of vessels and nodes can be depicted (Figure 1(c)). Two pelvic lymph nodes would have been considered normal using MRI without Combidex. With CEM, a clear‐cut distinction is seen between the normal node (black), and the node almost totally replaced with metastatic prostate cancer (Figure 1(d), node almost all white).
Figure 1.
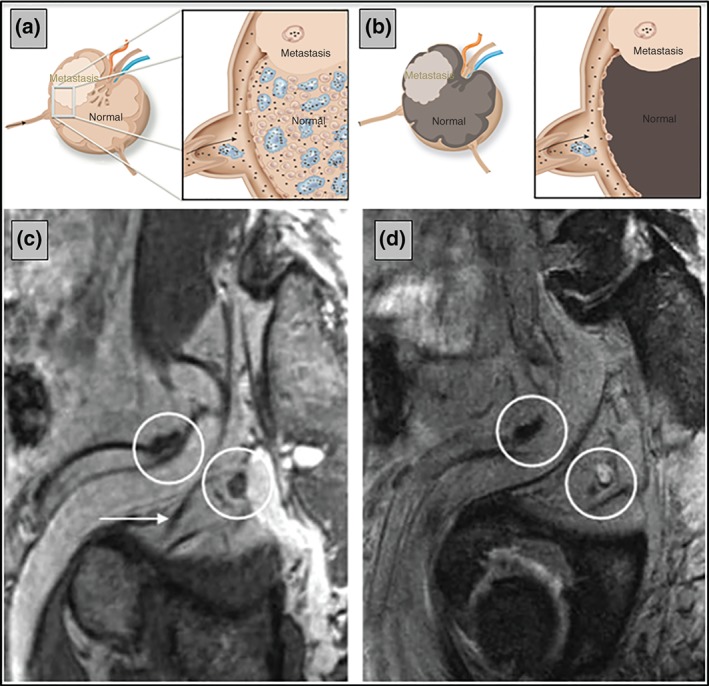
Mechanism of action of Combidex‐enhanced magnetic resonance imaging (MRI). In normal (parts of) healthy lymph nodes iron oxide nanoparticles accumulate (a), whereas metastases contain less nanoparticles 24–36 h after Combidex administration. In iron‐sensitive MRI scans, the nanoparticles cause the MR signal to disappear, while metastatic (parts of) lymph nodes retain MRI signal (b). On the T1‐weighted Combidex image of a patient with metastasized prostate cancer, two nodes (circles) are visible close to the obturator nerve (arrow in c). Both their sizes are normal. On the T2*‐weighted iron‐sensitive image, one of these nodes is black because of the presence of iron oxide nanoparticles, and thus is normal (left circle in d), whereas the metastatic node retained MR signal intensity and is white (right circle in d). Because of its position, 2 cm behind the obturator nerve, the metastatic node would not be removed during a typical pelvic lymph node dissection.
USPIO‐ENHANCED MRI: EARLIER RESULTS OF COMBIDEX
In the last 15 years, encouraging clinical results for CEM in patient use were shown with a pooled sensitivity of 90% and a pooled specificity of 96% for several types of cancer,42 including prostate cancer. The largest study with Combidex in prostate cancer was performed in 375 patients reporting sensitivity and specificity for the detection of lymph node metastasis of 82% and 93%, respectively, with histopathology of extended resections or guided lymph node biopsies as gold standard.43 All the studies evaluating CEM in prostate cancer were performed with an MRI system at a magnetic field strength of 1.5 T. A study comparing image quality of CEM at 1.5 T with 3.0 T showed improvements at the higher field strength,44 but the study was not designed to compare the detection of (small) metastatic lymph nodes. A Swiss research group published CEM studies combining prostate and bladder cancer using a field strength of 3 T.45, 46, 47 Their specificity of metastatic lymph node detection (87–96%) was in agreement with earlier results, but their sensitivity (65–80%) was lower, probably because of the use of a T2‐weighted, rather than a T2*‐weighted MRI sequence.48
Added clinical value of CEM was revealed in studies that showed positive lymph nodes outside the standard pelvic lymph node dissection area2 and standard radiation field49, 50 in a substantial number of patients (53–79%). A recent whole body MRI study for the assessment of metastatic spread in prostate cancer underlined these findings.4 With CEM an underestimation of metastatic disease, and consequent mismatch in treatment, can be avoided.
Several CEM studies emphasized the capability of finding metastases in normal‐sized lymph nodes.7, 8, 45, 49 This is a major step forward compared with conventional and established functional imaging techniques. Detecting small lymph node metastases or detecting limited metastatic disease provides opportunities for more patient‐tailored therapy. With CEM as a technique to (re)define oligometastatic disease, these therapies might even be given with curative intent.4, 5, 51, 52
Furthermore, knowledge on size of lymph node metastases can help predict prognosis. Patients with nodal metastases ≤8 mm versus larger nodal metastases have a significantly better 5‐year metastasis‐free survival (79% vs 16%) and overall survival (81% vs 36%).5 CEM can help to identify these small positive lymph nodes as the technique enables detection of (pathologic) lymph nodes as small as 2 mm.7
USPIOs BACK ON THE BLOCK: REINTRODUCTION PROCESS OF COMBIDEX
At present time, there is no alternative contrast agent or medical device that yields similar clinical results as CEM for detection of small metastatic lymph nodes in prostate2, 7, 8, 15, 43, 44, 49, 50, 51, 53, 54, 55, 56, 57, 58, 59 and other cancers.42 Radboudumc therefore decided to revive the production of Combidex and obtained all documents and rights for Combidex from its previous manufacturer, AMAG Pharma.
The first four production batches of Combidex for clinical patient use (intended for Radboudumc patients only) were produced and used as a legitimate exception of National and European Union (EU) legislation.60 Requirements for this category of products are presence of a sound rationale to use the product (motivation and justification of clinical need), lack of alternative medication with a marketing authorization, and preparation and control according to the European or National Pharmacopoeia.61 The drug has to be a medicinal product manufactured and supplied directly to the patients served by the pharmacy in question.60 The production of Combidex was outsourced to qualified and authorized companies [good manufacturing practice (GMP) production] under the supervision of the Department of Pharmacy of Radboudumc under Dutch law (Dutch Medicines Act and Directive 2001/83/EC). The department instituted a control process by performing audits and reviewing the required validation and stability protocols and reports. Specifications of Combidex regarding appearance, iron‐, dextran‐, citrate content, particle size, magnetic susceptibility, clustering of particles, pH value, sterility, endotoxins, and bioburden were reviewed by the qualified person of the Pharmacy Department of Radboudumc, who released the final product.
In 2015, all the rights were transferred to SPL Medical B.V. in Nijmegen, The Netherlands, who is currently the only rights owner and the only manufacturer of Combidex, and produces Combidex under GMP conditions.
CLINICAL USE OF COMBIDEX IN PROSTATE CANCER PATIENTS SINCE 2014
In 2014, Radboudumc launched ‘nano‐MRI,’ reintroducing CEM in patients with prostate cancer. Between January 2014 and July 2016, 310 men, with intermediate to high risk for lymph node metastases or suspicious for recurrent disease, underwent a 3‐T MRI after intravenous Combidex administration in our hospital (e.g., in Figure 2). Thirty of these patients underwent nano‐MRI twice. Demographic data of the patients are presented in Table 1.
Figure 2.
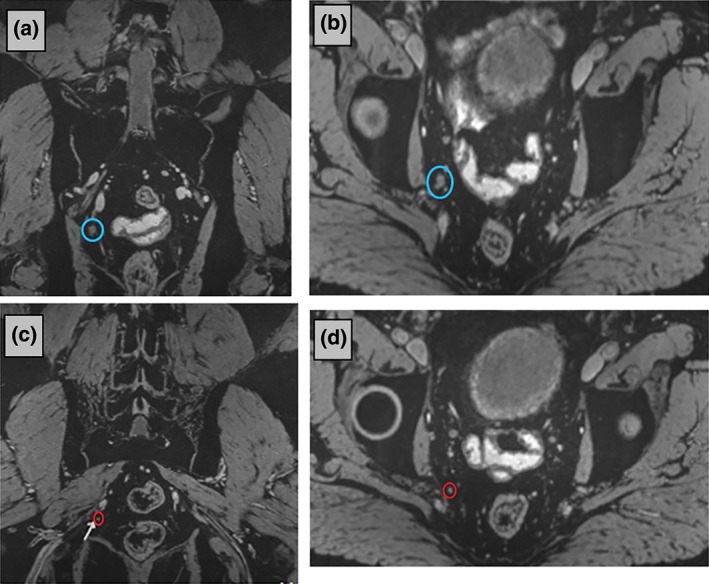
Nano‐magnetic resonance imaging (MRI) (Combidex‐enhanced MRI) at 3 T of a 53‐year‐old patient with recurrent prostate cancer after radical prostatectomy and radiotherapy (posttherapy PSA‐level 3.9 ng/mL). Twenty‐seven hours after administration of Combidex, metastatic lymph nodes remain white on the three‐dimensional (3D) iron‐sensitive MR‐images in contrast to benign lymph nodes who become black. A large (7 mm) metastatic lymph node was visible on nano‐MRI as a white spherical structure in two orthogonal planes through the node [blue circles in coronal (a) and axial images (b)]. A smaller metastatic white node (2 mm) is indicated with red circles in the coronal (c) and axial (d) reconstructions (orthogonal planes through the node of interest) of the 3D data set. The other small spherical structures are blood vessels, best appreciated when scrolling through the 3D image data set. Parameters: 3D T2*‐weighted MRI with echo time 12 ms (two combined echoes), resolution 0.85 × 0.85 × 0.85 mm, acquisition time 10:10 min.
Table 1.
Demographics of 310 Prostate Cancer Patients Studied with Nano‐Magnetic Resonance Imaging (January 2014–July 2016)
| Age (mean/median) | 64.7/65.0 years |
| Weight (mean/median) | 84.8/83.5 kg |
| Patients with prior allergic reactions | 7.4% |
| Patients with prior contrast reactions (iodine) | 1.0% |
| Newly diagnosed patients | 14.8% |
| Patients with PSA recurrence after therapy | 85.2% |
Patients were administered 2.6 mg Fe/kg Combidex, diluted in 100 mL 0.9% sodium chloride using a Minisart NML 0.22 µm pore size filter (Minisart NML Syringe Filters 16534‐k; Sartorius AG, Goettingen, Germany). Combidex was intravenously infused over at least 30 min. At the start of the infusion, a very slow infusion rate of 1 mL/min was used, which was increased after 5 min to an average infusion rate of 4 mL/min.
For patient safety, the contrast was given with direct access to supportive agents in case of severe or serious contrast reactions. A radiologist supervised each contrast administration procedure from the start of infusion until approximately 30 min after completion. If an adverse event was observed by the radiologist or reported by the patient, appropriate action was taken. Twenty‐four to 36 h later, when the patients returned for the MRI scan, patients were asked again about the occurrence of any adverse side effects. All adverse events were assessed by the radiologist and stratified into five grades according to the Common Terminology Criteria for Adverse Events,62 and noted in the patient's file and in a separate locked research database.
The radiologist made the judgment if the adverse effect was Combidex‐related. Technically, all nano‐MRI examinations were successful and of good quality, showing loss of MR signal because of Combidex in healthy lymph nodes in all patients, and showing white lymph nodes indicating metastases (Figure 2).
Adverse Effects
Eight of 310 patients (2.6%) had adverse effects. Seven of these patients (2.3%) were judged definitely (4) or possibly (3) contrast‐related. In four of these patients, the effects occurred during the beginning of the infusion (minor low back pain, flushing, and nausea). The infusion was temporarily discontinued in these patients whereupon the complaints disappeared. After several minutes, the infusion was restarted slowly and could be finished without any further complications. The other three patients complained of a dry mouth directly after completion of Combidex infusion. This disappeared in all before they left the department. As no treatment or intervention was needed, the adverse events were stratified as Grade 1 (Table 2).
Table 2.
Patients with Combidex Administration‐Related Adverse Events
| Type of Adverse Event, All Were Grade 1 | Number of Patients |
|---|---|
| Back pain | 2/310 (contrast‐related) |
| Nausea | 1/310 (contrast‐related) |
| Flushing/feeling hot | 1/310 (contrast‐related) |
| Dry mouth | 3/310 (possibly contrast‐related) |
Additionally, one patient with Cushing's disease had an acute adrenal crisis 2 h after Combidex infusion. He was treated successfully by outpatient care. The next day he underwent an uneventful nano‐MRI. The adrenal crisis was considered stress‐related, and not contrast agent‐related.
In 2009, Bernd et al. reported safety data of 37 clinical trials with in total 1663 patients who received Combidex.63 Adverse events were reported in 23% of patients subdivided into Grade 1 mild (55%), Grade 2 moderate (30%), Grade 4 severe (12%), and Grade 5 serious (3%).
Low back pain, flushing, and nausea (Table 2) occurred also in the earlier studies. The dry mouth reported in three of our patients was not reported before, but as it appeared immediately after the infusion was completed, it was considered possibly contrast‐related. The absence of moderate or serious (Grades 2–5) Combidex‐related adverse events in the current cohort is in agreement with a cohort study performed by our institute, published in 2008,43 where only mild (Grade 1) adverse events occurred in 21 of 375 (6%) patients. The explanation may be the similar way of the infusion: always diluted, very slow at the start, with immediate discontinuation of the infusion upon complaints. In the current study, we also added a filter in the infusion line.
FUTURE PERSPECTIVES FOR COMBIDEX
The Radboudumc spin‐off company SPL Medical B.V. was set up in 2015 with the aim to promote and to register Combidex in different regions in the world, and to make it available for as many patients as possible. Next to clinical use in prostate cancer patients in Radboudumc, clinical research is planned with the use of CEM in other types of cancer.
Recently our group succeeded in high‐resolution MR imaging of pelvic lymph nodes at a magnetic field strength of 7 T.64 Lymph nodes were clearly identifiable down to sizes of 1.5 mm. This opens possibilities for detection of even smaller metastatic lymph nodes with CEM at 7 T. Apart from oncologic applications, Combidex has shown value in preliminary studies in a multitude of diseases; promising applications that now can be explored again.
CONCLUSION
Radboudumc successfully reintroduced Combidex‐enhanced MRI as nano‐MRI, and currently uses it in patients with prostate cancer to detect small metastatic lymph nodes. A total of 310 patients had Combidex administered using a very slow drip infusion, and only in 2.6% of these patients mild Grade 1 Combidex‐related or possibly Combidex‐related adverse events occurred. The good safety profile and the rebirth of Combidex provide new opportunities for lymph node staging in patients with prostate cancer, and opens up possibilities for patients with other cancers and other diseases.
Conflicts of interest: J.O. Barentsz is a member of the advisory board of SPL Medical B.V., but has no financial interest in this company (SPL Medical is the owner of Combidex).
REFERENCES
- 1. Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent‐update 2013. Eur Urol 2014, 65:124–137. [DOI] [PubMed] [Google Scholar]
- 2. Heesakkers RA, Jager GJ, Hovels AM, de Hoop B, van den Bosch HC, Raat F, Witjes JA, Mulders PF, van der Kaa CH, Barentsz JO. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran‐10‐enhanced MR imaging. Radiology 2009, 251:408–414. [DOI] [PubMed] [Google Scholar]
- 3. Joniau S, Van den Bergh L, Lerut E, Deroose CM, Haustermans K, Oyen R, Budiharto T, Ameye F, Bogaerts K, Van Poppel H. Mapping of pelvic lymph node metastases in prostate cancer. Eur Urol 2013, 63:450–458. [DOI] [PubMed] [Google Scholar]
- 4. Larbi A, Dallaudiere B, Pasoglou V, Padhani A, Michoux N, Vande Berg BC, Tombal B, Lecouvet FE. Whole body MRI (WB‐MRI) assessment of metastatic spread in prostate cancer: therapeutic perspectives on targeted management of oligometastatic disease. Prostate 2016, 76:1024–1033. [DOI] [PubMed] [Google Scholar]
- 5. Meijer HJ, Debats OA, van Lin EN, Witjes JA, Kaanders JH, Barentsz JO. A retrospective analysis of the prognosis of prostate cancer patients with lymph node involvement on MR lymphography: who might be cured. Radiat Oncol 2013, 8:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fossati N, Willemse PM, van den Bergh RC, Van den Broeck T, Yuan CY, Briers E, Bellmunt J, Bolla M, Cornford P, De Santis M, et al. The benefits and harms of different extents of lymph node dissection during radical prostatectomy for prostate cancer: a systematic review. Eur Urol 2017. doi:http://dx.doi.org/10.1016/j.eururo.2016.12.00. [DOI] [PubMed] [Google Scholar]
- 7. Fortuin AS, Deserno WM, Meijer HJ, Jager GJ, Takahashi S, Debats OA, Reske SN, Schick C, Krause BJ, van Oort I, et al. Value of PET/CT and MR lymphography in treatment of prostate cancer patients with lymph node metastases. Int J Radiat Oncol Biol Phys 2012, 84:712–718. [DOI] [PubMed] [Google Scholar]
- 8. Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph‐node metastases in prostate cancer. N Engl J Med 2003, 348:2491–2499. [DOI] [PubMed] [Google Scholar]
- 9. Harisinghani MG, Barentsz JO, Hahn PF, Deserno W, de la Rosette J, Saini S, Marten K, Weissleder R. MR lymphangiography for detection of minimal nodal disease in patients with prostate cancer. Acad Radiol 2002, 9(suppl 2):S312–S313. [DOI] [PubMed] [Google Scholar]
- 10. Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, Severens JL, Barentsz JO. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta‐analysis. Clin Radiol 2008, 63:387–395. [DOI] [PubMed] [Google Scholar]
- 11. Dworak O. Number and size of lymph nodes and node metastases in rectal carcinomas. Surg Endosc 1989, 3:96–99. [DOI] [PubMed] [Google Scholar]
- 12. Harada T, Tanigawa N, Matsuki M, Nohara T, Narabayashi I. Evaluation of lymph node metastases of breast cancer using ultrasmall superparamagnetic iron oxide‐enhanced magnetic resonance imaging. Eur J Radiol 2007, 63:401–407. [DOI] [PubMed] [Google Scholar]
- 13. Kotanagi H, Fukuoka T, Shibata Y, Yoshioka T, Aizawa O, Saito Y, Tur GE, Koyama K. The size of regional lymph nodes does not correlate with the presence or absence of metastasis in lymph nodes in rectal cancer. J Surg Oncol 1993, 54:252–254. [DOI] [PubMed] [Google Scholar]
- 14. Rockall AG, Sohaib SA, Harisinghani MG, Babar SA, Singh N, Jeyarajah AR, Oram DH, Jacobs IJ, Shepherd JH, Reznek RH. Diagnostic performance of nanoparticle‐enhanced magnetic resonance imaging in the diagnosis of lymph node metastases in patients with endometrial and cervical cancer. J Clin Oncol 2005, 23:2813–2821. [DOI] [PubMed] [Google Scholar]
- 15. Fortuin AS, Meijer H, Thompson LC, Witjes JA, Barentsz JO. Ferumoxtran‐10 ultrasmall superparamagnetic iron oxide‐enhanced diffusion‐weighted imaging magnetic resonance imaging for detection of metastases in normal‐sized lymph nodes in patients with bladder and prostate cancer: do we enter the era after extended pelvic lymph node dissection? Eur Urol 2013, 64:961–963; discussion 963. [DOI] [PubMed] [Google Scholar]
- 16. Evangelista L, Zattoni F, Guttilla A, Saladini G, Zattoni F, Colletti PM, Rubello D. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta‐analysis. Clin Nucl Med 2013, 38:305–314. [DOI] [PubMed] [Google Scholar]
- 17. Evangelista L, Guttilla A, Zattoni F, Muzzio PC, Zattoni F. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate‐ to high‐risk prostate cancer: a systematic literature review and meta‐analysis. Eur Urol 2013, 63:1040–1048. [DOI] [PubMed] [Google Scholar]
- 18. Bluemel C, Krebs M, Polat B, Linke F, Eiber M, Samnick S, Lapa C, Lassmann M, Riedmiller H, Czernin J, et al. 68Ga‐PSMA‐PET/CT in patients with biochemical prostate cancer recurrence and negative 18F‐Choline‐PET/CT. Clin Nucl Med 2016, 41:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morigi JJ, Stricker PD, van Leeuwen PJ, Tang R, Ho B, Nguyen Q, Hruby G, Fogarty G, Jagavkar R, Kneebone A, et al. Prospective comparison of 18F‐fluoromethylcholine versus 68Ga‐PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med 2015, 56:1185–1190. [DOI] [PubMed] [Google Scholar]
- 20. Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, Bolton D, Lawrentschuk N. Sensitivity, specificity, and predictors of positive 68Ga‐prostate‐specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta‐analysis. Eur Urol 2016, 70:926–937. [DOI] [PubMed] [Google Scholar]
- 21. Jadvar H. Molecular imaging of prostate cancer with PET. J Nucl Med 2013, 54:1685–1688. [DOI] [PubMed] [Google Scholar]
- 22. Kahkonen E, Jambor I, Kemppainen J, Lehtio K, Gronroos TJ, Kuisma A, Luoto P, Sipila HJ, Tolvanen T, Alanen K, et al. In vivo imaging of prostate cancer using [68Ga]‐labeled bombesin analog BAY86‐7548. Clin Cancer Res 2013, 19:5434–5443. [DOI] [PubMed] [Google Scholar]
- 23. Budaus L, Leyh‐Bannurah SR, Salomon G, Michl U, Heinzer H, Huland H, Graefen M, Steuber T, Rosenbaum C. Initial experience of Ga‐PSMA PET/CT imaging in high‐risk prostate cancer patients prior to radical prostatectomy. Eur Urol 2015, 69:393–396. [DOI] [PubMed] [Google Scholar]
- 24. Afshar‐Oromieh A, Hetzheim H, Kratochwil C, Benesova M, Eder M, Neels OC, Eisenhut M, Kubler W, Holland‐Letz T, Giesel FL, et al. The theranostic PSMA ligand PSMA‐617 in the diagnosis of prostate cancer by PET/CT: biodistribution in humans, radiation dosimetry, and first evaluation of tumor lesions. J Nucl Med 2015, 56:1697–1705. [DOI] [PubMed] [Google Scholar]
- 25. Bourgeois S, Gykiere P, Goethals L, Everaert H, De Geeter FW. Aspecific uptake of 68Ga‐PSMA in Paget disease of the bone. Clin Nucl Med 2016, 41:877–878. [DOI] [PubMed] [Google Scholar]
- 26. Einspieler I, Tauber R, Maurer T, Schwaiger M, Eiber M. 68Ga prostate‐specific membrane antigen uptake in renal cell cancer lymph node metastases. Clin Nucl Med 2016, 41:e261–e262. [DOI] [PubMed] [Google Scholar]
- 27. Gykiere P, Goethals L, Everaert H. Healing sacral fracture masquerading as metastatic bone disease on a 68Ga‐PSMA PET/CT. Clin Nucl Med 2016, 41:e346–e347. [DOI] [PubMed] [Google Scholar]
- 28. Huang YT, Fong W, Thomas P. Rectal carcinoma on 68Ga‐PSMA PET/CT. Clin Nucl Med 2016, 41:e167–e168. [DOI] [PubMed] [Google Scholar]
- 29. Kanthan GL, Coyle L, Kneebone A, Schembri GP, Hsiao E. Follicular lymphoma showing avid uptake on 68Ga PSMA‐HBED‐CC PET/CT. Clin Nucl Med 2016, 41:500–501. [DOI] [PubMed] [Google Scholar]
- 30. Kanthan GL, Drummond J, Schembri GP, Izard MA, Hsiao E. Follicular thyroid adenoma showing avid uptake on 68Ga PSMA‐HBED‐CC PET/CT. Clin Nucl Med 2016, 41:331–332. [DOI] [PubMed] [Google Scholar]
- 31. Kanthan GL, Hsiao E, Kneebone A, Eade T, Schembri GP. Desmoid tumor showing intense uptake on 68Ga PSMA‐HBED‐CC PET/CT. Clin Nucl Med 2016, 41:508–509. [DOI] [PubMed] [Google Scholar]
- 32. Kanthan GL, Izard MA, Emmett L, Hsiao E, Schembri GP. Schwannoma showing avid uptake on 68Ga‐PSMA‐HBED‐CC PET/CT. Clin Nucl Med 2016, 41:703–704. [DOI] [PubMed] [Google Scholar]
- 33. Law WP, Fiumara F, Fong W, Miles KA. Gallium‐68 PSMA uptake in adrenal adenoma. J Med Imaging Radiat Oncol 2016, 60:514–517. [DOI] [PubMed] [Google Scholar]
- 34. Lawal I, Vorster M, Boshomane T, Ololade K, Ebenhan T, Sathekge M. Metastatic prostate carcinoma presenting as a superscan on 68Ga‐PSMA PET/CT. Clin Nucl Med 2015, 40:755–756. [DOI] [PubMed] [Google Scholar]
- 35. Noto B, Vrachimis A, Schafers M, Stegger L, Rahbar K. Subacute stroke mimicking cerebral metastasis in 68Ga‐PSMA‐HBED‐CC PET/CT. Clin Nucl Med 2016, 41:e449–e451. [DOI] [PubMed] [Google Scholar]
- 36. Sager S, Vatankulu B, Uslu L, Sonmezoglu K. Incidental detection of follicular thyroid carcinoma in 68Ga‐PSMA PET/CT imaging. J Nucl Med Technol 2016, 44:199–200. [DOI] [PubMed] [Google Scholar]
- 37. Turkbey B, Agarwal HK, Shih J, Bernardo M, McKinney YL, Daar D, Griffiths GL, Sankineni S, Johnson L, Grant KB, et al. A phase I dosing study of ferumoxytol for MR lymphography at 3 T in patients with prostate cancer. Am J Roentgenol 2015, 205:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McCulley L, Gelperin K, Bird S, Harris S, Wang C, Waldron P. Reports to FDA of fatal anaphylaxis associated with intravenous iron products. Am J Hematol 2016, 91:E496–E497. [DOI] [PubMed] [Google Scholar]
- 39. Medwatch . Feraheme (ferumoxytol): Drug safety communication – warnings strengthened and prescribing instructions changed. Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm440479.htm.
- 40. Debats OA, Fortuin AS, Meijer HJ, Hambrock T, Litjens GJ, Barentsz JO, Huisman HJ. Intranodal signal suppression in pelvic MR lymphography of prostate cancer patients: a quantitative comparison of ferumoxtran‐10 and ferumoxytol. PeerJ 2016, 4:e2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harisinghani MG, Saini S, Weissleder R, Hahn PF, Yantiss RK, Tempany C, Wood BJ, Mueller PR. MR lymphangiography using ultrasmall superparamagnetic iron oxide in patients with primary abdominal and pelvic malignancies: radiographic‐pathologic correlation. Am J Roentgenol 1999, 172:1347–1351. [DOI] [PubMed] [Google Scholar]
- 42. Wu L, Cao Y, Liao C, Huang J, Gao F. Diagnostic performance of USPIO‐enhanced MRI for lymph‐node metastases in different body regions: a meta‐analysis. Eur J Radiol 2011, 80:582–589. [DOI] [PubMed] [Google Scholar]
- 43. Heesakkers RA, Hovels AM, Jager GJ, van den Bosch HC, Witjes JA, Raat HP, Severens JL, Adang EM, van der Kaa CH, Futterer JJ, et al. MRI with a lymph‐node‐specific contrast agent as an alternative to CT scan and lymph‐node dissection in patients with prostate cancer: a prospective multicohort study. Lancet Oncol 2008, 9:850–856. [DOI] [PubMed] [Google Scholar]
- 44. Heesakkers RA, Futterer JJ, Hovels AM, van den Bosch HC, Scheenen TW, Hoogeveen YL, Barentsz JO. Prostate cancer evaluated with ferumoxtran‐10‐enhanced T2*‐weighted MR imaging at 1.5 and 3.0 T: early experience. Radiology 2006, 239:481–487. [DOI] [PubMed] [Google Scholar]
- 45. Birkhauser FD, Studer UE, Froehlich JM, Triantafyllou M, Bains LJ, Petralia G, Vermathen P, Fleischmann A, Thoeny HC. Combined ultrasmall superparamagnetic particles of iron oxide‐enhanced and diffusion‐weighted magnetic resonance imaging facilitates detection of metastases in normal‐sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol 2013, 64:953–960. [DOI] [PubMed] [Google Scholar]
- 46. Thoeny HC, Triantafyllou M, Birkhaeuser FD, Froehlich JM, Tshering DW, Binser T, Fleischmann A, Vermathen P, Studer UE. Combined ultrasmall superparamagnetic particles of iron oxide‐enhanced and diffusion‐weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal‐sized nodes of bladder and prostate cancer patients. Eur Urol 2009, 55:761–769. [DOI] [PubMed] [Google Scholar]
- 47. Triantafyllou M, Studer UE, Birkhauser FD, Fleischmann A, Bains LJ, Petralia G, Christe A, Froehlich JM, Thoeny HC. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur J Cancer 2013, 49:616–624. [DOI] [PubMed] [Google Scholar]
- 48. Fortuin AS, Barentsz JO. Comments on Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer, Triantafyllou et al., European Journal of Cancer, published online 22 October 2012. Eur J Cancer 2013, 49:1789–1790. [DOI] [PubMed] [Google Scholar]
- 49. Meijer HJ, Fortuin AS, van Lin EN, Debats OA, Alfred Witjes J, Kaanders JH, Barentsz JO. Geographical distribution of lymph node metastases on MR lymphography in prostate cancer patients. Radiother Oncol 2013, 106:59–63. [DOI] [PubMed] [Google Scholar]
- 50. Meijer HJ, van Lin EN, Debats OA, Witjes JA, Span PN, Kaanders JH, Barentsz JO. High occurrence of aberrant lymph node spread on magnetic resonance lymphography in prostate cancer patients with a biochemical recurrence after radical prostatectomy. Int J Radiat Oncol Biol Phys 2012, 82:1405–1410. [DOI] [PubMed] [Google Scholar]
- 51. Fortuin AS, Smeenk RJ, Meijer HJ, Witjes AJ, Barentsz JO. Lymphotropic nanoparticle‐enhanced MRI in prostate cancer: value and therapeutic potential. Curr Urol Rep 2014, 15:389. [DOI] [PubMed] [Google Scholar]
- 52. Hijazi S, Meller B, Leitsmann C, Strauss A, Meller J, Ritter CO, Lotz J, Schildhaus HU, Trojan L, Sahlmann CO. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga‐PSMA‐positron emission tomography/computerized tomography. Prostate 2015, 75:1934–1940. [DOI] [PubMed] [Google Scholar]
- 53. Barentsz JO, Futterer JJ, Takahashi S. Use of ultrasmall superparamagnetic iron oxide in lymph node MR imaging in prostate cancer patients. Eur J Radiol 2007, 63:369–372. [DOI] [PubMed] [Google Scholar]
- 54. Deserno WM, Harisinghani MG, Taupitz M, Jager GJ, Witjes JA, Mulders PF, Hulsbergen van der Kaa, Kaufmann D, Barentsz JO. Urinary bladder cancer: preoperative nodal staging with ferumoxtran‐10‐enhanced MR imaging. Radiology 2004, 233:449–456. [DOI] [PubMed] [Google Scholar]
- 55. Hovels AM, Heesakkers RA, Adang EM, Barentsz JO, Jager GJ, Severens JL. Cost‐effectiveness of MR lymphography for the detection of lymph node metastases in patients with prostate cancer. Radiology 2009, 252:729–736. [DOI] [PubMed] [Google Scholar]
- 56. Hovels AM, Heesakkers RA, Adang EM, Jager GJ, Barentsz JO. Cost‐analysis of staging methods for lymph nodes in patients with prostate cancer: MRI with a lymph node‐specific contrast agent compared to pelvic lymph node dissection or CT. Eur Radiol 2004, 14:1707–1712. [DOI] [PubMed] [Google Scholar]
- 57. Meijer HJ, Debats OA, Kunze‐Busch M, van Kollenburg P, Leer JW, Witjes JA, Kaanders JH, Barentsz JO, van Lin EN. Magnetic resonance lymphography‐guided selective high‐dose lymph node irradiation in prostate cancer. Int J Radiat Oncol Biol Phys 2012, 82:175–183. [DOI] [PubMed] [Google Scholar]
- 58. Meijer HJ, Debats OA, Roach M 3rd, Span PN, Witjes JA, Kaanders JH, van Lin EN, Barentsz JO. Magnetic resonance lymphography findings in patients with biochemical recurrence after prostatectomy and the relation with the Stephenson nomogram. Int J Radiat Oncol Biol Phys 2012, 84:1186–1191. [DOI] [PubMed] [Google Scholar]
- 59. Weidner AM, van Lin EN, Dinter DJ, Rozema T, Schoenberg SO, Wenz F, Barentsz JO, Lohr F. Ferumoxtran‐10 MR lymphography for target definition and follow‐up in a patient undergoing image‐guided, dose‐escalated radiotherapy of lymph nodes upon PSA relapse. Strahlenther Onkol 2011, 187:206–212. [DOI] [PubMed] [Google Scholar]
- 60. Dutch Medicines Act . Chap 4, par 1, art 40.1‐40.3a. Available at: http://wetten.overheid.nl/BWBR0021505/2009‐07‐01.
- 61. Decree Dutch Medicines Act . Par 2, art 2. Available at: http://wetten.overheid.nl/BWBR0021672/2007‐07‐01.
- 62. U.S. Department of Health and Human Services . Common terminology criteria for adverse events. Available at: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf.
- 63. Bernd H, De Kerviler E, Gaillard S, Bonnemain B. Safety and tolerability of ultrasmall superparamagnetic iron oxide contrast agent: comprehensive analysis of a clinical development program. Invest Radiol 2009, 44:336–342. [DOI] [PubMed] [Google Scholar]
- 64. Philips BWJ, Fortuin AS, Orzada S, Scheenen TWJ, Maas MC. High resolution MR imaging of pelvic lymph nodes at 7 Tesla. Magn Reson Med 2016. doi:10.1002/mrm.26498. [DOI] [PubMed] [Google Scholar]


