Abstract
Aim
To assess the lipid‐lowering efficacy and safety of alirocumab, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, in people with hypercholesterolaemia and prediabetes at baseline vs people with normoglycaemia at baseline in a pooled analysis of 10 ODYSSEY phase III trials.
Methods
People classified as having prediabetes had baseline HbA1c ≥39 mmol/mol (5.7%) and <48 mmol/mol (6.5%), or two baseline fasting plasma glucose values ≥5.6 mmol/l (100 mg/dl) but no more than one fasting plasma glucose value ≥7.0 mmol/l (126 mg/dl), or had specific terms reported in their medical history; people diagnosed with diabetes at baseline were excluded, and the remainder were classified as having normoglycaemia. Participants received alirocumab or control (placebo/ezetimibe) for 24–104 weeks, with maximally tolerated statin in most cases. The primary efficacy endpoint was LDL cholesterol reductions from baseline to week 24 in the intention‐to‐treat population using the mixed‐effect model with a repeated measures approach.
Results
Reductions in LDL cholesterol from baseline to week 24 with alirocumab were 44.0–61.8% (prediabetes group) and 45.8–59.5% (normoglycaemia group). In both subgroups, LDL cholesterol reductions were generally similar in those with and without baseline triglycerides ≥1.7 mmol/l (150 mg/dl). Alirocumab was not associated with changes in HbA1c or fasting plasma glucose over time in either subgroup (up to 24 months' follow‐up). Adverse event rates were generally similar in those with and without prediabetes.
Conclusions
Over a mean follow‐up of 24–104 weeks, alirocumab treatment resulted in significant LDL cholesterol reductions from baseline that were similar in participants with prediabetes and those with normoglycaemia at baseline, with no effect on glycaemia and a safety profile similar to that of the control.
What's new?
With statins being associated with an increased risk of diabetes (especially in individuals with risk factors for diabetes) and recent studies linking PCSK9 to glucose homeostasis, there is much interest in whether PCSK9 inhibitors affect diabetes risk.
We assessed the efficacy/safety of alirocumab, a PCSK9 inhibitor, vs control over a mean follow‐up of 24–104 weeks in people with hypercholesterolaemia and prediabetes vs people with normoglycaemia at baseline from 10 ODYSSEY phase III trials.
Our findings show alirocumab is generally well tolerated and significantly reduced LDL cholesterol levels to similar extents in individuals with prediabetes and those with normoglycaemia without any changes in measures of glycaemic control.
What's new?
With statins being associated with an increased risk of diabetes (especially in individuals with risk factors for diabetes) and recent studies linking PCSK9 to glucose homeostasis, there is much interest in whether PCSK9 inhibitors affect diabetes risk.
We assessed the efficacy/safety of alirocumab, a PCSK9 inhibitor, vs control over a mean follow‐up of 24–104 weeks in people with hypercholesterolaemia and prediabetes vs people with normoglycaemia at baseline from 10 ODYSSEY phase III trials.
Our findings show alirocumab is generally well tolerated and significantly reduced LDL cholesterol levels to similar extents in individuals with prediabetes and those with normoglycaemia without any changes in measures of glycaemic control.
Introduction
Prediabetes, defined as impaired fasting glucose and/or impaired glucose tolerance, increases risk of cardiovascular disease and Type 2 diabetes mellitus 1. People with prediabetes often have an abnormal lipid profile, evidenced by elevated levels of small dense LDL particles and triglycerides, and lower levels of HDL cholesterol 2. These individuals also frequently have visceral obesity and hypertension, and preventative strategies to manage these risk factors are recommended for them to lower their risks of diabetes and cardiovascular disease 1.
Statins, the first‐line therapy for lowering LDL cholesterol, have been shown to reduce the incidence of cardiovascular events in people with diabetes and prediabetes; however, statin treatment has been associated with an increased risk of transition to Type 2 diabetes, evidenced by increases in fasting plasma glucose (FPG) and HbA1c, especially in individuals with risk factors for the development of diabetes 3, 4. Nonetheless, because the benefits of statin therapy for the reduction of cardiovascular events are considered to far outweigh the risk of new‐onset diabetes 3, lipid levels in individuals with prediabetes are recommended to be managed with statins in the same manner as those with diabetes 1, 2, 4, 5.
Alirocumab is a fully human monoclonal antibody to proprotein convertase subtilisin/kexin type 9 (PCSK9), a major regulator of plasma LDL cholesterol metabolism 6. Alirocumab is approved in many countries, including the USA and across Europe, for the management of high‐risk patients with elevated LDL cholesterol despite maximally tolerated doses of statins and/or ezetimibe. Alirocumab, alone and in combination with other lipid‐lowering therapies, significantly reduced LDL cholesterol levels in phase III ODYSSEY clinical trials in people with hypercholesterolaemia 7, 8, 9, 10, 11, 12, 13, 14.
Recent evidence suggests that PCSK9 genetic variants associated with LDL cholesterol reductions are also associated with a small increase in risk of developing new‐onset diabetes in people with impaired FPG 15, or with an increased risk of Type 2 diabetes and higher FPG concentrations, but not HbA1c 16. The GLAGOV study of evolocumab, another PCSK9 inhibitor, found a small but statistically significant increase in FPG, but no change in HbA1c levels, at 78 weeks of treatment 17, although no effect of evolocumab on glycaemia was observed in a pooled analysis of phase III trials 18. A comparison of rates of adjudicated new‐onset diabetes cases in the recent FOURIER clinical outcomes trial showed no significant differences between evolocumab and placebo 19. In pooled or sub‐analyses of phase III trials, alirocumab did not affect mean HbA1c and FPG levels over 52–104 weeks of treatment in individuals with or without diabetes 20, 21, 22. Furthermore, no evidence of increased transition from normoglycaemia to prediabetes or new‐onset diabetes after alirocumab treatment was found in a pooled analysis of ~5000 individuals in 10 phase III trials of 24–104 weeks of double‐blind treatment 20.
In the present analysis, we assessed the lipid‐lowering efficacy and safety of alirocumab in people with prediabetes vs normoglycaemia at baseline using data from the same 10 phase III trials as used previously 20. People with diabetes at baseline were excluded.
Methods
Study design and participants
This post hoc analysis pooled data from 10 randomized, double‐blind, phase III trials studying alirocumab in individuals with hypercholesterolaemia and moderate to very high cardiovascular risk, with double‐blind trial durations of 24–104 weeks (Fig. 1). Methods and results for individual trials have been published 7, 8, 9, 10, 11, 12, 13, 14, 23. Trial protocols were approved by appropriate institutional review boards/independent ethics committees and trials were conducted in accordance with the Declaration of Helsinki and applicable amendments and International Conference on Harmonization guidelines for Good Clinical Practice. All trial participants provided written informed consent.
Figure 1.
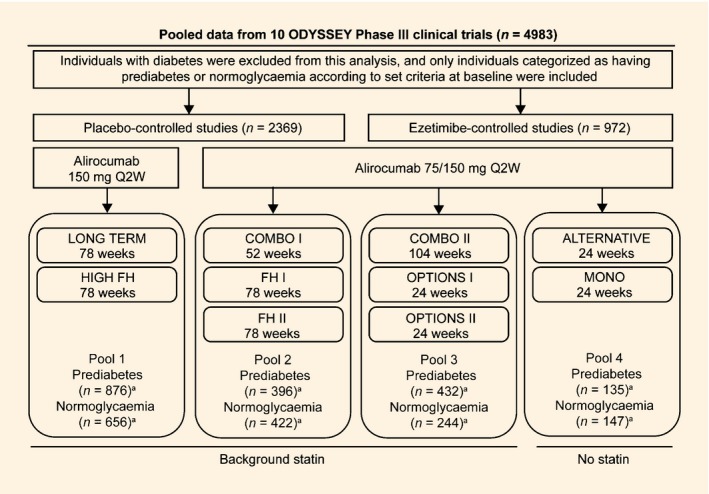
Overview of ODYSSEY trials included in this analysis (randomized population). FH, familial hypercholesterolaemia; HeFH, heterozygous familial hypercholesterolaemia; Q2W, every 2 weeks. aITT population. The FH I, FH II and HIGH FH studies recruited only individuals with HeFH. COMBO I and COMBO II recruited individuals with non‐FH at high cardiovascular risk. OPTIONS I, OPTIONS II and LONG TERM recruited both individuals with HeFH and non‐FH. ALTERNATIVE recruited individuals with moderate to high cardiovascular risk and not receiving a statin due to intolerance. Individuals recruited in the MONO study had moderate cardiovascular risk not receiving statins or other lipid‐lowering therapy. Further details of the ODYSSEY trials included in this analysis have been published previously: Clinicaltrials.gov identifiers: ALTERNATIVE, NCT01709513 11; COMBO I, NCT01644175 10; COMBO II, NCT01644188 13; FH I, NCT01623115; FH II, NCT01709500 9; HIGH FH, NCT01617655 23; LONG TERM, NCT01507831 12; MONO, NCT01644474 14; OPTIONS I, NCT01730040 7; OPTIONS II, NCT01730053 8.
For the purposes of the present analysis, efficacy data from the trials were compared between people with prediabetes and those with normoglycaemia at baseline in four pools according to alirocumab dose regimen, control and background statin use (Fig. 1). Pool 1 compared alirocumab 150 mg every 2 weeks vs placebo, with background statin. The other three pools included trials in which the alirocumab dose of 75 mg every 2 weeks could be increased (in a blinded fashion) to 150 mg (denoted as 75/150 mg) at week 12 if LDL cholesterol was ≥1.8 mmol/l (70 mg/dl) at week 8 [except for OPTIONS I and II and ALTERNATIVE trials, where the dose was increased if LDL cholesterol was ≥1.8 mmol/l or ≥2.6 mmol/l (100 mg/dl), depending on cardiovascular risk]. Pool 2 compared alirocumab 75/150 mg every 2 weeks vs placebo, with background statin. Pools 3 and 4 compared alirocumab 75/150 mg every 2 weeks vs ezetimibe; Pool 3 used background statin, and Pool 4 included trials performed without background statin.
Categorization of participants with prediabetes or normoglycaemia
Participants were categorized by diabetes status (diabetes, prediabetes or normoglycaemia) according to: (1) medical history prior to the trial; (2) baseline HbA1c (single measurement at baseline); and (3) FPG (two measures, one at screening and one at randomization). Oral glucose tolerance tests were not conducted in the trials.
Participants with diabetes at baseline, defined as (1) diagnosis of Type 1 or 2 diabetes in the medical history; (2) baseline HbA1c ≥48 mmol/mol (6.5%); or (3) two FPG values at screening or randomization ≥7.0 mmol/l (126 mg/dl), were excluded from this analysis. Efficacy and safety of alirocumab in participants with diabetes have been reported 20, 21, 22.
Prediabetes at baseline was defined as: (1) specific terms in the medical history (i.e. if a Custom Medical Dictionary of Regulatory Activities (MedDRA) Query of the medical history reported ‘impaired glucose control’, including preferred terms ‘glucose tolerance impaired’, ‘impaired fasting glucose’, ‘insulin resistance’ and ‘insulin resistance syndrome’); (2) baseline HbA1c 39 to <48 mmol/mol (5.7 to <6.5%); or (3) two FPG values at screening or randomization ≥5.6 mmol/l (≥100 mg/dl) but no more than one value ≥7.0 mmol/l (126 mg/dl). Participants who did not fulfill any of the above criteria at baseline were classified as having normoglycaemia.
Endpoints and assessments
Consistent with the parent trials, the primary efficacy endpoint was the percentage reduction in LDL cholesterol from baseline to week 24; other efficacy endpoints including percentage reductions in non‐HDL cholesterol, apolipoprotein (Apo) B and lipoprotein(a) [Lp(a)] from baseline to week 24. Efficacy was also assessed according to baseline triglyceride and HDL cholesterol levels.
Plasma lipid levels were assessed at weeks 0 (baseline), 4, 8, 12, 24, 36, 52, 64, 78 (or 76 in COMBO II), 88 and 104, depending on study duration, except ApoB and Lp(a), which were assessed at weeks 0, 12, 24, 52, 78 and 104, depending on study duration. All lipid measurements were conducted by a central laboratory (Medpace Reference Laboratories, Cincinnati, OH, and Leuven, Belgium, except for LONG TERM, which used Covance Central Laboratories, Indianapolis, IN, USA and Geneva, Switzerland). LDL cholesterol levels were calculated using the Friedewald equation unless triglyceride levels were >4.5 mmol/l (400 mg/dl), in which case it was determined by beta‐quantification. Non‐HDL cholesterol levels were calculated by subtracting HDL cholesterol from total cholesterol. Serum ApoB levels were determined from immunonephelometry. Serum Lp(a) was analysed as previously described 24, using an established immunoturbidimetric assay on a Siemens BNII (Siemens, Erlangen, Germany) validated against International Federation of Clinical Chemistry and WHO standards.
Baseline and safety data were compared in participants with prediabetes and those with normoglycaemia, in two pools according to placebo or ezetimibe control. Adverse events were encoded using standard MedDRA terms, and treatment‐emergent adverse events (TEAEs) were defined as events occurring from the first dose of study treatment to the last dose plus 70 days.
Time course measurements of HbA1c and FPG were compared in participants with prediabetes and those with normoglycaemia in two overall pooled groups of alirocumab and control. FPG levels were measured at screening and at weeks 0, 12, 24, 36, 52 and 64 (not in COMBO II), 78 (or 76 in COMBO II), and 104, depending on study duration. HbA1c levels were measured at screening (i.e. at 3 weeks prior to randomization; used for baseline HbA1c assessment) and at weeks 12 (LONG TERM, OPTIONS I, OPTIONS II and MONO only), 24, 52, and 78 (not in COMBO II), and 104, depending on study duration. HbA1c was measured by Covance Central Laboratory Services [a Level I National Glycohemoglobin Standardization Program (NGSP)‐certified laboratory) using the NGSP‐certified Bio‐Rad Variant II and Variant II Turbo systems, which are traceable to the Diabetes Control and Complications Trial Reference Method and Values.
Statistical methods
Efficacy analyses were performed in the intention‐to‐treat population, which included all randomized patients regardless of treatment discontinuation. Analyses of LDL cholesterol, non‐HDL cholesterol and ApoB used a mixed‐effect model with a repeated measures approach, which included fixed categorical effects of treatment group, randomization strata, time point, treatment‐by‐time point interaction, strata‐by‐time point interaction, treatment‐by‐subgroup interaction, time point‐by‐subgroup interaction and treatment‐by‐time point‐by‐subgroup interaction as well as the continuous fixed covariates of baseline values and baseline value‐by‐time point interaction 7, 8, 9, 10, 11, 12, 13, 14, 23. This model provided baseline‐adjusted least‐squares means estimates at week 24 with their corresponding 95% CIs and se values. The difference between these estimates are provided with their corresponding 95% CI and interaction P values.
Analyses of Lp(a) used a multiple imputation approach for the handling of missing values, followed by robust regression. Adjusted means and se values were obtained by combining adjusted means and se from robust regression model analyses of the different imputed datasets. The robust regression models include the fixed categorical effect of treatment group, study, randomization strata, subgroup (prediabetes or normoglycaemia) and subgroup‐by‐treatment interaction and the continuous fixed covariate of baseline (a) Lp value. No log transformation of Lp(a) was necessary using this method.
Baseline data (randomized population) were analysed using descriptive statistics. In addition, variables were compared between the participants with prediabetes and those with normoglycaemia using the appropriate statistical test as indicated in the results section below.
Safety data were analysed using descriptive statistics. The safety population included all randomized patients who received at least one dose or part of a dose of study treatment.
Results
Baseline categorization according to prediabetes status
Overall, 3341 participants were included in the analysis, including 1860 (56%) with prediabetes and 1481 (44%) with normoglycaemia. Baseline characteristics are shown in Table 1. Compared with those with normoglycaemia, those with prediabetes were slightly older, had higher BMI (both P<0.0001, Student's t‐test), had a higher proportion with a history of hypertension and atherosclerotic cardiovascular disease (both P<0.0001, chi‐squared test), and had slightly higher baseline triglyceride levels (P<0.0001, Wilcoxon test) and slightly lower baseline LDL cholesterol, HDL cholesterol, non‐HDL cholesterol and ApoB levels (all P<0.0001, Student's t‐test). Baseline Lp(a) levels were similar in participants with prediabetes and those with normoglycaemia (P=0.17, Wilcoxon test). Placebo‐controlled trials had a higher proportion of participants with heterozygous familial hypercholesterolaemia than ezetimibe‐controlled trials (Table 1) because of differences in trial inclusion criteria (Fig. 1).
Table 1.
Baseline characteristics and lipid levels, pooled by comparator and prediabetes status (randomized population)
| Placebo‐controlled pool | Ezetimibe‐controlled pool | |||||||
|---|---|---|---|---|---|---|---|---|
| Prediabetes (N = 1285) | Normoglycaemia (N = 1084) | Prediabetes (N = 575) | Normoglycaemia (N = 397) | |||||
| Alirocumab (N = 865) | Placebo (N = 420) | Alirocumab (N = 718) | Placebo (N = 366) | Alirocumab (N = 332) | Ezetimibe (N = 243) | Alirocumab (N = 223) | Ezetimibe (N = 174) | |
| Mean (sd) age, years | 59.8 (10.4) | 60.5 (10.2) | 54.1 (13.4) | 54.7 (12.8) | 62.0 (8.8) | 62.6 (9.3) | 60.5 (9.7) | 59.9 (10.8) |
| Men, n (%) | 551 (63.7) | 276 (65.7) | 435 (60.6) | 225 (61.5) | 241 (72.6) | 165 (67.9) | 145 (65.0) | 101 (58.0) |
| Mean (sd) BMI, kg/m2 | 29.9 (5.1) | 29.6 (5.1) | 28.0 (4.7) | 28.6 (4.8) | 29.9 (5.1) | 29.6 (5.4) | 27.7 (4.7) | 27.6 (4.1) |
| Mean (sd) HbA1c | ||||||||
| mmol/mol | 40 (3) | 40 (3) | 35 (2) | 35 (2) | 40 (3) | 40 (3) | 35 (2) | 35 (3) |
| % | 5.8 (0.3) | 5.8 (0.3) | 5.3 (0.2) | 5.4 (0.2) | 5.8 (0.3) | 5.8 (0.3) | 5.4 (0.2) | 5.4 (0.2) |
| Mean (sd) FPG | ||||||||
| mmol/l | 5.7 (0.6) | 5.7 (0.6) | 5.1 (0.5) | 5.1 (0.5) | 5.8 (0.8) | 5.7 (0.7) | 5.2 (0.5) | 5.1 (0.5) |
| mg/dl | 102.1 (11.4) | 102.1 (11.5) | 92.5 (9.5) | 92.3 (9.7) | 105.3 (14.5) | 102.8 (12.7) | 94.2 (9.2) | 92.6 (8.3) |
| Hypertension, n (%) | 547 (63.2) | 276 (65.7) | 351 (48.9) | 193 (52.7) | 246 (74.1) | 166 (68.3) | 127 (57.0) | 98 (56.3) |
| HeFH, n (%) | 346 (40.0) | 153 (36.4) | 393 (54.7) | 200 (54.6) | 8 (2.4) | 19 (7.8) | 27 (12.1) | 22 (12.6) |
| ASCVDa, n (%) | 676 (78.2) | 347 (82.6) | 459 (63.9) | 233 (63.7) | 269 (81.0) | 178 (73.3) | 160 (71.7) | 100 (57.5) |
| Any statin, n (%) | 865 (100.0) | 419 (99.8) | 717 (99.9) | 366 (100.0) | 268 (80.7) | 175 (72.0) | 152 (68.2) | 100 (57.5) |
|
High‐dose statinb, n (%) |
540 (62.4) | 261 (62.1) | 451 (62.8) | 244 (66.7) | 184 (55.4) | 116 (47.7) | 95 (42.6) | 57 (32.8) |
| Any other LLTs, n (%) | 338 (39.1) | 177 (42.1) | 318 (44.3) | 168 (45.9) | 34 (10.2) | 37 (15.2) | 43 (19.3) | 46 (26.4) |
| Baseline lipid levels, mean (sd) unless otherwise specified | ||||||||
| LDL cholesterol | ||||||||
| mmol/l | 3.3 (1.2) | 3.2 (1.1) | 3.5 (1.3) | 3.6 (1.3) | 3.1 (1.2) | 3.3 (1.5) | 3.6 (1.7) | 3.6 (1.7) |
| mg/dl | 126.9 (46.1) | 123.1 (40.7) | 136.8 (51.8) | 138.6 (50.8) | 119.6 (46.5) | 127.3 (57.3) | 140.8 (67.1) | 140.7 (65.8) |
| HDL cholesterol | ||||||||
| mmol/l | 1.3 (0.3) | 1.3 (0.3) | 1.4 (0.4) | 1.3 (0.4) | 1.3 (0.3) | 1.3 (0.4) | 1.4 (0.4) | 1.4 (0.4) |
| mg/dl | 50.0 (13.1) | 50.6 (12.9) | 52.7 (14.6) | 50.9 (13.6) | 48.8 (13.5) | 50.0 (14.1) | 53.4 (15.0) | 53.1 (15.3) |
| Fasting triglycerides, median (Q1:Q3) | ||||||||
| mmol/l | 1.4 (1.0: 2.0) | 1.4 (1.1: 2.0) | 1.2 (0.9: 1.7) | 1.2 (0.9: 1.8) | 1.5 (1.1: 2.2) | 1.5 (1.1: 2.2) | 1.3 (0.9: 1.8) | 1.3 (0.9: 2.0) |
| mg/dl | 126.5 (92.0: 176.0) | 126.5 (94.0: 173.0) | 106.6 (79.0: 149.0) | 109.7 (80.0: 162.0) | 135.0 (98.0: 191.5) | 135.0 (100.0: 197.0) | 114.0 (82.0: 161.0) | 112.0 (83.0: 178.0) |
| Non‐HDL cholesterol | ||||||||
| mmol/l | 4.0 (1.3) | 3.9 (1.1) | 4.2 (1.4) | 4.3 (1.4) | 3.9 (1.3) | 4.2 (1.8) | 4.4 (1.9) | 4.3 (1.8) |
| mg/dl | 155.4 (49.8) | 151.6 (44.1) | 161.4 (54.7) | 164.2 (54.3) | 150.4 (49.8) | 160.1 (69.9) | 168.0 (74.9) | 167.7 (68.1) |
| Apo B, mg/dl | 104.1 (28.9) | 102.0 (26.0) | 106.9 (31.1) | 109.3 (31.3) | 99.4 (28.2) | 102.8 (33.2) | 106.6 (36.9) | 108.9 (35.6) |
| Median (Q1:Q3) Lp(a), mg/dl | 29.0 (10.0: 75.0) | 25.0 (8.1: 71.3) | 24.5 (10.0: 70.6) | 24.4 (7.0: 77.5) | 25.0 (8.0: 75.0) | 24.0 (8.0: 59.0) | 24.0 (7.0: 64.0) | 22.0 (9.0: 55.0) |
Apo, apolipoprotein; ASCVD, atherosclerotic cardiovascular disease; FPG, fasting plasma glucose; HeFH, heterozygous familial hypercholesterolaemia; LLT, lipid‐lowering therapy; Lp(a) lipoprotein(a).
ASCVD included coronary heart disease, peripheral arterial disease and ischaemic stroke; for the FH II, ALTERNATIVE, OPTIONS I and OPTIONS II studies; transient ischaemic attack, carotid endarterectomy or carotid artery stent procedure, and renal artery stent procedure were also included.
High‐dose statin was defined as atorvastatin ≥ 40 mg, rosuvastatin ≥ 20 mg or simvastatin ≥ 80 mg.
Efficacy
In all pools, alirocumab significantly reduced LDL cholesterol levels from baseline to week 24 in both participants with prediabetes and those with normoglycaemia (Fig. 2); all LDL cholesterol reductions with alirocumab were significant vs control (based on 95% CI).
Figure 2.
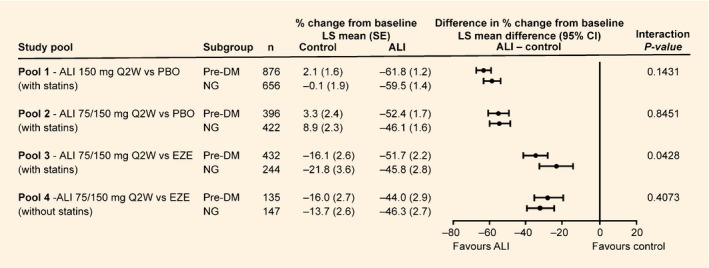
Percent change from baseline in calculated LDL cholesterol at week 24 according to prediabetes status (intention‐to‐treat analysis). ALI, alirocumab; DM, diabetes mellitus; EZE, ezetimibe; LS, least squares; NG, normoglycaemia; PBO, placebo; Q2W, every 2 weeks; LS means and se values taken from mixed‐effect model with repeated measures analysis.
As shown in Fig. 2, LDL cholesterol reductions from baseline to week 24 with alirocumab were 44.0–61.8% (prediabetes) and 45.8–59.5% (normoglycaemia). In each pool, differences in LDL cholesterol reductions between participants with prediabetes and those with normoglycaemia were not significant (interaction P values >0.05), except in Pool 3 (alirocumab 75/150 mg every 2 weeks vs ezetimibe with statins), which showed a somewhat greater reduction in the prediabetes than in the normoglycaemia group (interaction P value = 0.0428).
Changes in lipid levels in participants with prediabetes from baseline to week 24 in each pool according to baseline fasting triglyceride and HDL cholesterol levels are shown in Table S1. In all pools, LDL cholesterol reductions were similar in participants with prediabetes with baseline triglycerides < and ≥1.7 mmol/l (< and ≥150 mg/dl; interaction P values >0.05), except in Pool 1 (alirocumab 150 mg every 2 weeks vs placebo, with statins), which showed slightly higher LDL cholesterol reductions in participants with prediabetes and baseline triglycerides ≥1.7 vs <1.7 mmol/l (interaction P value = 0.0194). LDL cholesterol reductions were similar in participants with prediabetes with baseline HDL cholesterol < and ≥1.0 mmol/l (< and ≥40 mg/dl) in all pools (interaction P values >0.05). Comparable reductions in non‐HDL cholesterol, ApoB and Lp(a) were observed in participants with prediabetes regardless of baseline triglyceride and HDL cholesterol levels (interaction P values >0.05), except in Pool 4 (alirocumab 75/150 mg every 2 weeks vs ezetimibe without statins), which showed higher non‐HDL cholesterol and ApoB reductions in participants with prediabetes with HDL cholesterol <1.0 vs ≥1.0 mmol/l (interaction P values = 0.0288 for non‐HDL cholesterol and 0.0214 for ApoB).
An analysis of the proportion of participants with prediabetes in each of the two pools who required an alirocumab dose increase at week 12 stratified by baseline triglyceride and HDL cholesterol levels is shown in Fig. 3.
Figure 3.
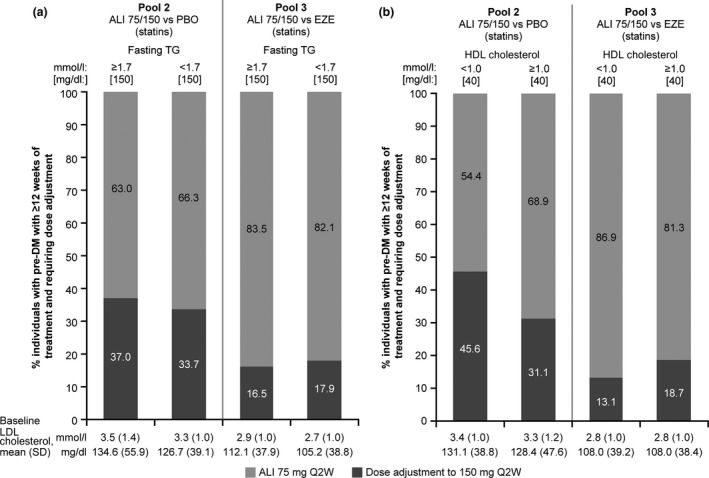
The proportion of individuals with prediabetes requiring dose adjustment at week 12 according to (a) fasting triglycerides and (b) HDL cholesterol levels (intention‐to‐treat analysis). ALI, alirocumab; DM, diabetes mellitus; EZE, ezetimibe; PBO, placebo; Q2W, every 2 weeks; TG, triglycerides. Only individuals with at least 12 weeks of alirocumab treatment duration were included. Dose adjustment data for Pool 4 (no statin) are not presented as there was an administrative error during the MONO study (dose adjustment threshold of 1.8 mmol/l [70 mg/dl] instead of 2.6 mmol/l [100 mg/dl] was utilized).
Safety
Alirocumab treatment was not associated with changes in mean HbA1c and FPG levels over time, regardless of prediabetes status (Fig. 4).
Figure 4.
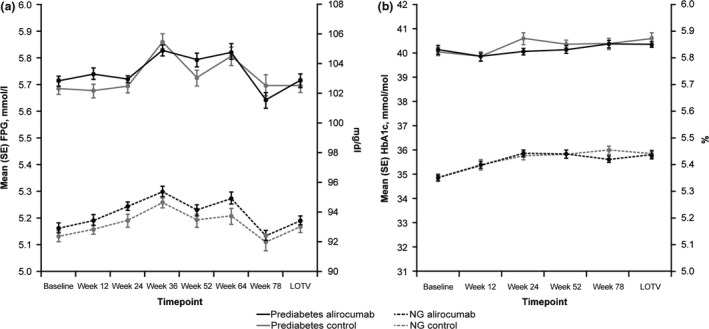
Mean (se) (a) FPG and (b) HbA1c over time in individuals with prediabetes or normoglycaemia in the pool of 10 phase III ODYSSEY studies (safety population). FPG, fasting plasma glucose; LOTV, last on‐treatment value; NG, normoglycaemia. LOTV defined as the last value collected up to 21 days after the last double‐blind investigational medical product injection. Approximate values of HbA1c in mmol/mol are shown on the left‐hand y‐axis in panel (b).
The TEAE frequencies were generally similar in participants with prediabetes and those with normoglycaemia, and in the alirocumab vs the control group (Table 2). The most common TEAEs in ≥5% of individuals varied across groups, but tended to include nasopharyngitis, myalgia, injection‐site reaction, upper respiratory tract infection and headache. The alirocumab‐treated groups had a slightly greater incidence of injection‐site reactions than the controls.
Table 2.
Safety analysis according to prediabetes status (safety population)
| n (%) | Placebo‐controlled trials | Ezetimibe‐controlled trials | ||||||
|---|---|---|---|---|---|---|---|---|
| Prediabetes (N = 1283) | Normoglycaemia (N = 1082) | Prediabetes (N = 574) | Normoglycaemia (N = 397) | |||||
| Alirocumab (N = 863) | Placebo (N = 420) | Alirocumab (N = 717) | Placebo (N = 365) | Alirocumab (N = 332) | Ezetimibe (N = 242) | Alirocumab (N = 223) | Ezetimibe (N = 174) | |
| TEAEs | 668 (77.4) | 335 (79.8) | 537 (74.9) | 281 (77.0) | 236 (71.1) | 173 (71.5) | 158 (70.9) | 119 (68.4) |
| Treatment‐emergent SAEs | 114 (13.2) | 60 (14.3) | 89 (12.4) | 35 (9.6) | 47 (14.2) | 32 (13.2) | 24 (10.8) | 14 (8.0) |
| TEAEs leading to death | 4 (0.5) | 4 (1.0) | 4 (0.6) | 2 (0.5) | 0 | 4 (1.7) | 1 (0.4) | 1 (0.6) |
| TEAEs leading to discontinuations | 37 (4.3) | 18 (4.3) | 37 (5.2) | 20 (5.5) | 27 (8.1) | 22 (9.1) | 23 (10.3) | 17 (9.8) |
| TEAEs by preferred term in ≥ 5% of individuals | ||||||||
| Nasopharyngitis | 112 (13.0) | 55 (13.1) | 82 (11.4) | 41 (11.2) | 18 (5.4) | 15 (6.2) | 18 (8.1) | 13 (7.5) |
| Myalgia | 39 (4.5) | 19 (4.5) | 42 (5.9) | 14 (3.8) | 19 (5.7) | 17 (7.0) | 24 (10.8) | 18 (10.3) |
| Injection‐site reaction | 62 (7.2) | 21 (5.0) | 72 (10.0) | 28 (7.7) | 11 (3.3) | 5 (2.1) | 7 (3.1) | 3 (1.7) |
| Upper respiratory tract infection | 60 (7.0) | 32 (7.6) | 31 (4.3) | 19 (5.2) | 23 (6.9) | 16 (6.6) | 12 (5.4) | 7 (4.0) |
| Headache | 39 (4.5) | 19 (4.5) | 46 (6.4) | 27 (7.4) | 19 (5.7) | 11 (4.5) | 8 (3.6) | 3 (1.7) |
| Influenza | 50 (5.8) | 19 (4.5) | 52 (7.3) | 21 (5.8) | 7 (2.1) | 5 (2.1) | 11 (4.9) | 4 (2.3) |
| Diarrhoea | 56 (6.5) | 21 (5.0) | 28 (3.9) | 11 (3.0) | 9 (2.7) | 7 (2.9) | 10 (4.5) | 5 (2.9) |
| Arthralgia | 43 (5.0) | 23 (5.5) | 33 (4.6) | 23 (6.3) | 18 (5.4) | 10 (4.1) | 8 (3.6) | 7 (4.0) |
| Back pain | 38 (4.4) | 25 (6.0) | 35 (4.9) | 20 (5.5) | 9 (2.7) | 7 (2.9) | 5 (2.2) | 9 (5.2) |
| Dizziness | 21 (2.4) | 18 (4.3) | 24 (3.3) | 13 (3.6) | 8 (2.4) | 14 (5.8) | 11 (4.9) | 5 (2.9) |
SAE, serious adverse event; TEAE, treatment emergent adverse event.
Discussion
This analysis pooled data from 10 phase III ODYSSEY trials to assess the efficacy and safety of alirocumab in people with hypercholesterolaemia categorized as having either prediabetes or normoglycaemia at baseline. In this analysis, alirocumab significantly reduced LDL cholesterol levels from baseline by a similar extent regardless of prediabetes status. These findings are consistent with previous sub‐analyses of these phase III trials by diabetes status, which showed no significant differences in LDL cholesterol reductions between individuals with vs without diabetes on alirocumab vs control (interaction P values >0.05; Table S2) 9, 10, 12, 22. Individuals with hyperglycaemia often have mixed dyslipidaemia, including elevated triglyceride and reduced HDL cholesterol levels, and reductions in ApoB and non‐HDL cholesterol levels may have stronger associations with cardiovascular risk than LDL cholesterol in such individuals 2. However, in the present analysis, improvements in LDL cholesterol, non‐HDL cholesterol, ApoB, and Lp(a) levels with alirocumab were generally comparable in participants with prediabetes with or without elevated triglyceride or reduced HDL cholesterol levels. Exceptions were seen in Pool 1 (alirocumab 150 mg every 2 weeks vs placebo, with statins), which showed slightly higher LDL cholesterol reductions in participants with prediabetes with baseline triglycerides ≥1.7 vs <1.7 mmol/l (≥ vs <150 mg/dl), and in Pool 4 (alirocumab 75/150 mg every 2 weeks vs ezetimibe, without statin), which showed higher reductions in non‐HDL cholesterol and ApoB levels in participants with prediabetes and baseline HDL cholesterol <1.0 vs ≥1.0 mmol/l (< vs ≥40 mg/dl); these sub‐group comparisons would be interesting to analyse further in the large clinical outcomes trial of alirocumab (ODYSSEY OUTCOMES; NCT01663402) 25. Alirocumab is also being studied specifically in people with Type 2 diabetes and mixed dyslipidaemia in the ODYSSEY DM‐DYSLIPIDEMIA trial (NCT02642159) 26.
Recent evidence suggested a link between PCSK9 genetic loss‐of‐function variants that reduce LDL cholesterol levels and increases in FPG levels and diabetes risk 15, 16. However, one prospective study found no association between the PCSK9 loss‐of‐function variant R46L and the risk of new‐onset Type 2 diabetes during a 9‐year follow‐up 27. In the present analysis, alirocumab did not appear to affect glucose homeostasis. Although HbA1c and FPG levels were higher in participants with prediabetes than in those with normoglycaemia (as would be expected), levels were not significantly altered in either group over the course of treatment with alirocumab or control for up to 78 weeks. These results supplement findings from pooled or sub‐analyses of people with vs without diabetes 21, 22 and a recent pooled analysis of the same 10 phase III ODYSSEY trials in individuals without diabetes 20, in which no significant changes over time in HbA1c and FPG levels were observed with alirocumab or control. Furthermore, alirocumab did not appear to increase transition from normoglycaemia at baseline to prediabetes [hazard ratio 1.20 (95% CI 0.96–1.49) vs placebo and 0.88 (95% CI 0.59–1.32) vs ezetimibe), nor from prediabetes at baseline to new‐onset diabetes [hazard ratio 0.90 (95% CI 0.63–1.29) vs placebo and 1.10 (95% CI 0.57–2.12) vs ezetimibe]; note that there were too few individuals who transitioned from normoglycaemia to new‐onset diabetes (three in total) to perform a statistical analysis. Development of diabetes based on adverse events related to the development or worsening of diabetes was reported in 2.8% vs 3.8% (alirocumab vs placebo) and 1.9% vs 1.6% (alirocumab vs ezetimibe) of participants with prediabetes at baseline; no individuals with normoglycaemia at baseline developed diabetes per adverse event (transition to diabetes was also based on laboratory data) 20. These findings highlight that, despite the potential link between PCSK9 genetic variants and the increase in diabetes risk, treatment with a PCSK9 inhibitor and monoclonal antibodies (that reduce plasma LDL cholesterol levels by binding extracellular PCSK9 and ultimately increasing the extracellular density of LDL receptors) may not have the same biological effects as life‐long exposure to decreased levels of LDL cholesterol due to PCSK9 genetic variants 15. Indeed, PCSK9 monoclonal antibodies affect the PCSK9 extracellular pathway without altering the PCSK9 intracellular pathway, which remains poorly characterized, especially in β cells 28.
To further investigate an effect of alirocumab on glycaemic control, an analysis with a longer follow‐up and a larger number of individuals is needed. The ongoing ODYSSEY OUTCOMES trial will capture diabetes and prediabetes reported by investigators, as well as by serial HbA1c and FPG measurements in >18 000 high‐risk individuals with 2–5 years of follow‐up, and will allow further analyses and provide longer‐term data on whether alirocumab has any effects on glycaemic measures and the development of diabetes 25.
The overall safety profile was similar across all groups, except for a slightly higher rate of injection‐site reactions in alirocumab‐treated individuals. Previous data have indicated that the majority of study participants and physicians have considered the alirocumab pre‐filled pen easy to use, and participants have shown a willingness to self‐inject 29; furthermore, high rates of alirocumab treatment adherence (~98%) were reported in phase III studies 10, 12. Safety was consistent with the known profile of alirocumab 30 and no unexpected TEAEs were identified.
Overall, the findings from this analysis suggest that alirocumab is a generally well tolerated and effective lipid‐lowering treatment for individuals with dyslipidaemia and prediabetes and normoglycaemia, without increased risk of loss of glucose homeostasis, over a mean follow‐up of 24–104 weeks.
Funding sources
The ODYSSEY studies and the presented post hoc analysis were funded by Sanofi and Regeneron Pharmaceuticals, Inc.
Competing interests
L.A.L. reports personal fees from Aegerion and Esperion, grants and personal fees from Amgen, AstraZeneca, Eli Lilly and Company, Merck, Regeneron Pharmaceuticals, Inc. and Sanofi, and grants from the Medicine Company and Pfizer, outside the submitted work. D.M.‐W. reports speaker's bureau and consultant/advisory board fees from Amgen, AstraZeneca, Boehringer Ingelheim, MSD (Merck), Novartis, Novo Nordisk and Sanofi. A.L. and M.T.B.‐D. are employees and stockholders of Sanofi. R.S. is an employee of and shareholder in Regeneron Pharmaceuticals, Inc. B.C. reports research funding and personal fees from Sanofi and Regeneron Pharmaceuticals, Inc. during the conduct of the study; research funding from Pfizer; honoraria from AstraZeneca, Pierre Fabre, Janssen, Eli Lilly and Company, MSD Merck & Co., Novo Nordisk, Sanofi, and Takeda, outside the submitted work.
Acknowledgements
The authors would like to thank the following people for their contributions to data collection and analysis, assistance with statistical analysis, or critical review of the manuscript: Tu Nguyen, Jay Edelberg and Michael Howard (Sanofi), Robert Pordy, Carol Hudson and Eva‐Lynne Greene (Regeneron Pharmaceuticals, Inc.). Medical writing support was provided by Grace Shim, PhD, and Rob Campbell, PhD, employees of Prime (Knutsford, UK), funded by Sanofi and Regeneron Pharmaceuticals, Inc. Responsibility for all opinions, conclusions, and interpretation of data lies with the authors.
Diabet. Med. 35, 121–130 (2018)
References
- 1. American Diabetes Association . Standards of Medical Care in Diabetes ‐ 2017. Diabetes Care 2017; 40(Suppl. 1): S1–S2.27979885 [Google Scholar]
- 2. Bays HE, Jones PH, Orringer CE, Brown WV, Jacobson TA. National Lipid Association Annual Summary of Clinical Lipidology 2016. J Clin Lipidol 2016; 10: S1–43. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet 2012; 380: 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kei A, Rizos EC, Elisaf M. Statin use in prediabetic patients: rationale and results to date. Ther Adv Chronic Dis 2015; 6: 246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maki KC, Ridker PM, Brown WV, Grundy SM, Sattar N. The Diabetes Subpanel of the National Lipid Association Expert P . An assessment by the Statin Diabetes Safety Task Force: 2014 update. J Clin Lipidol 2014; 8(Suppl 3): S17–29. [DOI] [PubMed] [Google Scholar]
- 6. Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res 2012; 53: 2515–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni‐Berthold I et al Alirocumab as add‐on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab 2015; 100: 3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farnier M, Jones P, Severance R, Averna M, Steinhagen‐Thiessen E, Colhoun HM et al Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis 2016; 244: 138–146. [DOI] [PubMed] [Google Scholar]
- 9. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R et al ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36: 2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U et al Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J 2015; 169: 906–915, e13. [DOI] [PubMed] [Google Scholar]
- 11. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ et al Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol 2015; 9: 758–769. [DOI] [PubMed] [Google Scholar]
- 12. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M et al Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 13. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R et al Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J 2015; 36: 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG et al Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double‐blind, randomized Phase 3 trial. Int J Cardiol 2014; 176: 55–61. [DOI] [PubMed] [Google Scholar]
- 15. Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR et al Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med 2016; 375: 2144–2153. [DOI] [PubMed] [Google Scholar]
- 16. Schmidt AF, Swerdlow DI, Holmes MV, Patel RS, Fairhurst‐Hunter Z, Lyall DM et al PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol 2016; 5: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ et al Effect of evolocumab on progression of coronary disease in statin‐treated patients: the GLAGOV randomized clinical trial. JAMA 2016; 316: 2373–2384. [DOI] [PubMed] [Google Scholar]
- 18. Sattar N, Preiss D, Robinson JG, Djedjos CS, Elliott M, Somaratne R et al Lipid‐lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta‐analysis of individual patient data. Lancet Diabetes Endocrinol 2016; 4: 403–410. [DOI] [PubMed] [Google Scholar]
- 19. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA et al Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 20. Colhoun HM, Ginsberg HN, Robinson JG, Leiter LA, Muller‐Wieland D, Henry RR et al No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J 2016; 37: 2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ginsberg HN, Farnier M, Robinson JG, Cannon CP, Sattar N, Baccara‐Dinet MT et al Efficacy and safety of alirocumab: pooled analyses of 1048 individuals with diabetes mellitus from five placebo‐controlled Phase 3 studies of at least 52 weeks duration. Circulation 2015; 132: A17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leiter LA, Zamorano JL, Bujas‐Bobanovic M, Louie MJ, Lecorps G, Cannon CP et al Lipid‐lowering efficacy and safety of alirocumab in patients with or without diabetes: a sub‐analysis of ODYSSEY COMBO II. Diabetes Obes Metab 2017; 19: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ginsberg HN, Rader DJ, Raal FJ, Guyton JR, Baccara‐Dinet MT, Lorenzato C et al Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL‐C of 160 mg/dl or higher. Cardiovasc Drugs Ther 2016; 30: 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gaudet D, Watts GF, Robinson JG, Minini P, Sasiela WJ, Edelberg J et al Effect of alirocumab on lipoprotein(a) over ≥1.5 years (from the Phase 3 ODYSSEY Program). Am J Cardiol 2017; 119: 40–46. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R et al Effect of alirocumab, a monoclonal antibody to PCSK9, on long‐term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J 2014; 168: 682–689. [DOI] [PubMed] [Google Scholar]
- 26. Müller‐Wieland D, Leiter LA, Cariou B, Letierce A, Colhoun HM, Del Prato S et al Design and rationale of the ODYSSEY DM‐DYSLIPIDEMIA trial: lipid‐lowering efficacy and safety of alirocumab in individuals with type 2 diabetes and mixed dyslipidemia at high cardiovascular risk. Cardiovasc Diabetol 2017; 16: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bonnefond A, Yengo L, Le May C, Fumeron F, Marre M, Balkau B et al The loss‐of‐function PCSK9 p. R46L genetic variant does not alter glucose homeostasis. Diabetologia 2015; 58: 2051–2055. [DOI] [PubMed] [Google Scholar]
- 28. Cariou B, Si‐Tayeb K, Le May C. Role of PCSK9 beyond liver involvement. Curr Opin Lipidol 2015; 26: 155–161. [DOI] [PubMed] [Google Scholar]
- 29. Roth EM, Bujas‐Bobanovic M, Louie MJ, Cariou B. Patient and physician perspectives on mode of administration of the PCSK9 monoclonal antibody alirocumab, an injectable medication to lower LDL‐C levels. Clin Ther 2015; 37: 1945–1954, e6. [DOI] [PubMed] [Google Scholar]
- 30. Jones PH, Bays HE, Chaudhari U, Pordy R, Lorenzato C, Miller K et al Safety of alirocumab (a PCSK9 monoclonal antibody) from 14 randomized trials. Am J Cardiol 2016; 118: 1805–1811. [DOI] [PubMed] [Google Scholar]


