ABSTRACT
Introduction: The effects of spinal bulbar muscular atrophy (SBMA) on quality of life (QoL) are not well understood. This study describes symptoms from the patient's perspective and the impact these symptoms have on QoL. Methods: We conducted open‐ended interviews with 21 adult men with genetically confirmed SBMA. Using a qualitative framework technique, we coded and analyzed interviews to identify symptoms and resulting themes. Results: From these interviews, 729 quotations were extracted. We identified 200 SBMA‐specific symptoms and 20 symptomatic themes. Weakness was mentioned by all interviewees. Symptoms within the domain of mental health and the specific themes of emotional issues and psychological impact were also frequently mentioned. Discussion: Numerous symptoms affect QoL for patients with SBMA. We identified previously unrecognized symptoms that are important to address in enhancing clinical care for patients with SBMA and in developing tools to evaluate efficacy in future clinical trials. Muscle Nerve 57: 40–44, 2018
Keywords: androgen receptor, inherited neuromuscular disease, Kennedy disease, motor neuron disease, open‐ended questions, quality of life, spinal and bulbar muscular atrophy
Abbreviations
- ALS
amyotrophic lateral sclerosis
- AR
androgen receptor
- CMT1A
Charcot–Marie–Tooth disease type 1A
- FSHD
facioscapulohumeral muscular dystrophy
- DM1
myotonic dystrophy type 1
- QoL
quality of life
- SBMA
spinal and bulbar muscular atrophy
- SF‐36v2
short‐form 36 version 2
Spinal and bulbar muscular atrophy (SBMA; Kennedy disease) is an adult onset, slowly progressive, X‐linked neuromuscular disease.1 SBMA results from a CAG trinucleotide expansion in the androgen receptor (AR) gene.2 Affected men usually present with weakness in both the distal and proximal extremities.3 Muscle cramping is also a common presenting symptom. In addition, patients develop bulbar dysfunction, fasciculations, hand tremor, erectile dysfunction, and gynecomastia.1, 4 There is wide variation in the symptoms identified in patients with SBMA. SBMA is a rare disorder with a prevalence estimated at 1 in 50,000–60,000.5, 6
Previous retrospective reviews and clinical trials have used general measures such as the short‐form 36v2 (SF‐36v2), Beck's Depression Inventory, and the International Index of Erectile Function to assess quality of life (QoL) for SBMA patients.3, 7, 8 These studies found that patients with SBMA have lower SF‐36v2 component (physical and mental) scores, higher rates of erectile dysfunction, and mild depression compared with their corresponding controls.3, 7, 8
There have been advances in QoL measurements for other neurological diseases. The goal has been to understand which issues are most important to patients and to develop clinically meaningful measurements for interventional trials. The first phase is focused on identifying symptoms and themes relevant to specific diseases, as was accomplished for Charcot–Marie–Tooth disease type 1A (CMT1A), facioscapulohumeral muscular dystrophy (FSHD), and early onset myotonic dystrophy type 1 (DM1).9, 10, 11 Some evaluations have broadened their analysis to investigate the importance of specific symptoms to patients with DM1 and 2.12, 13 One outcome of identifying what is most important to a select group of patients is the ability to include these items in a patient‐reported outcome measure designed to measure clinically relevant changes in clinical trials. A disease‐specific questionnaire would allow those caring for patients with SBMA to assess change in patient‐perceived disease burden, as was done with the Myotonic Dystrophy Health Index.14 When developed properly, these instruments have the capability to measure change serially in disease state over time and to correlate with a patient's overall functional status.15 Here we report symptoms that have the most impact on SBMA‐related QoL and describe the diagnostic odyssey of patients' experiences during the diagnostic process.
MATERIALS AND METHODS
Twenty‐one individuals who participated in a recent exercise trial agreed to participate in this study, with a sample size chosen for convenience.7 The study was performed with approval from the National Institutes of Health Combined Neuroscience Institutional Review Board. All participants had genetically confirmed SBMA, with AR repeat lengths of 38 or more CAGs. Study participants had a wide range of symptoms, disease progression, and level of functional ability.
Interview questions were developed and reviewed by an investigator with experience in administering and developing qualitative interviews (C.R.H.). After informed consent had been given, interviews were conducted by 1 of 3 investigators over the telephone or in person. Interviews were semistructured, with a specific list of open‐ended questions (Supp. Info. 1) to help guide the interviews and extract details regarding disease impact on the participant's QoL. Participants were allowed to expand on the questions to describe what was most important to their life, and they were provided with the opportunity to discuss additional symptoms and issues at the end of the interview that they considered relevant. Patients were also asked about their diagnostic odyssey and asked to describe the coping mechanisms that they use to deal with SBMA. All interviews were transcribed by Landmark Associates (Tempe, Arizona) for analysis.
Following transcription, 2 investigators analyzed the 21 interviews independently and together using a framework technique.16, 17 A team consensus approach was used to extract and analyze quotations and categorize them by symptom. The number of participants that mentioned each symptom and the frequency with which each symptom was mentioned were recorded. In some instances, a single quotation described multiple symptoms. Subjective interpretation by the investigators provided the opportunity to infer emotions alluded to in the quotations, whereas in other instances the emotion of the participant was clearly stated. The investigators categorized the symptoms into themes and broader domains representing physical, social, mental, and SBMA‐specific health.
RESULTS
Pertinent information was collected from the participants (Table 1). Altogether, 729 direct quotations were coded, and 200 symptoms of importance were identified. These symptoms represent 20 symptomatic themes and 4 major domains (Fig. 1, Supp. Info. Table 1). The most frequently mentioned SBMA themes were emotional issues (555 quotations), psychological impact (546 quotations), future/goal planning (305 quotations), and alterations in activities (245 quotations; Fig. 1B). Muscle weakness, which all patients reported, contributed to each of the themes. As an example of a quotation within the emotional issue theme, 1 patient reported that he does not allow the disease to control his life, “accepting limitations, and not allowing it to dictate life.” Another patient mentioned his physical and emotional challenges, “The other thing I think is there's just a general disappointment that you can't do the things you used to do. That gee, I'm stuck here, sitting down most of the time, dealing with not being able to—on my property I have to go down this big hill to just get my mail. I can't even do that, I can't even get my mail out of the mailbox. Thank God I have her to help me out with this stuff.”
Table 1.
Participant demographics
| Characteristic | Participant data | Range |
|---|---|---|
| Age, y, mean (SD) | 54.8 (8.6) | 42–74 |
| Age of onset of weakness, y, mean (SD) | 38.5 (10.0) | 21–56 |
| Ethnicity, % | Caucasian 87.7, Asian 4.7, Hispanic 4.7, Mixed 4.7 | |
| CAG repeat, mean (SD) | 46.6 (2.7) | 41–52 |
| Employment status, % | Employed 61.9, retired 28.6, disability 9.5 | |
| Education level, %a | Graduate 47.6, college 42.9, high school 4.7 | |
| Marital status, % | Married 76.2, divorced 9.5, separated 4.7, single 4.7, widower 4.7 |
One patient did not specify his education status.
Figure 1.
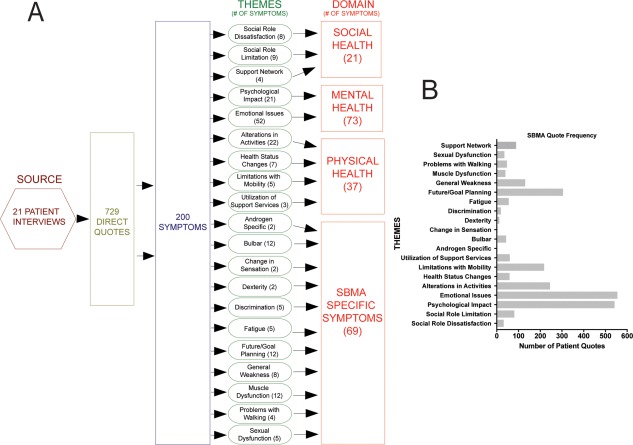
(A) Spinal and bulbar muscular atrophy conceptual domain model representing the schematic of symptoms and themes to generate the 4 domains. Number of symptom within each theme and domain is listed in parentheses. (B) Quotation frequency divided into each of the themes identified in A. SBMA, spinal and bulbar muscular atrophy.
When examining individual themes, the most frequently noted features were positive outlook, which consisted of both having a positive perspective and believing that you can achieve this positive mental status, (106 quotations), adapting to physical limitations (99), cognitive awareness of symptoms (88), changes in lifestyle due to the disease (86), adjusting to a “new normal” (81), and weakness (74). One participant described himself in the following way: “I had a lot of muscle spasms, stiffness, soreness, gradual—again, loss of strength, stamina; weakness.” Another patient who altered his lifestyle due to the disease stated “… stopped being on the ski patrol four years ago and I had to stop skiing two years ago, even though skiing was at the time a lot easier than walking.” A different participant had evidence of a positive outlook as demonstrated from the following quotation: “You can have a very productive and balanced life, given all the ramifications that the disease poses, by just being positive and looking for the things that you can control, and doing what you can about it, and then you can go on and have a lot of enjoyment.”
When asked about the initial diagnosis of SBMA, 4 of the 21 participants stated that they were initially diagnosed with amyotrophic lateral sclerosis (ALS). Nine participants presented with symptoms and a family history of SBMA, which helped in making the diagnosis. Only 2 of our participants mentioned speaking with a genetic counselor. One participant, who did not receive genetic counseling, discussed the importance of speaking to a counselor: “Very important, genetic counseling, I think. Because you want to know what's going to happen with your kids, especially if you're going to have a daughter.” A majority of the patients were diagnosed without any explanation of the disease, and only 3 patients were referred to a specialized neuromuscular clinic. A full list of symptoms with quotation frequency and theme classification is provided in Supporting Information Table 1.
DISCUSSION
In this study, we used in‐depth patient interviews to determine the most important themes and symptoms that affect the QoL of patients with SBMA. Our findings show that patients with SBMA alter their lifestyles to accommodate the progression of the disease and that a large majority of individuals maintain a positive outlook on life. Our study reports on a range of symptoms and issues that might not normally be identified in a clinical visit because of their nonphysical nature. Although these symptoms are not physical, they have a substantial impact on the QoL of patients with SBMA and may require clinical attention.
Our data support previous research indicating that patients experience a range of symptoms, with the majority indicating extremity weakness as a prevalent symptom but not all indicating bulbar involvement.3, 18 Weakness and inability to walk are described in many ways, given that these deficits not only have physical manifestations but also impair social and mental health. Awareness of these limitations and the identification of what one can no longer do is a prevalent issue in progressive neuromuscular disease.10, 12, 13, 19
Bulbar symptoms were described by a subset of our participants (29%). These symptoms have impacts outside of physical health, affecting work by making it difficult to communicate with coworkers and creating barriers when meeting new people. Similarly, previous reports identified problems with swallowing and choking and difficulty talking.9, 10, 11, 12, 13 Beyond the social aspect of bulbar symptoms, another potentially life‐threatening component of SBMA is laryngospasm, or dry drowning, a symptom present in our group and not seen in other neuromuscular disease symptom identification studies described above.9, 10, 11, 12, 13 Ten percent of the participants reported this symptom. Previous reports suggest that the prevalence is higher and may depend on disease progression.20, 21
The identified themes have both similarities and differences from those described in other neuromuscular diseases. Changes in housing are prevalent for patients with SBMA because approximately 80% of patients have difficulty with stairs compared with levels of 75% in FSHD, 38% in CMT1A, and 79% in DM1.9, 10, 12 Mental health symptoms are common in many diseases; however, there is a much higher frequency of quotations related to the domain of mental health in our population.9, 10 Cognitive impairment in FSHD and DM1 focuses on memory deficits,10, 12 and quotations on memory were not described by SBMA participants, resulting in the theme of psychological impact instead. Previous studies focusing on developing QoL measures for CMT1A, FSHD, DM1, and DM2 identified themes such as pain, sleep, and gustatory alterations that were not identified in our study.9, 10, 11, 12, 13 These themes were mentioned as symptoms but were not identified as overarching themes across our patient population. Some themes in our study (future/goal planning, utilization of support services, discrimination, and support network) were present in other diseases as symptoms but did not impact a sufficiently broad number of participants to be considered themes.
This study provides an important supplement to previous SBMA research, with a focus on allowing patients to identify the specific symptoms that are most important to them. Previous studies that used generalized measurements for QoL did not effectively provide a composite of all of the important symptoms in patients with SBMA.3, 4, 7, 8 As described in previous studies assessing QoL, it is helpful to prioritize symptoms based on importance to patients and to use that information to develop a QoL tool specific to SBMA.9, 14
The sample interviewed for our study was limited to a small number of participants who were concurrently participating in a clinical trial assessing the safety and efficacy of exercise in SBMA.7 There is potential for sample bias because selected participants were already physically active and motivated to participate in the clinical trial. Additionally, because the participants were enrolled in a clinical trial, they might have had a more positive outlook with hope for a treatment. Furthermore, the education level in our sample of participants was skewed in that half of the participants had graduate education, which is substantially more than the general US population in which only 12% are estimated to have graduate degrees.22 The symptoms we identified are based on the participants' own perspective of their disease status and challenges. We do not yet have a correlation between SBMA disease severity and symptom frequency.23 Future research will seek to incorporate functional rating scales to determine how the quotation frequencies correlate with objective measurements.
Our study interviews also examined the initial diagnostic experience of our patients and showed that 20% of participants were misdiagnosed with ALS. The rate of misdiagnosis is likely due to lack of awareness in the medical community and the relative rarity of SBMA and results in an extended time required for patients to be diagnosed. Misdiagnosis poses a problem with life planning and outlook because ALS has a much worse prognosis than SBMA.24 The results of our current study show that misdiagnosis leads to emotional problems such as anger, frustration, and fear. It is important to be aware of the potential for misdiagnosis with ALS because the diseases have shared features as well as distinguishing characteristics.25, 26
In conclusion, we used qualitative interviews to identify symptoms that most affect the QoL in patients with SBMA. Some of these symptoms have not been previously reported, and, until now, little attention has been given to the nonphysical aspects of the disease. These areas can serve as important symptoms to target in future therapeutic development and will form the basis for a questionnaire to test the generalizability of the findings to a larger group of SBMA patients.
The authors thank Elizabeth Hartnett, MSW (NINDS, NIH), for her help in scheduling participants during the study, the clinical staff of the outpatient NIH neurology clinic for their assistance during the participants' visits, the research participants who made this study possible, and Laura Braun for her help and editing the manuscript.
Ethical Publication Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Additional supporting information may be found in the online version of this article
Supporting Information 1
Supporting Information 2
Funding: This work was supported by intramural research funds from the National Institute of Neurological Disorders and Stroke.
Conflicts of Interest: None of the authors have any conflicts of interest to disclose.
REFERENCES
- 1. Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex‐linked recessive trait. Neurology 1968;18:671–680. [DOI] [PubMed] [Google Scholar]
- 2. La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X‐linked spinal and bulbar muscular atrophy. Nature 1991;352:77–79. [DOI] [PubMed] [Google Scholar]
- 3. Rhodes L, Freeman BK, Auh S, Kokkinis AD, La Pean A, Chen C, et al Clinical features of spinal and bulbar muscular atrophy. Brain 2009;132:3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atsuta N, Watanabe H, Ito M, Banno H, Suzuki K, Katsuno M, et al Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain 2006;129:1446–1455. [DOI] [PubMed] [Google Scholar]
- 5. Guidetti D, Sabadini R, Ferlini A, Torrente I. Epidemiological survey of X‐linked bulbar and spinal muscular atrophy, or Kennedy disease, in the province of Reggio Emilia, Italy. Eur J Epidemiol 2001;17:587–591. [DOI] [PubMed] [Google Scholar]
- 6. Fischbeck KH. Kennedy disease. J Inherit Metab Dis 1997;20:152–158. [DOI] [PubMed] [Google Scholar]
- 7. Shrader JA, Kats I, Kokkinis A, Zampieri C, Levy E, Joe GO, et al A randomized controlled trial of exercise in spinal and bulbar muscular atrophy Ann Clin Transl Neurol 2015;2:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fernández‐Rhodes LE, Kokkinis AD, White MJ, Watts CA, Auh S, Jeffries NO, et al Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomised placebo‐controlled trial. Lancet Neurol 2011;10:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson NE, Heatwole CR, Ferguson M, Sowden JE, Jeanat S, Herrmann DN. Patient identification of the symptomatic impact of Charcot–Marie–Tooth disease type 1A. J Clin Neuromuscul Dis 2013;15:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson NE, Quinn C, Eastwood E, Tawil R, Heatwole CR. Patient‐identified disease burden in facioscapulohumeral muscular dystrophy. Muscle Nerve 2012;46:948–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson NE, Luebbe E, Eastwood E, Chin N, Moxley RT, Heatwole CR. The impact of congenital and childhood myotonic dystrophy on quality of life: a qualitative study of associated symptoms. J Child Neurol 2014;29:983–986. [DOI] [PubMed] [Google Scholar]
- 12. Heatwole C, Bode R, Johnson N, Quinn C, Martens W, McDermott MP, et al Patient‐reported impact of symptoms in myotonic dystrophy type 1 (PRISM‐1). Neurology 2012;79:348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heatwole C, Johnson N, Bode R, Dekdebrun J, Dilek N, Hilbert JE, et al Patient‐reported impact of symptoms in myotonic dystrophy type 2 (PRISM‐2). Neurology 2015;85:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heatwole C, Bode R, Johnson N, Dekdebrun J, Dilek N, Heatwole M, et al Myotonic Dystrophy Health Index: initial evaluation of a disease‐specific outcome measure. Muscle Nerve 2014;49:906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heatwole C, Bode R, Johnson NE, Dekdebrun J, Dilek N, Eichinger K, et al Myotonic Dystrophy Health Index: correlations with clinical tests and patient function. Muscle Nerve 2016;53:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McColl E. Developing questionnaires In: Fayers PM, Hays RD, editors. Assessing quality of life in clinical trials: methods and practice, 2nd ed. Oxford, UK: Oxford University Press; 2005. p 9–25. [Google Scholar]
- 17. Ritchie J, Spencer L. Qualitative data analysis for applied policy research In: Bryman A, Burgess RG, editors. Analyzing qualitative data. London: Routledge; 1994. p 173–194. [Google Scholar]
- 18. Atsuta N, Watanabe H, Ito M, Banno H, Suzuki K, Katsuno M, et al Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain 2006;129:1446–1455. [DOI] [PubMed] [Google Scholar]
- 19. Johnson NE, Heatwole CR, Dilek N, Sowden J, Kirk CA, Shereff D, et al Quality‐of‐life in Charcot–Marie–Tooth disease: the patient's perspective. Neuromuscul Disord 2014;24:1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sperfeld A‐D, Hanemann CO, Ludolph AC, Kassubek J. Laryngospasm: an underdiagnosed symptom of X‐linked spinobulbar muscular atrophy. Neurology 2005;64:753–754. [DOI] [PubMed] [Google Scholar]
- 21. Tanaka S, Banno H, Katsuno M, Suzuki K, Suga N, Hashizume A, et al Distinct acoustic features in spinal and bulbar muscular atrophy patients with laryngospasm. J Neurol Sci 2014;337:193–200. [DOI] [PubMed] [Google Scholar]
- 22. Ryan CL, Bauman K. Educational attainment in the United States: 2015. Current Population Reports 2016. Available at: https://www.census.gov/content/dam/Census/library/publications/2016/demo/p20-578.pdf Accessed September 14, 2017.
- 23. Hashizume A, Katsuno M, Suzuki K, Banno H, Suga N, Mano T, et al A functional scale for spinal and bulbar muscular atrophy: cross‐sectional and longitudinal study. Neuromuscul Disord 2015;25:554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler 2009;10:310–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chahin N, Sorenson EJ. Serum creatine kinase levels in spinobulbar muscular atrophy and amyotrophic lateral sclerosis. Muscle Nerve 2009;40:126–129. [DOI] [PubMed] [Google Scholar]
- 26. Parboosingh JS, Figlewicz DA, Krizus A, Meininger V, Azad NA, Newman DS, et al Spinobulbar muscular atrophy can mimic ALS: the importance of genetic testing in male patients with atypical ALS. Neurology 1997;49:568–572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article
Supporting Information 1
Supporting Information 2


