Abstract
Purpose
To compare the additive effects and safety of 1% brinzolamide/0.5% timolol fixed combination (BTFC) versus the low‐dose regimen of 1% dorzolamide/0.5% timolol fixed combination (DTFC) in patients with open‐angle glaucoma and ocular hypertension (OAG/OH) following treatment with prostaglandin analogues (PGAs).
Methods
A prospective, randomized, double‐masked, multicentre, parallel‐group and active‐controlled study included 201 Japanese OAG/OH patients who had been treated with PGA. Efficacy was assessed as the change in intra‐ocular pressure (IOP) from baseline after weeks 4 and 8. Safety was assessed with adverse event rates, ocular discomfort score, blur scale, blood pressure and heart rates, best‐corrected visual acuity (BCVA) and slit lamp examinations.
Results
Intra‐ocular pressure (IOP) change from baseline at 9 AM/11 AM pooled over the 8 weeks was −3.3/−3.3 mmHg in the BTFC group and −2.9/−3.4 mmHg in the DTFC group, demonstrating non‐inferiority of BTFC to DTFC. Ocular irritation was frequently seen in DTFC group. Although blurred vision was frequently seen in BTFC group, it was transient and blurring became the equivalent 3 min after instillation between two groups. No noteworthy issue was observed in other safety outcome.
Conclusion
Non‐inferiority of BTFC to DTFC in IOP reduction was demonstrated after adding onto PGA therapy in Japanese OAG/OH patients. Although the score of blurred vision was transiently higher in BTFC than DTFC, treatment difference decreased and disappeared with time. Thus, BTFC can be considered as a safe and effective agent for glaucoma treatment.
Keywords: brinzolamide, dorzolamide, fixed combination, glaucoma, intra‐ocular pressure
Introduction
The only evidence‐based treatment for glaucoma involves lowering of IOP even in normal‐tension glaucoma (NTG) patients (Collaborative Normal‐Tension Glaucoma Study Group 1998a,b; The AGIS Investigators 2000), and IOP‐lowering agents are generally administered according to individual target IOP values. Various regimens are used for glaucoma treatment and are selected according to individual target pressures. Moreover, combination therapies are frequently administered with the expectation of further lowering of IOP levels.
Therapeutic challenges, such as inconvenience, poor adherence and ocular surface disease hamper eyedrop effectiveness, potentially contributing to poor prognosis in glaucoma patients (Hollo et al. 2014; Weinreb et al. 2014). Common barriers to instilling eyedrops include low self‐efficacy, forgetfulness and difficulty with drop administration and medication schedules (Kashiwagi & Furuya 2014; Newman‐Casey et al. 2015). In addition, multidrug therapies may induce chronic ocular surface disease (Baudouin et al. 2008; Skalicky et al. 2012). Therefore, single product combination medications are considered beneficial for glaucoma treatment, because they promote patient convenience and adherence.
Fixed combinations of a carbonic anhydrase inhibitor (CAI) and the beta‐blocker 0.5% timolol maleate are commonly used as second‐line treatments, and further IOP reductions are expected following insufficient efficacy of PGAs to achieve target pressure. Currently, 1% brinzolamide and 0.5% timolol maleate fixed combination (BTFC, AZARGA®, Japanese trade name AZORGA®; Alcon Laboratories, Inc., Fort Worth, TX, USA) and dorzolamide and 0.5% timolol combination (DTFC, COSOPT®; Merck & Co., Inc., Kenilworth, NJ, USA) are available in the global market. However, the concentration of dorzolamide in the Japanese COSOPT® preparation is 1%, but is 2% in foreign products.
Brinzolamide and 0.5% timolol maleate (BTFC) fixed combination and DTFC ophthalmic solutions containing 2% dorzolamide (2% DTFC) have been compared in terms of IOP‐lowering efficacy in other countries, and superior ocular comfort with BTFC has been demonstrated (Manni et al. 2009; Sezgin Akcay et al. 2013). However, IOP‐lowering effects and safety of BTFC have not been compared with those of DTFC containing 1% dorzolamide (1% DTFC) in Japan. Furthermore, the additive effects of BTFC or DTFC in PGA‐treated patients have not been compared. CAI/timolol fixed combination therapy is expected to have additive effects in Japanese patients who need lower target pressure, because the baseline pressure of OAG is lower in this population than in other countries, due to higher prevalence of NTG (Iwase et al. 2014). Therefore, we report a prospective, randomized, double‐masked, multicentre, parallel‐group and active‐controlled investigation of the efficacy, safety and the additive effects of BTFC and DTFC in PGA‐treated Japanese patients with OAG or OH.
Patients and Methods
This study was performed as a prospective, randomized, double‐masked, multicentre, parallel‐group and active‐controlled trial. The study protocol was reviewed and approved by the institutional review board of each participating institution and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before enrolment, and the study was registered with UMIN (http://www.umin.ac.jp), study number UMIN000017569.
Patients receiving PGA monotherapy for OAG or OH were recruited and included in analyses according to the following criteria: (1) 20 years of age or older, (2) currently receiving PGA monotherapy, PGA + alpha‐1/beta‐blocker, or PGA + alpha‐2 agonist at the screening visit, (3) able to receive only PGA for 4 weeks until the baseline visit, and (4) IOP ≥ 15 mmHg in at least one eye at both 9 and 11 AM baseline visits at baseline. Patients were excluded according to the following criteria: (1) history of hypersensitivity to any of the excipients in the study medications, (2) history of ocular trauma or intra‐ocular surgery in either eye within 6 months or experience of ocular laser surgery in either eye within 3 months of the screening examination, (3) ocular infection, ocular inflammation, history of or current clinically significant or progressive retinal disease, such as retinal degeneration, diabetic retinopathy (DR), or retinal detachment in either eye, (4) BCVA score of <0.2 (decimal visual acuity) in either eye, (5) any abnormality preventing reliable applanation tonometry of either eye, (6) angle grade 2 or less in either eye (Shaffer classification), (7) severe visual field loss in either eye (judged by the investigator), (8) previous or current use of any topical drug containing CAI, (9) use of any additional topical or systemic ocular hypotensive medication indicated for glaucoma or intra‐ocular hypertension during the study, (10) use of corticosteroid medications via ocular or systemic routes during the study, (11) pregnant or lactating, or intending to become pregnant during the study period, (12) previous or current therapy with another investigational agent within 30 days prior to the screening examination, (13) previous or current evidence of a severe illness or any other condition that could make the patient unsuitable for the study (judged by the investigator).
After obtaining informed consent from patients, inclusion and exclusion criteria were confirmed and screening examinations were performed. Subsequently, patients were provisionally enrolled in the study and the PGA run‐in period commenced with PGA monotherapy. Any IOP‐lowering medication used adjunctively to the PGA at screening was washed out over the four‐week run‐in period. After ≥4 weeks, baseline examinations were performed, and inclusion and exclusion criteria were again confirmed before assignment of medication regimens. A third‐party clinical research organization (CRO; Densuke Systems Co., Ltd., Tokyo, Japan) randomly assigned patients to BTFC or DTFC treatment groups using the permuted block method. Randomization codes were sealed and stored by the CRO until the key break. During the treatment period, PGA was continuously applied with assigned study medications (BTFC or DTFC) in both eyes two times daily (at 9 AM ± 30 min and 9 PM ± 30 min) for 8 weeks. PGA treatment times were the same as at baseline, and changes to other PGA or generic products with the same ingredients were not permitted. Intra‐ocular pressure (IOP) was measured immediately before treatments at 9 and 11 AM at baseline, at week 4 (28 ± 7 days) and week 8 (56 ± 7 days) examinations. We performed IOP measurements at 9 (just before eyedrop treatment) and 11 AM to assess IOP changes at peak and trough times when medication effects are expected to be lowest and highest, respectively. After baseline IOP measurements at 11 AM, study medications were prescribed and initial applications were performed immediately before assessing ocular discomfort score and blur scale questionnaires. Slit lamp examinations of superficial punctate keratitis (SPK, scores 0–2) and hyperaemia (scores 0–2), measurements of BCVA, blood pressure and heart rate, and observations of adverse events were performed at baseline and weeks 4 and 8. Slit lamp examinations were performed before measurements of IOP.
Intra‐ocular pressures of both eyes were measured twice using a Goldmann applanation tonometer. IOP data used for efficacy analysis were the mean of the two measurements. Efficacy outcomes were analysed using one eye (the target eye) from each patient. The target eye was defined as the eye that satisfied all inclusion/exclusion criteria, and if both eyes satisfied the criteria, the target eye was defined as that with higher IOP at 11 AM on baseline visit and was selected for analyses. When IOPs of both eyes were equal at 11 AM, the eye with higher IOP at 9 AM was selected, and when both eyes had equal IOPs at 9 and 11 AM, the right eye was selected for analysis.
The primary objective of this study was to demonstrate non‐inferiority of BTFC to DTFC for IOP‐lowering efficacy throughout the 8‐week study period. For this purpose, analysis was conducted as follows. Treatment means and differences in IOP changes from baseline were estimated with 95% confidence intervals using a mixed model for repeated measures (SAS Institute Inc. 2011). Non‐inferiority would be demonstrated when the upper limit of two‐sided 95% confidence interval of the treatment difference at 11 AM pooled over 8 weeks was below +1.5 mmHg, the non‐inferiority margin for this assessment. Statistical test was conducted first at 11 AM as primary analysis and then at 9 AM as secondary analysis based on a sequential testing procedure. When non‐inferiority was demonstrated at both time‐points, superiority would be tested at 11 AM and 9 AM sequentially and would be demonstrated when the confidence limit was below 0 mmHg. Among four hypothesis testing paradigms, one‐sided familywise error rate was controlled at one‐sided 2.5%. For other safety and other efficacy analysis, p‐values were provided for exploratory purposes only, without controlling for alpha and beta errors. Safety analysis was conducted using Safety data set. Efficacy analysis was conducted using full analysis set (FAS) and per protocol set (PPS). The data of all patients who received study medication were included in the Safety data set. The FAS included all randomized subjects who received study medication and who completed at least one scheduled on‐therapy study visit. The data of all patients who received study medication and met the inclusion and exclusion criteria were the PPS.
A target sample size of 86 was estimated for each treatment group. With 86 patients for each group, assuming that true treatment difference in IOP change from baseline is 0 mmHg and a common standard deviation is 3.0 mmHg, there is a 90% probability that the 97.5% one‐sided upper confidence limit of treatment difference falls below +1.5 mmHg. This non‐inferiority margin (1.5 mmHg) was selected from a previous study with a similar design (Manni et al. 2009).
Statistical analyses were conducted by the third‐party CRO (Densuke Systems Co., Ltd.) independent of investigational sites where patients were enrolled. The software used for statistical analysis was sas rel.9.3 (SAS Inc, Cary, NC, USA).
Results
A total of 201 patients were enrolled and randomized into treatment groups. Among these, 194 completed the study, and seven discontinued due to adverse events (five patients), exclusion by the investigator (one patient) or at the patient's request (one patient). Two patients had no IOP data after the baseline visit, and 199 patients were included in the FAS. However, 10 of these patients had protocol deviations due to personal reasons and the PPS comprised the remaining 189 patients and was used for primary analyses of 92 and 97 patients in BTFC and DTFC groups, respectively.
Patient backgrounds in the PPS are shown in Table 1. Numbers of patients diagnosed with OAG were 73/92 (79.3%) in the BTFC group and 81/97 (83.5%) in the DTFC group, and the remaining patients were diagnosed with OH. The PGA latanoprost was used by 66/92 (71.7%) patients of the BTFC group and by 52/97 (53.6%) patients in the DTFC group, and the PGA tafluprost was used in 14/92 (15.2%) and 24/97 (24.7%) patients in respective treatment groups.
Table 1.
Demographic data from the per protocol set
| BTFC | DTFC | |
|---|---|---|
| N | 92 | 97 |
| Sex, n (%) | ||
| Male | 43 (46.7) | 46 (47.4) |
| Female | 49 (53.3) | 51 (52.6) |
| Age, years | ||
| Mean ± SD | 62.5 ± 11.9 | 64.1 ± 12.4 |
| Range | 32–88 | 31–86 |
| Diagnosis, n (%) | ||
| OAG | 73 (79.3) | 81 (83.5) |
| OH | 19 (20.7) | 16 (16.5) |
| Concomitant prostaglandin analogue, n (%) | ||
| Latanoprost | 66 (71.7) | 52 (53.6) |
| Tafluprost | 14 (15.2) | 24 (24.7) |
| Travoprost | 11 (12.0) | 15 (15.5) |
| Bimatoprost | 1 (1.1) | 6 (6.2) |
| Best‐corrected visual acuity, LogMAR | ||
| Mean ± SD | −0.044 ± 0.098 | −0.033 ± 0.122 |
BTFC = brinzolamide/timolol fixed combination; DTFC = dorzolamide/timolol fixed combination; SD = standard deviation; OAG = open‐angle glaucoma; OH = ocular hypertension.
Descriptive statistics for IOP and IOP change from baseline in both groups from the PPS are tabulated in Table 2, and profiles of IOP over the study period are presented in Fig. 1. A mixed model for repeated measures was used to generate least square means of IOP and IOP change from baseline with confidence intervals (Table 3). IOP at 9 and 11 AM on the baseline day was 17.4 and 17.0 mmHg in the BTFC group and 17.3 and 17.0 mmHg in the DTFC group, respectively. Mean IOP changes from baseline at 9 and 11 AM on weeks 4 and 8 ranged from −3.5 to −3.0 mmHg in the BTFC group (p < 0.0001 at all measurement points) and from −3.4 to −2.7 mmHg in the DTFC group (p < 0.0001 at all measurement points). IOP changes from baseline at 11 AM and at 9 AM pooled over 8 weeks were −3.3 and −3.3 mmHg in the BTFC group and −3.4 and −2.9 mmHg in the DTFC group, and treatment differences with 95% two‐sided confidence intervals were 0.1 mmHg [−0.4, 0.6] at 11 AM and −0.4 mmHg [−0.9, 0.1] at 9 AM, respectively. According to the result that the upper side of 95% two‐sided confidence interval of treatment difference at 11 AM and 9 AM was less than the non‐inferiority criteria of +1.5 mmHg, non‐inferiority of BTFC to DTFC in IOP‐lowering efficacy at both time‐points was demonstrated. Moreover, similar results were generated using the FAS.
Table 2.
Descriptive statistics for IOP and IOP changes from baseline
| Visit | IOP (mmHg) | IOP change from baseline (mmHg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 weeks | 8 weeks | 4 weeks | 8 weeks | ||||||
| Time | 9 AM | 11 AM | 9 AM | 11 AM | 9 AM | 11 AM | 9 AM | 11 AM | 9 AM | 11 AM |
| BTFC | ||||||||||
| N | 92 | 92 | 91 | 91 | 90 | 90 | 91 | 91 | 90 | 90 |
| Mean | 17.4 | 17.0 | 14.4 | 13.9 | 14.0 | 13.5 | −3.1 | −3.0 | −3.5 | −3.5 |
| SD | 2.1 | 1.8 | 2.3 | 2.2 | 2.3 | 2.0 | 1.9 | 1.9 | 1.9 | 1.9 |
| Minimum | 15.0 | 15.0 | 8.0 | 8.0 | 8.0 | 9.0 | −8.5 | −8.0 | −8.0 | −9.0 |
| Maximum | 25.0 | 22.0 | 20.0 | 20.5 | 22.0 | 21.0 | 2.0 | 2.0 | 1.0 | 3.0 |
| DTFC | ||||||||||
| N | 97 | 97 | 96 | 96 | 97 | 97 | 96 | 96 | 97 | 97 |
| Mean | 17.3 | 17.0 | 14.6 | 13.6 | 14.3 | 13.5 | −2.7 | −3.3 | −3.0 | −3.4 |
| SD | 1.8 | 1.7 | 2.1 | 2.0 | 2.1 | 1.9 | 2.0 | 2.0 | 2.0 | 2.0 |
| Minimum | 15.0 | 15.0 | 10.0 | 9.0 | 10.0 | 9.0 | −7.0 | −9.0 | −7.0 | −9.5 |
| Maximum | 22.0 | 22.0 | 21.0 | 19.0 | 20.0 | 18.0 | 3.0 | 4.0 | 2.0 | 3.0 |
IOP = Intraocular pressure; BTFC = brinzolamide timolol fixed combination; DTFC = dorzolamide timolol fixed combination; SD = standard deviation.
Figure 1.
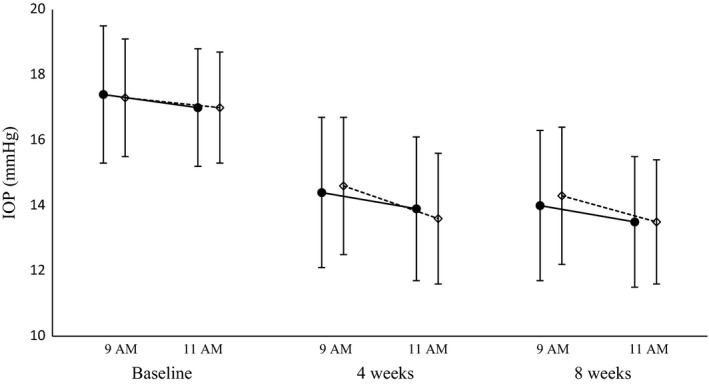
Changes in intra‐ocular pressure (IOP) following treatment with BTFC (●) or dorzolamide timolol fixed combination (DTFC) (♢) in pooled samples from baseline and 4‐ and 8‐week visits. Data are presented as means and standard deviations.
Table 3.
Least squares means and treatment differences in IOP and IOP changes from baseline
| Visit | IOP (mmHg) | IOP change from baseline | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Pooled | 4 week | 8 week | Pooled | 4 week | 8 week | ||||||||
| Time | 9 AM | 11 AM | 9 AM | 11 AM | 9 AM | 11 AM | 9 AM | 11 AM | 9 AM | 11 AM | 9 AM | 11 AM | 9 AM | 11 AM |
| BTFC | ||||||||||||||
| N | 92 | 92 | 92 | 92 | 91 | 91 | 90 | 90 | 92 | 92 | 91 | 91 | 90 | 90 |
| LSM | 17.4 | 17.0 | 14.2 | 13.7 | 14.4 | 13.9 | 14.0 | 13.4 | −3.3 | −3.3 | −3.1 | −3.0 | −3.5 | −3.5 |
| 95% CI | ||||||||||||||
| Lower | 17.1 | 16.6 | 13.8 | 13.3 | 14.0 | 13.5 | 13.6 | 13.0 | −3.6 | −3.6 | −3.5 | −3.4 | −3.9 | −3.9 |
| Upper | 17.8 | 17.3 | 14.6 | 14.1 | 14.8 | 14.4 | 14.4 | 13.9 | −2.9 | −2.9 | −2.7 | −2.6 | −3.1 | −3.1 |
| DTFC | ||||||||||||||
| N | 97 | 97 | 97 | 97 | 96 | 96 | 97 | 97 | 97 | 97 | 96 | 96 | 97 | 97 |
| LSM | 17.3 | 17.0 | 14.5 | 13.6 | 14.6 | 13.6 | 14.3 | 13.5 | −2.9 | −3.4 | −2.7 | −3.3 | −3.0 | −3.4 |
| 95% CI | ||||||||||||||
| Lower | 17.0 | 16.6 | 14.1 | 13.2 | 14.2 | 13.2 | 13.9 | 13.1 | −3.2 | −3.7 | −3.1 | −3.7 | −3.4 | −3.8 |
| Upper | 17.7 | 17.3 | 14.9 | 14.0 | 15.0 | 14.0 | 14.8 | 13.9 | −2.5 | −3.0 | −2.3 | −2.9 | −2.6 | −3.0 |
| Comparison between treatment | ||||||||||||||
| Differences | 0.1 | −0.0 | −0.3 | 0.1 | −0.2 | 0.3 | −0.3 | −0.1 | −0.4 | 0.1 | −0.3 | 0.3 | −0.5 | −0.1 |
| 95% CI | ||||||||||||||
| Lower | −0.4 | −0.5 | −0.8 | −0.4 | −0.8 | −0.3 | −0.9 | −0.7 | −0.9 | −0.4 | −0.9 | −0.2 | −1.0 | −0.7 |
| Upper | 0.7 | 0.5 | 0.3 | 0.7 | 0.4 | 0.9 | 0.3 | 0.5 | 0.1 | 0.6 | 0.2 | 0.9 | 0.1 | 0.5 |
IOP = Intra‐ocular pressure; BTFC = brinzolamide/timolol fixed combination; DTFC = dorzolamide/timolol fixed combination; LSM = least squares mean; CI = confidence interval.
Least squares means and confidence intervals were generated using a mixed model for repeated measures.
Discomfort scores were evaluated using a 5‐point scale (0–4) immediately after 11 AM baseline eyedrop applications (Table 4). Although no differences in ocular pain and foreign body sensations were observed, a 0.73 treatment difference in blurred vision (p < 0.0001) and a slight −0.19 difference in irritation (p = 0.0415) were identified.
Table 4.
Discomfort scores
| Assessment | Treatment | N | Mean | SD | Minimum | Maximum | Comparisons between treatments | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Differences | 95% CI | p Valuea | ||||||||
| Lower | Upper | |||||||||
| Ocular pain | BTFC | 98 | 0.08 | 0.31 | 0 | 2 | −0.05 | −0.15 | 0.06 | 0.3829 |
| DTFC | 101 | 0.13 | 0.44 | 0 | 3 | |||||
| Irritation | BTFC | 98 | 0.28 | 0.51 | 0 | 3 | −0.19 | −0.37 | −0.01 | 0.0415 |
| DTFC | 101 | 0.47 | 0.77 | 0 | 3 | |||||
| Foreign body sensation | BTFC | 98 | 0.06 | 0.24 | 0 | 1 | 0.03 | −0.03 | 0.09 | 0.2896 |
| DTFC | 101 | 0.03 | 0.17 | 0 | 1 | |||||
| Blurred vision | BTFC | 98 | 1.59 | 0.91 | 0 | 4 | 0.73 | 0.49 | 0.97 | <.0001 |
| DTFC | 101 | 0.86 | 0.84 | 0 | 3 | |||||
SD = standard deviation; CI = confidence interval; BTFC = brinzolamide/timolol fixed combination; DTFC = dorzolamide/timolol fixed combination.
Unpaired t‐test.
Changes in blurred vision with time from just after eyedrop application to 3 min later were investigated using a 51‐point scale (0–50; Table 5 and Fig. 2). Although mean blurred vision scores were transiently higher in the BTFC group than in the DTFC group (>9 points at 0 min, p < 0.0001), treatment difference decreased and almost disappeared at 3 min.
Table 5.
Blur scale
| Time after instillation (min) | BTFC | DTFC | Comparisons between treatments (p valuea) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Median | Minimum | Maximum | N | Mean | SD | Median | Minimum | Maximum | ||
| 0 | 98 | 19.8 | 15.6 | 20.0 | 0 | 50 | 101 | 10.4 | 13.8 | 0.0 | 0 | 40 | <0.0001 |
| 1 | 98 | 13.1 | 12.8 | 10.0 | 0 | 50 | 101 | 6.8 | 10.2 | 0.0 | 0 | 40 | 0.0001 |
| 2 | 98 | 7.7 | 9.6 | 3.0 | 0 | 40 | 101 | 3.9 | 7.3 | 0.0 | 0 | 30 | 0.0019 |
| 3 | 98 | 3.7 | 7.4 | 0.0 | 0 | 40 | 101 | 2.4 | 5.8 | 0.0 | 0 | 30 | 0.1573 |
BTFC = brinzolamide/timolol fixed combination; DTFC = dorzolamide/timolol fixed combination; SD = standard deviation.
t‐test based on a mixed model for repeated measures.
Figure 2.
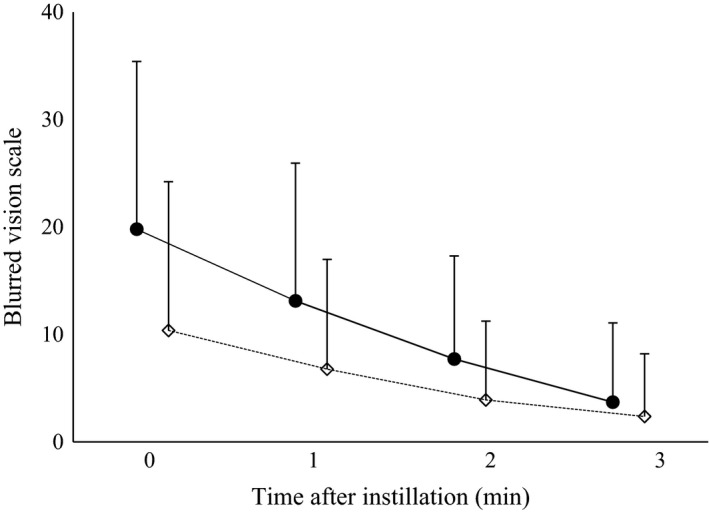
Changes in blur scale after instillation of BTFC (●) or DTFC (♢). Data are presented as means and standard deviations.
Drug‐related adverse events (Table 6) occurred in 26.5% (26/98) of patients in the BTFC group and in 20.4% of patients (21/103) in the DTFC group. Eye local drug‐related adverse events with greater than 2% incidence in the BTFC group included blurred vision (20.4%; 20/98) and eye irritation (2.0%; 2/98), whereas blurred vision and eye irritation were observed in 8.7% (9/103) and 9.7% (10/103) of patients in the DTFC group, respectively. No serious study drug‐related adverse events were recorded.
Table 6.
Drug‐related adverse events
| BTFC | DTFC | |||
|---|---|---|---|---|
| N = 98 | N = 103 | |||
| N | % | N | % | |
| Patients with drug‐related adverse events | 26 | 26.5 | 21 | 20.4 |
| Eye disorders | ||||
| Blurred vision | 20 | 20.4 | 9 | 8.7 |
| Eye irritation | 2 | 2.0 | 10 | 9.7 |
| Corneal disorder | 1 | 1.0 | 0 | 0.0 |
| Eye pruritus | 1 | 1.0 | 0 | 0.0 |
| Conjunctival hyperaemia | 1 | 1.0 | 1 | 1.0 |
| Punctate keratitis | 1 | 1.0 | 1 | 1.0 |
| Iritis | 1 | 1.0 | 0 | 0.0 |
| Keratitis | 0 | 0.0 | 1 | 1.0 |
| Administration site irritation | 0 | 0.0 | 1 | 1.0 |
| Administration site pain | 0 | 0.0 | 2 | 1.9 |
| Systemic disorders | ||||
| Bradycardia | 1 | 1.0 | 0 | 0.0 |
| Arrhythmia | 1 | 1.0 | 0 | 0.0 |
| Dysgeusia | 1 | 1.0 | 1 | 1.0 |
BTFC = brinzolamide/timolol fixed combination; DTFC = dorzolamide/timolol fixed combination.
Visual acuity (VA) did not vary in both study groups, no difference were found at any time‐point, and no differences in blood pressure or pulse were identified. Moreover, changes in SPK and hyperaemia scores from baseline did not differ between treatment groups at weeks 4 and 8, and anterior chambers had normal appearance in both treatment groups at all time‐points.
Discussion
The present multicentre prospective study is the first to compare the additive IOP‐lowering effects of BTFC and DTFC on top of a PGA in Japanese OAG/OH patients.
In the present study, patients applied CAI/BB eyedrops at 9:00 AM and 9 PM and we performed IOP measurements at 9 (just before eyedrop treatment) and 11 AM to assess IOP changes at trough and peak times when medication effects are expected to be lowest and highest, respectively. The non‐inferiority of BTFC to DTFC was demonstrated in IOP change from baseline both at 11 AM (peak time) and 9 AM (trough time) pooled over weeks.
Previous comparisons of IOP reductions by BTFC and DTFC have only been performed in studies of non‐Japanese subjects treated with 2% DTFC, which has differing dorzolamide content to the Japanese DTFC formulation (Manni et al. 2009). In a study by Manni et al., BTFC or 2% DTFC was applied alone to the eyes of patients with OH or OAG. These patient groups included subjects with pseudoexfoliation glaucoma and pigmentary glaucoma, and relatively high IOPs of 24–36 and 21–36 mmHg were observed at 8:00 and 10:00 AM. In agreement with the present data, these investigators reported IOP‐lowering non‐inferiority immediately before eyedrop treatments at 8:00 AM and at 2 and 8 hr later, with a non‐inferiority margin of 1.5 mmHg in all cases. In Japan, two studies indicate no significant changes in IOP after switching from 1% DTFC to BTFC in patients receiving PGA treatments (Inoue et al. 2015; Shimizu et al. 2015). However, these data may be compromised by the impact of treatment adherence before and after switching regimens and by IOP fluctuations with the timing of eyedrop administration. The present study was designed and implemented using a randomized, double‐masked and parallel‐group design to avoid bias and similarly demonstrated that IOP reductions following treatment with BTFC are not inferior to those following DTFC treatment.
In the present study, we included patients with ≥15 mmHg IOP while being treated with a PGA, and mean 9‐AM IOP values at baseline did not differ significantly between BTFC and 1% DTFC groups. Moreover, mean IOP values at 9 and 11 AM on baseline at weeks 4 and 8 were similar in both treatment groups, and both treatments successfully reduced IOP by ≥ 2 mmHg from baseline values. Accordingly, the early manifest glaucoma trial demonstrated a relationship between IOP and progression of visual field defects in glaucoma and showed that minimal IOP changes of 1 mmHg were accompanied by approximately 10% changes in the risk of progression (Leske et al. 2003). Similarly, the present IOP data show that addition of BTFC or DTFC may be sufficient for PGA‐treated patients requiring additional IOP reductions.
To assess ocular irritation, eye pain, foreign body sensation and blurred vision immediately after eyedrop administration, we compared discomfort scores between BTFC‐ and DTFC‐treated patients using a questionnaire with a five‐point scale. In these assessments, ocular irritation and blurred vision scores differed between BTFC and DTFC groups.
Similar comparisons of usability and preference between BTFC and DTFC have been reported in Japan and other countries. Specifically, Vold et al. (2008) reported that ocular discomfort scores for symptoms such as irritation and burning sensation immediately after eyedrop administration were lower in patients treated with BTFC than in those treated with DTFC. In addition, Sezgin Akcay et al. (2013) compared blurred vision, ocular irritation, eye pain and foreign body sensations after administration of eyedrops using their respective 0–4 score scales and reported higher incidence of blurred vision in the BTFC group, but more frequent ocular irritation, eye pain and foreign body sensation in the DTFC group. Similarly, Mundorf et al. (2008) investigated preferences of BTFC and DTFC using a crossover study design and reported preference for BTFC. Moreover, in a Japanese study, Shimizu et al. (2015) compared the usability of drugs after switching from DTFC to BTFC and showed increased incidence of blurred vision in those switching to BTFC, but reduced ocular irritation and burning sensation.
Differences in sensations during application of the eyedrop formulations, such as ocular irritation and burning, may reflect pH, because the Japanese 1% DTFC preparation has a pH of 5.5–5.8, whereas BTFC has a comparatively neutral pH of 6.7–7.7, which is similar to that of tear fluid. In the present study, differences in discomfort scores for blurred vision and irritation were identified, and as reported previously, these were worse in BTFC for blurred vision and DTFC for irritation, respectively. In contrast, ocular irritation, eye pain and foreign body sensations were better in our DTFC group than has been reported in previously reported studies, potentially reflecting sensory familiarity of patients with previously established eyedrop regimens for glaucoma. Because the discomfort questionnaires were scheduled after the 11 AM IOP measurement, anaesthetic effects from eyedrop treatment procedures may have remained during completion of the questionnaire. However, surveys of patients in the BTFC group were performed under the same conditions. Hence, although comparisons of discomfort scores were used as exploratory end‐points in the present study, further investigation of these parameters is required following administration of eyedrops.
In this study, we determined degrees of blurred vision for 3 min from immediately after eyedrop application using a 51‐point scale, and differences between treatment groups were recorded for 2 min, but were not identified after 3 min. Although BTFC often caused blurred vision as an adverse reaction in this and other clinical trials as it is an ophthalmic suspension, no previous studies report chronological evaluations of blurred vision after eyedrop application, and the duration of this adversity may be clinically insignificant in comparison with that of DTFC. However, although blurred vision following BTFC treatment diminished within 3 min, this symptom was so often intolerable in the initial treatment that complete initial explanation is quite important to keep adherence (Park et al. 2013).
Finally, local drug‐related adverse eye events with greater than 2% prevalence in the BTFC group included blurred vision and ocular irritation, but did not require treatment. Moreover, these adverse effects are clearly stated on BTFC package inserts. Moreover, no abnormalities of corrected VA blood pressure, pulse and slit lamp microscope examinations were identified in either treatment group.
In conclusion, in this study we demonstrated that 1% BTFC is not inferior to 1% DTFC when administered in combination with PGA to Japanese OAG/OH patients. Both BTFC and DTFC reduced IOP effectively, and among the various discomfort assessments, temporarily blurred vision was more prevalent in BTFC than DTFC patients. However, the treatment difference decreased and almost disappeared with time. Taken together, the present data warrant further consideration of 1% BTFC as a safe and effective additional therapy for glaucoma treatment.
Supporting information
Appendix: Members involved in study.†
Data S1.: Funding information.
Contributor Information
Makoto Aihara, Email: aihara-tky@umin.ac.jp.
the JAC Study group†:
Yohei Chikaraishi, Jun Ueda, Yuta Sakaue, Akiko Ohta, Motohiro Shirakashi, Kiyoshi Yaoeda, Takayuki Tanaka, Tokuhide Oyama, Shigeru Hoshiai, Shunei Gen, Shuuko Fujitani, Arata Sekine, Masahiro Fujita, Yukihiro Matsumoto, Hirotaka Suzumura, Takuji Kato, Sakae Matsuzaki, Setsuko Hashida, Shinpei Sato, Isao Sato, Tomoyuki Muramatsu, Akira Ando, Masamitsu Takeuchi, Shingo Onoe, Shinichi Manabe, Yumi Oda, Kumi Horikawa, Kaori Kifuku, Ken Hayashi, Mineo Ozaki, Saya Ishii, Akiko Matsuyama, Takashi Komizo, Kazunori Miyata, Shinichiro Otani, Fumie Kagaya, Junko Hanaya, Kazuhiko Unoki, Shigeko Unoki, Takehiro Yamashita, and Toyomi Yamashita
References
- Baudouin C, Liang H, Hamard P, Riancho L, Creuzot‐Garcher C, Warnet JM & Brignole‐Baudouin F (2008): The ocular surface of glaucoma patients treated over the long term expresses inflammatory markers related to both T‐helper 1 and T‐helper 2 pathways. Ophthalmology 115: 119–115. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal‐Tension Glaucoma Study Group (1998a): The effectiveness of intraocular pressure reduction in the treatment of normal‐tension glaucoma. Am J Ophthalmol 126: 498–505. [DOI] [PubMed] [Google Scholar]
- Collaborative Normal‐Tension Glaucoma Study Group (1998b): Comparison of glaucomatous progression between untreated patients with normal‐tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 126: 487–497. [DOI] [PubMed] [Google Scholar]
- Hollo G, Topouzis F & Fechtner RD (2014): Fixed‐combination intraocular pressure‐lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother 15: 1737–1747. [DOI] [PubMed] [Google Scholar]
- Inoue K, Shiokawa M, Ishida K & Tomita G (2015): Safety and efficacy of switching from dorzolamide 1.0%/timolol maleate 0.5% eye drops to brinzolamide 1.0%/timolol maleate 0.5% eye drops. Clin Ophthalmol 9: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase A, Suzuki Y, Araie M & Tajimi Study Group (2014): Characteristics of undiagnosed primary open‐angle glaucoma: the Tajimi Study. Ophthalmic Epidemiol 21: 39–44. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K & Furuya T (2014): Persistence with topical glaucoma therapy among newly diagnosed Japanese patients. Jpn J Ophthalmol 58: 68–74. [DOI] [PubMed] [Google Scholar]
- Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L & Komaroff E (2003): Early Manifest Glaucoma Trial G: factors for glaucoma progression and the effect of treatment: the early manifest glaucoma trial. Arch Ophthalmol 121: 48–56. [DOI] [PubMed] [Google Scholar]
- Manni G, Denis P, Chew P et al. (2009): The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination versus dorzolamide 2%/timolol 0.5% in patients with open‐angle glaucoma or ocular hypertension. J Glaucoma 18: 293–300. [DOI] [PubMed] [Google Scholar]
- Mundorf TK, Rauchman SH, Williams RD, Notivol R & Brinzolamide/Timolol Preference Study Group (2008): A patient preference comparison of Azarga (brinzolamide/timolol fixed combination) vs Cosopt (dorzolamide/timolol fixed combination) in patients with open‐angle glaucoma or ocular hypertension. Clin Ophthalmol 2: 623–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman‐Casey PA, Robin AL, Blachley T, Farris K, Heisler M, Resnicow K & Lee PP (2015): The most common barriers to glaucoma medication adherence: a cross‐sectional survey. Ophthalmology 122: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Kang KD, Moon J & Korean Glaucoma Compliance Study Group (2013): Noncompliance with glaucoma medication in Korean patients: a multicenter qualitative study. Jpn J Ophthalmol 57: 47–56. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (2011): SAS/STAT® 9.3 user's guide. Chapter 58 The MIXED procedure. Cary, NC: SAS Institute Inc. [Google Scholar]
- Sezgin Akcay BI, Guney E, Bozkurt KT, Unlu C & Akcali G (2013): The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination versus dorzolamide 2%/timolol 0.5% in patients with open‐angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther 29: 882–886. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Nakakura S, Nishiyama M, Tabuchi H & Kiuchi Y (2015): Efficiency, safety, and patient preference of switching from dorzolamide 1%/timolol 0.5% to brinzolamide 1%/timolol 0.5% while maintaining the prostaglandin F2alpha analog. Clin Ophthalmol 9: 475–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalicky SE, Goldberg I & McCluskey P (2012): Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol 153: 1.e2–9.e2. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators (2000): The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol 130: 429–440. [DOI] [PubMed] [Google Scholar]
- Vold SD, Evans RM, Stewart RH, Walters T & Mallick S (2008): A one‐week comfort study of BID‐dosed brinzolamide 1%/timolol 0.5% ophthalmic suspension fixed combination compared to BID‐dosed dorzolamide 2%/timolol 0.5% ophthalmic solution in patients with open‐angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther 24: 601–605. [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T & Medeiros FA (2014): The pathophysiology and treatment of glaucoma: a review. JAMA 311: 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix: Members involved in study.†
Data S1.: Funding information.


