Abstract
Background
A relevant proportion of allergic rhinoconjunctivitis (ARC) patients experience recurrent symptoms after successfully completing allergen immunotherapy (AIT). This prospective, controlled, noninterventional study used internationally standardized instruments to determine the clinical effects of a preseasonal, ultra‐short‐course booster AIT on clinical outcome parameters.
Methods
This two‐arm study included patients aged ≥12 years with recurrent grass pollen‐induced seasonal AR who had completed a successful course of any grass pollen AIT at least 5 years before enrolment. Overall, 56 patients received one preseasonal short‐course booster AIT using tyrosine‐absorbed grass pollen allergoids containing the adjuvant monophosphoryl lipid A (MPL ®); 51 control patients received symptomatic medication. The combined symptom and medication score (CSMS) was recorded in the (peak) grass pollen season. Furthermore, concomitant (antiallergic) medication use, the patients’ state of health, Mini Rhinoconjunctivitis Quality of Life Questionnaire (MiniRQLQ) results and safety/tolerability of the treatment were assessed.
Results
The CSMS in the peak grass pollen season was significantly lower in the booster AIT group (Δ=38.4%, P<.01). Moreover, significantly more patients in this group used no concomitant antiallergic medication throughout the peak grass pollen season. Twice as many patients in the booster AIT group as in the control group reported having a better state of health than in the preceding season. MiniRQLQ results showed significant differences favouring the booster AIT. The booster AIT was generally well tolerated, with only two patients reporting mild, grade 1 systemic adverse events.
Conclusion
Booster AIT using tyrosine‐absorbed allergoids containing the adjuvant MPL ® effectively prevents re‐occurrence of symptoms in patients with grass pollen‐induced ARC.
Keywords: booster, combined symptom and medication score, grass pollen allergy, revaccination, subcutaneous allergen immunotherapy
Abbreviations
- AE
adverse event
- AIT
allergen immunotherapy
- ARC
allergic rhinoconjunctivitis
- CSMS
combined symptom and medication score
- dMS
daily medication score
- dSS
daily symptom score
- EAACI
European Academy of Allergy and Clinical Immunology
- MiniRQLQ
Mini Rhinoconjunctivitis Quality of Life Questionnaire
- MPL
monophosphoryl lipid A
- RCSS
rhinoconjunctivitis symptom score
- RQLQ
Rhinoconjunctivitis Quality of Life Questionnaire
- SAE
severe adverse event
- SCIT
subcutaneous immunotherapy
- SLIT
sublingual immunotherapy
1. INTRODUCTION
Allergen immunotherapy (AIT) constitutes the only disease‐modifying treatment option for patients with IgE‐mediated allergic diseases.1, 2, 3, 4 Several clinical trials and meta‐analyses have yielded clear evidence for the clinical efficacy and safety of both sublingual (SLIT) and subcutaneous (SCIT) immunotherapy.5, 6, 7 However, conventional SCIT treatment still requires up to 90 injections over 3‐5 years.8, 9 For patients enrolled in conventional SCIT treatments, the withdrawal and noncompliance rates were found to be quite high, ranging from 11% to 50%.10 Among the causes for withdrawal, SCIT patients reported inconvenience as being one of the most relevant disadvantages of this form of AIT.
Nevertheless, SCIT is a highly effective treatment for both intermittent and persistent allergic rhinitis 1, 11 and has also been shown to have long‐term clinical benefits for several years after completion.12, 13, 14 In addition, new developments in SCIT have recently been made (eg, by making molecular modifications or by using novel adjuvant‐containing preparations of vaccines), aiming to optimize its clinical efficacy and safety.15, 16, 17 One example of a successfully used adjuvant is monophosphoryl lipid A (MPL®), which is a detoxified, attenuated form of the lipid A component of the lipopolysaccharide of Salmonella minnesota.18 Use of this adjuvant aims to enhance the immunological effects of SCIT, such as augmenting the pro‐tolerogenic Treg as well as Th1 response, and therefore to shorten the whole treatment course in favour of a preseasonal short schedule of four injections only.9 The clinical efficacy of this ultra‐short‐course SCIT has been broadly demonstrated in several pivotal trials in pollen‐allergic patients.19, 20, 21, 22, 23
Although many aspects of SCIT have been thoroughly investigated and AIT vaccines have been optimized throughout recent decades,2, 3, 5 a relevant proportion of patients still experience recurrent symptoms in subsequent years after cessation of AIT even though they initially reported clinical improvement.24, 25 However, only two older studies have indeed evaluated the efficacy of a new course of SCIT (revaccination) in patients with recurrent pollen allergy.24, 26 Although these trials have demonstrated the clinical benefit of revaccination, there is a clear unmet need to assess its clinical efficacy using internationally standardized instruments to determine the effect on clinical outcome parameters such as those proposed by a Task Force of the European Academy of Allergy and Clinical Immunology (EAACI).27
This prospective, controlled, noninterventional study therefore aimed to investigate the clinical effects of a preseasonal, ultra‐short‐course booster AIT using tyrosine‐absorbed grass pollen allergoids containing the adjuvant MPL®. The study planned to include adolescent and adult patients with recurrent grass pollen‐induced seasonal allergic rhinoconjunctivitis and to assess state‐of‐the‐art clinical endpoints in AIT trials. All patients had to have completed a successful course of any AIT for grass pollen at least five years before study enrolment.
2. MATERIALS AND METHODS
2.1. Study design
The study was designed as a prospective, controlled, nonrandomized, patient‐preference, noninterventional study and planned to include 150 patients: 100 patients choosing booster AIT plus symptomatic medication during the grass pollen season as required and 50 control patients choosing only symptomatic treatment in approximately 20 study centres across Germany. The study was scheduled to be conducted from March to September 2015, thus during the peak of the grass pollen season. The study was carried out in accordance with the German Medicinal Products Act (Arzneimittelgesetz, AMG), Section 67 subsection 6. After counselling on professional regulations, it was approved by the competent ethics committee of the state of Hesse in Frankfurt, Germany, and registered under the number FF 10/2015. The study was further registered with ClinicalTrials.gov under the registry number NCT02579720.
2.2. Participants
Eligible patients were adults and adolescents aged 12 years or older with recurrent grass pollen‐induced allergic rhinoconjunctivitis (ARC) who had completed AIT (SLIT or SCIT; any product) that had successfully reduced the symptoms in the patients at least five years prior to enrolment. Patients with grass pollen‐related ARC who had not previously received AIT with a grass pollen extract or who had completed such an AIT course less than five years before enrolment in the study were not included. Patients with coexisting perennial and seasonal allergies were enrolled in the study if these allergies did not cause any symptoms during the season observed.
2.3. Study treatments
All participants were allowed to choose their preferred treatment option at Visit 1 before the onset of the grass pollen count when they sought medical advice in the investigators’ practices because of their recurrent allergy symptoms in the previous season. Thus, one group (“booster AIT group”) received four preseasonal injections of the AIT (POLLINEX® Quattro Plus 1.0 mL; Bencard® Allergie GmbH, Munich, Germany) at the standard application doses of 300 SU, 800 SU and 2000 SU (twice) according to the Summary of Product Characteristics 28 plus symptomatic medication during the grass pollen season as required. The booster AIT group completed a total of seven visits, which included four preseasonal visits at which the booster injections were administered, followed by three visits at the beginning, during the peak and at the end of the grass pollen season, respectively (Figure 1).
Figure 1.
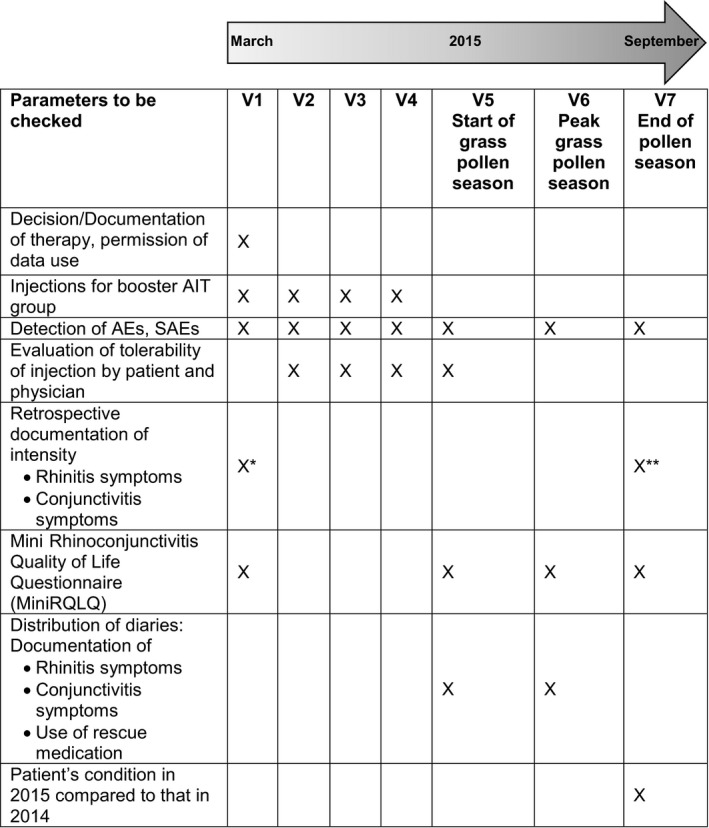
Study overview. AIT, allergen immunotherapy; AE, adverse event; SAE, severe adverse event. *Intensity of symptoms in 2014. **Intensity of symptoms in 2015
The other group (“control group”) received exclusively symptomatic medication during the grass pollen season. Patients in this group, alongside the inclusion Visit 1, completed three visits during the grass pollen season and therefore had a total of four visits (Figure 1).
The following rescue medication was provided to the patients free of charge via an Internet pharmacy upon request: advanced, second‐generation, nonsedative oral antihistamines, intranasal glucocorticosteroids and oral glucocorticosteroids. In cases of non‐controlled asthma, combinations of inhaled long‐acting β2‐agonists and glucocorticosteroids were prescribed.
2.4. Peak pollen period
The regional peak pollen period was defined as those 30 consecutive days per centre with the highest local grass pollen counts, starting with the first onset of moderate‐to‐high pollen concentrations (stages 2‐3 according to the German Weather Service, Medicine Meteorology [Deutscher Wetterdienst Medizin‐Meteorologie], http://www.dwd.de) in the nearest pollen count station in that region. The symptom data and medication use data of these centre‐specific, 30‐day periods were matched for all patients.
2.5. Instruments and assessments
The diary provided data for a total of three scores: the daily symptom score (dSS), the daily medication score (dMS) and the combined symptom and medication score (CSMS).27 The dSS included the patients’ daily ratings of six rhinoconjunctivitis symptoms: sneezing, runny nose, itchy nose, blocked nose, itchy eyes and watery eyes on a scale from 0 to 3, where 0=“no symptoms,” 1=“mild symptoms,” 2=“moderate symptoms” and 3=“severe symptoms.” For each patient, all six single scores were added together, yielding the daily rhinoconjunctivitis symptom score (RCSS). The dMS calculated the daily intake of symptomatic medication for each patient for 30 continuous days during the peak of the grass pollen season, yielding a final score of 1 to 3 (1=“intake of oral antihistamines,” 2=“use of steroidal nasal spray alone or in combination with oral antihistamines” and 3=“intake of steroidal tablets alone or in combination with steroidal nasal spray and/or oral antihistamines”).
Finally, the dSS and the dMS were added together to obtain a daily CSMS with a maximum score of 6:
Additionally, patients from both study groups were asked to rank the intensity of their allergy complaints on a scale from 0 to 3, where 0=“no symptoms,” 1=“mild symptoms,” 2=“moderate symptoms” and 3=“severe symptoms” at Visit 1 (for symptoms in 2014, before the trial) and Visit 7 (for symptoms in 2015, during the trial).29
To evaluate the clinical outcome of the tested ultra‐short‐course AIT, any symptomatic medication use in both groups was documented for the grass pollen season in the year before and during the study. This scale ranged from 0 to 4, where 0=“no use,” 1=“rare use,” 2=“<4 weeks’ use,” 3=“≥4 weeks’ use” and 4=“constant use.”
At the last visit (Visit 7), the state of health of the patients in both study groups was evaluated by patients and investigators individually using a scale from 1 to 4, where 1=“significantly better,” 2=“slightly better,” 3=“unchanged” and 4=“worse,” comparing the year before AIT treatment and the year of the AIT treatment.30
Furthermore, patients in both study groups evaluated their quality of life at Visits 1, 5, 6 and 7 using the Mini Rhinoconjunctivitis Quality of Life Questionnaire (MiniRQLQ) according to Juniper.31 The questionnaire consisted of 14 questions on possible interferences caused by the ARC with regard to work or leisure time and health conditions. Patients assessed how greatly they felt affected by each impairment on a scale from 0 to 6, where 0=“not troubled,” 1=“hardly troubled at all,” 2=“somewhat troubled,” 3=“moderately troubled,” 4=“quite a bit troubled,” 5=“very troubled” to 6=“extremely troubled.”
The tolerability of each single injection was rated by the patients in the booster AIT group and the investigators individually on a scale from 1 to 4, where 1=“very good,” 2=“good,” 3=“moderate” and 4=“poor” after each injection visit.
Adverse events (AEs) and serious adverse events (SAEs) were recorded at each visit and included the diagnosis, the beginning and end of the events, their frequency, their intensity and recovery from such events. The investigator had to decide whether the AE was an SAE, which was defined as death or a life‐threatening event, persistent or significant disability, congenital anomaly or birth defect, patient hospitalization or important medical conditions such as anaphylactic shocks.
2.6. Statistical analysis
Data entry and analysis were carried out using the statistical analysis program SPSS version 23 (IBM Corp., Armonk, NY, USA). Demographic and other baseline characteristics as well as all efficacy and safety variables were analysed using descriptive statistics for each treatment group. Continuous data were described by the number of valid or missing values, mean, median, standard deviation, minimum and maximum. Categorical data were expressed as absolute or percentage frequency. Scores for ordinal variables were considered as continuous. Group comparisons of continuous outcomes and factors were performed using a t test if these followed a normal distribution, or the Mann‐Whitney U test if they did not. Results were considered statistically significant if P<.05. With regard to the main parameter CSMS, the patients’ diaries were only considered if the participants had evaluated at least 20% of the 30 days of the peak pollen season. In the case of missing values, the biometric techniques last value carried forward (LVCF) or first value carried backwards (FVCB) were applied to complete the data set.
3. RESULTS
3.1. Study population
The planned sample size of 150 participants was not reached due to the high specificity of the inclusion criteria: patients had to have completed an AIT at least five years prior to enrolment and had to have experienced a reduction in grass allergy symptoms thereafter, but before enrolment had to have reported suffering from recurrent symptoms. At the end, however, 107 patients (71.3% of the planned sample size) were included in this prospective, controlled, nonrandomized, noninterventional patient‐preference study. Of these, 56 patients (52.3%) chose to receive preseasonal subcutaneous injections and, if needed, to use additional symptomatic medications during the pollen season, and 51 patients (47.7%) chose to use exclusively symptomatic medication.
The study groups comprised a total of 57 female and 50 male individuals aged 15‐75 years. The booster AIT group included 3.6% more male than female patients, whereas in the control group, the female population was 17.6% greater than the male one (Table 1). The mean age was 40.46 years in the booster AIT group and 38.02 years in the control group (Table 1). Regarding the allergy status of the patients, 50.0% of all booster AIT patients and 49.0% of the control patients were monosensitized to grass pollen.
Table 1.
Demographic data
| Total | Treatment | ||
|---|---|---|---|
| Booster AIT Group | Control Group | ||
| Age, y | |||
| N | 107 | 56 | 51 |
| Mean±SD | 39.30±14.87 | 40.46±14.28 | 38.02±15.53 |
| Median (IQR 25‐75) | 39 (28‐49) | 40 (32‐49) | 34 (26‐49) |
| Sex, N (%) | |||
| Female | 57 (53.3%) | 27 (48.2%) | 30 (58.8%) |
| Male | 50 (46.7%) | 29 (51.8%) | 21 (41.2%) |
AIT, allergen immunotherapy; N, number of patients; SD, standard deviation; IQR, interquartile range.
The two study groups were compared with respect to symptomatic medication use and the intensity of allergy symptoms in the year before this study. Symptomatic medication use was almost identical in both groups: 96.2% of the patients in the booster AIT group vs 96.0% in the control group. The mean score of the intensity of allergy symptoms was 4.33 in the booster AIT group and 3.92 in the control group. However, the difference was not statistically significant. Therefore, both study groups were comparable at baseline with regard to demographic data and allergy status.
Of the planned 20 study centres, 14 study centres indicated being able to provide eligible patients and therefore participated in this study. In the end, only eight of those 14 study centres included eligible patients. The allowed number of about 10 patients per study centre was adjusted to a maximum of 32 patients per centre.
Overall, the AIT treatment course took place from 16 March to 22 June 2015. One patient in the booster AIT group skipped the fourth injection due to severe seasonal allergic discomfort but continued to participate in the study. Four patients (three in the booster AIT group and one in the control group) dropped out during the course of the study: one patient after the third injection due to personal reasons, two patients after Visit 5 and one patient before Visit 6 after providing diary data in the pollen peak (Figure 2).
Figure 2.
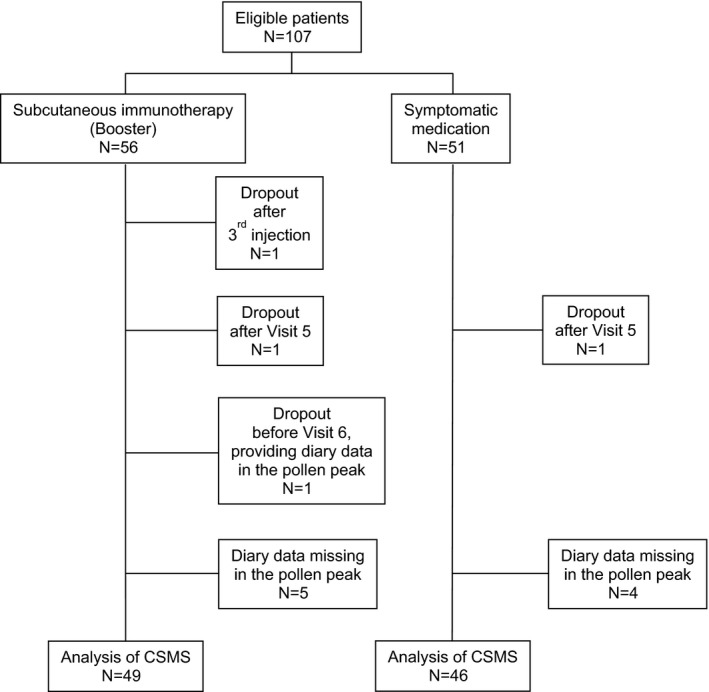
CONSORT flow diagram
3.2. Outcomes
In 2015, the start of the 30‐day peak of the grass pollen season in the different study centres was from 18 May to 11 June. Mean pollen counts in this time period ranged from 2.08 to 2.37 and were therefore comparable between the study centres. The CSMS of the booster AIT group was consistently lower than that of the control group, and the average daily mean CSMS was 38.4% lower in the booster AIT group than that in the control group (Figure 3; Table 2). The reduction in the dSS was 29.3% and in the dMS 46.9%. When comparing the booster AIT group and the control group, the differences in the mean CSMS (P<.01), dSS (P<.05) and dMS (P<.01) were statistically significant (Table 2).
Figure 3.
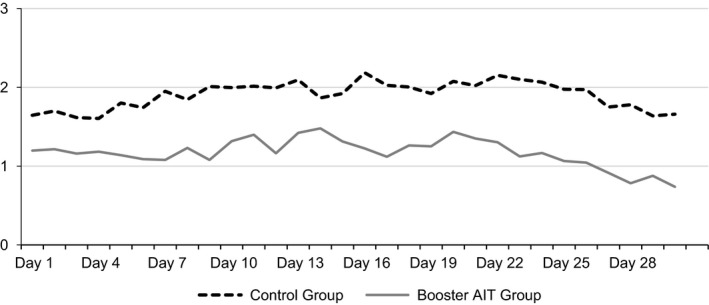
Daily mean of the CSMS during the peak of the grass pollen season of 30 days in 2015
Table 2.
CSMS, dSS, dMS and MiniRQLQ. P values comparing the two treatment groups
| Treatment group | CSMS daily mean 30 days | dSS daily mean 30 days | dMS daily mean 30 days | MiniRQLQ | |||
|---|---|---|---|---|---|---|---|
| Visit 1 | Visit 5 | Visit 6 | Visit 7 | ||||
| Control group | |||||||
| Number of patients | |||||||
| Valid | 46 | 46 | 46 | 43 | 46 | 42 | 40 |
| Missing | 5 | 5 | 5 | 8 | 5 | 9 | 11 |
| Mean±SD | 1.9±1.14 | 0.92±0.57 | 0.98±0.80 | 1.66±1.50 | 1.88±1.35 | 1.43±1.20 | 0.94±1.22 |
| Median (IQR 25‐75) | 1.96 (1.21‐2.48) | 0.88 (0.36‐1.33) | 0.87 (0.36‐1.42) | 1.71 (0.21‐2.64) | 1.68 (0.70‐2.64) | 1.29 (0.43‐2.30) | 0.39 (0.16‐1.07) |
| Booster AIT group | |||||||
| Number of patients | |||||||
| Valid | 49 | 49 | 49 | 53 | 51 | 48 | 50 |
| Missing | 7 | 7 | 7 | 3 | 5 | 8 | 6 |
| Mean±SD | 1.17±0.93 | 0.65±0.49 | 0.52±0.68 | 1.27±1.26 | 0.99±0.85 | 0.87±0.89 | 0.48±0.72 |
| Median (IQR 25‐75) | 1.07 (0.33‐1.95) | 0.54 (0.2‐1) | 0.3 (0‐0.85) | 0.43 (0.07‐2.57) | 0.79 (0.21‐1.57) | 0.5 (0.09‐1.46) | 0.14 (0‐0.52) |
| P value | .001 | .022 | .001 | .255 | .001 | .013 | .013 |
AIT, allergen immunotherapy; CSMS, combined symptom and medication score; dSS, daily symptom score; dMS, daily medication score; MiniRQLQ, Mini Rhinoconjunctivitis Quality of Life Questionnaire; SD, standard deviation; IQR, interquartile range.
The study also allowed a retrospective assessment of the intensity of rhinitis and conjunctivitis symptoms. Compared with 2014, the intensity decreased in 2015 by 57% in the booster AIT group and by 30% in the control group. Both reductions were statistically significant (P<.001 for both). Additionally, the result in a drop of symptoms in the booster AIT group was almost twice as much as that in the control group and was statistically significant (P<.05).
The number of patients not using any symptomatic medication in the booster AIT group increased from 3.8% in 2014 to 34.0% in 2015. Thus, one‐third of the patients in the booster AIT group did not need symptomatic medication. In contrast, the percentage of patients in the control group not using any symptomatic medication was similar in 2014 (4.0%) and 2015 (2.0%). The difference between both groups in 2015 was statistically significant (P<.001).
The mean score for the patients’ state of health in 2015 in the booster AIT group was 1.52, whereas the score in the control group was 2.52. This difference was statistically significant (P<.001). In the booster AIT group, 92% of the patients evaluated their state of health in 2015 as “significantly better” or “slightly better,” whereas 46% of the patients in the control group gave the same ratings (Table 3).
Table 3.
Patients’ evaluation of their state of health during the grass pollen season 2015
| Total | Treatment | ||
|---|---|---|---|
| Booster AIT Group | Control Group | ||
| State of health 2015, N (%) | |||
| 1=significantly better | 32 (32.6%) | 29 (58.0%) | 3 (6.3%) |
| 2=slightly better | 36 (36.7%) | 17 (34.0%) | 19 (39.6%) |
| 3=unchanged | 27 (27.6%) | 3 (6.0%) | 24 (50.0%) |
| 4=worse | 3 (3.1%) | 1 (2.0%) | 2 (4.2%) |
AIT, allergen immunotherapy; N, number of patients.
The quality of life assessment showed that both study groups had similar symptom scores before the start of the grass pollen season (1.27 in the booster AIT group and 1.66 in the control group). During and at the end of the pollen season (Visits 5, 6 and 7), the patients in the booster AIT group reported continuously better quality of life results (0.99, 0.87 and 0.48, respectively) than did the patients in the control group (1.88, 1.43 and 0.94, respectively). These findings were statistically significant at Visits 5, 6 and 7 (P<.01 at Visit 5, and P<.05 at both Visits 6 and 7) (Table 2).
The majority of patients in the booster AIT group evaluated the tolerability of all four injections as “very good” or “good” (77% to 84% for each injection), and no patient judged the tolerability as “poor.” Also, the large proportion of investigators rated the tolerability as “very good” or “good” (84% to 95% for each injection).
The majority of the booster AIT patients (71.4%) did not suffer from any complaints at the injection site, while 28.6% of subjects reported expected AEs such as itching and swelling. Two AEs reported by two different patients (3.6%) were related to the treatment medication but did not occur at the injection site: rhinitis‐related discomfort and unspecific headache. A large proportion of the booster AIT patients (96.4%) did not suffer from any AE other than complaints at the injection site. There were no reports of SAEs, cases of fatality, anaphylaxis or the need for adrenaline injections.
4. DISCUSSION
This is the first study to evaluate the efficacy of preseasonal, optimized, ultra‐short‐course SCIT with MPL® as a “booster treatment” for patients with recurrent seasonal allergic rhinoconjunctivitis caused by grass pollen after a successful first treatment course (SLIT or SCIT; any product) at least five years prior to this new course of AIT. Moreover, this investigation analysed the CSMS and other outcome parameters as recommended by the EAACI as clinical endpoints in field trials of AIT.27
POLLINEX® Quattro products are designed to be used as (short‐course) preseasonal vaccines and have demonstrated their efficacy in several pivotal trials with pollen‐allergic patients.19, 20, 21, 22, 23 Therefore, these vaccines may be ideal candidates for booster therapy of patients with recurrent symptoms. In our trial, the efficacy of this booster AIT was demonstrated by a 38.4% reduction in the CSMS in the booster AIT group compared with the control group throughout the entire 30 days of the peak grass pollen season. This effect on the CSMS was mirrored in the percentage of patients not needing to take any symptomatic medication. During the grass pollen season of 2015, 34% of the booster AIT group required no such medication in contrast to only 2% of patients in the control group.
Clinical data on revaccinated AIT patients are indeed limited. One long‐term prospective study over 15 years evaluated the efficacy of sublingual revaccination in patients with recurrent allergy symptoms.32 These first clinical data on SLIT revaccination indicated that patients responded to a second course of SLIT more rapidly than to the first. Other studies investigating revaccination with SCIT clearly indicated a similar benefit in patients who had received SCIT in the past. In 1994, Ebner et al. 24 conducted a study on revaccinating patients who had recurrent grass/rye pollen allergy symptoms after they had previously undergone successful immunotherapy for an average of 3 years. Actively treated patients received a booster immunotherapy with six low‐dose injections or 11 high‐dose injections. Although limited in its study design, their results confirmed an improvement of up to 70.8% in the symptom scores for the actively treated groups. Another study by Horak et al.26 evaluated the “booster effect” of adsorbed grass pollen L‐tyrosine as a way to complete a previous AIT with a pollen mix extract in order to gain full tolerance to grass pollen. Whereas patients who were treated using a specific hyposensitization solution reported an improvement of 10%, patients receiving the additional L‐tyrosine booster reported an “add‐on” benefit of a further 10%. In line with the results from the studies discussed above, we found a 57% reduction in the retrospective assessment of the intensity of rhinitis and conjunctivitis symptoms in patients who received ultra‐short‐course booster therapy because of recurrent allergic symptoms.
Patient‐reported outcomes, such as the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ), are regarded as another important outcome parameter in clinical trials on AIT.27 Our study yielded significant differences in the MiniRQLQ favouring booster AIT over pharmacotherapy alone. This analysis clearly demonstrates that patients with recurrent symptoms (after initially improving under previous AIT) benefit from ultra‐short‐course booster therapy already in the first year after revaccination with respect to their daily routine and activities. These results are in line with those for the patients’ (general) state of health: 92% of the patients after booster therapy reported a “significantly better” or “slightly better” state of health in 2015 than in the year before, which was only reported by 46% of the patients in the control group.
Another aspect of this trial focused on the safety and tolerability of booster therapy with an MPL®‐enhanced SCIT allergoid product in patients with recurrent symptoms. Previous clinical trials investigating the same product have confirmed its good safety profile.9, 19, 20, 21, 33, 34, 35 In a double‐blind, placebo‐controlled trial, DuBuske et al. found no major differences between actively treated and placebo‐treated grass pollen‐allergic patients with regard to systemic AEs.22 The safety of allergoids was also confirmed by a large prospective survey investigating the risk factors of allergen preparations in paediatric and adult patients.36, 37 The reported good safety profile is most probably due to the reduction in the allergenicity of the allergoids in comparison with the native allergens, while the impact of these allergoids on the immunogenic processes remains intact and is enhanced by MPL®.38 In our trial, two systemic reactions (in two actively treated patients) were found, both of which were mild. Furthermore, the patients themselves rated the tolerability of the active treatment as “very good” or “good” (77% to 84% for each injection), which corresponds to the patient‐rated tolerability results for MPL®‐enhanced SCIT of “very good” or “good” (93% to 97%) reported in double‐blind, placebo‐controlled trials.9, 21 Taken together, this open trial did not reveal any relevant increase in AEs under MPL®‐enhanced booster SCIT.
One limitation of this study is the lack of randomization. Instead, patients chose the therapy based on their personal experience and preference. Randomization aims to reduce baseline discrepancies between groups. Surprisingly, although random allocation did not take place, the two groups showed no major differences regarding demographic data and disease‐related baseline parameters. The preponderance of male patients in the AIT group has been described before.39 Another limitation is that symptomatic medication use in the grass pollen season in the year before and in the year during the study was enquired retrospectively. Thus, the accuracy could be restricted as it is sometimes difficult for patients to remember a special situation in the past.
The strength of this noninterventional study lies in the availability of a control group. The booster AIT group and the control group were well balanced, and the number of patients in both groups delivering diary data for the CSMS analysis was almost identical. Although both groups received highly efficient symptomatic medication during the pollen season, the booster AIT group still showed a lower CSMS during the pollen peak, demonstrating the efficacy of the ultra‐short‐course booster therapy.
In conclusion, this noninterventional study clearly indicates that patients with recurrent grass pollen allergy symptoms and a history of a previous AIT of any route or course that at first successfully reduced the symptoms benefit from a new round of ultra‐short‐course SCIT (booster) compared with patients using standard pharmacotherapy alone. Although the influence of the booster injection on immunological parameters could not be investigated in this noninterventional study, the clinical effects of this ultra‐short‐course booster therapy have been demonstrated using state‐of‐the‐art endpoints as recommended and standardized by the EAACI.27 Moreover, this booster treatment is safe and its tolerability is comparable to that found after short‐course treatment with a first round of AIT.
AUTHOR CONTRIBUTIONS
OP developed the idea of the study and contributed to the study design. RM developed the study design together with OP, MK and LK. UPF, FG and YR were responsible for the acquisition of the data. AA and KS conducted the statistical analysis. OP, RM, SL and UPF interpreted the data. SL performed the research and together with UPF drafted and wrote the manuscript. All authors critically revised the manuscript draft and gave final approval of the submitted version of the manuscript.
ACKNOWLEDGMENTS
We would like to thank Gena Kittel and Marie‐Josefine Joisten for their proofreading and editorial assistance.
CONFLICTS OF INTEREST
RM reports personal fees from ALK, grants from ASIT biotech, personal fees from Allergopharma, personal fees from Allergy Therapeutics, grants and personal fees from Bencard, grants from Leti, grants, personal fees and non‐financial support from Lofarma, non‐financial support from Roxall, grants and personal fees from Stallergenes, grants from Optima, personal fees from Friulchem, personal fees from Hexal, personal fees from Servier, personal fees from Klosterfrau, non‐financial support from Atmos, personal fees from Bayer, non‐financial support from Bionorica, personal fees from FAES, personal fees from GSK, personal fees from MSD, personal fees from Johnson&Johnson, personal fees from Meda, personal fees and non‐financial support from Novartis, non‐financial support from Otonomy, personal fees from Stada, personal fees from UCB, non‐financial support from Ferrero, grants from BitopAG, grants from Hulka, personal fees from Nuvo, grants from Ursapharm, outside the submitted work. OP reports personal fees from Bencard Allergie GmbH during the conduct of the study; grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL‐Allergy Holding B.V./HAL‐Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from Novartis Pharma, personal fees from MEDA Pharma, grants and personal fees from Anergis S.A., personal fees from Sanofi US Services, personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Pohl‐Boskamp, outside the submitted work. LK reports grants and personal fees from ALK Abelló, Denmark, personal fees from Meda, Sweden, grants and personal fees from Novartis, Switzerland, grants and personal fees from Allergopharma, Germany, grants and personal fees from Bionorica, Germany, personal fees from Boehringer Ingelheim, Germany, grants and personal fees from GSK, Great Britain, grants and personal fees from Lofarma, Italy, grants from Biomay, Austria, grants from HAL, Netherlands, grants from LETI, Spain, grants from Roxall, Germany, grants from Bencard, Great Britain, outside the submitted work. MK is an employee of Bencard. SL, UPF, AA, FG, YR and KS have nothing to disclose.
Pfaar O, Lang S, Pieper‐Fürst U, et al. Ultra‐short‐course booster is effective in recurrent grass pollen‐induced allergic rhinoconjunctivitis. Allergy. 2018;73:187–195. https://doi.org/10.1111/all.13240
Funding information
This trial was sponsored by Bencard® Allergie GmbH, Munich, Germany. The sponsor also paid for open access of the article (OnlineOpen publication).
The results of this study were presented in part as poster presentations at the Congress of the European Academy of Allergy and Clinical Immunology (EAACI) in Vienna, Austria, in June 2016, and at the German Allergy Congress in Berlin, Germany, in September 2016.
Edited by: Wytske Fokkens
REFERENCES
- 1. Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556‐568. [DOI] [PubMed] [Google Scholar]
- 2. Noon L. Prophylactic inoculation against hay fever. Lancet. 1911;177:1572‐1573. [Google Scholar]
- 3. Durham SR, Leung DY. One hundred years of allergen immunotherapy: time to ring the changes. J Allergy Clin Immunol. 2011;127:3‐7. [DOI] [PubMed] [Google Scholar]
- 4. Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: A systematic review and meta‐analysis. Allergy. 2017;72:1597‐1631. [DOI] [PubMed] [Google Scholar]
- 5. Pfaar O, Bachert C, Bufe A, et al. Guideline on allergen‐specific immunotherapy in IgE‐mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto‐Rhino‐Laryngology, Head and Neck Surgery (DGHNO‐KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV‐HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23:282‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calderon MA, Alves B, Jacobson M, Hurwitz B, Sheikh A, Durham S. Allergen injection immunotherapy for seasonal allergic rhinitis. Cochrane Database Syst Rev. 2007;CD001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Compalati E, Penagos M, Tarantini F, Passalacqua G, Canonica GW. Specific immunotherapy for respiratory allergy: state of the art according to current meta‐analyses. Ann Allergy Asthma Immunol. 2009;102:22‐28. [DOI] [PubMed] [Google Scholar]
- 8. Bousquet J, Lockey R, Malling HJ, et al. Allergen immunotherapy: therapeutic vaccines for allergic diseases. World Health Organization. American academy of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1998;81:401‐405. [DOI] [PubMed] [Google Scholar]
- 9. Rosewich M, Lee D, Zielen S. Pollinex Quattro: an innovative four injections immunotherapy in allergic rhinitis. Hum Vaccin Immunother. 2013;9:1523‐1531. [DOI] [PubMed] [Google Scholar]
- 10. Hsu NM, Reisacher WR. A comparison of attrition rates in patients undergoing sublingual immunotherapy vs subcutaneous immunotherapy. Int Forum Allergy Rhinol. 2012;2:280‐284. [DOI] [PubMed] [Google Scholar]
- 11. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008;63(Suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
- 12. Durham SR, Penagos M. Sublingual or subcutaneous immunotherapy for allergic rhinitis? J Allergy Clin Immunol. 2016;137:339‐349. [DOI] [PubMed] [Google Scholar]
- 13. Di Bona D, Plaia A, Leto‐Barone MS, La Piana S, Di Lorenzo G. Efficacy of subcutaneous and sublingual immunotherapy with grass allergens for seasonal allergic rhinitis: a meta‐analysis‐based comparison. J Allergy Clin Immunol. 2012;130:1097‐1107. [DOI] [PubMed] [Google Scholar]
- 14. Durham SR, Walker SM, Varga EM, et al. Long‐term clinical efficacy of grass‐pollen immunotherapy. N Engl J Med. 1999;341:468‐475. [DOI] [PubMed] [Google Scholar]
- 15. Pfaar O, Cazan D, Klimek L, Larenas‐Linnemann D, Calderon MA. Adjuvants for immunotherapy. Curr Opin Allergy Clin Immunol. 2012;12:648‐657. [DOI] [PubMed] [Google Scholar]
- 16. Valenta R, Campana R, Focke‐Tejkl M, Niederberger V. Vaccine development for allergen‐specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol. 2016;137:351‐357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Casale TB, Stokes JR. Future forms of immunotherapy. J Allergy Clin Immunol. 2011;127:8‐15. [DOI] [PubMed] [Google Scholar]
- 18. von Baehr V, Hermes A, von Baehr R, et al. Allergoid‐specific T‐cell reaction as a measure of the immunological response to specific immunotherapy (SIT) with a Th1‐adjuvanted allergy vaccine. J Investig Allergol Clin Immunol. 2005;15:234‐241. [PubMed] [Google Scholar]
- 19. McCormack PL, Wagstaff AJ. Ultra‐short‐course seasonal allergy vaccine (Pollinex Quattro). Drugs. 2006;66:931‐938. [DOI] [PubMed] [Google Scholar]
- 20. Baldrick P, Richardson D, Wheeler AW, Woroniecki SR. Safety evaluation of a new allergy vaccine containing the adjuvant monophosphoryl lipid A (MPL) for the treatment of grass pollen allergy. J Appl Toxicol. 2004;24:261‐268. [DOI] [PubMed] [Google Scholar]
- 21. Drachenberg KJ, Pröll S, Urban E, Woroniecki SR. Single‐course specific immunotherapy with mixed pollen allergoids: results of a multi‐centre study. Allergol Immunopathol (Madr). 2003;31:77‐82. [DOI] [PubMed] [Google Scholar]
- 22. DuBuske LM, Frew AJ, Horak F, et al. Ultrashort‐specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:239‐247. [DOI] [PubMed] [Google Scholar]
- 23. Drachenberg KJ, Wheeler AW, Stuebner P, Horak F. A well‐tolerated grass pollen‐specific allergy vaccine containing a novel adjuvant, monophosphoryl lipid A, reduces allergic symptoms after only four preseasonal injections. Allergy. 2001;56:498‐505. [DOI] [PubMed] [Google Scholar]
- 24. Ebner C, Kraft D, Ebner H. Booster immunotherapy (BIT). Allergy. 1994;49:38‐42. [DOI] [PubMed] [Google Scholar]
- 25. Pipet A, Botturi K, Pinot D, Vervloet D, Magnan A. Allergen‐specific immunotherapy in allergic rhinitis and asthma. Mechanisms and proof of efficacy. Respir Med. 2009;103:800‐812. [DOI] [PubMed] [Google Scholar]
- 26. Horak F, Jäger S. Skoda‐Türk R [Reduced allergen immunotherapy of grass pollinosis (author's transl)]. Wien Klin Wochenschr Suppl. 1980;117:36‐38. [PubMed] [Google Scholar]
- 27. Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854‐867. [DOI] [PubMed] [Google Scholar]
- 28. Summary of Product Characteristics . POLLINEX® Quattro Plus 1.0 ml. In. https://www.bencard.de/fileadmin/PDF/Download-Center/Pollinex/PQ_Fachinformation.pdf.
- 29. Sieber J, Shah‐Hosseini K, Mösges R. Specific immunotherapy for allergic rhinitis to grass and tree pollens in daily medical practice‐symptom load with sublingual immunotherapy compared to subcutaneous immunotherapy. Ann Med. 2011;43:418‐424. [DOI] [PubMed] [Google Scholar]
- 30. Shah‐Hosseini K, Krudewig EM, Hadler M, Karagiannis E, Mösges R. Management of Grass Pollen Allergy with 5‐Grass Pollen Tablet: Results of a 2‐Year Real‐Life Study. Adv Ther. 2017;34:1382‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Development and validation of the Mini Rhinoconjunctivitis Quality of Life Questionnaire. Clin Exp Allergy. 2000;30:132‐140. [DOI] [PubMed] [Google Scholar]
- 32. Marogna M, Spadolini I, Massolo A, Canonica GW, Passalacqua G. Long‐lasting effects of sublingual immunotherapy according to its duration: a 15‐year prospective study. J Allergy Clin Immunol. 2010;126:969‐975. [DOI] [PubMed] [Google Scholar]
- 33. Puggioni F, Durham SR, Francis JN. Monophosphoryl lipid A (MPL) promotes allergen‐induced immune deviation in favour of Th1 responses. Allergy. 2005;60:678‐684. [DOI] [PubMed] [Google Scholar]
- 34. Patel P, Salapatek AM. Pollinex Quattro: a novel and well‐tolerated, ultra short‐course allergy vaccine. Expert Rev Vaccines. 2006;5:617‐629. [DOI] [PubMed] [Google Scholar]
- 35. Gawchik SM, Saccar CL. Pollinex Quattro Tree: allergy vaccine. Expert Opin Biol Ther. 2009;9:377‐382.19216626 [Google Scholar]
- 36. Calderón MA, Vidal C, Rodríguez Del Río P, et al. European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): a real‐life clinical assessment. Allergy. 2017;72:462‐472. [DOI] [PubMed] [Google Scholar]
- 37. Rodríguez del Río P, Vidal C, Just J, et al. The European Survey on Adverse Systemic Reactions in Allergen Immunotherapy (EASSI): A paediatric assessment. Pediatr Allergy Immunol. 2017;28:60‐70. [DOI] [PubMed] [Google Scholar]
- 38. Worm M, Ernst D, Kraller M, Babina M. The Impact on Allergy‐Related Cells of a Birch Pollen Allergoid, with and without Monophosphoryl Lipid A, in Comparison with the Native Equivalent. Int Arch Allergy Immunol. 2017;172:20‐26. [DOI] [PubMed] [Google Scholar]
- 39. Manzotti G, Pappacoda A, Dimatteo M, Scolari C, Riario‐Sforza GG, Incorvaia C. Ultra short pre‐seasonal subcutaneous immunotherapy and pre‐coseasonal sublingual immunotherapy for pollen allergy: an evaluation of patient's preference in real life. Eur Ann Allergy Clin Immunol. 2013;45:138‐143. [PubMed] [Google Scholar]


