Abstract
The aim of this review was to provide strong clinical evidence of the efficacy of deep brain stimulation (DBS) of the globus pallidus internus (GPi) in isolated inherited or idiopathic dystonia. Eligible studies were identified after a systematic literature review of the effects of bilateral GPi‐DBS in isolated dystonia. Absolute and percentage changes from baseline in the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) motor and disability scores were pooled, and associations between treatment effect and patient characteristics were explored using meta‐regression. In total, 24 studies were included in the meta‐analysis, comprising 523 patients. The mean absolute and percentage improvements in BFMDRS motor score at the last follow‐up (mean 32.5 months; 24 studies) were 26.6 points [95% confidence interval (CI), 22.4–30.8] and 65.2% (95% CI, 59.6–70.7), respectively. The corresponding changes in disability score at the last follow‐up (mean 32.9 months; 14 studies) were 6.4 points (95% CI, 5.0–7.8) and 58.6% (95% CI, 50.3–66.9). Multivariate meta‐regression of absolute scores indicated that higher BFMDRS motor and disability scores before surgery, together with younger age at time of surgery, were the main factors associated with significantly better DBS outcomes at the latest follow‐up. Reporting of safety data was frequently inconsistent and could not be included in the meta‐analysis. In conclusion, patients with isolated inherited or idiopathic dystonia significantly improved after GPi‐DBS. Better outcomes were associated with greater dystonia severity at baseline. These findings should be taken into consideration for improving patient selection for DBS.
Keywords: deep brain stimulation, dystonia, globus pallidus, surgery
Introduction
Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures or both 1. The disorder can induce severe disability and have an important impact on patients’ quality of life (QoL) 2, 3, 4. When available medical treatment has failed, bilateral deep brain stimulation (DBS) of the globus pallidus internus (GPi) is considered safe and effective, in particular in patients with isolated inherited or idiopathic (formerly primary) segmental or generalized dystonia 5, 6, 7.
To date, three meta‐analyses have assessed the effects of DBS in patients with isolated dystonia 8, 9, 10. These analyses calculated the change in score from before surgery to after surgery as a percentage of the baseline score. This value traditionally gives results that are considered clinically relevant for physicians, easily understood by patients and do not depend on the actual scale used to measure the outcome. However, the percentage change is highly dependent on the patient's baseline score. For the same absolute number of points gained on a scale, a patient with a higher baseline score will mathematically have a smaller percentage improvement than a patient with a lower baseline. Similarly, the same percentage could represent vastly different changes on the actual measurement scale, depending on where the patients started. Therefore, interpreting percentage change without knowledge of the population's baseline characteristics does not reflect the role of the initial dystonia severity in prognosis for DBS. Statistically, a method that has higher statistical power would be better, such as the absolute change in score, which is not sensitive to changes in baseline characteristics and is mathematically more appropriate in the context of a meta‐analysis 11. The absolute change better reflects improvements in health experienced by patients with dystonia after treatment with DBS, as it shows the actual number of points by which patients have improved, whereas the percentage change is skewed in favor of a lower baseline dystonia status.
Therefore, we analyzed the available data primarily using absolute changes from baseline, but also reporting the corresponding percentage change, to provide statistical validity as well as ‘practical’ and comparative clinical relevance for physicians. Our aims were to: (i) systematically identify and summarize all existing evidence on the efficacy and safety of bilateral GPi‐DBS in isolated dystonia; (ii) synthesize the available evidence with statistical meta‐analytic methods where feasible; and (iii) explore the effects of study‐level covariates on study results.
Methods
Systematic review
The systematic review was based on a literature search conducted in PubMed, Embase, Medscape and the Cochrane Central Register of Controlled Trials from January 1990 to November 2015. The search dates pre‐date the publication of the new classification of dystonia 1, so the classical definition of ‘primary’ dystonia from 1998 12 was used for study identification and selection. Primary dystonia studies were reclassified using the new classification as isolated (inherited or idiopathic) dystonia, with further specification of idiopathic or inherited types where applicable.
Publications reporting efficacy and/or safety and/or QoL outcomes for patients with isolated dystonia treated with DBS were selected. Editorials, letters, case reports, non‐systematic reviews and papers published before 1990, in a language other than English, German, French, Italian or Spanish, or reporting results on <10 patients were excluded. Further search strategy details and the selection criteria are in Appendix S1. Precautions were taken to avoid inclusion of studies reporting results on the same patients. Authors were contacted in case of doubt.
Statistical analysis
Data on study and patient characteristics, efficacy, QoL and safety parameters were extracted. For accurate calculation of absolute changes from baseline, studies were included in the meta‐analysis only if sufficient data using relevant statistical elements were reported, i.e. means and measures of precision (e.g. SDs or CIs for continuous outcomes; frequencies for binary outcomes). For studies not reporting results at study level, or when the study only reported mean changes in score but not the precision, reported individual patient data were used to derive aggregated results.
As we aimed to show corresponding percentage changes for each study included, missing data on percentage score were imputed. Mean percentage changes in scores were calculated by dividing the mean absolute change in score by the mean baseline score and multiplying by 100. The missing precision could not be approximated using available data; therefore, the median precision observed in other studies of the dataset for the same outcome was used as a proxy (a commonly used approach).
Individual study results for each outcome were pooled at 6 and 12 months and at the last mean follow‐up. Fixed‐effect and random‐effect models were applied, using the inverse variance approach. The most appropriate model in each meta‐analysis was chosen based on the presence of heterogeneity (i.e. ‘statistical heterogeneity’ is present when observed intervention effects are more different from each other than one would expect due to random error/chance alone 13), assessed by Cochran's Q test and I 2 statistics. The latter measure can be regarded as the percentage of the total variability in a set of effect sizes due to true heterogeneity and interpreted according to the classification of Higgins and Thompson in 2002 (25%, low level of heterogeneity; 50%, moderate; 75%, high) 14. In the presence of significant heterogeneity, the random‐effects analysis is the preferred approach.
The effect of study characteristics on heterogeneity in the observed effects was explored using meta‐regression. Based on previous literature findings and clinician input, as well as data available in identified studies, the characteristics included in the meta‐regression were: duration of follow‐up, baseline scores, study location, publication year, gender split, mean age at onset of disease and surgery, duration of disease, proportion of life lived with dystonia (PLD) (defined as duration of disease divided by age at baseline, multiplied by 100), and proportion of dystonia 1 protein (DYT1)‐positive patients. Due to lack of reporting in the identified studies, it was not possible to include type of dystonia, comorbidities (e.g. depression, anxiety), dystonia‐related physical complications (e.g. skeletal deformities, fixed positions), medication and stimulation parameters. Meta‐regression was carried out on absolute changes at the last follow‐up (due to their greater statistical power 11). Both univariate and multivariate models are presented. All analyses were implemented in stata SE version 8.2 (Stata Corp., College Station, TX, USA).
Results
Systematic review
In total, the literature search revealed 2501 citations (initial and update search), resulting in identification of 58 relevant articles corresponding to 54 distinct studies (Fig. 1). Most were bilateral DBS of the GPi. All were uncontrolled, apart from two studies that started as randomized controlled trials for the first 3 months post‐surgery before being extended as observational studies 5, 15. Another study was not a randomized controlled trial, but included a double‐blind evaluation with and without stimulation 3 months post‐surgery (patients served as their own control) 6.
Figure 1.
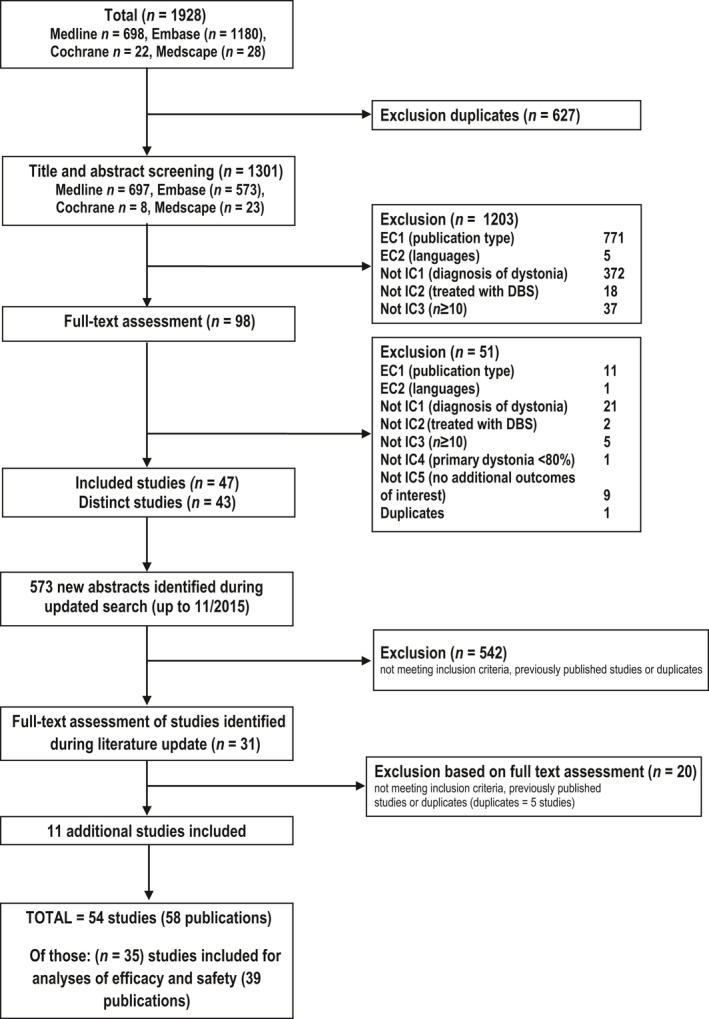
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flowchart. DBS, deep brain stimulation. EC, Exclusion criteria; IC, inclusion criteria.
Of the 54 studies identified, 24 reported usable data on the Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) 16; all 24 studies included movement scores (range 0–120), whereas 14 also reported disability scores (range 0–30) (see Table S1 for patient characteristics). Data of efficacy from 12 studies were not usable, as relevant data and/or statistics were insufficiently reported or not reported at all.
Ten studies reported results on the Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) 17. However, in most cases, these were poorly reported, with insufficient details on changes in scores. Therefore, TWSTRS was not included in the meta‐analysis.
Similarly, evidence on patient‐reported outcomes (e.g. QoL) was insufficient for inclusion in the meta‐analysis; only six studies reported QoL data and those that did used different outcomes and timepoints. The collected QoL data are provided in Table S2.
Safety outcomes were reported in 33 included studies and details are outlined in Table S3. To summarize, the most commonly reported adverse events (AEs) were device‐ or surgery‐related complications/failures (e.g. electrode or cable fracture) (reported in 23 studies), stimulation‐related AEs (18 studies) and wound problems or infections (15 studies). Intracranial hemorrhages/bleedings, mostly asymptomatic, were reported in five studies. Potentially life‐threatening AEs were reported in two studies, acute relapse of dystonia in four patients due to loss of stimulation following hardware issues. However, reporting of safety outcomes was generally inconsistent and not systematic, preventing detailed analysis of severity, time to resolution, relation to the procedure, device, stimulation or pre‐existing condition. Therefore, safety data were excluded from the meta‐analysis.
In total, the 24 studies in the meta‐analysis included 523 patients (average 22 patients per study, range 10–54) with an average follow‐up of 32.5 months (range 6–72 months). The BFMDRS mean score at time of surgery was 44.0 (SD 13.6; range 16.8–72.2) for movement and 11.3 (SD 3.4; range 4.7–16.5) for disability. Both genders were equally represented. Patients were on average 35.1 years old (SD 15.8; range 12.3–64.5) at time of surgery, and had on average been suffering from dystonia for 12.6 years (SD 4.8; range 5.1–24). Two studies focused only on pediatric patients (<18 years at time of surgery), but children were present in eight other studies in the meta‐analysis (Table S1).
Efficacy outcomes (meta‐analysis)
BFMDRS motor scores
Both absolute and percentage changes showed that DBS significantly improved motor scores at 6 months post‐surgery, with continued improvement until the last follow‐up. Mean absolute changes increased from a 23.8‐point improvement (95% CI, 18.5–29.1) at 6 months to 26.6 points (95% CI, 22.4–30.8) at the last follow‐up (mean 32.5 months, range 6–72 months) (Fig. 2a). The mean percentage improvement also increased from 59.0% (95% CI, 51.2–66.7) at 6 months to 65.2% (95% CI, 59.6–70.7) at the last follow‐up (Fig. 2a). A summary of the BFMDRS motor scores at 6 months and the latest timepoints are outlined in Table 1, with full details (all timepoints) in Appendix S2.
Figure 2.
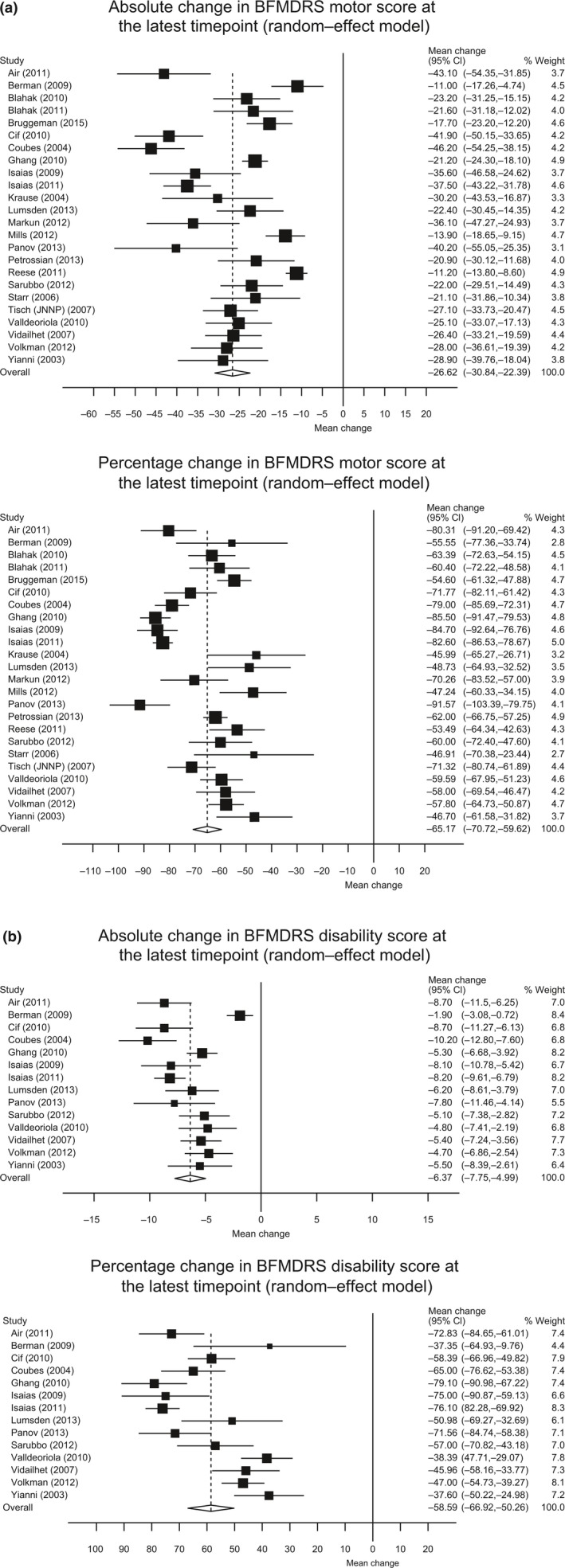
Forest plots of changes in Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) at last follow‐up. (a) Absolute (top) and percentage (bottom) change in motor; (b) absolute (top) and percentage (bottom) change in disability. CI, confidence interval. Negative changes indicate improvement.
Table 1.
Absolute and percentage changes in Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) 16 motor and disability scores (random‐effects model)
| Analysis | BFMDRS motor score | BFMDRS disability score | ||||||
|---|---|---|---|---|---|---|---|---|
| No. of studies | Months after surgery (mean/median) (range) | Pooled absolute mean change from meta‐analysis (95% CI) | Pooled percentage mean change from meta‐analysis, (%) (95% CI) | No. of studies | Months after surgery (mean/median) (range) | Pooled absolute mean change from meta‐analysis (95% CI) | Pooled percentage mean change from meta‐analysis, (%) (95% CI) | |
| At last follow‐up | 24 | 32.5/29.5 (6–72) | −26.6 (−30.8 to −22.4) | −65.2 (−70.7 to −59.6) | 14 | 32.9/30 (8–72) | −6.4 (−7.8 to −5.0) | −58.6 (−66.9 to −50.3) |
| At 6 months | 10 | 6.6/6 (6–9) | −23.8 (−29.1 to −18.5) | −59.0 (−66.7 to −51.2) | 6 | 6.8/6 (6–9) | −4.8 (−6.6 to −3.1) | −44.2 (−48.1 to −40.3) |
CI, confidence interval.
BFMDRS disability scores
Deep brain stimulation significantly improved BFMDRS disability scores in dystonia at 6 months post‐surgery, and patients continued to improve until the last follow‐up. Mean absolute changes increased from a 4.8‐point improvement (95% CI, 3.1–6.6) at 6 months to 6.4 points (95% CI, 5.0–7.8) at the last follow‐up (mean 32.9 months, Fig. 2b). The mean percentage improvement also increased from 44.2% (95% CI, 40.3–48.1) at 6 months to 58.6% (95% CI, 50.3–66.9) at the last follow‐up (Fig. 2b). A summary of the BFMDRS disability scores at 6 months and the latest timepoints are outlined in Table 1, with full details (all timepoints) in Appendix S2.
Effect of covariates (univariate and multivariate meta‐regression)
Covariates used in meta‐regression models are included in Table S1. After univariate meta‐regression, significantly greater improvements in BFMDRS motor and disability scores post‐DBS were associated with higher BFMDRS motor and disability scores (i.e. greater impairment) at baseline, younger age at time of surgery, younger age at disease onset, greater PLD and DYT1‐positive status (Tables 2 and 3). When combined in a multivariate model (apart from DYT1‐positive status, which was reported in only a subset of studies), only higher BFMDRS baseline scores remained significant, thus identifying them as the main predictors of response. As an aside, a multivariate model restricted to the three age‐related covariates showed that only age at surgery remained significant.
Table 2.
Impact of covariates on efficacy outcomes using univariate meta‐regression of absolute changes in Burke–Fahn–Marsden Dystonia Rating Scale (BFMDRS) 16 motor and disability scores at last follow‐up (mean 32.5 months for motor score, 32.9 months for disability score)
| Covariate | BFMDRS motor score | BFMDRS disability score | ||||
|---|---|---|---|---|---|---|
| No. of studies | Effect (95% CI) | P‐value | No. of studies | Effect (95% CI) | P‐value | |
| Baseline BFMDRS motor score | 23/24 | −0.55 (−0.74 to −0.36) | <0.001 | 13/14 | −0.49 (−0.72 to −0.26) | <0.001 |
| Age at onset | 21/24 | 0.41 (0.20 to 0.62) | <0.001 | 13/14 | 0.10 (0.02 to 0.17) | 0.010 |
| Age at surgery | 24/24 | 0.42 (0.23 to 0.62) | <0.001 | 14/14 | 0.11 (0.04 to 0.17) | 0.001 |
| Percentage of life with the disease | 21/24 | −0.38 (−0.61 to −0.14) | 0.002 | 13/14 | −0.07 (−0.16 to 0.01) | 0.080a |
| Percentage of DYT1 + patients | 17/24 | −0.23 (−0.33 to −0.12) | <0.001 | 11/14 | −0.04 (−0.07 to −0.02) | 0.002 |
A significance level of 10% was used due to the small number of data points in the model. CI, confidence interval; DYT1, dystonia 1 protein.
Table 3.
Clinical relevance of the impact of covariates on outcomes with deep brain stimulation (DBS) in dystonia (interpretation of univariate meta‐regression using absolute scores)
| Covariate | Change in covariate | Clinical relevance to the outcome of DBS in dystonia | |
|---|---|---|---|
| Effect on average BFMDRS motor score | Effect on average BFMDRS disability score | ||
| Impairment at baseline | Increase of 1 point on the average BFMDRS score at baseline | 0.55‐point decrease | 0.49‐point decrease |
| Age at onset of disease | Increase of 1 year in the average age at time of onset | 0.41‐point decrease | 0.10‐point decrease |
| Age at surgery | Increase of 1 year in the average age at time of surgery | 0.42‐point decrease | 0.11‐point decrease |
| PLD | Increase of 1% of life lived with the disease | 0.38‐point decrease | 0.07‐point decrease |
| DYT1‐positive status | Increase of 1% of patients who are DYT1‐positive | 0.23‐point decrease | 0.04‐point decrease |
BFMDRS, Burke–Fahn–Marsden Dystonia Rating Scale 16; DYT1, dystonia 1 protein; PLD, proportion of life lived with dystonia.
Discussion
This systematic review and meta‐analysis provides up‐to‐date evidence of the efficacy of bilateral GPi‐DBS in patients with isolated dystonia. GPi‐DBS significantly improved both motor function and ability to perform activities of daily living in patients with inherited or idiopathic isolated dystonia, measured by absolute and percentage changes in the BFMDRS score from baseline. Large effect sizes were consistently reported, in particular with regard to the BFMDRS scores (mean changes in the BFMDRS movement score: −11.0 to −46.2 points and 46.0–91.6%; mean changes in the BFMDRS disability score: −1.9 to −10.2 points and 37.3–79.1%), and effects were maintained for up to 72 months. Furthermore, both univariate and multivariate meta‐regression indicated a significant positive correlation between a greater effect of DBS and younger age at time of surgery and greater impairment at baseline (i.e. higher BFMDRS motor and disability scores). When interpreting the results, it should be considered that patients with initial higher BFMDRS scores had more room for improvement, whereas the effect in less impaired patients might have been skewed according to a floor effect. It should also be noted that BFMDRS scores may not adequately reflect severity of disease in focal or segmental dystonia. Only the univariate meta‐regression showed a positive, significant relationship with greater PLD and DYT1‐positive status.
Our findings support and expand results from previous analyses, which found consistent and large benefits across studies. One review separated patients with dystonia into two subtypes, one with good evidence of favorable outcome and one with less predictable outcome, and found that GPi‐DBS was more effective long‐term in patients with severe isolated (inherited or idiopathic) generalized and cervical dystonia 10. Another meta‐analysis showed that the mean percentage change in the BFMDRS score from baseline was 51.8% (range –34% to 100%) post‐surgery (although it is unclear at what timepoint the data were analyzed) 8.
Although data on improvement in the TWSTRS scores in patients with cervical dystonia could not be meta‐analyzed due to insufficient details on changes in scores, individual study results support the positive impact of DBS in patients with cervical dystonia, with up to 40–70% improvements in the total TWSTRS scores.
Our univariate meta‐regression showed that absolute BFMDRS motor and disability scores decreased more after DBS with greater PLD. This differs from previous analysis in children demonstrating a negative correlation between PLD and percentage BFMDRS 18. It was argued that measuring absolute changes in BFMDRS after DBS would be a fairer representation of the effects of DBS on the most severely affected cases 18. The patients included in our large meta‐analysis were on average 35.1 years old (SD 15.8; range 12.3–64.5) at time of surgery, and had been suffering from dystonia for an average of 12.6 years (SD 4.8; range 5–24).
Although younger age at DBS surgery confers an advantage for DBS dystonia reduction, thus strongly supporting DBS in childhood, DBS is also effective in adults. Indeed, our univariate analysis demonstrated that patients with severe dystonia who have lived with dystonia for many years responded well to DBS. Other results are in contrast with previous analyses. One study found that shorter dystonia duration and lower severity score at surgery were associated with significantly greater improvements post‐DBS 9, whereas in another study longer duration of symptoms correlated negatively with surgical outcome, with no effect on outcome of DYT1 8. However, our systematic review is more extensive than those previously published (a wider search and larger selection of databases). A novel feature of our review is that it also includes both individual patient data and results aggregated at study level; therefore, meta‐analysis was the most appropriate statistical tool. Furthermore, it analyzed absolute and percentage changes.
The main limitation of our analysis is linked to the quality of data reported in available publications, which led to 38% of identified studies being excluded at the outset. In particular, no data on QoL effects could be included due to the lack of quality reporting. In addition, some efficacy data were excluded from our meta‐analysis as no precision (i.e. SD, standard error or CI) was provided around the mean estimates, or results were reported as medians and ranges. Furthermore, details on patient enrolment (timing, centers) in some studies were poorly reported and, despite precautions (contact with authors, omission of papers with obvious large patient overlap), some partial overlaps, albeit very limited, may exist. The safety analysis presented specific challenges due to wide disparities in the information reported. Some studies focused on the number of events, others on the number of patients experiencing them, and others described the evolution of a few clinically interesting patients. This lack of consistency across studies, as well as the lack of detail on the events reported (type, severity, resolution, relation to surgery, device or condition), prevented meta‐analysis and limited reporting to a very generic outline. The limited data also restricted the type of analyses, and subgroup analyses (e.g. by type of dystonia, adult/pediatric populations) could not be performed. Multivariate meta‐regression models assessing the impact of all study characteristics were implemented. However, the overall number of studies was limited and ideally more data are necessary to properly assess the joint effect of these variables, i.e. their impact on outcome as well as their correlation with each other. Meta‐regression results should only be considered as leads for further investigations and not used to draw definite conclusions.
This review has important implications for future research. In particular, improved and consistent reporting of results, including provision of precision estimates, mean and median values and ranges, is vital for inclusion of studies into meta‐analyses. In addition, consistent and thorough reporting of safety data is essential. Meta‐regression also indicates the need to investigate associations between DBS benefits with specific population characteristics. Finally, data gaps related to patient‐reported outcomes such as QoL, which were rarely assessed 19, indicate the need for more comprehensive assessment of the impact of dystonia and DBS from the patient's perspective.
Conclusions
This meta‐analysis adds further strong evidence that bilateral GPi‐DBS has a significant beneficial clinical impact on patients with isolated dystonia, reflected by both absolute and percentage changes in BFMDRS from baseline. Moreover, benefits continue for successive years over a 3‐year follow‐up period. Greater impairment at baseline and younger age at DBS surgery were positively related to better outcomes with GPi‐DBS. These findings have relevance in clinical practice regarding the timing and selection of patients with dystonia for DBS.
Disclosure of conflicts of interest
E. Moro has received honoraria from Medtronic for consulting services and lecturing. C. LeReun received consulting fees from Medtronic International Sàrl for her work on this project. J. K. Krauss is a consultant to Medtronic and to Boston Scientific. He received honoraria from AbbVie and fees for speaking from St Jude. A. Albanese received speaker's honoraria from Ipsen, Merz, Medtronic and Boston Scientific. J.‐P. Lin has received a New Service and Innovation Grant from Guy's and St Thomas’ Charity G, Dystonia Society UK grants, an Action Medical Research grant, unrestricted education grants from Medtronic Ltd for educational meetings and consultancy honoraria from Medtronic Ltd. M. Vidailhet is on a Merz scientific advisory board. She has received unrestricted research grants from ANR (French National Institute) and from AMADYS and Alliance France Dystonie (patient associations). S. Walleser Autiero and T.C. Brionne are employees of Medtronic International Sàrl.
Supporting information
Table S1. Patient characteristics at baseline of all studies included in the efficacy and safety meta‐analyses.
Table S2. Quality of life after deep brain stimulation of the globus pallidus internus.
Table S3. Safety outcomes reported in bilateral deep brain stimulation of the globus pallidus internus studies.
Appendix S1. Detailed search strategy.
Appendix S2. Full results of meta‐analyses regarding efficacy of deep brain stimulation in dystonia at 6, 12 and 24–36 months and at last follow‐up.
Acknowledgements
The study was funded by Medtronic International Sàrl, including the services of the medical writer, D. Nock (Medical WriteAway, Norwich, UK). Medtronic International Sàrl provided funding for the services of an independent outcomes research consultancy (Optum Insight) to undertake the systematic literature search and review, and for the services of an independent statistician (Corinne LeReun) to undertake the statistical analyses. Medtronic International Sàrl had the initial idea for the study design; the consultants undertook data collection and analyses, and wrote the study reports, without Medtronic's influence.
References
- 1. Albanese A, Bhatia K, Bressman SB, et al Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013; 28: 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Camfield L, Ben‐Shlomo Y, Warner T. Impact of cervical dystonia on quality of life. Mov Disord 2002; 17: 838–841. [DOI] [PubMed] [Google Scholar]
- 3. Page D, Butler A, Jahanshahi M. Quality of life in focal, segmental, and generalized dystonia. Mov Disord 2007; 22: 341–347. [DOI] [PubMed] [Google Scholar]
- 4. Lin JP, Lumsden DE, Gimeno H, Kaminska M. The impact and prognosis for dystonia in childhood including dystonic cerebral palsy: a clinical and demographic tertiary cohort study. J Neurol Neurosurg Psychiatry 2014; 85: 1239–1244. [DOI] [PubMed] [Google Scholar]
- 5. Kupsch A, Benecke R, Mueller J, et al Pallidal deep‐brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006; 355: 1978–1990. [DOI] [PubMed] [Google Scholar]
- 6. Vidailhet M, Vercueil L, Houeto J, et al Bilateral deep‐brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005; 352: 459–467. [DOI] [PubMed] [Google Scholar]
- 7. Moro E, Gross R, Krauss J. What's new in surgical treatment for dystonia? Mov Disord 2013; 28: 1013–1020. [DOI] [PubMed] [Google Scholar]
- 8. Holloway K, Baron M, Brown R, Cifu D, Carne W, Ramakrishnan V. Deep brain stimulation for dystonia: a meta‐analysis. Neuromodulation 2006; 9: 253–261. [DOI] [PubMed] [Google Scholar]
- 9. Andrews C, Aviles‐Olmos I, Hariz M, Foltynie T. Which patients with dystonia benefit from deep brain stimulation? A metaregression of individual patient outcomes. J Neurol Neurosurg Psychiatry 2010; 81: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 10. Vidailhet M, Jutras M‐F, Grabli D, Roze E. Deep brain stimulation for dystonia. J Neurol Neurosurg Psychiatry 2013; 84: 1029–1042. [DOI] [PubMed] [Google Scholar]
- 11. Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol 2001; 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fahn S, Bressman S, Marsden C. Classification of dystonia. Adv Neurol 1998; 78: 1–10. [PubMed] [Google Scholar]
- 13. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. http://handbook.cochrane.org/ (accessed 25/01/2017). [Google Scholar]
- 14. Higgins J, Thompson S. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 15. Volkmann J, Mueller J, Deuschl G, et al Pallidal neurostimulation in patients with medication‐refractory cervical dystonia: a randomised, sham‐controlled trial. Lancet Neurol 2014; 13: 875–884. [DOI] [PubMed] [Google Scholar]
- 16. Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985; 35: 73–77. [DOI] [PubMed] [Google Scholar]
- 17. Consky E, Lang A. Clinical assessments of patients with cervical dystonia In: Jankovic J, Hallett M, eds. Therapy with Botulinum Toxin. New York, NY: Marcel Dekker, 1994: 211–237. [Google Scholar]
- 18. Lumsden D, Kaminska M, Gimeno H, et al Proportion of life lived with dystonia inversely correlates with response to pallidal deep brain stimulation in both primary and secondary childhood dystonia. Dev Med Child Neurol 2013; 55: 567–574. [DOI] [PubMed] [Google Scholar]
- 19. Cano S, Warner T. Dystonia and quality of life In: Warner T, Bressman S, eds. Clinical Diagnosis and Management of Dystonia. Boca Raton, FL: CRC Press, 2007: 241–247. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient characteristics at baseline of all studies included in the efficacy and safety meta‐analyses.
Table S2. Quality of life after deep brain stimulation of the globus pallidus internus.
Table S3. Safety outcomes reported in bilateral deep brain stimulation of the globus pallidus internus studies.
Appendix S1. Detailed search strategy.
Appendix S2. Full results of meta‐analyses regarding efficacy of deep brain stimulation in dystonia at 6, 12 and 24–36 months and at last follow‐up.


