Abstract
Background
Allergy immunotherapy (AIT) is the only treatment for allergic rhinitis (AR) and/or allergic asthma (AA) with long‐term efficacy. However, there are few real‐life data on the progression of AR and/or AA in patients receiving AIT.
Objectives
To assess the real‐world, long‐term efficacy of grass pollen sublingual immunotherapy (SLIT) tablets in AR and their impact on asthma onset and progression.
Methods
In a retrospective analysis of a German longitudinal prescription database, AR patients treated with grass pollen SLIT tablets were compared with a control group not having received AIT. Multiple regression analysis was used to compare changes over time in rescue symptomatic AR medication use after treatment cessation, asthma medication use, and the time to asthma onset in the two groups.
Results
After applying all selection criteria, 2851 SLIT and 71 275 control patients were selected for the study. After treatment cessation, AR medication use was 18.8 percentage points lower (after adjustment for covariates, and relative to the pretreatment period) in SLIT tablet group than in the non‐AIT group (P<.001). Asthma onset was less frequent in SLIT tablet group than in non‐AIT group (odds ratio: 0.696, P=.002), and time to asthma was significantly longer (hazard ratio: 0.523; P=.003). After SLIT cessation, asthma medication use fell by an additional 16.7 percentage points (relative to the pretreatment period) in the SLIT tablet group vs the non‐AIT group (P=.004).
Conclusions
Real‐world treatment of AR patients with grass pollen SLIT tablets was associated with slower AR progression, less frequent asthma onset, and slower asthma progression.
Keywords: allergic rhinitis, asthma, database analysis, grass pollen, sublingual immunotherapy
1. INTRODUCTION
Allergic rhinitis (AR) is a common, chronic, inflammatory illness characterized by the presence of rhinorrhoea, nasal congestion, sneezing, nasal and ocular itching, and/or watery eyes. Worldwide, approximately 500 million people suffer from AR,1 including over 100 million in Europe2 and about 60 million in the USA.3, 4 The estimated prevalence of AR in the US general population is 30.2%.5 Grass pollen is the disease‐inducing allergen in an estimated 62.1% of cases of AR.6 Moderate‐to‐severe symptoms of AR have a negative impact on quality of life, workplace productivity, and school performance.7, 8, 9 AR is also associated with an increased risk or worsening of allergic asthma.10, 11, 12, 13 The prevalence of allergic asthma is higher in individuals with AR than in individuals without AR.14, 15, 16
Although symptomatic medications such as antihistamines and corticosteroids provide temporary relief, allergen immunotherapy (AIT) is currently the only treatment with long‐term efficacy. A large body of evidence from meta‐analyses and double‐blind, placebo‐controlled, randomized clinical trials (DBPC RCTs) shows that AIT is associated with significantly less severe AR symptoms and with lower rescue medication use. In patients with moderate‐to‐severe AR, grass pollen sublingual immunotherapy (SLIT) tablets have also demonstrated (i) a sustained clinical effect after 3 years of treatment and (ii) a long‐term effect after treatment cessation.17 Although severe asthma is a contraindication for AIT, there is some evidence to suggest that this treatment provides symptom relief in patients with mild‐to‐moderate allergic asthma.17, 18, 19, 20, 21, 22, 23
AIT's impact on the “allergic march” has also been assessed. In particular, several studies have investigated the prevention of allergic asthma in AR patients treated with AIT. The PAT study showed that a 3‐year course of subcutaneous allergen immunotherapy (SCIT) had long‐term clinical effects, and potentially prevented the development of asthma for up to 7 years after treatment cessation.24, 25, 26 Two open‐label studies have reported similar effects of SLIT on the development of asthma.27, 28
More recently, the GRAZAX® Asthma Prevention (GAP) trial (a large DBPC RCT: https://clinicaltrials.gov/ct2/show/NCT01061203) fails to prevent asthma, defined as reversible impairment of lung function but reported favourable results in reducing the risk of symptoms.29 Administration of timothy pollen SLIT tablets was associated with a relative reduction (vs placebo) in the proportion of children experiencing asthma symptoms or using asthma medication. This effect was still observed 2 years after treatment cessation.
Lastly, Schmitt et al.30 concluded that AIT effectively prevents asthma in patients with AR in a real‐world setting. Although the study considered many different allergens, the dataset contained relatively few prescriptions of SLIT formulations. Furthermore, the study was limited to a single region of Germany. Given the absence of other primary analyses of real‐life settings with sufficiently high numbers of SLIT prescriptions, this study therefore sought to assess the long‐term effects of a single type of AIT formulation (grass pollen SLIT tablets) on the progression of AR, the progression of existing asthma, and new asthma onset in patients with AR, relative to symptomatic medication use alone (ie, a control group). To this end, the real‐life longitudinal prescription data in a large, German, nationwide database were retrospectively analyzed.31
2. METHODS
2.1. Overall study design
The study data were extracted from a German longitudinal prescription database (LRx, IMS Health, Frankfurt am Main, Germany).31 Germany was chosen because it was the first European country to authorize the marketing of the grass pollen SLIT tablet formulations and thus provides the longest period for data analysis. A timothy grass pollen tablet (Grazax®; ALK, Hørsholm, Denmark32, 33) had received marketing authorization in Germany in November 2006, and a five‐grass pollen SLIT tablet (Oralair®; Stallergenes Greer, Antony, France17, 34) had become available in Germany in August 2008. In a retrospective analysis of prescription data on symptomatic medications for AR and asthma, a group of AR patients, as defined by ARIA classification, having received a grass pollen SLIT tablet (Oralair® or Grazax®) was compared with a control, non‐AIT group of AR patients having received symptomatic medications only. The LRx database does not contain clinical information (such as diagnoses); hence, patient profiles (eg, the presence and/or progression of grass pollen‐induced AR and/or asthma) have to be inferred from proxy prescription data. As observed in previous epidemiology researches, results of studies conducted with LRx database in other therapeutic areas are generally in line with published reports when a selection of drugs reflects standard management of a disease. In some cases, noticeable discrepancies between LRx results and literature were found (eg, prevalence in epilepsy, persistence in osteoporosis). In these cases, reasons of discrepancies were presented and discussed.31 Prescriptions selected as proxy of AR and asthma are described in the next paragraph. The overall analysis period ranged from January 2009 to February 2016.
2.2. Datasets and proxy clinical data
The LRx database contains information on around 60% of all prescriptions reimbursed by statutory health insurance funds in Germany.31 It was created in January 2008 and is updated monthly. Each prescription is associated with an individual, fully anonymized patient ID number allowing individual patient histories to be followed up over time. For each prescription, the LRx database provides the exact dispensing date, the prescribing physician's speciality, and full details of the medication (brand, formulation, active compound, dose level, strength, package size, etc.). The patients’ basic demographic characteristics (age and gender) are known in most cases. In line with the German legislation on anonymised database analysis, informed consent was not required.
As mentioned above, longitudinal prescription data were used as a proxy for clinical status. Over‐the‐counter medications are also used as treatments for AR but, by definition, are not recorded in the LRx prescription database. However, patients receiving SLIT tablets have moderate‐to‐severe AR and are therefore more likely to receive prescription medication. Preliminary analyses of another database (Pharma Scope; IMS Health) showed that the only widely prescribed, reimbursed, AR‐specific class of prescription medication is nasal corticosteroids (INSs, ATC R01A1). Hence, the prescription of this drug class at least once over the grass pollen season for three consecutive years was used to identify a set of control (non‐AIT) AR patients. Overall symptomatic medication use (ie, oral/systemic antihistamines [ATC R06A0], ophthalmic corticosteroids [ATC S01B0 and ophthalmic corticosteroid/antibiotic combinations; ATC S01C1], and INSs) was tracked and scored to assess AR progression in the selected patients. Only AR prescriptions occurring 1 month before and during the grass pollen season in Germany (May‐August) were analyzed.35
Similarly, the occurrence and progression of asthma were estimated from prescriptions of guidelines‐recommended medications: inhaled short‐acting β‐agonists (SABAs; ATC R03A2 and R03A4) and inhaled corticosteroids (ICSs; ATC R03D1).36 The presence of asthma was defined as at least two prescriptions of these medications in the same year or in two successive calendar years. The date of new asthma onset was defined as the date of the first prescription of SABAs or ICSs. Asthma progression was estimated by tracking prescriptions for long‐acting β‐agonists (LABAs; ATC R03A3), combinations of LABAs and ICSs (ATC R03F1), methylxanthines (R03B2), leukotriene antagonists (ATC R03J2), and depot formulations of systemic corticosteroids (ATC H02A1) as well as SABAs and ICSs. Depot formulations of systemic corticosteroids are not a recommended option, but are used as treatment for asthma. A sensitivity analysis excluding those was performed.
2.3. Analytical time periods
The index date was defined as the date of the first SLIT tablet prescription (for the SLIT tablet group) or the date of the second INS prescription (for the non‐AIT group, as mentioned above, patients included in the control group were requested to have a prescription of this drug class at least once over the grass pollen season for three consecutive years, with the first year before index as the preindex period and the two subsequent years representing the treatment period). The preindex period was defined as the 365‐day period before the index date. The treatment period stretched from the index date to the expiry date of the last SLIT prescription (for the SLIT tablet group) or the last AR prescription in a subsequent pollen season (for the non‐AIT group). The follow‐up period stretched from the end of the treatment period to the end of the study. The full analysis period combined the treatment and follow‐up periods.
2.4. Study population and inclusion/exclusion criteria
Patients in the SLIT tablet group were selected on the basis of a SLIT tablet prescription in at least two successive treatment cycles (Figure 1). Patients with an index date from 2009 to 2012 were included, thus ensuring that the patient was observable for at least 365 days prior to this date. The other main inclusion criteria were as follows: age over 5 years at the index date; at least one prescription of INS in the 365 days prior to the index date; and at least 2 years of follow‐up after the expiry of the last SLIT tablet prescription. The main exclusion criteria were as follows: no record of any AIT formulation other than a grass pollen SLIT tablet; severe asthma (defined as at least one prescription of omalizumab [Xolair®, Novartis, East Hanover, NJ, USA]), perennial asthma (defined as at least three prescriptions of inhaled corticosteroids: ICSs, ICS/LABA combinations, or depot formulations), or methylxanthine use over three successive 4‐month periods (January‐April, May‐August, and September‐December) before or during the year of the index date.
Figure 1.
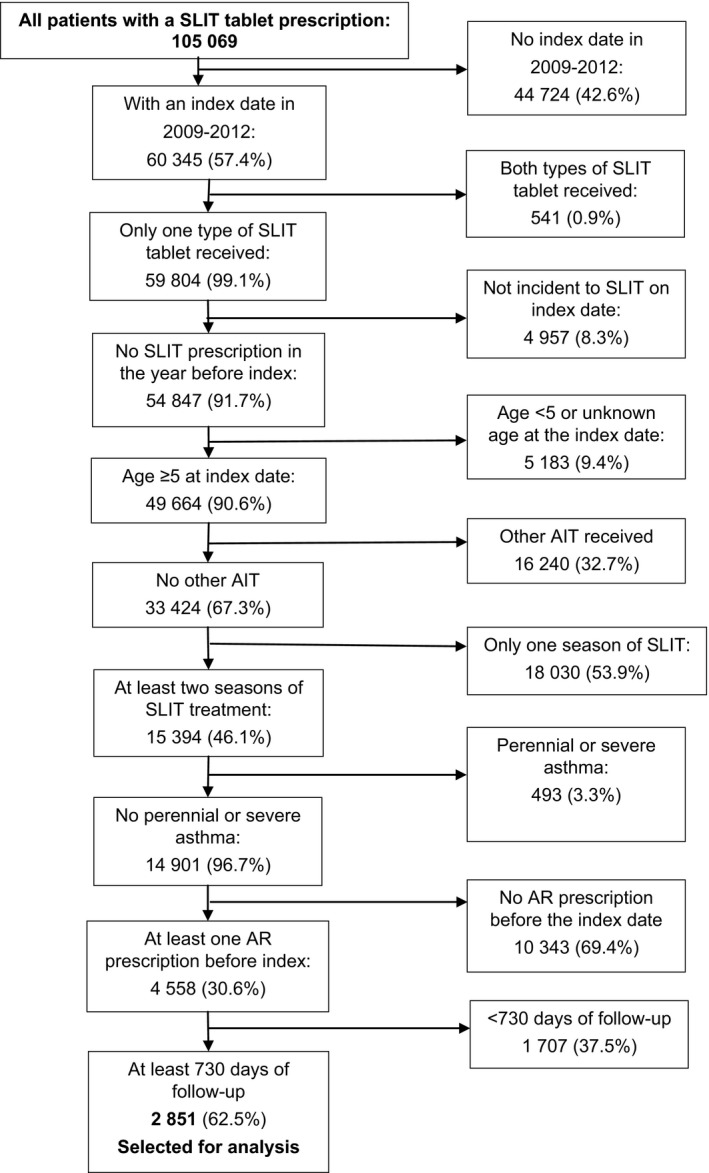
The patient selection process for the sublingual immunotherapy (SLIT) tablet group (the percentages refer to the proportion of the previous n)
Patients in the non‐AIT group were selected on the basis of at least one INS prescription during the grass pollen season or in the month before the grass pollen season in three consecutive years (with the first year being 2008, 2009, 2010, or 2011; Figure 2). The other main inclusion criteria were as follows: age over 5 years at the index date; at least 2 years of follow‐up after the expiry of the last AR prescription. The main exclusion criteria were as follows: no record of any AIT formulation (including but not restricted to grass pollen SLIT tablets) and severe or perennial asthma (defined as for the SLIT tablet group).
Figure 2.
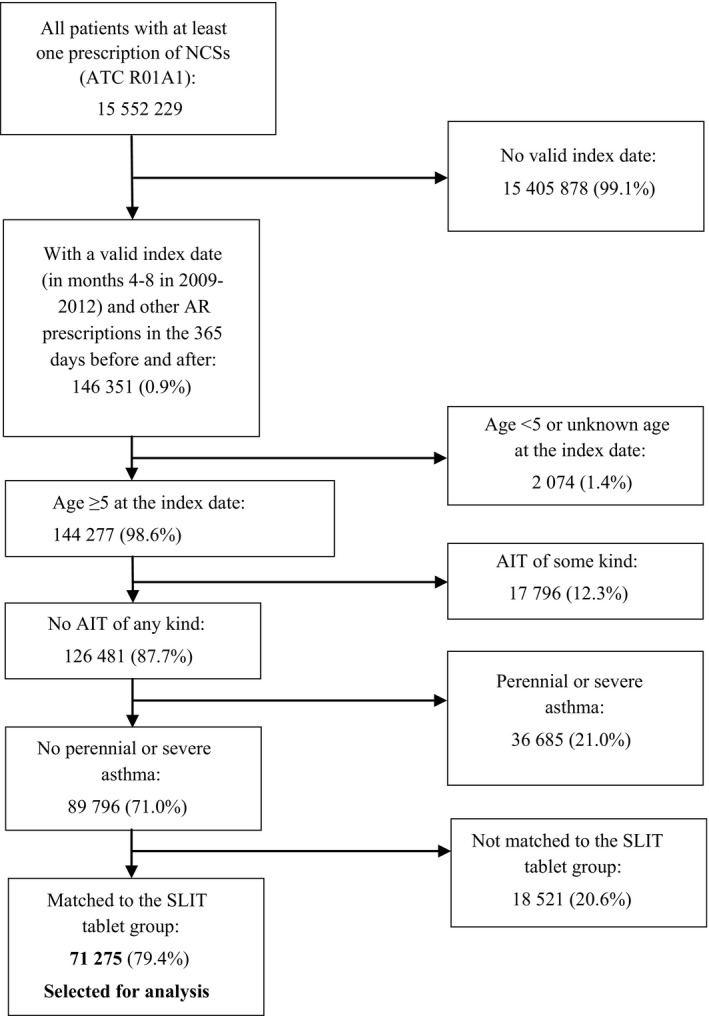
The patient selection process for the non‐AIT (AIT) group (the percentages referred to the proportion of the previous n)
In order to avoid confounding bias due to differences in the length and intensity of the grass pollen season in different years, patients in the SLIT tablet group and the non‐AIT group were matched by index year. The process was repeated until no eligible non‐AIT patients remained (final matching ratio: 25:1). Possible confounders other than index year (patient gender, patient age group at the index date, main prescriber, asthma status at the index date, severity of AR before the index date, and the number of years of SLIT treatment) were not used as matching criteria but were subsequently corrected for in all analyses by multiple regressions (see Appendix S1).
2.5. Study endpoints
The primary endpoint was the change over time in prescriptions of AR symptomatic medications after treatment cessation. The secondary endpoints were new asthma onset, defined as the time to the first prescription of SABAs or ICSs, during treatment and after treatment cessation (as a binary [yes/no] variable) in patients without asthma at the index date, and the change over time in asthma medication prescriptions during the treatment and follow‐up periods in patients with asthma at the index date. To this end, the total number of prescriptions per time period was summed and divided by the length of the time period (in years), in order to compare values for patients with time periods of different lengths. To correct for differences in treatment intensity at the index date, the outcome variable was defined as follows:
The progressions of AR and asthma were analyzed using linear regression. In secondary analyses, time to new asthma onset was assessed in a Cox proportional hazard (PH) regression. Interactions between independent variables were not included in the regression.
Allergic rhinitis progression was only analyzed for the follow‐up period because the structure of the selection process distorted this variable during the treatment period. All the patients in the non‐AIT group had received AR medications during the treatment period, whereas some patients in the SLIT tablet may only have received AR medications before the index date (this was an inclusion criterion) and not during the SLIT treatment period. In contrast, the asthma analyses were performed for the treatment, follow‐up, and full analysis periods.
All analyses were performed using SAS software (version 9.3; SAS Institute, Inc., Cary, NC, USA). The threshold for statistical significance was set to P<.05 in all cases.
3. RESULTS
3.1. Characteristics of the study population
The database contained 105 069 patients having received at least one prescription of SLIT tablets, and 15 552 229 non‐AIT patients having received AR treatment. After application of the inclusion and exclusion criteria, 2851 SLIT tablet patients (Oralair®: 1466 patients; Grazax®: 1385 patients) and 71 275 control non‐AIT patients were selected for analysis (Table 1). In the SLIT tablet group, most exclusions (Figure 1) were due to the index year (excluding 42.6% of all SLIT patients), the need for SLIT to have been administered in at least two successive grass pollen allergy treatment cycles (excluding 53.9%), and the requirement for at least one AR prescription in the year before the index date (excluding 69.4%). In the non‐AIT group (Figure 2), 99.1% of the excluded patients were eliminated due to the lack of an index date.
Table 1.
Demographic and prescription‐related characteristics of the patients in the SLIT and control (non‐AIT) groups at the index date or during the preindex period
| Parameters | SLIT group, n=2851 | non‐AIT group, n=71 275 |
|---|---|---|
| Patient gender (n, %) | ||
| Male | 1070 (37.5%) | 25 286 (35.5%) |
| Female | 984 (34.5%) | 26 585 (37.3%) |
| Unknown | 797 (28.0%) | 19 404 (27.2%) |
| Patient age group (n, %) | ||
| 5‐17 y | 1386 (48.6%) | 5327 (7.5%) |
| 18+ y | 1465 (51.4%) | 65 948 (92.5%) |
| Main prescriber (n, %) | ||
| ENT specialist | 1088 (38.2%) | 26 109 (36.6%) |
| Dermatologist | 522 (18.3%) | 946 (1.3%) |
| Pulmonologist | 197 (6.9%) | 1371 (1.9%) |
| Paediatrician | 631 (22.1%) | 1528 (2.1%) |
| Internal medicine specialist | 67 (2.4%) | 9150 (12.8%) |
| General practitioner | 305 (10.7%) | 31 370 (44.0%) |
| Other speciality | 41 (1.4%) | 801 (1.1%) |
| Asthma status (n, %) | ||
| No asthma | 2191 (76.9%) | 53 718 (75.4%) |
| With asthma | 604 (21.2%) | 14 954 (21.0%) |
| With old asthma (absent for >1 y before the index date) | 56 (2.0%) | 2603 (3.7%) |
| AR prescriptions per patient per year in the preindex period (n=2851; mean±SD, range) | 2.01±1.68 (1‐19) | 3.65±2.83 (1‐73) |
| Asthma prescriptions per patient per year in the preindex period (n=604; mean±SD, range) | 3.38±2.72 (1‐28) | 3.00±2.30 (1‐40) |
AIT, allergy immunotherapy; AR, allergic rhinitis; SLIT, sublingual immunotherapy.
Although the gender distribution was very similar in the two groups, the age profiles at the index date differed markedly. For example, the proportion of under‐18 patients was 48.6% in the SLIT tablet group and 7.5% in the non‐AIT group. Accordingly, the proportion of patients with a paediatrician as the main prescriber was higher in the SLIT tablet group than in the non‐AIT group (22.1% and 2.1%, respectively). Importantly, the proportion of asthma‐free patients at the index date was similar in the SLIT and non‐AIT groups (76.9% and 75.4%, respectively; Table 1).
3.2. Progression of AR after treatment cessation
In both groups, the mean number of AR prescriptions was lower after treatment cessation than during the preindex period. However, the relative decrease in the mean±SD number of AR prescriptions per year was greater in the SLIT tablet group (from 2.01±1.68 to 0.23±0.67, ie, almost a ninefold decrease) than in the non‐AIT group (from 3.65±2.83 to 1.10±1.82, ie, a threefold decrease). After adjustment for covariates, a linear regression analysis of the change in AR prescriptions confirmed that the decrease was significantly greater in the SLIT tablet group (Table 2; regression coefficient [95% confidence interval, CI]=0.188 [0.222‐0.155]; P<.001).
Table 2.
Allergic rhinitis (AR) progression (measured as the intensity of AR medication use) in the SLIT and non‐AIT groups over the follow‐up period: regression coefficients; 95% confidence intervals (CI) and P‐values of the factors included in the linear regression model
| Factors | Regression coefficient | 95% CI | P‐value |
|---|---|---|---|
| Intercept | 0.358 | 0.305‐0.41 | <.001 |
| SLIT treatment (vs control) | −0.188 | −0.222 to −0.155 | <.001 |
| Age <18 y (vs age 18+ y) | −0.127 | −0.145 to −0.11 | <.001 |
| Male (vs unknown) | −0.081 | −0.092 to −0.07 | <.001 |
| Female (vs unknown) | −0.056 | −0.067 to −0.045 | <.001 |
| ENT specialist (vs GP) | −0.037 | −0.047 to −0.028 | <.001 |
| Dermatologist (vs GP) | −0.075 | −0.106 to −0.043 | <.001 |
| Pneumologist (vs GP) | −0.025 | −.055 to 0.005 | .105 |
| Paediatrician (vs GP) | −0.045 | −0.074 to −0.015 | .003 |
| Internal specialist (vs GP) | 0.003 | −0.011 to 0.016 | .690 |
| Other speciality (vs GP) | 0.204 | 0.163‐0.244 | <.001 |
| With asthma before index (vs no asthma) | 0.035 | 0.027‐0.043 | <.001 |
| Number of years SLIT treatment (p.a.) | 0.037 | 0.011‐0.062 | .005 |
AIT, allergen immunotherapy; SLIT, sublingual immunotherapy.
3.3. New asthma onset
In the full analysis period, the proportion of initially asthma‐free patients with new asthma onset was lower in the SLIT tablet group (n=208, 9.5%) than in the non‐AIT group (n=6222, 11.6%). After adjustment for covariates, the odds ratio [95% CI] for new asthma onset evidenced a reduction in the risk of asthma onset in the SLIT tablet group in all three analytical time periods (Table 3). The relative risk reduction was around 30% during treatment and around 40% during follow‐up.
Table 3.
Asthma occurrence in the various time periods (logistic regression for the SLIT tablet group vs the non‐AIT group)
| Factors | Odds ratio | 95% CI | P‐value |
|---|---|---|---|
| Treatment period | |||
| Intercept | 0.063 | 0.044‐0.092 | <.001 |
| SLIT treatment (vs control) | 0.714 | 0.547‐0.932 | .013 |
| Age <18 y (vs age 18+ y) | 1.102 | 0.963‐1.260 | .159 |
| Male (vs unknown) | 0.875 | 0.803‐0.952 | .002 |
| Female (vs unknown) | 1.068 | 0.984‐1.159 | .117 |
| ENT specialist (vs GP) | 0.681 | 0.631‐0.736 | <.001 |
| Dermatologist (vs GP) | 1.054 | 0.838‐1.325 | .654 |
| Pneumologist (vs GP) | 2.898 | 2.314‐3.630 | <.001 |
| Paediatrician (vs GP) | 1.472 | 1.182‐1.832 | .001 |
| Internal specialist (vs GP) | 0.977 | 0.880‐1.084 | .659 |
| Other speciality (vs GP) | 1.125 | 0.837‐1.514 | .435 |
| Number of years SLIT treatment (p.a.) | 0.813 | 0.664‐0.994 | .044 |
| Level of AR treatment before index | 0.977 | 0.965‐0.990 | <.001 |
| Length of individual observation period | 1.752 | 1.569‐1.957 | <.001 |
| Follow‐up period | |||
| Intercept | 0.018 | 0.008‐0.040 | <.001 |
| SLIT treatment (vs control) | 0.575 | 0.372‐0.888 | .013 |
| Age <18 y (vs age 18+ y) | 0.868 | 0.729‐1.033 | .110 |
| Male (vs unknown) | 0.805 | 0.728‐0.890 | <.001 |
| Female (vs unknown) | 0.984 | 0.893‐1.085 | .751 |
| ENT specialist (vs GP) | 0.820 | 0.750‐0.896 | <.001 |
| Dermatologist (vs GP) | 0.742 | 0.530‐1.038 | .082 |
| Pneumologist (vs GP) | 2.136 | 1.550‐2.943 | <.001 |
| Paediatrician (vs GP) | 0.930 | 0.664‐1.302 | .672 |
| Internal specialist (vs GP) | 1.010 | 0.892‐1.143 | .876 |
| Other speciality (vs GP) | 1.282 | 0.924‐1.779 | .138 |
| Number of years SLIT treatment (p.a.) | 0.981 | 0.679‐1.418 | .919 |
| Level of AR treatment before index | 1.014 | 1.000‐1.027 | .049 |
| Length of individual observation period | 1.311 | 1.252‐1.372 | <.001 |
| Full analysis period (treatment+follow‐up) | |||
| Intercept | 0.044 | 0.030‐0.064 | <.001 |
| SLIT treatment (vs control) | 0.696 | 0.552‐0.877 | .002 |
| Age <18 y (vs age 18+ y) | 0.973 | 0.872‐1.085 | .620 |
| Male (vs unknown) | 0.835 | 0.781‐0.893 | <.001 |
| Female (vs unknown) | 1.024 | 0.960‐1.093 | .470 |
| ENT specialist (vs GP) | 0.738 | 0.696‐0.784 | <.001 |
| Dermatologist (vs GP) | 0.905 | 0.746‐1.099 | .313 |
| Pneumologist (vs GP) | 2.655 | 2.181‐3.232 | <.001 |
| Paediatrician (vs GP) | 1.257 | 1.042‐1.517 | .017 |
| Internal specialist (vs GP) | 0.991 | 0.912‐1.076 | .827 |
| Other speciality (vs GP) | 1.178 | 0.937‐1.480 | .161 |
| Number of years SLIT treatment (p.a.) | 1.089 | 0.922‐1.285 | .315 |
| Level of AR treatment before index | 0.998 | 0.989‐1.008 | .720 |
| Length of individual observation period | 1.202 | 1.166‐1.240 | <.001 |
AIT, allergen immunotherapy; AR, allergic rhinitis; SLIT, sublingual immunotherapy.
3.4. Time to asthma onset
The analysis of the time to first prescription of SABAs or ICSs was unclear when comparing the SLIT tablet and non‐AIT groups during the first year of treatment: The Kaplan‐Meier curves for the two groups were essentially superimposed until 10 months after the index date (Figure 3). Thereafter, the curves diverged, and the SLIT tablet group's curve was consistently above that of the non‐AIT group. The difference between the two curves could not be analyzed in a valid way using Cox regression, due to a significant violation of the PH assumption (P=.02 in a supremum test). When the analysis was restricted to the follow‐up period (Figure 4), the PH assumption was not violated significantly (P=.46 in a supremum test), and so Cox regression could be applied. Cox regression over the follow‐up period showed that patients in the SLIT tablet group without asthma at the end of treatment period had a significantly lower risk of developing asthma after treatment cessation, relative to patients in the non‐AIT group (hazard ratio [95% CI]=0.523 [0.341‐0.803], P=.003).
Figure 3.
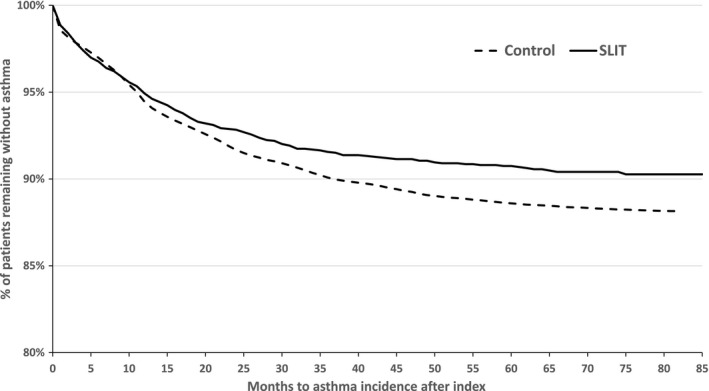
Time to asthma onset, defined as time to the date of first prescriptions of short‐acting β‐agonists or inhaled corticosteroids for sublingual immunotherapy (SLIT) and non‐AIT groups during the full analysis period, in patients without asthma at the index date (note the offset of the y‐axis)
Figure 4.
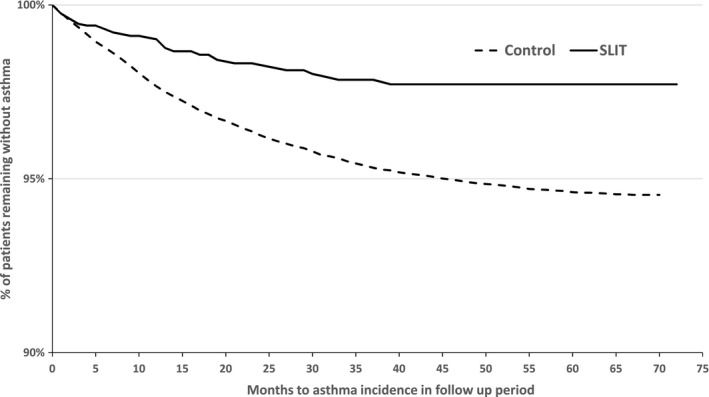
Time to asthma onset, defined as time to the date of first prescriptions of short‐acting β‐agonists or inhaled corticosteroids for sublingual immunotherapy (SLIT) and non‐AIT groups during the follow‐up period, in patients without asthma at the end of treatment period (note the offset of the y‐axis)
3.5. Progression of asthma
In patients with asthma during the preindex period (Table 1), the mean number of asthma prescriptions was slightly higher in the SLIT tablet group (3.38 per year) than in the non‐AIT group (3.00 per year). During the treatment period, the values in the two groups were very similar (Table 4). During the follow‐up period, there were notably fewer asthma prescriptions in the SLIT tablet group (0.68 per year) than in the non‐AIT group (1.13 per year). Lastly, during the full analysis period, the number of prescriptions was slightly lower in the SLIT tablet group. Relative to the preindex period, the fold change in the SLIT tablet group was 1.066 for the treatment period, 0.261 for the follow‐up period, and 0.537 for the full analysis period. The equivalent values for the non‐AIT group were, respectively, 1.180, 0.448, and 0.614.
Table 4.
Asthma progression (measured as the intensity of asthma medication use) in the SLIT and non‐AIT groups over the various time periods
| Asthma prescriptions per patient per annum | Mean±SD | Range | Median [IQR] |
|---|---|---|---|
| Preindex period | |||
| SLIT group | 3.38±2.72 | 1‐28 | 3 [2‐4] |
| Non‐AIT group | 3.00±2.30 | 1‐40 | 2 [1‐4] |
| Treatment period | |||
| SLIT group | 2.97±3.46 | 0‐23.43 | 1.84 [0.66‐4.08] |
| Non‐AIT group | 2.94±2.82 | 0‐45.30 | 2.27 [1.00‐4.14] |
| Follow‐up period | |||
| SLIT group | 0.68±1.63 | 0‐12.99 | 0 [0‐0.53] |
| Non‐AIT group | 1.13±2.32 | 0‐98.83 | 0.41 [0‐1.29] |
| Full analysis period (treatment+follow‐up) | |||
| SLIT group | 1.47±1.94 | 0‐16.59 | 0.78 [0.31‐1.84] |
| Non‐AIT group | 1.55±2.10 | 0‐75.58 | 0.92 [0.41‐1.93] |
AIT, allergy immunotherapy; SLIT, sublingual immunotherapy.
As preceding, data were adjusted for covariates. Once data adjusted, a linear regression showed that the progression of asthma was consistently and significantly slower in the SLIT tablet group (Table 5; treatment period: slope [95% CI]=−0.206 [−0.351 to −0.061], P=.005, vs the non‐AIT group; follow‐up period: slope [95% CI]=−0.167 [−0.279 to −0.055], P=.004; full analysis period: slope [95% CI]=−0.126 [−0.227 to −0.025], P=.014). In patients with asthma during the preindex period, the administration of grass pollen SLIT tablets was associated with a difference in asthma progression (vs non‐AIT patients) of around 20% during the treatment period and around 17% during the post‐treatment period.
Table 5.
Asthma progression (measured as the intensity of asthma medication use) in the SLIT and non‐AIT groups over the follow‐up period: regression coefficients; 95% confidence intervals (CI) and P‐values of the factors included in the linear regression model
| Factors | Regression coefficient | 95% CI | P‐value |
|---|---|---|---|
| Treatment period | |||
| Intercept | 1.319 | 1.090‐1.548 | <.001 |
| SLIT treatment (vs control) | −0.206 | −0.351 to −0.061 | .005 |
| Age <18 y (vs age 18+ y) | 0.012 | −0.060 to 0.085 | .743 |
| Male (vs unknown) | −0.031 | −0.078 to 0.016 | .198 |
| Female (vs unknown) | −0.029 | −0.075 to 0.016 | .202 |
| ENT specialist (vs GP) | −0.202 | −0.246 to −0.158 | <.001 |
| Dermatologist (vs GP) | −0.101 | −0.256 to 0.055 | .205 |
| Pneumologist (vs GP) | 0.246 | 0.167‐0.325 | <.001 |
| Paediatrician (vs GP) | 0.039 | −0.066 to 0.145 | .465 |
| Internal specialist (vs GP) | −0.014 | −0.069 to 0.042 | .633 |
| Other speciality (vs GP) | 0.004 | −0.155 to 0.164 | .958 |
| Number of years SLIT treatment (p.a.) | 0.009 | −0.103 to 0.121 | .876 |
| Level of AR treatment before index | −0.025 | −0.031 to −0.018 | <.001 |
| Follow‐up period | |||
| Intercept | 0.349 | 0.172‐0.526 | .000 |
| SLIT treatment (vs control) | −0.167 | −0.279 to −0.055 | .004 |
| Age <18 y (vs age 18+ y) | −0.171 | −0.227 to −0.115 | <.001 |
| Male (vs unknown) | −0.108 | −0.144 to −0.072 | <.001 |
| Female (vs unknown) | −0.088 | −0.123 to −0.053 | <.001 |
| ENT specialist (vs GP) | −0.047 | −0.081 to −0.013 | .007 |
| Dermatologist (vs GP) | −0.174 | −0.294 to −0.053 | .005 |
| Pneumologist (vs GP) | 0.005 | −0.057 to 0.066 | .881 |
| Paediatrician (vs GP) | −0.003 | −0.084 to 0.079 | .951 |
| Internal specialist (vs GP) | 0.005 | −0.038 to 0.048 | .818 |
| Other speciality (vs GP) | 0.036 | −0.088 to 0.159 | .570 |
| Number of years SLIT treatment (p.a.) | 0.091 | 0.005‐0.178 | .039 |
| Level of AR treatment before index | 0.004 | −0.001 to 0.009 | .082 |
| Full analysis period (treatment+follow‐up) | |||
| Intercept | 0.446 | 0.286‐0.605 | <.001 |
| SLIT treatment (vs control) | −0.126 | −0.227 to −0.025 | .014 |
| Age <18 y (vs age 18+ y) | −0.145 | −0.196 to −0.094 | <.001 |
| Male (vs unknown) | −0.096 | −0.129 to −0.063 | <.001 |
| Female (vs unknown) | −0.080 | −0.112 to −0.049 | <.001 |
| ENT specialist (vs GP) | −0.078 | −0.109 to −0.047 | <.001 |
| Dermatologist (vs GP) | −0.161 | −0.269 to −0.053 | .004 |
| Pneumologist (vs GP) | 0.058 | 0.003‐0.114 | .038 |
| Paediatrician (vs GP) | 0.004 | −0.070 to 0.077 | .923 |
| Internal specialist (vs GP) | −0.002 | −0.041 to 0.036 | .909 |
| Other speciality (vs GP) | 0.032 | −0.079 to 0.143 | .574 |
| Number of years SLIT treatment (p.a.) | 0.132 | 0.054‐0.210 | .001 |
| Level of AR treatment before index | 0.000 | −0.004 to 0.005 | .963 |
AIT, allergen immunotherapy; AR, allergic rhinitis; SLIT, sublingual immunotherapy.
4. DISCUSSION
In the SLIT tablet group, the intensity of AR treatment with symptomatic medications decreased after AIT initiation. This finding is in line with the reduction in medication scores generally observed in DBPC RCTs of grass pollen SLIT tablet.17 However, the absolute reductions in medication use seen in DBPC RCTs and in dataset analyses cannot be compared directly.
As mentioned in the Introduction, a few clinical studies have investigated the preventive effect of AIT on asthma onset in AR patients.24, 25, 26, 27, 28, 29, 30 Novembre et al.27 randomly assigned 113 children with grass pollen‐induced AR to either 3 years of SLIT or 3 years of standard pharmacotherapy alone. At the end of the treatment period, the proportion of patients having developed asthma was significantly lower (P=.0412) in the SLIT group (18%) than in the pharmacotherapy‐only group (40%). Similarly, Marogna et al.28 assigned 216 children with AR to either 3 years of SLIT or 3 years of pharmacotherapy alone. Again, the proportion of patients with asthma after 3 years was significantly lower in the SLIT group than in the pharmacotherapy‐only group (1.5% vs 29% for persistent asthma; P<.001).
The results of the PAT study showed that a 3‐year course of SCIT with standardised allergen (grass pollen and birch) in children was associated with a significantly lower incidence of asthma (relative to symptomatic medication alone) at the end of the treatment period and even 5 and 7 years after treatment cessation.24, 25, 26 In contrast, the GAP study in children failed to observe a relative reduction in the time to diagnosis of a reversible impairment of lung function.29 Nevertheless, there was a clinically meaningful treatment effect on asthma symptoms in patients having developed the condition. Similarly, Schmitt et al.'s recent retrospective cohort analysis of a German regional prescriptions database showed that AIT (all types pooled) decreased the incidence of asthma in patients with AR30 in a population of both children and adults. Our database analysis provides similar outcomes in a population of adults and children.
This therapeutic area has been addressed in various meta‐analyses and systematic reviews; such studies provide unique insights into the comparative effectiveness of a therapeutic intervention (eg, AIT) vs a control (eg, placebo or other pharmacological treatments). One recent meta‐analysis found a low level of evidence to support the concept whereby AIT prevents the onset of new allergen sensitisations, and another found that AIT did not result in a statistically significant reduction in the risk of developing a first allergic disease.37, 38 However, it must be borne in mind that (i) these analyses may not be able to draw valid conclusions when the data show extremely high clinical and methodological heterogeneity and (ii) not all the studies included in these systematic reviews had the same primary outcome.
4.1. Study limitations and strengths
The present study had some limitations.
Firstly, the LRx prescription database lacked direct, clinical information such as the diagnostic methodology and the sensitisation status, but as per SmPCs of both tablets, SLIT tablets can be prescribed if grass pollen allergy is “confirmed by a positive cutaneous test and/or a positive titer of the specific IgE to the grass pollen.” Furthermore, not reporting sensitization status in the database would not impact the results of the study as both tablets showed a similar treatment effect in mono‐ and polysensitised patients. Some of the patients included in the control non‐AIT group may have suffered from AR induced by an allergen other than grass pollen (ie, an allergen whose season overlapped with that of grass pollen). However, given the predominance of grass pollen AR, this source of bias would have been minimized. Besides, ATC drug class codes were used as proxies for the diagnosis and treatment of AR and asthma. This approach can be a concern when drugs in the class have multiple indications (eg, in both COPD and asthma). The choice of INSs as the AR‐specific drug class eliminated the risk of including patients not suffering from AR. The LRx database is limited to reimbursed prescriptions, and so OTC medications were not tracked. However, around 90% of INS packs delivered in Germany are prescribed (source: Pharma Scope, IMS Health), which should have minimized bias. In contrast, only 35% of oral antihistamine packs in Germany are delivered with a prescription; this would lead to underestimation of the intensity of AR treatment. However, there is no reason to believe that this potential bias would have affected the SLIT tablet group more than the non‐AIT group. Furthermore, all antihistamine prescriptions for children up to and including the age of 12 are reimbursed in Germany. Given that the proportion of young patients was greater in the SLIT tablet group, any bias would have led to underestimation (and not overestimation) of SLIT's effect.
Secondly, the two‐grass pollen SLIT tablet formulations analyzed here differ in their allergen composition and recommended regimen. Oralair® contains pollen extract from five species of grass, whereas Grazax® contains timothy pollen extract only. A three‐season pre‐ and co‐seasonal regimen is recommended for Oralair®, whereas 3 years of continuous treatment is recommended for Grazax®. Future research in this field could compare the two tablets.
Thirdly, the SLIT tablet and non‐AIT groups were only matched for the treatment index year and so differed in some important respects (reflecting real life). The proportion of children and adolescents was markedly higher in the SLIT tablet group, as observed in an earlier German observational study;30 these observations suggest that physicians in Germany are more likely to prescribe SLIT tablets to younger patients. Overall, specialist physicians accounted for a higher proportion of main prescribers in the SLIT tablet group than in the non‐AIT group; this is not unexpected, as the prescription of SLIT requires experience in allergology. In Germany, allergology is an additional medical qualification (gained typically by dermatologists, ENT specialists, and pulmonologists), rather than being a separate medical speciality per se. The higher proportion of paediatricians as main prescribers in the SLIT tablet group reflected the age difference. As part of the allergic march, allergic asthma tends to emerge more frequently in childhood and adolescence than in adulthood.13 Hence, one would expect to see more asthma onset in a younger population. However, patient age was one of the covariates controlled for in our analyses and did not influence asthma onset. Furthermore, any bias due to patient age in the present study would tend to reduce the effect of SLIT and not increase it. Accordingly, the significant differences in favour of SLIT observed here are likely to be genuine, and the true, underlying effect may be greater still.
Lastly, regarding asthma definition, as mentioned in the method section, depot formulations of systemic corticosteroids are used to measure asthma progression. A sensitivity analysis excluding those was performed and did not impact the results.
The present study also had a number of strengths—the most important of which is its use of real‐world data. After a medication has been granted marketing authorization, the reimbursement authorities increasingly request evidence of real‐world effectiveness for confirming the efficacy assessed in RCTs. The patient populations in real‐world data analyses are more representative than those in RCTs. Furthermore, the present study enabled us to assess clinically relevant endpoints and long‐term benefits and to compare an intervention with a standard of care—as observed in real life. Nationwide databases constitute an intervention‐free source of real‐world data from a broad population; as such, they are increasingly taken into account by health technology assessment agencies.
The present analysis was based on 2851 patients receiving SLIT tablets and 71 275 patients with AR due to grass pollen, with up to 8 years of real‐life data. This constitutes a large sample in a major European country and enabled a comparison of SLIT and the standard of care with regard to pragmatic endpoints reflecting long‐term benefit. As the effect size indicates a potential reduction of 18.8% for the AR progression, 42.5% of asthma occurrence, and 16.7% of asthma progression after treatment cessation, we consider the results as of clinical meaningful. This result also showed economic impact. The present results confirm and reinforce clinical outcomes and provided an assessment of additional benefits for asthma that had previously been investigated in specific populations in controlled environments (RCTs). The present findings are also in line with the results of similar observational studies that (with one exception30) were based only on a few hundred patients.
5. CONCLUSION
The present real‐life retrospective analysis is the first to have analyzed a database large enough to allow an assessment of the benefits of a treatment group as small as grass pollen SLIT tablets (with only two products currently on the market). Earlier studies were based on much smaller patient counts or failed to include enough patients taking this specific SLIT formulation. The present findings indicate the overall long‐term clinical value of SLIT tablets by showing a post‐treatment effect of at least 2 years of grass pollen SLIT tablets (relative to symptomatic medication alone). This may be translated into clinical practice as a slower progression of AR, a preventive effect on asthma (with a reduced risk of new asthma onset in nonasthmatic population and a slower asthma progression in the asthmatic population) in routine use.
5.1. Future research
Using the same dataset, future research could focus on the real‐life, long‐term impact of each of the two SLIT tablets (Oralair® and Grazax®) on AR and asthma. The number of patients included in the present study should be sufficient for assessing the long‐term effect on AR. However, larger samples sizes would be required for reliable assessment of the impact on asthma (ie, new asthma onset in initially asthma‐free patients, and the progression of asthma in patients with current asthma). Given that Oralair® and Grazax® have different administration protocols, exposure could be considered as a variable in a specific analysis. It would also be interesting to assess the impact of grass pollen SLIT tablets on conjunctivitis during treatment and after cessation. Lastly, the same methodology could be applied to studies of SLIT with other allergen sources (eg, birch pollen).
CONFLICT OF INTEREST
Stefan Zielen received fees for lectures and advisory boards from the following companies: IMS Health GmbH & Co. OHG (www.imshealth.com), Bene Arzneimittel GmbH (http://www.bene-arzneimittel.de), Vifor Pharma Deutschland GmbH (http://www.viforpharma.de), Novartis AG (https://www.novartis.com), GlaxoSmithKline GmbH (http://www.glaxosmithkline.de), ALK‐Abelló Arzneimittel GmbH (http://www.alk-abello.com), Allergy Therapeutics (http://www.allergytherapeutics.com), Boehringer Ingelheim (https://www.boehringer-ingelheim.de), Allergopharma GmbH (https://www.allergopharma.de), and Biotest (https://www.biotest.com). Philippe Devillier received fees for lectures and advisory boards from the following companies: ALK‐Abelló, Astra Zeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, IMS Health GmbH & Co, Meda Pharma, Menarini, Novartis, Nycomed‐Takeda, Sandoz, Stallergenes Greer. Joachim Heinrich received consulting fees from IMS, Kabi. Hartmut Richter is an employee of QuintilesIMS. Ulrich Wahn has received consulting fees from Allergopharma, Danone, Hipp, Merck, Novartis, and Stallergenes Greer; honoraria for lectures from ALK‐Abelló, Allergopharma, Allergy Therapeutics, LETI, MSD, Nestlé, Novartis, Nutricia, and Stallergenes Greer; and research funding from Stallergenes Greer.
AUTHOR CONTRIBUTIONS
Stefen Zielen, Philippe Devillier, Joachim Heinrich, Hartmut Richter, and Ulrich Wahn all made substantial contributions to conception and design of the study and acquisition, analysis, and/or interpretation of data. They all contributed to drafting the article or revising it critically for important intellectual content and have given their final approval of the version submitted for consideration for publication.
Supporting information
ACKNOWLEDGMENTS
We thank David Fraser PhD (Biotech Communication SARL, France) for copyediting assistance.
Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long‐term relief in allergic rhinitis and reduces the risk of asthma: A retrospective, real‐world database analysis. Allergy. 2018;73:165–177. https://doi.org/10.1111/all.13213
Edited by: De Yun Wang
REFERENCES
- 1. Ozdoganoglu T, Songu M. The burden of allergic rhinitis and asthma. Ther Adv Respir Dis. 2012;6:11‐23. [DOI] [PubMed] [Google Scholar]
- 2. EFA Book on Respiratory Allergies raise awareness, relieve the burden; Edited by Erkaa Valovirta. http://www.theipcrg.org/download/attachments/689587/EFABookonRespiratoryAllergiesFINAL.pdf?version=1%26modificationDate=1332965739000. Accessed April 23, 2017 [Google Scholar]
- 3. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(Suppl 2):S1‐S84. [DOI] [PubMed] [Google Scholar]
- 4. Meltzer EO, Blaiss MS, Naclerio RM, et al. Burden of allergic rhinitis: allergies in America, Latin America, and Asia‐Pacific adult surveys. Allergy Asthma Proc. 2012;33(Suppl 1):S113‐S141. [DOI] [PubMed] [Google Scholar]
- 5. Nathan RA, Meltzer EO, Derebery J, et al. The prevalence of nasal symptoms attributed to allergies in the United States: findings from the burden of rhinitis in an America survey. Allergy Asthma Proc. 2008;29:600‐608. [DOI] [PubMed] [Google Scholar]
- 6. Bachert C, van Cauwenberge P, Olbrecht J, van Schoor J. Prevalence, classification and perception of allergic and nonallergic rhinitis in Belgium. Allergy. 2006;61:693‐698. [DOI] [PubMed] [Google Scholar]
- 7. Lamb CE, Ratner PH, Johnson CE, et al. Economic impact of workplace productivity losses due to allergic rhinitis compared with select medical conditions in the United States from an employer perspective. Curr Med Res Opin. 2006;22:1203‐1210. [DOI] [PubMed] [Google Scholar]
- 8. Blaiss MS. Allergic rhinitis in schoolchildren consensus G. Allergic rhinitis and impairment issues in schoolchildren: a consensus report. Curr Med Res Opin. 2004;20:1937‐1952. [DOI] [PubMed] [Google Scholar]
- 9. Walker S, Khan‐Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case‐control study. J Allergy Clin Immunol. 2007;120:381‐387. [DOI] [PubMed] [Google Scholar]
- 10. Simons FE. Allergic rhinobronchitis: the asthma‐allergic rhinitis link. J Allergy Clin Immunol. 1999;104:534‐540. [DOI] [PubMed] [Google Scholar]
- 11. Greiner AN, Hellings PW, Rotiroti G, Scadding GK. Allergic rhinitis. Lancet. 2011;378:2112‐2122. [DOI] [PubMed] [Google Scholar]
- 12. Van Bever HP, Samuel ST, Lee BW. Halting the allergic march. World Allergy Organ J. 2008;1:57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shaker M. New insights into the allergic march. Curr Opin Pediatr. 2014;26:516‐520. [DOI] [PubMed] [Google Scholar]
- 14. Settipane RJ, Hagy GW, Settipane GA. Long‐term risk factors for developing asthma and allergic rhinitis: a 23‐year follow‐up study of college students. Allergy Proc. 1994;15:21‐25. [DOI] [PubMed] [Google Scholar]
- 15. Pariente PD, LePen C, Los F, Bousquet J. Quality‐of‐life outcomes and the use of antihistamines in a French national population‐based sample of patients with perennial rhinitis. Pharmacoeconomics. 1997;12:585‐595. [DOI] [PubMed] [Google Scholar]
- 16. Leynaert B, Bousquet J, Neukirch C, Liard R, Neukirch F. Perennial rhinitis: an independent risk factor for asthma in nonatopic subjects: results from the European Community Respiratory Health Survey. J Allergy Clin Immunol. 1999;104:301‐304. [DOI] [PubMed] [Google Scholar]
- 17. Didier A, Wahn U, Horak F, Cox LS. Five‐grass‐pollen sublingual immunotherapy tablet for the treatment of grass‐pollen‐induced allergic rhinoconjunctivitis: 5 years of experience. Expert Rev Clin Immunol. 2014;10:1309‐1324. [DOI] [PubMed] [Google Scholar]
- 18. Calamita Z, Saconato H, Pela AB, Atallah AN. Efficacy of sublingual immunotherapy in asthma: systematic review of randomized‐clinical trials using the Cochrane Collaboration method. Allergy. 2006;61:1162‐1172. [DOI] [PubMed] [Google Scholar]
- 19. Canonica GW, Passalacqua G. Sublingual immunotherapy in the treatment of adult allergic rhinitis patients. Allergy. 2006;61(Suppl 81):20‐23. [DOI] [PubMed] [Google Scholar]
- 20. Erny‐Albrecht K, Valentine W, Christensen J, Vestenbaek U, Palmer AJ. Sublingual immunotherapy in allergic rhinitis and asthma: a review of recent clinical evidence. J Appl Res. 2007;7:17‐31. [Google Scholar]
- 21. Incorvaia C, Di Rienzo A, Celani C, Makri E, Frati F. Treating allergic rhinitis by sublingual immunotherapy: a review. Ann Ist Super Sanita. 2012;48:172‐176. [DOI] [PubMed] [Google Scholar]
- 22. Aboshady OA, Elghanam KM. Sublingual immunotherapy in allergic rhinitis: efficacy, safety, adherence and guidelines. Clin Exp Otorhinolaryngol. 2014;7:241‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dranitsaris G, Ellis AK. Sublingual or subcutaneous immunotherapy for seasonal allergic rhinitis: an indirect analysis of efficacy, safety and cost. J Eval Clin Pract. 2014;20:225‐238. [DOI] [PubMed] [Google Scholar]
- 24. Niggemann B, Jacobsen L, Dreborg S, et al. Five‐year follow‐up on the PAT study: specific immunotherapy and long‐term prevention of asthma in children. Allergy. 2006;61:855‐859. [DOI] [PubMed] [Google Scholar]
- 25. Moller C, Dreborg S, Ferdousi HA, et al. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT‐study). J Allergy Clin Immunol. 2002;109:251‐256. [DOI] [PubMed] [Google Scholar]
- 26. Jacobsen L, Niggemann B, Dreborg S, et al. Specific immunotherapy has long‐term preventive effect of seasonal and perennial asthma: 10‐year follow‐up on the PAT study. Allergy. 2007;62:943‐948. [DOI] [PubMed] [Google Scholar]
- 27. Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851‐857. [DOI] [PubMed] [Google Scholar]
- 28. Marogna M, Tomassetti D, Bernasconi A, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008;101:206‐211. [DOI] [PubMed] [Google Scholar]
- 29. Valovirta E, Cronjager R, Petersen T, et al. Top‐line results from the five‐year landmark GRAZAX® Asthma Prevention (GAP) trial in children.. EAACI 2016 Conference; June 13, 2016; Vienna, Austria.: Allergy; 2016:98‐99.
- 30. Schmitt J, Schwarz K, Stadler E, Wustenberg EG. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: results from a large retrospective cohort study. J Allergy Clin Immunol. 2015;136:1511‐1516. [DOI] [PubMed] [Google Scholar]
- 31. Richter H, Dombrowski S, Hamer H, Hadji P, Kostev K. Use of a German longitudinal prescription database (LRx) in pharmacoepidemiology. Ger Med Sci. 2015;13:Doc14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Senna GE, Calderon M, Milani M. Allergy immunotherapy tablet: Grazax(R) for the treatment of grass pollen allergy. Expert Rev Clin Immunol. 2011;7:21‐27. [DOI] [PubMed] [Google Scholar]
- 33. Nelson H, Lehmann L, Blaiss MS. Treatment of seasonal allergic rhinoconjunctivitis with a once‐daily SQ‐standardized grass allergy immunotherapy tablet. Curr Med Res Opin. 2012;28:1043‐1051. [DOI] [PubMed] [Google Scholar]
- 34. Koberlein J, Mosges R. Oralair((R)): a causal treatment for grass pollen‐induced allergic rhinoconjunctivitis. Immunotherapy. 2013;5:13‐21. [DOI] [PubMed] [Google Scholar]
- 35. Stiftung Deutscher Informationsdienst . Indication of flowering periods of various allergenic pollen types in Germany 2007‐2011. 2011. http://www.pollenstiftung.de/pollenvorhersage/pollenflug-kalender/. Accessed December 18, 2015.
- 36. Global Initiative for Asthma . Global strategy for asthma management and prevention. 2015. www.ginasthma.org. Accessed December 18, 2015.
- 37. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Macchia L, Di Lorenzo G. Efficacy of allergen immunotherapy in reducing the likelihood of developing new allergen sensitizations: a systematic review. Allergy. 2017; 72:691‐704. [DOI] [PubMed] [Google Scholar]
- 38. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta‐analysis. Pediatr Allergy Immunol. 2017;28:18‐29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


