Abstract
Approximately 2 million Japanese individuals are infected with hepatitis C virus and are at risk for cirrhosis, end‐stage liver disease, and hepatocellular carcinoma. Patients in whom interferon (IFN)/ribavirin (RBV) therapy has failed remain at risk as effective therapeutic options are limited. This phase 2, randomized, open‐label study evaluated an IFN‐ and RBV‐free regimen of once‐daily ombitasvir (ABT‐267), an NS5A inhibitor, plus paritaprevir (ABT‐450), an NS3/4A protease inhibitor dosed with ritonavir (paritaprevir/ritonavir), in pegylated IFN/RBV treatment–experienced Japanese patients with hepatitis C virus subtype 1b or genotype 2 infection. Patients without cirrhosis (aged 18‐75 years) with subtype 1b infection received ombitasvir 25 mg plus paritaprevir/ritonavir 100/100 mg or 150/100 mg for 12 or 24 weeks; patients with genotype 2 infection received ombitasvir 25 mg plus paritaprevir/ritonavir 100/100 mg or 150/100 mg for 12 weeks. Sustained virologic response (SVR) at posttreatment week 24 (SVR24) was the primary endpoint. Adverse events were collected throughout the study. One hundred ten patients received ≥1 dose of study medication. In the subtype 1b cohort, SVR24 rates were high (88.9%‐100%) regardless of paritaprevir dose or treatment duration. In the genotype 2 cohort, SVR24 rates were 57.9% and 72.2% with 100 mg and 150 mg of paritaprevir, respectively. The SVR24 rate was higher in patients with subtype 2a (90%) than 2b (27%). Concordance between SVR12 and SVR24 was 100%. The most common adverse events overall were nasopharyngitis (29%) and headache (14%). Conclusion: In this difficult‐to‐treat population of patients in whom prior pegylated IFN/RBV had failed, ombitasvir/paritaprevir/ritonavir demonstrated potent antiviral activity with a favorable safety profile among Japanese patients with hepatitis C virus genotype 1b or 2a infection. (Hepatology 2015;61:1523–1532)
Abbreviations
- AE
adverse event
- CI
confidence interval
- DAA
direct‐acting antiviral agent
- HCV
hepatitis C virus
- LLOQ
lower limit of quantification
- pegIFN
pegylated interferon
- RAV
resistance‐associated variant
- RBV
ribavirin
- SAE
serious adverse event
- SVR
sustained virologic response (SVR4, SVR12, and SVR24, SVR 4, 12, and 24 weeks after end of treatment)
Chronic hepatitis C viral (HCV) infection is a significant global health problem affecting approximately 170 million people worldwide and causing almost 500,000 deaths each year from HCV–related liver disease.1 Phylogenetic studies suggest that the HCV epidemic was introduced in waves across the globe; HCV began to infect large numbers of Japanese youth in the 1920s, southern Europeans in the 1940s, and North Americans in the 1960s and 1970s.2 Although HCV seroprevalence is similar among these geographic areas, the impact of HCV‐related morbidity and mortality has been highest among the Japanese population, where HCV‐associated hepatocellular carcinoma rates are three‐fold higher than in Italy and six‐fold higher than in the United States. Of the 2 million Japanese patients who are seropositive, roughly 70% are infected with subtype 1b, 20% with subtype 2a, and the remainder with subtype 2b.3 In contrast to the United States and many parts of Europe, only 1%‐2.5% of Japanese patients carry subtype 1a.4
Because the risk of HCV‐related morbidity is clearly linked to duration of infection, there is an urgent need for potent therapeutic interventions among Japanese patients. Although the addition of the first‐generation protease inhibitors telaprevir and boceprevir improved the overall efficacy rates of pegylated interferon (pegIFN) plus ribavirin (RBV) in patients with genotype 1 infection in the United States, Europe, Asia, and Japan, the adverse event (AE) profile was also additive.5, 6, 7, 8, 9 In addition to the well‐known side effects of IFN‐based therapy, rash was seen in 50% of patients receiving telaprevir, and the risk of anemia increased significantly among patients who received either telaprevir or boceprevir. Although the protease inhibitor simeprevir was shown to be efficacious and better tolerated in Japanese patients with genotype 1 infection in combination with pegIFN/RBV, patients still experienced the AEs associated with an IFN‐based therapeutic backbone, such as severe fatigue, depression, poor appetite, and weight loss.10, 11
In addition, the effect of triple therapy on efficacy was blunted among those with a history of prior treatment failure with pegIFN/RBV therapy. For example, in Japanese patients with genotype 2 infection, the rate of sustained virologic response (SVR) with IFN‐based regimens ranged from 64% and 82% in treatment‐naive patients12, 13, 14 compared with only 40% in treatment‐experienced patients.12 Response also varied by subtype, with subtype 2a having higher SVR rates compared with subtype 2b.12, 13
Combination therapy with direct‐acting antiviral (DAA) agents with different mechanisms of action have demonstrated promising efficacy rates and favorable tolerability profiles in patients with HCV.15, 16, 17 Ombitasvir (formerly ABT‐267) is an HCV NS5A inhibitor dosed once daily. Paritaprevir (formerly ABT‐450) is an HCV NS3/4A protease inhibitor that is administered with low‐dose ritonavir (paritaprevir/ritonavir) to increase paritaprevir plasma levels and half‐life, enabling once‐daily dosing.18 Both ombitasvir and paritaprevir have potent in vitro antiviral activity against multiple subtypes, including 1a, 1b, 2a, 2b, 3a, 4a, and 6a.19, 20 In this study, we examined the efficacy and safety of an all‐oral pegIFN‐ and RBV‐free regimen of ombitasvir/paritaprevir/ritonavir in pegIFN/RBV treatment–experienced Japanese patients with HCV subtype 1b or genotype 2 without cirrhosis.
Patients and Methods
Study Design
This was a phase 2, randomized, open‐label, parallel‐arm, dose‐ and duration‐finding study (ClinicalTrials.gov identifier NCT01672983) (Fig. 1). The study was approved by all institutional review boards and conducted in accordance with the International Conference on Harmonisation guidelines and the Declaration of Helsinki. Written informed consent was obtained from all patients before enrollment.
Figure 1.
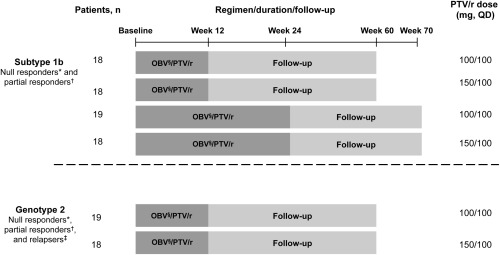
Study design. *Patients not achieving a 2 log10 IU/mL reduction in HCV RNA at week 12 after ≥10 weeks of pegIFN/RBV. †Patients who achieved a ≥2 log10 IU/mL reduction in HCV RNA at week 12 after ≥20 weeks of pegIFN/RBV but had HCV RNA levels above the lower limit of detection at treatment end. ‡Patients with undetectable levels of HCV RNA after one or more courses of pegIFN/RBV treatment who had detectable HCV RNA within 24 weeks. §Dose: 25 mg QD. Abbreviations: OBV, ombitasvir; PTV, paritaprevir; QD, once daily; r, ritonavir.
Patient Population
Patients were enrolled from August 2012 to December 2012 at 18 sites in Japan. Eligible patients were adults (aged 18‐75 years) with chronic HCV subtype 1b or genotype 2 infection and HCV RNA levels >10,000 IU/mL without cirrhosis who previously failed pegIFN/RBV therapy. The HCV genotype was assessed at screening using the Versant HCV Genotype Inno‐LiPA Assay (LiPA, v. 2.0 or higher; Siemens Healthcare Diagnostics, Tarrytown, NY).
Patients with subtype 1b were eligible if they were null responders (i.e., did not achieve a 2 log10 IU/mL reduction in HCV RNA levels at week 12 after at least 10 weeks of treatment with pegIFN/RBV) or partial responders (i.e., achieved at least a 2 log10 IU/mL reduction in HCV RNA at week 12 after a minimum of 20 weeks of treatment with pegIFN/RBV but had HCV RNA levels above the lower limit of detection at the end of treatment). Patients with genotype 2 were eligible if they were null or partial responders or relapsers (i.e., patients with undetectable levels of HCV RNA at the end of at least one course of pegIFN/RBV and whose levels became detectable within 24 weeks after the end of that treatment). Patients with human immunodeficiency virus or hepatitis B virus coinfection or any cause of liver disease other than chronic HCV infection were excluded. Full inclusion and exclusion criteria are in Supporting Table 1.
Study Medication
Patients with subtype 1b infection were randomized in a 1:1:1:1 ratio by interactive response technology to once‐daily ombitasvir 25 mg plus paritaprevir/ritonavir 100/100 mg for 12 weeks, ombitasvir 25 mg plus paritaprevir/ritonavir 150/100 mg for 12 weeks, ombitasvir 25 mg plus paritaprevir/ritonavir 100/100 mg for 24 weeks, or ombitasvir 25 mg plus paritaprevir/ritonavir 150/100 mg for 24 weeks. Patients with genotype 2 infection were randomized in a 1:1 ratio to once‐daily ombitasvir 25 mg plus paritaprevir/ritonavir 100/100 mg for 12 weeks or ombitasvir 25 mg plus paritaprevir/ritonavir 150/100 mg for 12 weeks (Fig. 1). For each genotype, the randomization schedule was stratified by prior treatment response (null responders, partial responders for subtype 1b, and null responders, partial responders, and relapsers for genotype 2).
Efficacy
Plasma HCV RNA levels were determined by a central laboratory, using the COBAS TaqMan real‐time reverse‐transcriptase polymerase chain reaction assay 2.0 (Roche, Nutley, NJ), which has a lower limit of detection of 15 IU/mL for genotype 1 and 5.6 IU/mL for genotype 2 and a lower limit of quantitation (LLOQ) of 25 IU/mL for both genotypes.
The primary efficacy endpoint was the percentage of patients who achieved SVR (HCV RNA <25 IU/mL) 24 weeks after the last dose of study drug (SVR24). Secondary efficacy endpoints were the percentage of patients with SVR 12 weeks after the last dose of study drug (SVR12) and the percentage of those patients with an end‐of‐treatment response (HCV RNA <25 IU/mL at week 12 for the 12‐week treatment arms or at week 24 for the 24‐week treatment arms). The percentage of patients with SVR 4 weeks after the last dose of study drug (SVR4) and the percentage who achieved rapid virologic response (HCV RNA <25 IU/mL at treatment week 4) were also determined.
On‐treatment virologic failure included rebound (two consecutive HCV RNA measurements greater than or equal to LLOQ after achieving HCV RNA levels less than LLOQ during treatment or an increase in HCV RNA levels >1 log10 IU/mL from nadir in two consecutive measurements at any time point during treatment) or failure to suppress (all on‐treatment HCV RNA values ≥ LLOQ) with at least 6 weeks of treatment. Relapse was defined as two consecutive HCV RNA measurements greater than or equal to LLOQ between the final treatment visit and posttreatment week 24 and included patients who completed treatment (at least 77 days of study drug for the 12‐week arms and at least 161 days of study drug for the 24‐week arms) and had HCV RNA levels less than LLOQ at the final treatment visit.
Because of known limitations of the LiPA 2.0 assay (i.e., an inability to universally determine subtypes for genotype 2 virus), a 329‐nucleotide region of HCV NS5B in baseline samples from all patients with genotype 2 infection was sequenced and used to perform a phylogenetic analysis.21 All efficacy and safety analyses by HCV genotype were based on the LiPA 2.0 assay unless otherwise stated. Patients with virologic failure had resistance‐associated variants determined for HCV NS3/4A and NS5A at baseline and at the time of failure by population nucleotide sequencing. Translated amino acid sequences were used to identify resistance‐associated variants.
Safety
At each study visit AEs were evaluated. Data on serious AEs (SAEs) were collected throughout the study period. Data on nonserious AEs were collected during the treatment‐emergent period: from study drug initiation to 30 days after treatment cessation. All AEs were coded using the Medical Dictionary for Regulatory Activities. Data on treatment‐emergent AEs are reported.
Statistical Analyses
A minimum of 96 subjects (16 subjects per treatment arm) and no more than 120 subjects (20 subjects per arm) were planned to be enrolled. A sample size of 16 patients per treatment arm would provide a two‐sided 95% confidence interval (CI) of 54.4%‐96.0% using binomial exact methodology, assuming an observed SVR24 rate of 80%. All randomized patients who received at least one dose of study drug were included in the intent‐to‐treat and safety populations. Analyses of rapid virologic response, end‐of‐treatment response, and all SVR endpoints were performed on the intent‐to‐treat population. A two‐sided 95% binomial exact CI was calculated for the SVR24 rate for each treatment regimen. The effects of treatment duration and paritaprevir dose on SVR24 in the subtype 1b cohort were assessed using the stratum‐adjusted Mantel‐Haenszel method, with adjustment for treatment duration (when testing for paritaprevir dose), paritaprevir dose (when testing for treatment duration), and prior treatment response (null response, partial response). In the genotype 2 cohort, the effect of paritaprevir dose on SVR24 rate was evaluated using the Fisher exact test. The SVR24 rates and corresponding 95% exact binomial CIs were calculated within subgroups based on prior HCV treatment response and, for the genotype 2 cohort, HCV subtype determined by LiPA 2.0 and phylogenetic analysis. All statistical tests and CIs were two‐sided, with a significance level of 0.05. Additional details of the statistical analysis of the primary endpoint are available in the Supporting Information.
Results
Patient Characteristics
Of 144 patients screened, 110 (subtype 1b, n = 73; genotype 2, n = 37) were randomized and received at least one dose of study medication (Fig. 2). Baseline characteristics are presented in Table 1. Among all patients combined, 46.4% were male, the mean age was 59.2 years, and the mean body mass index was 23.6 kg/m2. Most patients in the subtype 1b cohort were null responders (66.7%‐72.2%), whereas patients who relapsed were predominant in the genotype 2 cohort (89.5%‐94.4%). The phylogenetic analysis identified 20 patients with subtype 2a and 15 patients with subtype 2b infection. In addition, two patients initially identified as having genotype 2 based on the LiPA 2.0 assay were identified as having subtype 1b based on phylogenetic analysis (Supporting Table 2). A sensitivity analysis of SVR24, which was performed using HCV genotype assignments as determined by phylogenetic analysis, yielded SVR24 rates that were similar to those using the LiPA 2.0 assay. Therefore, the two patients initially identified as having genotype 2 did not affect the overall results in patients with subtype 1b.
Figure 2.
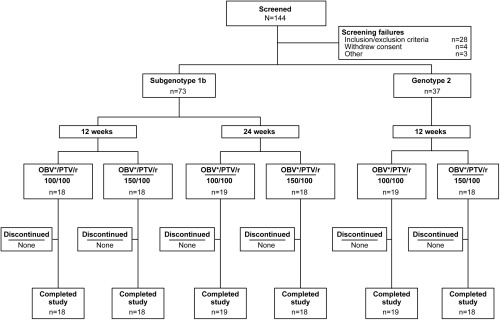
Patient disposition. Abbreviations: OBV, ombitasvir; PTV, paritaprevir; r, ritonavir. *Dose: 25 mg.
Table 1.
Baseline Demographics and Clinical Characteristics
| Parameter | HCV Subtype 1b Cohort | Genotype 2 Cohort | ||||
|---|---|---|---|---|---|---|
| 12 Weeks | 24 Weeks | 12 Weeks | ||||
| OBVa/PTV/r 100/100 (n = 18) | OBVa/PTV/r 150/100 (n = 18) | OBVa/PTV/r 100/100 (n = 19) | OBVa/PTV/r 150/100 (n = 18) | OBVa/PTV/r 100/100 (n = 19) | OBVa/PTV/r 150/100 (n = 18) | |
| Gender, male n (%) | 10 (55.6) | 11 (61.1) | 7 (36.8) | 8 (44.4) | 7 (36.8) | 8 (44.4) |
| Age, years median (range) | 59.5 (24‐70) | 58.5 (33‐72) | 63.0 (46‐72) | 59.0 (31‐69) | 65.0 (46‐74) | 65.5 (49‐71) |
| IL‐28B rs12979860 genotype CC, n (%) | 1 (5.6) | 0 | 1 (5.3) | 1 (5.6) | 14 (73.7) | 16 (88.9) |
| HCV subtype 1b or 2bb, n (%) | 18 (100) | 18 (100) | 19 (100) | 18 (100) | 8 (42.1) | 8 (44.4) |
| HCV subtype 1b or 2b,c, n (%) | ||||||
| 1b | NA | NA | NA | NA | 1 (5.3) | 1 (5.6) |
| 2a | NA | NA | NA | NA | 11 (57.9) | 9 (50.0) |
| 2b | NA | NA | NA | NA | 7 (36.8) | 8 (44.4) |
| Fibrosis stage, n/N (%)d | ||||||
| F0–F1 | 3/9 (33.3) | 3/8 (37.5) | 3/8 (37.5) | 3/8 (37.5) | 4/9 (44.4) | 8/11 (72.7) |
| F2 | 4/9 (44.4) | 3/8 (37.5) | 4/8 (50.0) | 3/8 (37.5) | 4/9 (44.4) | 2/11 (18.2) |
| F3 or higher | 2/9 (22.2) | 2/8 (25.0) | 1/8 (12.5) | 2/8 (25.0) | 1/9 (11.1) | 1/11 (9.1) |
| HCV RNA, mean ± SD, log10 IU/mL, | 6.5 ± 0.6 | 6.7 ± 0.4 | 6.5 ± 0.5 | 6.5 ± 0.4 | 6.9 ± 0.4 | 6.7 ± 0.6 |
| Platelet count, mean ± SD, × 109 cells/L | 205.4 ± 64.4 | 201.4 ± 40.4 | 202.6 ± 46.9 | 195.4 ± 41.5 | 191.7 ± 48.5 | 194.9 ± 34.3 |
| ALT, mean ± SD, U/L | 51.4 ± 31.8 | 68.7 ± 64.9 | 70.4 ± 52.3 | 72.5 ± 53.8 | 32.0 ± 23.4 | 29.7 ± 12.3 |
| Prior pegIFN/RBV response, n (%) | ||||||
| Null responder∥ | 13 (72.2) | 12 (66.7) | 13 (68.4) | 13 (72.2) | 0 | 0 |
| Partial responder | 5 (27.8) | 6 (33.3) | 6 (31.6) | 5 (27.8) | 2 (10.5) | 1 (5.6) |
| Relapser | 0 | 0 | 0 | 0 | 17 (89.5) | 17 (94.4) |
Dose: 25 mg.
Genotype per Inno‐LiPA 2.0 assay.
Genotype per phylogenic analysis.
Fibrosis stage was not available for all patients because it was obtained based on results of liver biopsy, FibroTest, and FibroScan. Patients with only a discriminant score were not included.
∥Although genotype 2–infected null responders were eligible, none were enrolled.
Abbreviations: ALT, alanine transaminase; IL, interleukin; NA, not available; OBV, ombitasvir; PTV, paritaprevir; r, ritonavir.
Virologic Response
On‐ and posttreatment virology results for patients with subtype 1b and genotype 2 infection are shown in Fig. 3A and B, respectively. Patients with subtype 1b had SVR24 rates of 88.9%‐100% in the 100‐mg paritaprevir dosing arm and the 150‐mg paritaprevir dosing arm, respectively. Two subtype 1b patients (both of whom received paritaprevir/ritonavir 150/100 mg for 12 weeks) did not achieve SVR24; one relapsed at posttreatment week 2, and one discontinued study treatment due to an AE. No significant differences in SVR24 rates were observed between treatment durations (24 versus 12 weeks: difference, 5.00; 95% CI −6.85 to 16.84) or paritaprevir doses (150 mg versus 100 mg: difference, −5.00; 95% CI −16.84 to 6.85). The SVR24 rates were 100% (51/51) among prior null responders and 90.9% (20/22) among prior partial responders with subtype 1b (Table 2).
Figure 3.
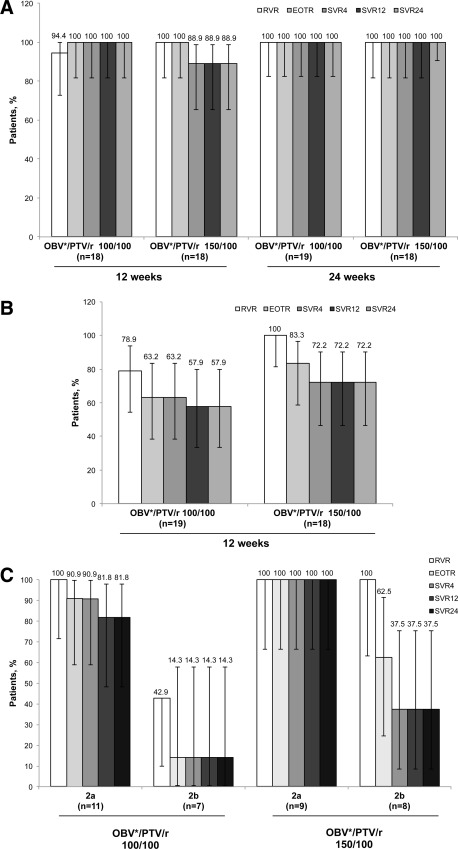
Efficacy of ombitasvir/paritaprevir/ritonavir in patients with HCV infection. Virologic response by treatment group for patients classified according to LiPA 2.0 analysis as having HCV subtype 1b (A) or HCV genotype 2 (B) infection and by subtype of genotype 2 according to phylogenetic analysis (C). Error bars represent 95% confidence intervals. Two patients identified as genotype 2 by LiPA 2.0 analysis were found to be HCV subtype 1b during phylogenetic analysis; both of these patients achieved SVR12 and SVR24. *Dose: 25 mg. Abbreviations: EOTR, end‐of‐treatment response; GT1b, HCV subtype 1b; OBV, ombitasvir; PTV, paritaprevir; r, ritonavir; RVR, rapid virologic response.
Table 2.
SVR24 by Prior pegIFN/RBV Response, n/N (%, 95% CI)
| Prior pegIFN/RBV Response | HCV Subtype 1b Cohort | Genotype 2 Cohort | ||||
|---|---|---|---|---|---|---|
| 12 Weeks | 24 Weeks | 12 Weeks | ||||
| OBVa/PTV/r 100/100 (n = 18) | OBVa/PTV/r 150/100 (n = 18) | OBVa/PTV/r 100/100 (n = 19) | OBVa/PTV/r 150/100 (n = 18) | OBVa/PTV/r 100/100 (n = 19) | OBVa/PTV/r 150/100 (n = 18) | |
| Null responders | 13/13 (100, 75.3‐100) | 12/12 (100, 73.5‐100) | 13/13 (100, 75.3‐100) | 13/13 (100, 75.3‐100) | NA | NA |
| Partial responders | 5/5 (100, 47.8‐100) | 4/6 (66.7, 22.3‐95.7) | 6/6 (100, 54.1‐100) | 5/5 (100, 47.8‐100) | 2/2 (100, 15.8‐100) | 1/1 (100, 2.5‐100) |
| Relapsers | NA | NA | NA | NA | 9/17 (52.9, 27.8‐77.0) | 12/17 (70.6, 44.0‐89.7) |
Dose: 25 mg.
Abbreviations: NA, not applicable; OBV, ombitasvir; PTV, paritaprevir; r, ritonavir.
In patients with genotype 2 infection, overall SVR24 rates were 72.2% with paritaprevir/ritonavir 150/100 mg and 57.9% with paritaprevir/ritonavir 100/100 mg (Fig. 3B). Reasons for not achieving SVR24 were on‐treatment failure (paritaprevir/ritonavir 100/100 mg arm: n = 7/19, 36.8%; paritaprevir/ritonavir 150/100 mg arm: n = 3/18, 16.7%) and relapse by posttreatment week 24 (paritaprevir/ritonavir 100/100 mg arm: n = 1/12, 8.3%; paritaprevir/ritonavir 150/100 mg arm: n = 2/15, 13.3%). All partial responders (3/3) achieved SVR24; the SVR24 rate in relapsers was 62% (21/34) (Table 2). By phylogenetic analysis, the SVR24 rate was higher in patients with subtype 2a (n = 18/20, 90%) than 2b (n = 4/15, 27%) (Supporting Table 2, Fig. 3C).
Complete concordance (100%) was observed between SVR12 and SVR24. Further, all patients who achieved SVR24 maintained the response throughout the follow‐up period (posttreatment week 48).
Virologic Failure
No patient with subtype 1b infection had on‐treatment virologic failure; one patient experienced posttreatment relapse. The patient who relapsed had resistance‐associated variants (RAVs) in NS3 (D168V) and NS5A (Y93H) at the time of virologic failure. The NS5A RAV, Y93H, was present at baseline in four patients with subtype 1b infection; all achieved SVR24. In patients with genotype 2 infection, on‐treatment virologic failure was more frequent in those who received paritaprevir/ritonavir 100/100 mg (36.8% [7/19]; 95% CI 16.29‐61.64) than paritaprevir/ritonavir 150/100 mg (16.7% [3/18]; 95% CI 3.58‐41.42). It was also more frequent among those with phylogenetically determined subtype 2b (11/15 [73%]) versus subtype 2a infection (2/20 [10%]). All genotype 2 patients with virologic failure had RAVs in NS3 and/or NS5A at the time of virologic failure, most commonly D168V or D168Y in NS3 and L28F in NS5A. The presence of RAVs in NS3 and NS5A at baseline did not impact response.
Safety
Rates of any reported treatment‐emergent AEs were similar across treatment arms (73.7%‐88.9%) (Table 3, Supporting Table 3), and most were mild in severity. The most common treatment‐emergent AEs were nasopharyngitis (29.1%) and headache (13.6%) (Table 3). Five SAEs (n = 5/110, 4.5%) were reported: autoimmune hepatitis, fluid retention, femoral fracture, tibia fracture, and ischemic colitis. Autoimmune hepatitis and fluid retention were considered by the investigator as having a reasonable possibility of being treatment‐related. One patient (n = 1/110, 0.9%) discontinued study drug because of an SAE of fluid retention.
Table 3.
Treatment‐Emergent AEs
| Parameter, n (%) | HCV Subtype 1b Cohort | Genotype 2 Cohort | ||||
|---|---|---|---|---|---|---|
| 12 Weeks | 24 Weeks | 12 Weeks | ||||
| OBVa/PTV/r 100/100 (n = 18) | OBVa/PTV/r 150/100 (n = 18) | OBVa/PTV/r 100/100 (n = 19) | OBVa/PTV/r 150/100 (n = 18) | OBVa/PTV/r 100/100 (n = 19) | OBVa/PTV/r 150/100 (n = 18) | |
| Any AE | 14 (77.8) | 15 (83.3) | 16 (84.2) | 15 (83.3) | 14 (73.7) | 16 (88.9) |
| Any SAE | 0 | 2 (11.1) | 2 (10.5) | 0 | 1 (5.3) | 0 |
| AE leading to study drug discontinuation | 0 | 1 (5.6) | 0 | 0 | 0 | 0 |
| Common AEsb | ||||||
| Nasopharyngitis | 1 (5.6) | 6 (33.3) | 4 (21.1) | 9 (50.0) | 5 (26.3) | 7 (38.9) |
| Headache | 2 (11.1) | 1 (5.6) | 3 (15.8) | 1 (5.6) | 3 (15.8) | 5 (27.8) |
| Back pain | 3 (16.7) | 1 (5.6) | 2 (10.5) | 2 (11.1) | 0 | 0 |
| Diarrhea | 3 (16.7) | 0 | 1 (5.3) | 1 (5.6) | 0 | 0 |
| Vomiting | 3 (16.7) | 0 | 0 | 0 | 0 | 0 |
| Rash | 0 | 0 | 2 (10.5) | 3 (16.7) | 0 | 1(5.6) |
Dose: 25 mg.
Incidence >15% in any group.
Abbreviations: OBV, ombitasvir; PTV, paritaprevir; r, ritonavir.
There were no grade 3 or 4 abnormalities in hemoglobin, alkaline phosphatase, or total bilirubin during treatment (Supporting Table 4). Two patients experienced grade 3 alterations in liver enzymes. One patient experienced a grade 3 alanine aminotransferase level at day 156. This patient was retrospectively found to have laboratory values at screening and baseline suggestive of previously undiagnosed autoimmune disease. Another patient, who had a grade 3 aspartate aminotransferase level (206 IU/mL) at baseline, experienced a grade 3 aspartate aminotransferase level of 171 U/L on day 7. Both patients were asymptomatic, completed treatment, and achieved SVR24. One patient (1/110, 0.9%) experienced a grade 2 abnormality in hemoglobin, and two patients (2/110, 1.8%) experienced a grade 2 abnormality in bilirubin during treatment. The grade 2 bilirubin abnormality was not associated with an increase in transaminase, peaked within the first 2 weeks of treatment, and resolved spontaneously with continuation of treatment.
Discussion
Patients with HCV infection who have a history of prior IFN/RBV treatment failure are a difficult‐to‐treat population, particularly those who are prior null responders. In this randomized phase 2 study of an IFN‐ and RBV‐free regimen of ombitasvir/paritaprevir/ritonavir in pegIFN/RBV treatment–experienced Japanese patients, high SVR rates were observed in patients with HCV subtype 1b infection, regardless of paritaprevir dose or treatment duration. Among patients with genotype 2 infection, SVR rates were higher in those receiving paritaprevir 150 mg and those with subtype 2a infection. Together, these results show promising antiviral activity for this all‐oral 2 DAA combination regimen.
These results, in previously treated Japanese patients with HCV subtype 1b, compare favorably with published reports of other IFN‐free and RBV‐free regimens and IFN‐free, RBV‐containing regimens in treatment‐experienced non‐Japanese populations with genotype 1 infection. Among 12‐week treatment regimens that contained two DAAs alone or in combination with RBV, SVR12 rates were 82% with daclatasvir/asunaprevir in null responders or those who were intolerant of IFN22 and between 94% and 96% with sofosbuvir plus ledipasvir in treatment‐experienced patients.23 In addition, SVR12 rates with sofosbuvir/ledipasvir were lower in patients with subtype 1b infection versus those with 1a infection. In trials evaluating regimens of the three DAAs ombitasvir/paritaprevir/ritonavir and dasabuvir with or without RBV, SVR12 rates of between 96.6% and 100% were achieved in patient populations with HCV genotype 1b infection.15, 17, 24, 25, 26 Rates of SVR12 with ombitasvir/paritaprevir/ritonavir and dasabuvir were not greatly influenced by prior IFN‐treatment response17, 24 but were slightly higher in patients with HCV subtype 1b versus 1a infection.15, 17, 25, 26
The efficacy of IFN‐free DAA regimens has also been studied in genotype 2 patients. In the POSITRON trial, 92% of patients without cirrhosis but with genotype 2 HCV infection for whom IFN treatment was not an option achieved SVR12 after 12 weeks of treatment with sofosbuvir plus RBV.27 In the FUSION trial, a similar SVR12 rate (96%) was achieved in treatment‐experienced patients, most of whom were prior relapsers.27 In the VALENCE trial, an SVR12 rate of 93% was observed with a 12‐week sofosbuvir plus RBV regimen. The percentage of patients with HCV genotype 2 infection who achieved SVR12 was high in treatment‐experienced (approximately 90%) and treatment‐naive (approximately 98%) patients. Unfortunately, this study did not evaluate SVR rates by subgenotype or response to prior IFN therapy.28 In the current study of treatment‐experienced patients with genotype 2 infection, response rates ranged from 100% in partial responders to 52.9%‐70.6% among relapsers and were greater with paritaprevir 150 mg and among patients with subtype 2a infection. These differences in response rates for genotype 2 may be related to NS3/4A or NS5A polymorphisms between subtypes 2a and 2b. In a small study performed in the United States, high SVR rates were achieved with ombitasvir/paritaprevir/ritonavir when combined with RBV (eight of 10 patients) and without RBV (six of 10 patients) among treatment‐naive patients with HCV genotype 2.29 Although the data set is limited, this small study suggests that RBV may offer additional benefit in patients with genotype 2 infection.
This study also indicates that the safety and tolerability of the ombitasvir/paritaprevir/ritonavir combination regimen compare favorably to pegIFN‐based therapy and are similar to other IFN‐free regimens. No grade 3 or 4 abnormalities in hemoglobin, alkaline phosphatase, or total bilirubin were observed. Although the mean age of the study population was 59.2 years, clinically significant reductions in hemoglobin were observed in only one patient (0.9%). Transient grade 2 increases in bilirubin were observed infrequently during the first 2 weeks of treatment, were not accompanied by alanine aminotransferase elevations, and improved with continuation of treatment. These results are consistent with the known class effect of HCV protease inhibitors on the bilirubin transporter organic anion‐transporting protein B1.24
The strengths of this study are that it was conducted in a recognized difficult‐to‐treat patient population and that the study population was representative of patients with HCV infection in Japan.30 However, there were some limitations, such as the exclusion of patients with cirrhosis and the relatively small number of patients in each treatment arm, which limits the ability to assess differences in treatment response between subgroups.
In conclusion, in light of the promising results of this phase 2b study and recent results from studies in Western patients with HCV genotype 1 infection,16, 24, 25, 31 future trials should be undertaken to evaluate the efficacy and safety of ombitasvir 25 mg plus paritaprevir/ritonavir 150/100 mg in treatment‐naive and treatment‐experienced Japanese patients with HCV infection, with and without compensated cirrhosis.
Supporting information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.27705/suppinfo.
Supplementary Information
Acknowledgment
We acknowledge Travis Yanke, Melissa Cook, Christine Collins, Rajeev M. Menon, Gretja Schnell, Rakesh Tripathi, Jill Beyer, Thomas Reisch, and Takuma Matsuda at AbbVie for assistance provided in the preparation and execution of the study. Editorial and medical writing support was provided by Jillian Gee, Ph.D., C.M.P.P., at Complete Publication Solutions, LLC; this study was funded by AbbVie.
Potential conflict of interest: Dr. Chayama consults for and received grants from Bristol‐Myers Squibb. He advises and received grants from Eisai. He consults for AbbVie. He received grants from Ajinomoto, Astellas, AstraZeneca, Chugai, Daiichi Sankyo, Dainippon Sumitomo, GlaxoSmithKline, Janssen, Kowa, Kyorin, Mitsubishi Tanabe, MSD, Nippon Kayaku, Nippon Seiyaku, Nippon Shinyaku, Roche, Takeda, Teijin, Toray, Torii, Tsumura, and Zeria. Dr. Kurosaki is on the speakers' bureau for MSD, Daiichi Sankyo, Bristol‐Myers Squibb, GlaxoSmithKline, Toray, Janssen, Otsuka, and Chugai. Dr. Kumada consults for Dainippon Sumitomo, Toray, MSD, Bristol‐Myers Squibb, and Mitsubishi Tanabe. He holds intellectual property rights with SRL. Dr. Sato is on the speakers' bureau for Bristol‐Myers Squibb, Janssen, and Chugai. He received grants from Dainippon Sumitomo, MSD, Daiichi Sankyo, and AbbVie. Dr. Badri is employed by and owns stock in AbbVie. Dr. Pilot‐Matias is employed by and owns stock in AbbVie. Dr. Rodrigo is employed by and owns stock in AbbVie. Dr. Setze is employed by and owns stock in AbbVie. Dr. Vilchez is employed by and owns stock in AbbVie.
Sponsored by AbbVie, which contributed to the design and conduct of the study, data management, data analysis, interpretation of the data, and preparation and approval of the manuscript.
References
- 1. Dore GJ, Ward J, Thursz M. Hepatitis C disease burden and strategies to manage the burden (Guest Editors Mark Thursz, Gregory Dore and John Ward). J Viral Hepat 2014;21(Suppl. 1):1‐4. [DOI] [PubMed] [Google Scholar]
- 2. El‐Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 2012;142:1264‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chung H, Ueda T, Kudo M. Changing trends in hepatitis C infection over the past 50 years in Japan. Intervirology 2010;53:39‐43. [DOI] [PubMed] [Google Scholar]
- 4. Wu S, Kanda T, Nakamoto S, Jiang X, Miyamura T, Nakatani SM, et al. Prevalence of hepatitis C virus subgenotypes 1a and 1b in Japanese patients: ultra‐deep sequencing analysis of HCV NS5B genotype‐specific region. PLoS One 2013;8:e73615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1207‐1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011;364:2405‐2416. [DOI] [PubMed] [Google Scholar]
- 7. Kumada H, Toyota J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment‐naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol 2012;56:78‐84. [DOI] [PubMed] [Google Scholar]
- 8. Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med 2011;364:2417‐2428. [DOI] [PubMed] [Google Scholar]
- 9. Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1195‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashi N, Izumi N, Kumada H, Okanoue T, Tsubouchi H, Yatsuhashi H, et al. Simeprevir with peginterferon/ribavirin for treatment‐naive hepatitis C genotype 1 patients in Japan: CONCERTO‐1, a phase III trial. J Hepatol 2014;61:219‐227. [DOI] [PubMed] [Google Scholar]
- 11. Hayashi N, Seto C, Kato M, Komada Y, Goto S. Once‐daily simeprevir (TMC435) with peginterferon/ribavirin for treatment‐naive hepatitis C genotype 1–infected patients in Japan: the DRAGON study. J Gastroenterol 2014;49:138‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kawaoka T, Hayes CN, Ohishi W, Ochi H, Maekawa T, Abe H, et al. Predictive value of the IL28B polymorphism on the effect of interferon therapy in chronic hepatitis C patients with genotypes 2a and 2b. J Hepatol 2011;54:408‐414. [DOI] [PubMed] [Google Scholar]
- 13. Sakamoto N, Nakagawa M, Tanaka Y, Sekine‐Osajima Y, Ueyama M, Kurosaki M, et al. Association of IL28B variants with response to pegylated‐interferon alpha plus ribavirin combination therapy reveals intersubgenotypic differences between genotypes 2a and 2b. J Med Virol 2011;83:871‐878. [DOI] [PubMed] [Google Scholar]
- 14. Akuta N, Suzuki F, Seko Y, Kawamura Y, Sezaki H, Suzuki Y, et al. Association of IL28B genotype and viral response of hepatitis C virus genotype 2 to interferon plus ribavirin combination therapy. J Med Virol 2012;84:1593‐1599. [DOI] [PubMed] [Google Scholar]
- 15. Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, et al. Treatment of HCV with ABT‐450/r‐ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1594‐1603. [DOI] [PubMed] [Google Scholar]
- 16. Kowdley KV, Lawitz E, Poordad F, Cohen DE, Nelson DR, Zeuzem S, et al. Phase 2b trial of interferon‐free therapy for hepatitis C virus genotype 1. N Engl J Med 2014;370:222‐232. [DOI] [PubMed] [Google Scholar]
- 17. Poordad F, Hezode C, Trinh R, Kowdley KV, Zeuzem S, Agarwal K, et al. ABT‐450/r‐ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370:1973‐1982. [DOI] [PubMed] [Google Scholar]
- 18. Menon RM, Klein CE, Lawal AA, Chiu YL, Awni WM, Podsadecki TJ, et al. Pharmacokinetics and tolerability of the HCV protease inhibitor ABT‐450 following single ascending doses in healthy adult volunteers with and without ritonavir (HEP DART 2009 abstract 57). Global Antiviral Journal 2009;5:53. [Google Scholar]
- 19. Krishnan P, Beyer J, Mistry N, Koev G, Reisch T, DeGoey D, et al. In vitro and in vivo antiviral activity and resistance profile of ombitasvir, an inhibitor of HCV NS5A. Antimicrob Agents Chemother 2014. doi:10.1128/AAC.04226-14. [DOI] [PMC free article] [PubMed]
- 20. Pilot‐Matias T, Tripathi R, Cohen D, Gaultier I, Dekhtyar T, Lu L, et al. In vitro and in vivo antiviral activity and resistance profile of the hepatitis C virus NS3/4A protease inhibitor ABT‐450. Antimicrob Agents Chemother 2014. doi:10.1128/AAC.04227-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koletzki D, Dumont S, Vermeiren H, Fevery B, De Smet P, Stuyver LJ. Development and evaluation of an automated hepatitis C virus NS5B sequence‐based subtyping assay. Clin Chem Lab Med 2010;48:1095‐1102. [DOI] [PubMed] [Google Scholar]
- 22. Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, et al. All‐oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet 2014;384:1597‐1605. [DOI] [PubMed] [Google Scholar]
- 23. Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483‐1493. [DOI] [PubMed] [Google Scholar]
- 24. Andreone P, Colombo MG, Enejosa JV, Koksal I, Ferenci P, Maieron A, et al. ABT‐450, ritonavir, ombitasvir, and dasabuvir achieves 97% and 100% sustained virologic response with or without ribavirin in treatment‐experienced patients with HCV genotype 1b infection. Gastroenterology 2014;147:359‐365. [DOI] [PubMed] [Google Scholar]
- 25. Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, et al. ABT‐450/r‐ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med 2014;370:1983‐1992. [DOI] [PubMed] [Google Scholar]
- 26. Zeuzem S, Jacobson IM, Baykal T, Marinho RT, Poordad F, Bourliere M, et al. Retreatment of HCV with ABT‐450/r‐ombitasvir and dasabuvir with ribavirin. N Engl J Med 2014;370:1604‐1614. [DOI] [PubMed] [Google Scholar]
- 27. Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez‐Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867‐1877. [DOI] [PubMed] [Google Scholar]
- 28. Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370:1993‐2001. [DOI] [PubMed] [Google Scholar]
- 29. Lawitz E, Sullivan G, Rodriguez‐Torres M, Bennett M, Poordad F, Kapoor M, et al. Exploratory trial of ombitasvir and ABT‐450/r with or without ribavirin for HCV genotype 1, 2, and 3 infection. J Infect 2014. doi: 10.1016/j.jinf.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 30. Moriya T, Koyama T, Tanaka J, Mishiro S, Yoshizawa H. Epidemiology of hepatitis C virus in Japan. Intervirology 1999;42:153‐158. [DOI] [PubMed] [Google Scholar]
- 31. Lawitz E, Hezode C, Varunok P, Thuluvath PJ, Baykal T, Kapoor M, et al. Interferon‐ and ribavirin‐free regimen of ABT‐450/r + ABT‐267 in HCV genotype 1b–infected treatment‐naïve patients and prior null responders [Abstract]. Hepatology 2013;58:244A. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.27705/suppinfo.
Supplementary Information


