Abstract
An approach is presented for the structure determination of clathrates using NMR spectroscopy of enclathrated xenon to select from a set of predicted crystal structures. Crystal structure prediction methods have been used to generate an ensemble of putative structures of o‐ and m‐fluorophenol, whose previously unknown clathrate structures have been studied by 129Xe NMR spectroscopy. The high sensitivity of the 129Xe chemical shift tensor to the chemical environment and shape of the crystalline cavity makes it ideal as a probe for porous materials. The experimental powder NMR spectra can be used to directly confirm or reject hypothetical crystal structures generated by computational prediction, whose chemical shift tensors have been simulated using density functional theory. For each fluorophenol isomer one predicted crystal structure was found, whose measured and computed chemical shift tensors agree within experimental and computational error margins and these are thus proposed as the true fluorophenol xenon clathrate structures.
Keywords: computational chemistry, crystal structure prediction, density functional theory, first principles modelling, Xe NMR spectroscopy
Introduction
Over the past few years, the combined use of solid‐state nuclear magnetic resonance (NMR) and computational methods has developed into a practical method for determining crystal structures.1 This is due to the sensitivity of NMR chemical shifts to the molecular environment in a crystal, and the reliability of methods for predicting the relative NMR shielding of atoms in different environments. The approach involves first predicting the full set of low energy crystal structures available to a molecule, followed by chemical shielding calculations, which are matched against the measured chemical shifts of a material. Applications to organic crystals have found that, given a set of predicted crystal structures, the isotropic 1H chemical shifts are usually sufficient to identify which of the predicted structures corresponds to the material under investigation.2
Kazankin et al.3, 4 reported that xenon clathrates can be formed from several monosubstituted phenol compounds. With the exception of phenol,5 hydroquinone,5, 6, 7, 8 p‐cresol,9 and p‐fluorophenol,10 Xe clathrates have not been described in the English literature and have remained relatively unknown in the West. The crystal structures of o‐ and m‐fluorophenol xenon clathrates have, to our knowledge, never previously been determined. The NMR properties of Xe included in porous structures gives a potentially sensitive probe of the clathrate structure, which is explored here as a means of determining the structures of the o‐ and m‐fluorophenol xenon clathrates.
The first 129Xe NMR experiments were performed in 1951,11 but only a modest number of further studies appeared until the early 1980s when Ripmeester and Davidson investigated enclathrated xenon12 and Ito and Fraissard proposed the use of 129Xe NMR spectroscopy for probing the properties of zeolites.13 Today, the application range of 129Xe NMR spectroscopy extends from materials property studies of micro‐ and mesoporous solids through polymers, liquid crystals, proteins, and biosensors, to magnetic resonance imaging (MRI) for medical purposes.14, 15, 16, 17
The reason for using xenon to probe the properties of various materials arises from the high sensitivity of the shielding of 129Xe to its local environment, and that the variability in the shielding stems exclusively from environmental effects.18, 19, 20 Another reason is the good NMR receptivity of 129Xe, which is about 33 times that of 13C. A disadvantage in some cases may be the long 129Xe spin‐lattice relaxation time T 1, which is, for example, several minutes in xenon clathrate hydrate.12 The application range of 129Xe NMR spectroscopy and MRI further widened when the so‐called optical pumping or hyperpolarization technique was invented,21 enabling the increase of the 129Xe polarization by up to four or five orders of magnitude and making experiments possible with very small amounts of xenon gas.
The work of Ripmeester and Davidson12 revealed the potential of 129Xe NMR spectroscopy in studies of clathrates. The 129Xe NMR spectrum of xenon clathrate hydrate consists of two broad resonance lines: one at 152±2 ppm and the other at 242±2 ppm downfield (smaller shielding) from the low pressure gas peak. The former resonance arises from xenon in large cages, and the latter from xenon in small cages. Thus, the 129Xe chemical shift relates to the size of cages.22 Furthermore, the resonance at 152 ppm displays a cylindrically symmetric powder lineshape with a chemical shift anisotropy of 32±3 ppm; the resonance lineshape relates to the shape of the cage accommodating the xenon atoms. The line intensities in turn relate to the occupation number. An illustrative example is the distribution of xenon atoms in the alpha cages of the NaA zeolite.23 The 129Xe NMR spectrum of xenon in NaA consists of several distinct signals, the chemical shift being determined by the occupation number of a cage.
Modeling of 129Xe NMR spectra of clathrate cages dates back to the studies of chemical shifts24 and NMR line shapes25 inside clathrate hydrate structures I and II, which were modeled as molecular clusters extracted from the clathrate structures. Similar quantum chemical cluster models were also applied in studies of Xe inside fullerene cages,26, 27 for which both relativistic28, 29 as well as dynamical and environmental29, 30 effects were studied. Current cluster modeling makes use of recent advances in the development of relativistic quantum chemistry methods, which have enabled very demanding studies of large heavy‐element systems such as cryptophanes31 and self‐organizing metallo‐supramolecular cages.32
Diffraction is the usual method for crystal structure solution. Structure determination of clathrates by diffraction methods can, however, be hindered by their instability. For the materials studied here, it was not possible to obtain a powder X‐ray diffraction (PXRD) pattern from the m‐fluorophenol clathrate and only a low resolution diffraction pattern could be obtained from the o‐fluorophenol clathrate, from which structure determination would not be possible. Therefore, 129Xe NMR spectra cannot be combined with PXRD experiments as usually done for identifying new clathrate phases.33, 34 The exceptional sensitivity of xenon's chemical shift anisotropy to its environment should enable the distinction between different candidate clathrate structures. This hypothesis was investigated by comparing experimentally observed and quantum chemically modeled 129Xe NMR isotropic and anisotropic shift parameters in clathrate structures obtained by computational crystal structure prediction (CSP).
CSP has until recently been focused primarily on predicting the single thermodynamically stable structure, or possibly a few low‐energy polymorphs. The lowest energy crystal structures available to a given molecule are, in all but very rare exceptions,35 close‐packed, leaving no room for the inclusion of guest molecules. There have been few reports of the prediction of porous molecular crystals36, 37, 38 or solvates.39, 40, 41 The empty host molecule frameworks of observed inclusion structures have been shown to often exist as local minima on the lattice energy surface, albeit sometimes at relatively high energies compared to close‐packed alternative structures.41, 42, 43 This suggests an efficient approach to the discovery of inclusion structures: searching for stable, empty frameworks using CSP methods and subsequently inserting the guest. This method should be particularly suited to weakly interacting guests such as Xe, where inclusion is expected to leave the host framework relatively unperturbed. The approach should be more efficient than searching the dramatically larger multi‐component phase space defined by the host and guest together.
In this study, CSP calculations were performed for o‐ and m‐fluorophenol with the specific aim of predicting realistic xenon clathrate complexes for which calculated 129Xe NMR parameters can be compared to measured spectra. Advanced first principles density functional theory (DFT) electronic structure calculations of the 129Xe NMR shielding tensors, with a proper inclusion of electron correlation as well as relativistic, periodic, and dynamical effects, have been carried out for a set of predicted structures. A comparison of the simulated isotropic and anisotropic Xe chemical shift parameters with experimentally observed solid state 129Xe NMR data allows the identification of the crystal structures of these elusive clathrates.
Experimental Section
NMR Spectroscopy
Preparation of samples
Clathrate samples were prepared from commercially available o‐ and m‐fluorophenol (Aldrich, 98 % assay) and used directly without purification. The substances were transferred into pyrex glass tubes (4 mm outer diameter, 0.8 mm walls) and connected to a volume‐calibrated vacuum line. Two cycles of freeze–thaw were applied to reduce oxygen content. Isotopically enriched 129Xe gas (Chemgas, 89 % enrichment) was then frozen into the evacuated tubes. The amount of transferred gas was controlled by the pressure drop in the vacuum line, measured with a digital pressure gauge (1 hPa precision). The amount of gas inserted into the tubes was chosen to be sufficient for saturation of a 3:1 host–guest clathrate stoichiometry and with excess to pressurize the sample tube so as to maintain clathrate structural stability at the desired experimental temperature of 250 K.3, 4 The glass sample tubes were flame sealed, cooled in liquid nitrogen, and equilibrated for a period of two weeks at ca. 243 K prior to NMR measurements.
NMR experiments
The 129Xe NMR spectrum of xenon in m‐fluorophenol was measured at 251 K on a Bruker DSX300WB spectrometer (129Xe Larmor frequency 83.03 MHz) using a 7 mm variable angle spinning (VAS) probe head (DOTY Scientific, Inc., USA), without spinning. The static powder spectrum (the axis of the solenoid coil was set perpendicular to the external magnetic field) was observed while applying cross‐polarization (CP) and proton decoupling. The following acquisition parameters were used: 129Xe pulse width 5 μs, 1H decoupling pulse width 16 μs, mixing time 5.4 ms, repetition time 35 s, strength of the 1H decoupling field 34 kHz, and number of collected free induction decay (FID) signals 220. The aim of applying proton decoupling was to diminish the effect of the 129Xe–1H dipolar coupling on the line width. The 129Xe chemical shift was measured relative to the isotropic 129Xe chemical shift in hydroquinone, in which the shift relative to zero‐pressure xenon is known to be 222.1 ppm. Temperature calibration was based on the measurement of the 1H chemical shift difference in a separate methanol sample.44 Prior to Fourier transformation the FID signal was multiplied by an exponentially decaying apodization function leading to ≈100 Hz line broadening.
The 129Xe NMR spectrum of xenon in o‐fluorophenol was in turn measured at 253 K on a Bruker DPX400 spectrometer (129Xe Larmor frequency 110.70 MHz) using a 5 mm broad band observe (BBO) probe head. 129Xe chemical shift is given with respect to the signal of zero‐pressure xenon gas, which was determined using two samples with known xenon pressure and extrapolation using the second virial coefficient. Temperature calibration was in this case performed using 1H chemical shifts in methanol, but with methanol placed in the annulus of a double tube system (outer tube 5 mm, inner tube 4 mm). A 129Xe pulse width of 29.5 μs was applied. Prior to Fourier transformation, the FID signal was multiplied by an exponentially decaying apodization function leading to 50 Hz line broadening.
The elements of the chemical shift tensors were determined in both cases using Dmfit.45 The uncertainties were estimated to be ±0.2 ppm, ±0.4 ppm, and <0.04, respectively, in the three adjusted NMR parameters: isotropic chemical shift (CS) δ≡δiso= −σiso referenced to the zero‐pressure limit Xe gas, chemical shift anisotropy (CSA) Δδ=δzz−(δxx+δyy)/2 with positive/negative values for prolate/oblate spheroids along the z‐direction and asymmetry parameter η=(δyy−δxx)/(δzz−δiso) with the value 0 for an axially symmetric tensor (with respect to z) and 1 for the fully asymmetric case, when δyy=δiso. The isotropic Xe nuclear shielding constant is the trace of the shielding tensor, σiso=(σxx+σyy+σzz)/3. The components of the principal axis system (PAS) of the Xe shift tensor follow Haeberlen's convention:46 |δzz−δiso|≥|δxx−δiso|≥|δyy−δiso|. A relatively large contribution to the uncertainties arises from the wavy background in the experimental spectra, which was therefore eliminated.
Powder X‐ray diffraction
Samples of both o‐ and m‐fluorophenol were prepared in glass capillaries. Xenon gas was frozen into the evacuated capillaries, which were flame‐sealed after submerging in liquid N2. Capillaries containing the samples were kept immersed in liquid N2 prior to mounting in a pre‐cooled single‐crystal diffractometer. Diffraction data were collected at 100 K using graphite monochromated MoKα radiation.
Crystal structure prediction
Crystal structure prediction (CSP) calculations involved five steps: i) the lowest energy conformations of the isolated molecules were identified; ii) hypothetical crystal packings were generated and then lattice energy minimized using a simple force field and rigid‐molecule constraints; iii) a subset of the lowest energy predicted crystal structures were re‐optimized using an anisotropic atom–atom force field; iv) intramolecular flexibility was introduced, allowing the hydroxyl group to reorient in response to packing forces in each low energy crystal structure; v) porous structures were identified, xenon atoms inserted into the pores, and the structures once again re‐optimized.
Molecular geometries, energies, and charge densities, calculated by DFT using Gaussian 0947 with the B3LYP48, 49 functional and 6‐311G(d,p) basis set, were used throughout the CSP calculations. Two stable, planar conformers of each molecule were identified (Figure 1) and DFT predicts that their energies are sufficiently close that either could form low energy crystal structures.50 Therefore, both conformers of both molecules were included in the CSP study.
Figure 1.
Conformers of the two fluorophenol isomers. a) cis‐ and b) trans‐o‐fluorophenol c) cis‐ and d) trans‐m‐fluorophenol.
Hypothetical crystal structures were generated with the rigid DFT‐optimized molecular geometries using Monte Carlo simulated annealing51 with Materials Studio. Searches were performed in the most commonly observed space groups of known molecular organic crystal structures; 25 space groups were searched with one molecule in the asymmetric unit (Z′=1) and 5 space groups with Z′=2, including all combinations of the two conformers in the asymmetric unit.
Structures with a lattice energy within a 15 kJ mol−1 window from the lowest energy structure were further refined with an anisotropic, atomic multipole‐based intermolecular atom–atom potential model,52 combined with a DFT treatment of intramolecular energies and geometries. Duplicate crystal structures were removed using the Compack53 program and the resulting unique structures were re‐optimized using the CrystalOptimizer54 program to treat flexibility of the hydroxyl group within each crystal structure. Full details of the conformational analysis and CSP methods are provided in the Supporting Information.
Selection of likely clathrate host structures
Lattice energy differences between polymorphs are usually very small and rarely exceed 8 kJ mol−1.55 Since voids in crystal structures are thermodynamically unfavorable,42, 56 structures within a larger energy range than in the usual application of CSP to polymorph prediction were considered as putative inclusion frameworks. Crystal structures within an energy cutoff of 13 kJ mol−1 above the global minimum for each molecule were considered.
To guide the selection of potential clathrate host frameworks, the guest‐to‐host volume ratio Rg in 31 representative clathrate structures, taken from the Cambridge Structural Database (CSD), were examined (see Supporting Information for details). The volume ratio Rg is calculated with Equation (1):
| (1) |
where Vg is the van der Waals volume of the guest molecule and VH is an individual void's contact volume.57
One xenon atom was inserted at each cavity's centroid coordinates and the resulting xenon clathrate structures were geometry‐optimized as described above, assuming rigid molecules, imposing no space group symmetry and using an ad hoc exp‐6 potential for xenon.
129Xe NMR shielding calculations
The three 129Xe NMR parameters of the predicted structures were modeled and compared with the experimental data in several stages. In NMR modeling the chemical shift reference is a free Xe atom. In the first step, all predicted clathrate structures were subjected to nonrelativistic (NR) NMR modeling of cluster models. The results from the NR cluster models were used to identify likely candidates for the experimentally observed clathrate structures. These candidates were then further studied using DFT calculations on their fully periodic models. Finally, a few crystal structures that agreed best with experimental NMR results were chosen for more detailed modeling. Full details are given in the Supporting Information.
Screening of structures by cluster modeling
The NMR parameters of probable clathrate structures were first modeled using clusters consisting of a single xenon‐occupied cavity, including the Xe atom and all nearby fluorophenol molecules. Nonrelativistic Xe shielding tensor calculations were performed on the cluster models using Turbomole.58 The BHandHLYP59, 60 hybrid functional, including 50 % of exact Hartree–Fock (HF) exchange (EEX), was chosen based on benchmark calculations on the xenon–benzene system, where high quality nonrelativistic ab initio Xe chemical shifts are reproduced reasonably well.29 BHandHLYP has been shown to slightly underestimate Xe chemical shift and anisotropy, leaving room for improvements in the modeling by approaching the experimental values from below for Xe‐containing molecules,61, 62, 63 Xe atoms moving freely inside buckminster fullerenes,29, 30 and in self‐organizing metallo‐supramolecular cages.32 Those studies show that the typical overestimation of Xe CS and CSA by pure generalized gradient approximation (GGA) DFT functionals, such as PBE64 and BLYP,59, 65 is partly compensated when the amount of exact HF exchange is increased in B3LYP (EEX=20 %) and BHandHLYP (EEX=50 %) hybrid functionals. Calculations have been performed with a mixed basis set denoted as MHA/SVP (see Supporting Information for details).
Periodic modeling of likely candidates
Five o‐fluorophenol and seven m‐fluorophenols crystal structures, with screening level NMR parameters close to the experimental values, were chosen for further scrutiny. These structures were optimized with respect to both atomic positions and lattice parameters with planewave, periodic DFT with the PBE functional and Tkatchenko–Scheffler (TS) dispersion correction,66 denoted as PBE‐TS structures from now on.
Following optimization, NMR shielding tensors were computed with the PBE functional using the gauge‐including projector augmented wave (GIPAW) method67, 68 (see Figures 6 and 7 below). These periodic DFT results include the scalar relativistic (SR) effects on Xe shielding at the 1‐component zeroth‐order regular approximation (ZORA)69, 70 level of theory. Details of the periodic calculations with CASTEP71, 72 are in the Supporting Information.
Figure 6.
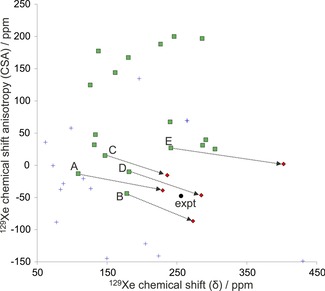
129Xe NMR parameters computed at screening (nonrelativistic DFT/BHandHLYP/MHA) level for cluster models of the predicted clathrate structures of o‐fluorophenol. Structures with asymmetry η>0.04 are shown as blue crosses, whereas structures with symmetric chemical shift tensors (η≤0.04) are shown as green squares. The experimental data (expt, black filled circle) is included for comparison. Structures that were considered for further study are connected with the black arrows to the periodic GIPAW/PBE calculation of Xe shielding from periodic PBE‐TS optimized structures of the corresponding crystal (red diamonds). The labels A–E correspond to structures oF_A to oF_E in the text.
Figure 7.
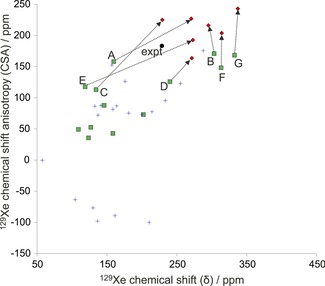
129Xe NMR parameters computed at screening (nonrelativistic DFT/BHandHLYP/MHA) level for cluster models of the predicted clathrate structures of m‐fluorophenol. Structures with asymmetry η>0.04 are shown as blue crosses, whereas structures with symmetric chemical shift tensors (η≤0.04) are shown as green squares. The experimental data (expt, black filled circle) is included for comparison. Structures that were considered for further study are connected with the black arrows to the periodic GIPAW/PBE calculation of Xe shielding from periodic PBE‐TS optimized structures of the corresponding crystal (diamonds). The labels A–G correspond to structures mF_A to mF_G in the text.
Detailed modeling of the most probable structures
After periodic NMR calculations of the DFT optimized structures, the most probable clathrate structures were selected for more detailed DFT NMR modeling using the Amsterdam Density Functional (ADF) program.73, 74 In addition to the NR and SR‐ZORA quantum chemistry, ADF provides a 2‐component spin‐orbit SO‐ZORA method also including SR effects.69, 75
A much wider range of DFT functionals than is currently available in periodic CASTEP can be used in ADF. Calculations were performed with the jcpl/TZP mixed basis set76 (see Supporting Information for details) on clusters comprising a single cavity to test the influence of correlation treatment and the amount of EEX on NMR parameters using the PBE, BLYP, B3LYP and BHandHLYP functionals at the SR‐ZORA level. By using the results in scaling of the periodic PBE results, estimates of SR periodic BLYP, B3LYP, and BHandHLYP results were obtained. It is expected that, as the electron correlation description is improved by an increasing portion of EEX, the calculated NMR parameters for the true crystal structures should approach the experimental results. Hence, the BHandHLYP‐scaled periodic PBE results provide the best estimation of 129Xe NMR parameters and lack only contributions due to molecular dynamics and relativistic SO effects. The latter were treated as an additive correction obtained from the difference of the static SR‐ and SO‐ZORA cluster calculations with the BHandHLYP functional.
The effect of Xe dynamics at T=300 K for 129Xe NMR shielding parameters was modeled for the few most probable clathrates by canonical NVT Metropolis Monte Carlo (MC‐NVT) of Xe motion on a potential energy surface inside a cluster cavity with fixed PBE‐TS optimized geometry. The temperature effects on Xe chemical shift and anisotropy were calculated as the difference between the thermally averaged shielding tensor and the reference tensor with Xe at the center of the cage.
For further details, see the Supporting Information, which includes a detailed description of crystal structure prediction (CSP) and its performance for the known high density crystal forms of the two fluorophenols, as well as the method to compute guest to host volume ratios. Revised Williams99 parameters for H…A interactions, as well as potential parameters for F and Xe are described. Also included are: details of DFT structure optimization, Xe NMR calculations, and modeling of Xe dynamics; Xe NMR results at different levels and their sensitivity to structure; details of powder X‐ray diffraction (PXRD) measurements and results, and a number of clathrate structures in CIF format.
Results and Discussion
Experimental NMR results
Both isomers formed clathrates with xenon under the experimental conditions and allowed recording of properly shaped powder patterns without active mixing and crushing of crystals during their formation. A powder‐like appearance and lack of macroscopic crystallites was confirmed by visual inspection and the fit to spectra. There is no preferential growth imposed by tube walls as this would not allow for proper random averaging of orientations of crystallites in relation to the magnetic field of the spectrometer.
Axially symmetric powder spectra have been observed for both clathrates (Figure 2), which confirms the existence of highly symmetric voids occupied by xenon atoms. High values of 129Xe chemical shift for both clathrates, δ=228.5 ppm for m‐fluorophenol and δ=256.0 ppm for o‐fluorophenol, suggest tight cages with only one occupational site. In such cases, where 129Xe NMR powder patterns are expected to reflect only interactions of xenon atoms with the immediately adjacent host molecules, the influence of neighboring cages with xenon is expected to be negligible.77
Figure 2.
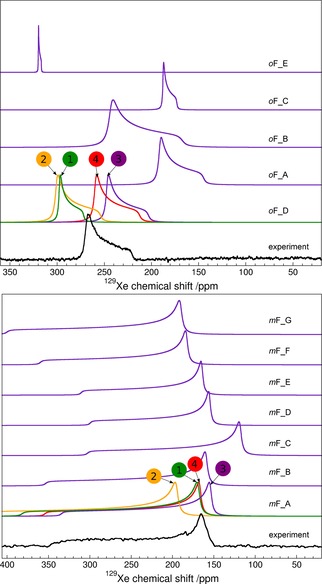
Experimental (black) and calculated (indigo) 129Xe NMR powder spectra of o‐fluorophenol (top) and m‐fluorophenol (bottom) with 129Xe gas reference at zero. Calculated spectra for all structures were obtained from BHandHLYP‐scaled periodic PBE NMR parameters at PBE‐TS optimized geometry (method/structure=BHandHLYP/PBE‐TS). For the most probable candidates, oF_D and mF_A, results from different periodic modeling levels are displayed: (1, green) PBE/CSP, (2, orange) PBE/PBE‐TS, (3, indigo) BHandHLYP/PBE‐TS and (4, red) BHandHLYP/PBE‐TS with effects due to Xe dynamics at T=300 K (see text for details).
The spectrum of m‐fluorophenol (Figure 2) resembles that of β‐hydroquinone,8 β‐phenol, and p‐fluorophenol, with one notable difference; it has the largest observed value of 129Xe NMR CSA among known inclusion compounds: Δδ=183.3 ppm versus 161.9, 171, and 164 ppm for hydroquinone,78 phenol,5 and p‐fluorophenol,79 respectively. Small additional features in the observed spectrum of m‐fluorophenol are attributed to amorphous host substance and xenon atoms adsorbed in this amorphous phase.
The 129Xe NMR spectrum of o‐fluorophenol shows negative CSA (Figure 2), Δδ=−47.5 ppm, in contrast to all previously known solid‐state 129Xe NMR powder patterns of phenol clathrates for which positive CSA is observed.5, 6, 7, 8, 9, 10 Changes in the sign of the CSA can be observed in a group of porous channel‐like dipeptides,80 where CSA changes from positive to negative with increasing xenon gas pressure, and therefore with pronounced Xe–Xe interactions playing a greater role. No changes of the NMR spectrum of o‐fluorophenol clathrate have been observed when repeating the experiment with samples at different pressures. The small negative CSA may result from an axially symmetric but oblate environment around the xenon atom in this clathrate.
Powder X‐ray diffraction results
A PXRD pattern could not be obtained from m‐fluorophenol clathrate, which transformed too quickly to the known high density form when transferred to the diffractometer. This is confirmed by comparison of the measured PXRD pattern to that simulated from the known crystal structure. For o‐fluorophenol, a broad powder pattern was obtained that does not correspond to either known crystal structures (see Supporting Information). The pattern is too broad to index, so it could not be used to determine the structure.
Crystal structure prediction results
CSP resulted in an exceptionally large number of low energy crystal structures for each molecule (Figures 3 and 4).
Figure 3.
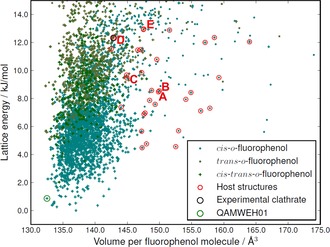
The crystal energy landscape of o‐fluorophenol. Each point represents one crystal structure and is colored according to the molecular conformation (cis‐trans‐o‐fluorophenol refers to Z′=2 structures containing both conformers). Structures with a lattice energy within 13 kJ mol−1 from the lowest energy structure and having cavities of suitable size for xenon absorption (see text) are encircled in red. The experimentally known high‐pressure polymorph (CSD refcode QAMWEH01) is encircled in green. The labels A–E correspond to structures oF_A to oF_E in the text.
Figure 4.
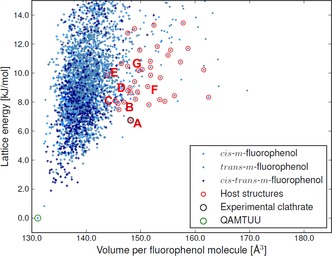
The crystal energy landscape of m‐fluorophenol. Each point represents one crystal structure and is colored according to the molecular conformation (cis‐trans‐m‐fluorophenol refers to Z′=2 structures containing both conformers). Structures with a lattice energy within 13 kJ mol−1 from the lowest energy structure and having cavities of suitable size for xenon absorption (see text) are encircled in red. The experimentally known stable polymorph (CSD refcode QAMTUU) is encircled in green. The labels A–G correspond to structures mF_A to mF_G in the text.
For o‐fluorophenol, Z′=2 crystal structures with both molecules in the cis‐conformation are energetically favored. The known81 high‐pressure polymorph II (CSD refcode QAMWEH01) is located in the search, 0.8 kJ mol−1 above the global minimum (Figure 3) and with a slightly higher density, as expected for a high pressure polymorph. The known low‐temperature polymorph I81 (CSD refcode QAMWEH) is a disordered Z′=1.5 structure and could not be predicted with the methods employed here.
One crystal structure of m‐fluorophenol is known81 (CSD refcode QAMTUU) and corresponds to the global lattice energy minimum from the search. The structure is geometrically well reproduced (with a deviation in atomic positions in a 15‐molecule cluster taken from predicted and X‐ray crystal structures of RMSD15=0.203 Å).
Overlays of both matches are included in the Supporting Information. The successful reproduction of the known crystal structures of both molecules amongst the lowest energy predicted structures provides confidence in the sampling of crystal structures and of their lattice energy rankings.
Selection rules for clathrate structures
Because the inclusion of guest molecules in clathrates can significantly stabilize the structure, using the lattice energy of the empty host alone is not useful for selection of promising structures. The analysis of known clathrates found that the guest‐to‐host volume ratio Rg is normally distributed with a mean of 59 % and a standard deviation of 8 percentage units, in good agreement with Rebek's “55 % solution”82 and providing further evidence for the empirical rule that Rg in observed inclusion structures should fall in this limited range.82, 83
Of the predicted crystal structures within 13 kJ mol−1 of the global minimum for o‐fluorophenol and m‐fluorophenol, 230 and 223 are porous (having cavities >10 Å3). 33 (o‐fluorophenol) and 32 (m‐fluorophenol) of the predicted structures have cavities of a suitable volume to accommodate xenon as a guest, which were take to be those with Rg within three standard deviations (±3σ) for observed clathrates. These structures are encircled in red in Figures 3 and 4. The lowest energy of these structures are 4.5 and 6.7 kJ mol−1 above the global minima for o‐ and m‐fluorophenol, respectively.
Computational NMR results
Screening of structures by cluster models
Examples of cluster models used for initial screening are shown in Figure 5. As can be seen in Figures 6 and 7, even for a set of structures with similar void volumes and packing energies, the computed NMR tensor parameters for 129Xe vary widely in the initial screening using cluster models of the predicted structures. This demonstrates the exceptional sensitivity of the 129Xe NMR parameters to small differences in cavity size and shape.
Figure 5.
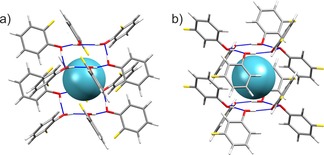
Examples of clusters used in the initial screening NMR calculations of the predicted clathrate structures. The clusters shown correspond to the structures selected as best models of the observed clathrates: a) o‐fluorophenol structure oF_D, b) m‐fluorophenol structure mF_A. Atoms are colored by element: carbon (grey); oxygen (red); hydrogen (white); fluorine (yellow).
Calculated NMR parameters for the 33 o‐ and 32 m‐fluorophenol clathrate structures obtained with nonrelativistic DFT cluster models are tabulated in Tables S4 and S5 (Supporting Information). Many of these structures, including all predicted Z′=2 structures, have chemical shift tensors with significant asymmetry; these voids are clearly not compatible with the experimental spectra, in which η<0.04. At this stage, only the structures with axially symmetric chemical shift tensors (green squares in Figures 6 and 7) are kept as possible candidates for the experimental structures.
Optimization of the clathrate structure after insertion of the xenon into the host allowed the structures to relax in response to guest insertion. Despite very small structural changes, this significantly affected the calculated NMR parameters (Figure S7 in the Supporting Information).
Because the screening level of theory is expected to underestimate both CS and CSA by a few tens of ppm, structures with both properties of the correct sign and underestimated were focused on, also including a few structures with overestimated CS for m‐fluorophenol and slightly positive CSA for o‐fluorophenol, for higher level calculations. This excluded all but five o‐fluorophenol and seven m‐fluorophenol structures; hereafter, these structures are referred to as oF_A to oF_E (in order of increasing energy, Figure 3) and mF_A to mF_G (Figure 4). All of these candidate clathrate structures have space group symmetry R .
NMR chemical shift parameters from periodic DFT calculations
NMR shielding tensors were calculated by periodic DFT for the five plausible o‐fluorophenol and seven m‐fluorophenol clathrate structures. Periodic modeling was found to be necessary and the geometry relaxation with periodic PBE‐TS shifts the CS and CSA towards larger magnitudes, corresponding to more elliptic, prolate (Δδ>0) and oblate (Δδ<0) m‐ and o‐fluorophenol cavities, respectively.
NMR parameters from SR GIPAW/PBE calculations at the PBE‐TS optimized geometries are tabulated in Tables S6 and S7 in the Supporting Information, and are displayed in Figures 6 and 7 as red diamonds connected with arrows to the corresponding screening cluster result (green squares). It should be noted that the periodic results set upper limits on 129Xe CS and CSA, as the PBE functional (and all pure GGA DFT functionals) overestimates the magnitudes of both parameters.29, 32, 61, 62, 63 Although the disagreement in CS and CSA of some of the candidate structures now makes them unlikely (such as oF_C, oF_E, and mF_G), all were kept for further analysis with more detailed NMR calculations.
Results of detailed NMR calculation of the most likely candidates
Typically, the crystal lattice effect, that is, the difference of 129Xe NMR parameters between the periodic and cluster models of the same PBE‐TS optimized clathrate, increases both CS and CSA as seen in Tables 1 and 2. The extent of the change is, however, very case‐specific and unforeseeable, which makes periodic modeling essential, in one form or another, in the present search for a best candidate of an unknown clathrate stucture.
Table 1.
Theoretically modelled 129Xe NMR chemical shift (δ) and anisotropy (CSA, Δδ) in ppm for the five most likely o‐fluorophenol CSP candidates in their PBE‐TS optimized structures, unless stated otherwise.
| oF_A | oF_B | oF_C | oF_D | oF_E | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Method | δ | Δδ | δ | Δδ | δ | Δδ | δ | Δδ | δ | Δδ |
| Cluster model [a] | ||||||||||
| PBE | 218.7 | −14.3 | 245.0 | −79.3 | 215.1 | 4.6 | 261.5 | −38.6 | 370.5 | 7.7 |
| BLYP | 226.5 | −14.6 | 253.4 | −81.8 | 222.5 | 4.1 | 270.9 | −40.2 | 381.5 | 7.9 |
| B3LYP | 195.9 | 23.1 | 223.1 | −75.6 | 195.9 | 0.9 | 240.8 | −36.6 | 338.1 | 13.1 |
| BHandHLYP | 165.8 | −21.3 | 190.7 | −68.3 | 162.6 | −1.2 | 209.9 | −35.8 | 288.5 | 18.3 |
| BHandHLYP[b] | 145.8 | −20.5 | 175.0 | −61.8 | 143.2 | 0.9 | 189.4 | −31.6 | 262.0 | 14.4 |
| BHandHLYP[c] | 174.9 | −11.6 | 192.3 | −71.2 | 171.3 | 6.7 | 216.7 | −31.6 | 292.0 | 31.1 |
| Periodic model [d] | ||||||||||
| PBE[e] | 195.1 | −28.1 | 272.1 | −66.4 | 238.7 | 7.0 | 288.8 | −26.2 | 362.1 | 23.1 |
| PBE | 229.8 | −38.9 | 273.2 | −86.7 | 236.4 | −15.4 | 285.0 | −46.5 | 402.7 | 2.0 |
| BLYP[f] | 237.3 | −39.1 | 281.5 | −89.3 | 243.6 | −15.9 | 294.3 | −48.1 | 413.6 | 2.1 |
| B3LYP[f] | 206.3 | −48.0 | 250.8 | −82.9 | 216.7 | −19.3 | 263.8 | −44.5 | 369.5 | 7.5 |
| BHandHLYP[f] | 175.6 | −46.3 | 217.7 | −75.4 | 182.6 | −21.5 | 232.3 | −43.6 | 318.8 | 12.7 |
| BHandHLYP[g] | 236.2 | −82.4 | 243.7 | −46.8 | ||||||
| BHandHLYP[h] | 237.8 | −85.4 | 250.5 | −42.6 | ||||||
| Experimental | 256.0 | −47.5 | ||||||||
[a] Scalar relativistic SR‐ZORA calculations of a cluster model of one clathrate cavity with ADF code73, 74 using Xe/other=jcpl/TZP basis sets.76 129Xe chemical shift (δ=σXe−atom−σ) with respect to free Xe atom shielding values (σXe−atom): PBE/BLYP/B3LYP/BHandHLYP=5752.2/5752.6/5752.1/5752.0 ppm. Δδ (CSA) defined in principal axis system (PAS) along the unique axis (perpendicular to the plane of O−H rings). Asymmetry parameter η=0 in all cavities due to the cylindrical symmetry. [b] As footnote [a], but at nonrelativistic (NR) level of theory with σXe−atom(BHandHLYP)=5643.4 ppm. [c] As footnote [a], but at relativistic spin‐orbit SO‐ZORA level of theory with σXe−atom(BHandHLYP)=6609.3 ppm. [d] Scalar relativistic GIPAW results obtained with CASTEP code.71, 72 All periodic 129Xe chemical shifts referenced to CASTEP/PBE value: σXe−atom=5926.3 ppm. [e] In the CSP optimized crystal geometry. [f] As footnote [d], but estimated by scaling GIPAW/PBE PAS components with factors obtained at SR‐ZORA level for cluster model resulting in data in footnote [a]. [g] Correction added to result of footnote [f] due to thermal averaging (AVE) over Xe motion at T=300 by MC‐NVT simulation (see text for details). [h] Relativistic spin‐orbit (SO) corrections obtained as difference of SO‐ZORA and SR‐ZORA calculations with ADF code added to result of footnote [g].
Table 2.
Theoretically modelled 129Xe NMR chemical shift (δ) and anisotropy (CSA, Δδ) in ppm for the seven most likely m‐fluorophenol CSP candidates in their PBE‐TS optimized structures, unless stated otherwise.
| mF_A | mF_B | mF_C | mF_D | mF_E | mF_F | mF_G | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Method | δ | Δδ | δ | Δδ | δ | Δδ | δ | Δδ | δ | Δδ | δ | Δδ | δ | Δδ |
| Cluster model [a] | ||||||||||||||
| PBE | 259.6 | 219.9 | 272.1 | 188.5 | 216.5 | 198.5 | 256.3 | 146.6 | 256.7 | 187.1 | 297.6 | 187.9 | 316.1 | 220.4 |
| BLYP | 264.8 | 222.0 | 278.7 | 189.5 | 224.1 | 201.3 | 262.7 | 147.5 | 262.0 | 189.1 | 305.4 | 189.3 | 323.3 | 222.1 |
| B3LYP | 236.9 | 197.2 | 240.0 | 172.1 | 193.6 | 182.4 | 225.6 | 135.5 | 233.0 | 167.1 | 265.1 | 173.8 | 282.1 | 204.0 |
| BHandHLYP | 204.6 | 174.7 | 202.4 | 166.9 | 167.5 | 159.2 | 189.8 | 127.8 | 198.4 | 141.9 | 227.2 | 161.4 | 240.9 | 188.1 |
| BHandHLYP[b] | 182.3 | 165.8 | 189.8 | 159.5 | 152.2 | 147.7 | 176.7 | 122.6 | 177.1 | 135.1 | 210.6 | 153.5 | 223.9 | 179.9 |
| BHandHLYP[c] | 211.2 | 176.3 | 197.4 | 162.1 | 170.1 | 156.2 | 186.4 | 124.6 | 204.7 | 139.0 | 224.0 | 157.0 | 237.2 | 182.1 |
| Periodic model [d] | ||||||||||||||
| PBE[e] | 239.7 | 213.1 | 428.8 | 226.8 | 198.7 | 162.1 | 343.8 | 166.0 | 193.2 | 156.9 | 436.1 | 194.5 | 467.0 | 225.7 |
| PBE | 270.6 | 226.7 | 295.2 | 216.0 | 229.2 | 224.8 | 271.4 | 163.4 | 272.9 | 192.4 | 314.1 | 203.7 | 337.2 | 242.9 |
| BLYP[f] | 275.5 | 228.9 | 301.6 | 217.0 | 236.7 | 227.6 | 277.7 | 164.3 | 277.9 | 194.4 | 321.7 | 205.1 | 344.2 | 244.5 |
| B3LYP[f] | 247.3 | 203.4 | 262.3 | 199.3 | 205.7 | 208.3 | 240.0 | 152.0 | 248.6 | 171.8 | 280.7 | 189.4 | 302.3 | 226.1 |
| BHandHLYP[f] | 214.2 | 180.2 | 223.8 | 194.1 | 179.1 | 184.5 | 203.3 | 144.2 | 213.1 | 145.9 | 241.9 | 176.7 | 260.1 | 209.9 |
| BHandHLYP[g] | 229.3 | 187.3 | 229.7 | 200.4 | 227.4 | 152.3 | 250.2 | 191.2 | ||||||
| BHandHLYP[h] | 235.9 | 188.9 | 224.7 | 195.6 | 233.8 | 149.4 | 247.0 | 186.7 | ||||||
| Experimental | 228.5 | 183.3 | ||||||||||||
[a] Scalar relativistic SR‐ZORA calculations of a cluster model of one clathrate cavity with ADF code73, 74 using Xe/other=jcpl/TZP basis sets.76 129Xe chemical shift (δ=σXe−atom−σ) with respect to free Xe atom shielding values (σXe−atom): PBE/BLYP/B3LYP/BHandHLYP=5752.2/5752.6/5752.1/5752.0 ppm. Δδ (CSA) defined in principal axis system (PAS) along the unique axis (perpendicular to the plane of O−H rings). Asymmetry parameter η=0 in all cavities due to the cylindrical symmetry. [b] As footnote [a], but at nonrelativistic (NR) level of theory with σXe−atom(BHandHLYP)=5643.4 ppm. [c] As footnote [a], but at relativistic spin‐orbit SO‐ZORA level of theory with σXe−atom(BHandHLYP)=6609.3 ppm. [d] Scalar relativistic GIPAW results obtained with CASTEP code.71, 72 All periodic 129Xe chemical shifts referenced to CASTEP/PBE value: σXe−atom=5926.3 ppm. [e] In the CSP optimized crystal geometry. [f] As footnote [d], but estimated by scaling GIPAW/PBE PAS components with factors obtained at SR‐ZORA level for cluster model resulting in data in footnote [a]. [g] Correction added to result of footnote [f] due to thermal averaging (AVE) over Xe motion at T=300 K by MC‐NVT simulation (see text for details). [h] Relativistic spin‐orbit (SO) corrections obtained as difference of SO‐ZORA and SR‐ZORA calculations with ADF code added to result of footnote [g].
The ADF/SR‐ZORA results for PBE‐TS optimized cluster models were used to extract correction factors (see details in Supporting Information) by which the periodic, SR GIPAW/PBE results were scaled in order to obtain periodic estimates of 129Xe NMR parameters with BLYP, B3LYP, and BHandHLYP functionals. The estimated periodic data for the five and seven candidates of o‐fluorophenol and m‐fluorophenol clathrates are listed in Tables 1 and 2 as well as displayed in Figure 8 and 9, respectively. The best BHandHLYP‐scaled periodic estimates bring one clathrate structure for each of the fluorophenol isomers into excellent agreement with the experimental NMR: oF_D and mF_A. For o‐fluorophenol, the other CSP candidates in the PBE‐TS crystal geometry have quite different periodic BHandHLYP 129Xe CS and/or CSA values as compared to the experimental ones, whereas for m‐fluorophenol the closest alternative candidates overestimate either the CS (mF_F) or CSA (mF_B). The BHandHLYP functional is expected to provide reasonable approximation for both quantities due to benchmarking against ab initio calculations.61, 62, 63
Figure 8.
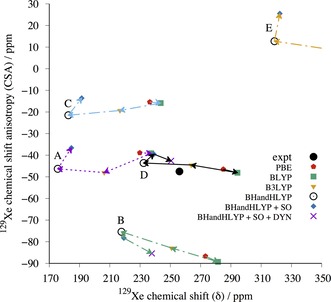
The periodic GIPAW results for the five most likely o‐fluorophenol structures optimized at the periodic PBE‐TS level of theory. The labels A–E correspond to structures oF_A to oF_E in the text. The correct structure is expected to approach the experimental (expt) 129Xe NMR parameters, when the computed GIPAW/PBE result is scaled with factors obtained using different pure and hybrid DFT functionals with increasing amount of exact exchange in the series of PBE→BLYP(0 %)→B3LYP(20 %)→BHandHLYP(50 %). The SO correction is added to BHandHLYP values of all structures (blue diamonds). For structures oF_B and oF_D, the final points (indigo crosses) include also the effect of Xe dynamics (DYN) at T=300 K.
Figure 9.
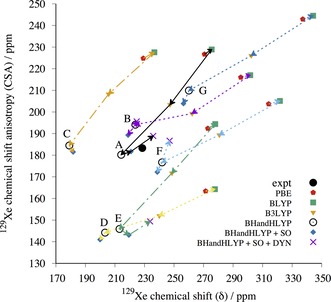
The periodic GIPAW results for the seven m‐fluorophenol structures optimized at the periodic PBE‐TS level of theory. The labels A–G correspond to structures mF_A to mF_G in the text. The correct structure is expected to approach the experimental (expt) 129Xe NMR parameters, when the computed GIPAW/PBE result is scaled with factors obtained using different pure and hybrid DFT functionals with increasing amount of exact exchange in the series of PBE→BLYP(0 %)→B3LYP(20 %)→BHandHLYP(50 %). The SO correction is added to BHandHLYP values of all structures (blue diamonds). For structures mF_A, mF_B, mF_E, and mF_F, the final point (indigo crosses) include also the effect of Xe dynamics (DYN) at T=300 K.
In addition, the relativistic SO correction, obtained as the difference between SO‐ and SR‐ZORA cluster calculations with ADF (see Tables 1 and 2), is added to the static BHandHLYP results of all structures (Figure 8 and Figure 9). Due to its different physical origin, the SO effect is case‐specific and may either increase or decrease the CS and CSA, although the latter is affected slightly more. The magnitude of the SO correction is, however, smaller than the dynamical correction (see below) and, hence, does not alter the identification of the best clathrate candidates.
The temperature effect of Xe dynamics at T=300 K was modeled for the two (oF_B and oF_D) and four (mF_A, mF_B, mF_E, and mF_F) most relevant clathrate candidates. The dynamical (DYN) correction was added to the SO corrected BHandHLYP results (BHandHLYP+SO). In all cases, the thermal averaging increases the magnitudes of both CS and CSA of 129Xe. As seen in Figures 8 and 9, the inclusion of DYN correction confirms the most probable clathrate structure of both isomers. The effect on the simulated spectra is also shown in Figure 2.
It is evident from Figure 8 and 9 (see also Tables S6 and S7 in the Supporting Information) that in all probability the crystal structures for o‐ and m‐fluorophenol clathrates are oF_D and mF_A. For them, the 129Xe NMR isotropic chemical shift and chemical shift anisotropies at different levels of theory encompass the experimental data. For both structures, the experimental NMR parameters are approached as the computational level is improved. The approximated periodic BHandHLYP results at PBE‐TS optimized structures only slightly underestimate the chemical shift, leaving room for improvements by dynamic and relativistic SO corrections. Astonishingly, the most accurate level of theoretical modeling almost quantitatively reproduces the two NMR parameters that are clearly specific for a given clathrate. The remaining differences between experimental and computational data may be attributed to deficiencies in the structure as well as treatments of electron correlation and thermal averaging of the whole system.
The PXRD results provide further evidence for the CSP‐129Xe NMR determined structure of the o‐fluorophenol clathrate; the simulated diffraction pattern from oF_D is similar to the PXRD obtained from the clathrate sample (see Figure S11 in Supporting Information). PXRD of the present quality cannot, however, be used to unambiquously distinquish between the candidates structures.
Description of the proposed clathrate structures
The crystal structures that were proposed as the fluorophenol xenon clathrates belong to space group R , with lattice parameters shown in Table 3. Both structures have three‐fold screw axes parallel to the c lattice vector, resulting in six‐membered rings of host molecules held together with strong hydrogen bonds formed by practically ideal OH⋅⋅⋅O interactions. The hydrogen bonding forms a (12) graph set,84 which seems to be a characteristic feature in clathrates of phenol derivatives. Packing diagrams are displayed in Figures 10 and Figure 11. Crystallographic information files (CIFs) are included in the Supporting Information. Both structures have three cavities per unit cell, with volumes 61.4 and 88.5 Å3 each for o‐ and m‐fluorophenol, respectively, resulting in Rg values of 68.7 and 47.7 % for xenon, both within 1.5 standard deviations from the ideal ratio of 59 %.
Table 3.
Unit cell parameters (after PBE‐TS geometry optimization) for the proposed xenon clathrate structures of o‐ and m‐fluorophenol.
| Structure | space group | a [Å] | b [Å] | c [Å] | γ[°] | |
|---|---|---|---|---|---|---|
| oF_D |
R
|
22.5225 | 22.5225 | 5.6879 | 120 | |
| mF_A |
R
|
22.5526 | 22.5526 | 5.6998 | 120 |
Figure 10.
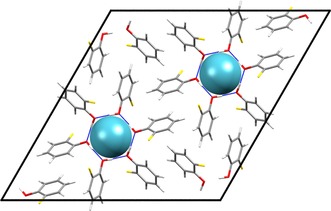
Packing diagram of the PBE‐TS optimized o‐fluorophenol xenon clathrate structure oF_D viewed down the c‐axis. Space group R and with (12) hydrogen bonds encasing the xenon atoms. See also Figure 5 a.
Figure 11.
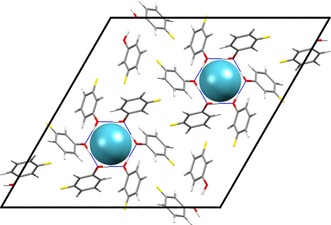
Packing diagram of the PBE‐TS optimized m‐fluorophenol xenon clathrate mF_A viewed down the c‐axis. Space group R and with (12) hydrogen bonds encasing the xenon atoms. See also Figure 5 b.
Despite the same hydrogen bonding and crystal symmetry, the voids in the two structures are of quite different geometry. The void in oF_D has an oblate shape with ratios of the principal moments of the free volume (calculated from a grid sampling in Platon)85 of 1.00:1.00:0.85, with the short dimension oriented along the crystallographic c‐axis. In contrast, mF_A has a prolate shape with calculated ratio of dimensions of 1.00:1.00:1.20, elongated along the crystallographic c‐axis. Thus, the sign of the CSA relates to the shape of the void, as expected, with an oblate cavity leading to a negative CSA and prolate cavity yielding a positive CSA.
The proposed structure of the o‐fluorophenol Xe clathrate (oF_D) is one of the highest energy and one of the densest structures that was considered as a possible clathrate (Figure 3). The stabilization of this structure must derive from the interactions of Xe with the host structure, which should be large, due to the tight fit of Xe to the host cavities.
In contrast, the most likely structure of the m‐fluorophenol Xe clathrate (mF_A) corresponds to the most stable structure on the crystal structure landscape (Figure 4) that contains cavities suitable for Xe enclathration; this structure's stability relates, in part, to the stability of the host framework.
Conclusion
Detailed first principles NMR calculations have been used on candidates from CSP to propose structures for the xenon clathrates of o‐ and m‐fluorophenol. The exceptional sensitivity of the 129Xe chemical shift tensor to its local environment allows comparisons between observed and calculated NMR chemical shift parameters that have been used to directly confirm or reject hypothetical clathrate structures. Based on these comparisons, likely crystal structures were proposed for the two clathrates. The proposed structures strongly resemble a previously known β‐hydroquinone xenon clathrate6 and have similar (12) hydrogen bonding motifs.84
The unusual o‐fluorophenol 129Xe NMR powder spectrum, with its negative CSA, was initially thought to suggest a structural motif different from the known and common (12) hydrogen‐bonded double sandwich. Our results, however, confirm that the (12) motif is an important feature for clathrates of phenol derivatives that is present in both materials studied here.
Static solid‐state NMR spectroscopy has clear benefits over high‐frequency magic‐angle spinning NMR spectroscopy, that would only result in isotropic chemical shifts rather than the complete chemical shift tensors. In this study, using isotropic shifts only would not have allowed the identification of the experimental clathrate structures among the predicted candidates.
The method presented here is a powerful approach for structure determination of porous materials, which is particularly useful in cases where powder diffraction patterns either cannot be obtained, or are insufficient for structure determination. The success of the method is related to the high sensitivity of the 129Xe NMR chemical shift and chemical shift anisotropy to minute details of the cavity geometry, such that even structurally very similar clathrates can have vastly different chemical shift tensors. This sensitivity, however, also requires highly detailed NMR calculations; in addition to the proper treatment of electron correlation and relativistic phenomena, the inclusion of explicit crystal lattice effects, as well as Xe dynamics, was necessary in order to precisely reproduce experimental NMR values. Developments in this area, along with progress in CSP algorithms, open up new possibilities for the prediction and characterization of new porous materials by combining structure prediction with computational and experimental 129Xe NMR spectroscopy.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
The work reported here has been financially supported by the European Research Council under the European Union's Seventh Framework Programme (FP/2007‐2013)/ERC Grant Agreement no. 307358, ERC‐stG‐2012‐ANGLE (J.N., G.D.), by the Magnus Ehrnrooth Foundation and NGS‐NANO (M.S.), and by the Academy of Finland project no.s. 125316, 218191, 255641, and 285666 (P.L.). Computational resources for the NMR part of the article were partially provided by CSC–IT Center for Science Ltd, Espoo and Kajaani, Finland. Parts of the calculations have been carried out using resources provided by Wrocław Centre for Networking and Supercomputing (http://wcss.pl). The crystal structure prediction was carried out using the IRIDIS high performance computing facility at the University of Southampton. M.S. acknowledges the great help of Anu M. Kantola in performing solid‐state NMR measurements.
M. Selent, J. Nyman, J. Roukala, M. Ilczyszyn, R. Oilunkaniemi, P. J. Bygrave, R. Laitinen, J. Jokisaari, G. M. Day, P. Lantto, Chem. Eur. J. 2017, 23, 5258.
Contributor Information
Prof. Graeme M. Day, Email: g.m.day@soton.ac.uk.
Dr. Perttu Lantto, Email: perttu.lantto@oulu.fi.
References
- 1. Baias M., Dumez J.-N., Svensson P. H., Schantz S., Day G. M., Emsley L., J. Am. Chem. Soc. 2013, 135, 17501–17507. [DOI] [PubMed] [Google Scholar]
- 2. Baias M., Widdifield C. M., Dumez J.-N., Thompson H. P. G., Cooper T. G., Salager E., Bassil S., Stein R. S., Lesage A., Day G. M., Emsley L., Phys. Chem. Chem. Phys. 2013, 15, 8069–8080. [DOI] [PubMed] [Google Scholar]
- 3. Kazankin Y. N., Palladiev A. A., Trofimov A. M., Zh. Obshch. Khim. 1972, 42, 2368–2370. [Google Scholar]
- 4. Kazankin Y. N., Palladiev A. A., Trofimov A. M., Zh. Obshch. Khim. 1973, 43, 2670–2671. [Google Scholar]
- 5. Ripmeester J. A., J. Am. Chem. Soc. 1982, 104, 289–290. [Google Scholar]
- 6. Birchall T., Frampton C. S., Schrobilgen G. J., Valsdóttir J., Acta Crystallogr. Sect. C 1989, 45, 944–946. [Google Scholar]
- 7. Mandelcorn L., Chem. Rev. 1959, 59, 827–839. [Google Scholar]
- 8. Ilczyszyn M., Selent M., Ilczyszyn M. M., J. Phys. Chem. A 2012, 116, 3206–3214. [DOI] [PubMed] [Google Scholar]
- 9. Kang S.-P., Lee H., J. Chem. Eng. Data 1997, 42, 467–469. [Google Scholar]
- 10. Barrer R. M., Shanson V. H., J. Chem. Soc. Faraday Trans.1 1976, 72, 2348–2354. [Google Scholar]
- 11. Proctor W., Yu F., Phys. Rev. 1951, 81, 20–30. [Google Scholar]
- 12. Ripmeester J., Davidson D., J. Mol. Struct. 1981, 75, 67–72. [Google Scholar]
- 13. Ito T., Fraissard J. in Proceedings of the Fifth International Conference on Zeolites (Ed.: L. V. C. Rees), Heyden, London, 1980, p. 510. [Google Scholar]
- 14. Bartik B., Choquet P., Constantinesco A., Duhamel G., Fraissard J., Hyacinthe J. N., Jokisaari J., Locci E., Lowery T. J., Luhmer M., Meersmann T., Moudrakovski I. L., Pavlovskaya G. E., Pierce K. L., Pines A., Ripmeester J. A., Telkki V.-V., Veeman W. S., Actual. Chim. 2005, 287, 16–34. [Google Scholar]
- 15. Raftery D., in Annual Reports on NMR Spectroscopy, Volume 57 (Ed.: G. Webb), Academic Press, 2006, pp. 205–270. [Google Scholar]
- 16. Spence M. M., Rubin S. M., Dimitrov I. E., Ruiz E. J., Wemmer D. E., Pines A., Yao S. Q., Tian F., Schultz P. G., Proc. Natl. Acad. Sci. USA 2001, 98, 10654–10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mugler J. P., Altes T. A., Ruset I. C., Dregely I. M., Mata J. F., Miller G. W., Ketel S., Ketel J., Hersman F. W., Ruppert K., Proc. Natl. Acad. Sci. USA 2010, 107, 21707–21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davidson D. W., Ripmeester J. A. in Inclusion Compounds, Volume 3: Physical Properties and Applications (Eds. J. L. Atwood, J. E. D. Davies, D. D. MacNicol), Academic Press, London, 1984, pp. 69–128. [Google Scholar]
- 19. Dybowski C., Bansal N., Duncan T. M., Annu. Rev. Phys. Chem. 1991, 42, 433–464. [Google Scholar]
- 20. Ratcliffe C. I. in Annual Reports on NMR Spectroscopy Volume 36 (Ed.: G. A. Webb), Academic Press, 1998, pp. 123–221. [Google Scholar]
- 21. Walker T. G., Happer W., Rev. Mod. Phys. 1997, 69, 629. [Google Scholar]
- 22. Ripmeester J., Ratcliffe C., Tse J., J. Chem. Soc. Faraday Trans. 1 1988, 84, 3731–3745. [Google Scholar]
- 23. Jameson C. J., Jameson A. K., Baello B. I., Lim H., J. Chem. Phys. 1994, 100, 5965–5976. [Google Scholar]
- 24. Stueber D., Jameson C. J., J. Chem. Phys. 2004, 120, 1560–1571. [DOI] [PubMed] [Google Scholar]
- 25. Jameson C. J., Stueber D., J. Chem. Phys. 2004, 120, 10200–10214. [DOI] [PubMed] [Google Scholar]
- 26. Bühl M., Patchkovskii S., Thiel W., Chem. Phys. Lett. 1997, 275, 14–18. [Google Scholar]
- 27. Sears D. N., Jameson C. J., J. Chem. Phys. 2003, 118, 9987–9989. [Google Scholar]
- 28. Autschbach J., Zurek E., J. Phys. Chem. A 2003, 107, 4967–4972. [Google Scholar]
- 29. Straka M., Lantto P., Vaara J., J. Phys. Chem. A 2008, 112, 2658–2668. [DOI] [PubMed] [Google Scholar]
- 30. Standara S., Kulhánek P., Marek R., Straka M., J. Comput. Chem. 2013, 34, 1890–1898. [DOI] [PubMed] [Google Scholar]
- 31. Bagno A., Saielli G., Chem. Eur. J. 2012, 18, 7341–7345. [DOI] [PubMed] [Google Scholar]
- 32. Roukala J., Zhu J., Giri C., Rissanen K., Lantto P., Telkki V.-V., J. Am. Chem. Soc. 2015, 137, 2464–2467. [DOI] [PubMed] [Google Scholar]
- 33. Ripmeester J. A., Tse J. S., Ratcliffe C. I., Powell B. M., Nature 1987, 325, 135–136. [Google Scholar]
- 34. Yang L., Tulk C. A., Klug D. D., Moudrakovski I. L., Ratcliffe C. I., Ripmeester J. A., Chakoumakos B. C., Ehm L., Martin C. D., Parise J. B., Proc. Natl. Acad. Sci. USA 2009, 106, 6060–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flippen J. L., Karle J., Karle I. L., J. Am. Chem. Soc. 1970, 92, 3749–3755. [DOI] [PubMed] [Google Scholar]
- 36. Jones J. T. A., Hasell T., Wu X., Bacsa J., Jelfs K. E., Schmidtmann M., Chong S. Y., Adams D. J., Trewin A., Schiffman F., Cora F., Slater B., Steiner A., Day G. M., Cooper A. I., Nature 2011, 474, 367–371. [DOI] [PubMed] [Google Scholar]
- 37. Pyzer-Knapp E. O., Thompson H. P. G., Schiffmann F., Jelfs K. E., Chong S. Y., Little M. A., Cooper A. I., Day G. M., Chem. Sci. 2014, 5, 2235–2245. [Google Scholar]
- 38. Slater A. G., Little M. A., Pulido A., Chong S. Y., Holden D., Chen L., Morgan C., Wu X., Cheng G., Clowes R., Briggs M. E., Hasell T., Jelfs K. E., Day G. M., Cooper A. I., Nat. Chem. 2017, 9, 17–25. [DOI] [PubMed] [Google Scholar]
- 39. Cruz-Cabeza A. J., Day G. M., Jones W., Chem. Eur. J. 2008, 14, 8830–8836. [DOI] [PubMed] [Google Scholar]
- 40. Cruz-Cabeza A. J., Karki S., Fabian L., Friscic T., Day G. M., Jones W., Chem. Commun. 2010, 46, 2224–2226. [DOI] [PubMed] [Google Scholar]
- 41. Braun D. E., Gelbrich T., Kahlenberg V., Griesser U. J., CrystEngComm 2015, 17, 2504–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cruz-Cabeza A. J., Day G. M., Jones W., Chem. Eur. J. 2009, 15, 13033–13040. [DOI] [PubMed] [Google Scholar]
- 43. Cruz Cabeza A. J., Day G. M., Motherwell W. D. S., Jones W., J. Am. Chem. Soc. 2006, 128, 14466–14467. [DOI] [PubMed] [Google Scholar]
- 44. Webb A., Annu. Rep NMR Spectro. 2002, 45, 1–67. [Google Scholar]
- 45. Massiot D., Fayon F., Capron M., King I., Le Calvé S., Alonso B., Durand J.-O., Bujoli B., Gan Z., Hoatson G., Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar]
- 46. Haeberlen U., High Resolution NMR in Solids Selective Averaging: Supplement 1 Advances in Magnetic Resonance, Elsevier, 2012. [Google Scholar]
- 47.Gaussian 09 Revision D.01, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian Inc. Wallingford CT, 2009.
- 48. Becke A., J. Chem. Phys. 1993, 98, 5648. [Google Scholar]
- 49. Stephens P. J., Devlin F. J., Chabalowski C. F., Frisch M. J., J. Phys. Chem. 1994, 98, 11623. [Google Scholar]
- 50. Thompson H. P., Day G. M., Chem. Sci. 2014, 5, 3173–3182. [Google Scholar]
- 51. Karfunkel H., Gdanitz R., J. Comput. Chem. 1992, 13, 1171–1183. [Google Scholar]
- 52. Pyzer-Knapp E. O., Thompson H. P. G., Day G. M., Acta Crystallogr. Sect. A 2016, 72, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chisholm J. A., Motherwell S., J. Appl. Crystallogr. 2005, 38, 228–231. [Google Scholar]
- 54. Kazantsev A. V., Karamertzanis P. G., Adjiman C. S., Pantelides C. C., J. Chem. Theory Comput. 2011, 7, 1998–2016. [DOI] [PubMed] [Google Scholar]
- 55. Nyman J., Day G. M., CrystEngComm 2015, 17, 5154–5165. [Google Scholar]
- 56. Kitaigorodsky A., Molecular Crystals and Molecules, Academic Press, 1973. [Google Scholar]
- 57. Barbour L. J., Chem. Commun. 2006, 1163–1168. [DOI] [PubMed] [Google Scholar]
- 58.TURBOMOLE V6.6 2014, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989–2007, TURBOMOLE GmbH, since 2007; available from http://www.turbomole.com.
- 59. Becke A. D., Phys. Rev. A 1988, 38, 3098. [DOI] [PubMed] [Google Scholar]
- 60. Becke A. D., J. Chem. Phys. 1993, 98, 1372. [Google Scholar]
- 61. Lantto P., Vaara J., J. Chem. Phys. 2007, 127, 084312. [DOI] [PubMed] [Google Scholar]
- 62. Straka M., Lantto P., Rasanen M., Vaara J., J. Chem. Phys. 2007, 127, 234314. [DOI] [PubMed] [Google Scholar]
- 63. Lantto P., Standara S., Riedel S., Vaara J., Straka M., Phys. Chem. Chem. Phys. 2012, 14, 10944–10952. [DOI] [PubMed] [Google Scholar]
- 64. Perdew J. P., Burke K., Ernzerhof M., Phys. Rev. Lett. 1996, 77, 3865. [DOI] [PubMed] [Google Scholar]
- 65. Lee C., Yang W., Parr R., Phys. Rev. B 1988, 37, 785. [DOI] [PubMed] [Google Scholar]
- 66. Tkatchenko A., Scheffler M., Phys. Rev. Lett. 2009, 102, 073005. [DOI] [PubMed] [Google Scholar]
- 67. Pickard C. J., Mauri F., Phys. Rev. B 2001, 63, 245101. [Google Scholar]
- 68. Yates J. R., Pickard C. J., Mauri F., Phys. Rev. B 2007, 76, 024401. [Google Scholar]
- 69. van Lenthe E., Baerends E. J., Snijders J. G., J. Chem. Phys. 1993, 99, 4597. [Google Scholar]
- 70. Wolff S. K., Ziegler T., van Lenthe E., Baerends E. J., J. Chem. Phys. 1999, 110, 7689–7698. [Google Scholar]
- 71. Clark S. J., Segall M. D., Pickard C. J., Hasnip P. J., Probert M. I. J., Refson K., Payne M. C., Z. Kristallogr. - Cryst. Mater. 2005, 220, 567–570. [Google Scholar]
- 72. McNellis E. R., Meyer J., Reuter K., Phys. Rev. B 2009, 80, 205414. [Google Scholar]
- 73. te Velde G., Bickelhaupt F. M., Baerends E. J., Guerra C. Fonseca, van Gisbergen S. J. A., Snijders J. G., Ziegler T., J. Comput. Chem. 2001, 22, 931–967. [Google Scholar]
- 74.ADF2016, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, http://www.scm.com.
- 75. van Lenthe E., Baerends E. J., Snijders J. G., J. Chem. Phys. 1994, 101, 9783. [Google Scholar]
- 76. van Lenthe E., Baerends E. J., J. Comput. Chem. 2003, 24, 1142. [DOI] [PubMed] [Google Scholar]
- 77. Jameson C. J., de Dios A. C., J. Chem. Phys. 2002, 116, 3805–3821. [Google Scholar]
- 78. Moudrakovski I., Ratcliffe C., Ripmeester J., J. Am. Chem. Soc. 2001, 123, 2066–2067. [DOI] [PubMed] [Google Scholar]
- 79. Davidson D. W., Garg S. K., Gough S. R., Handa Y. P., Ratcliffe C. I., Tse J. S., Ripmeester J. A. in Clathrate Compounds, Molecular Inclusion Phenomena, and Cyclodextrins (Eds.: J. L. Atwood, J. E. D. Davis, T. Osa), Springer, Netherlands, 2012, pp. 231–238. [Google Scholar]
- 80. Soldatov D. V., Moudrakovski I. L., Grachev E. V., Ripmeester J. A., J. Am. Chem. Soc. 2006, 128, 6737–6744. [DOI] [PubMed] [Google Scholar]
- 81. Oswald I. D. H., Allan D. R., Motherwell W. D. S., Parsons S., Acta Crystallogr. Sect. A 2005, 61, 69–79. [DOI] [PubMed] [Google Scholar]
- 82. Mecozzi S., J. Rebek Jr. , Chem. Eur. J. 1998, 4, 1016–1022. [Google Scholar]
- 83. Nakano K., Sada K., Kurozumi Y., Miyata M., Chem. Eur. J. 2001, 7, 209–220. [DOI] [PubMed] [Google Scholar]
- 84. Etter M. C., J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar]
- 85. Spek A. L., J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


