SUMMARY
The human gut is colonized by a large number of microorganisms (~1013 bacteria) that support various physiologic functions. A perturbation in healthy gut microbiome might leads to the development of inflammatory diseases including multiple sclerosis (MS). Therefore, gut commensals can provide promising therapeutic options for treating autoimmune diseases such as MS. We report identification of human gut–derived commensal bacteria, Prevotella histicola, which can suppress an autoimmune disease in HLA class-II transgenic model of experimental autoimmune encephalomyelitis (EAE); an animal model of MS. P. histicola suppresses disease through modulation of systemic immune responses. P. histicola challenge led to a decrease in pro-inflammatory Th1 and Th17 cells, and increase in the frequencies of CD4+FoxP3+ regulatory T cells, tolerogenic dendritic cells, and suppressive macrophage. Our study provides evidence that administration of gut commensals may regulate a systemic immune response and may, therefore, have a possible role in the treatment strategies for MS.
INTRODUCTION
The human gut is colonized by a large number of microorganisms (~1013 bacteria) that support various physiologic functions (Sender et al., 2016). Recent research envisages humans as holobionts having evolved with our microbiome with our gut microbiome being vital to human health (Charbonneau et al., 2016; Honda and Littman, 2016). The increased prevalence of inflammatory diseases in developed countries has been attributed to an altered gut microbiome that is characteristically linked with the disease state. Therefore, gut commensals capable of restoring the healthy microbiome could be a promising therapy for treating inflammatory diseases such as multiple sclerosis (MS). Although several therapies are available, none cure the disease and many are not well tolerated.
The hypothesis that MS is an autoimmune disease caused by myelin-specific CD4 T-cells comes from experimental autoimmune encephalomyelitis (EAE), an animal model of MS (Gold et al., 2000). CD4 T cell repertoire is selected in humans by human leukocyte antigen (HLA)-class II molecules, and MS patients show increased frequency of particular HLA class II haplotypes such as HLA-DR2/DQ6, DR3/DQ2, and DR4/DQ8 (Dyment et al., 2005; Zivadinov et al., 2007). We have used transgenic mice expressing human class II genes and lacking endogenous class II genes to identify disease-susceptible and resistant class II alleles (Luckey et al.; Mangalam et al., 2008). Transgenic mice expressing HLA-DR3 and DQ8 genes (HLA-DR3.DQ8) develop EAE and have severe brain and spinal cord pathology (Mangalam et al., 2009a; Mangalam et al., 2004).
During recent decades, the incidence of autoimmune diseases in developed countries has increased steadily (Bach, 2002; Okada et al., 2010). Numerous hypotheses have been suggested to explain this phenomenon, including alterations of the gut microbiome due to decreased exposure to parasites, antibiotics, western diet, and other environmental factors (Rook, 2012). Additionally, western diets are associated with an abundance of the Bacteroides enterotype, whereas the Prevotella enterotype is more prevalent in persons with a carbohydrate-rich, high-fiber, agrarian diet (Wu et al., 2011).
As certain commensal bacteria residing in the intestine might have immunomodulatory properties, we cultured proximal small bowel biopsies from Coeliac disease patients to isolate and characterize gut commensal with the ability to modulate immune response (Marietta et al., 2016). We isolated Prevotella histicola (P. histicola), Prevotella melaninogenica (P. melaninogenica), and Capnocytophaga sputigena (C. sputigena) and investigated the ability of these anaerobic, gram-negative Bacteroidetes commensals to modulate PLP91–110-induced EAE in HLA-DR3.DQ8 transgenic mice. Here, we report that a strain of P. histicola can suppress or prevent disease in EAE. We further show that P. histicola suppresses disease through downregulation of pro-inflammatory Th1/Th17 response and induction of regulatory CD4+FoxP3+ regulatory T cells (Tregs).
RESULTS
Treatment with P. histicola suppressed PLP91–110-induced EAE in HLA-DR3.DQ8 transgenic mice
Previously, we showed that double-transgenic mice expressing human HLA-DR3 and DQ8 (HLA-DR3.DQ8) develop EAE with CNS pathology (Mangalam et al., 2009a). Therefore, we tested the immunomodulatory capabilities of P. histicola, P. melaninogenica, and C. sputigena in HLA-DR3.DQ8 transgenic mice (See Figure S1A). Among three bacteria tested, P. histicola treated group had higher levels of IL-10 and TGF-β compared to medium or C. sputigena. Mice treated with P. melaninogenica had higher levels of IL-10 but no change in levels of TGF-β or TNF-α. Next, we tested if these commensals modulated EAE in HLA-DR3.DQ8 transgenic mice.
EAE was induced in HLA-DR3.DQ8 transgenic mice (Mangalam et al., 2009a) and 7 days post-immunization, animals were challenged with 1×109 CFU/ml bacteria or medium (Lavasani et al., 2010). Mice were gavaged every other day with P. histicola, P. melaninogenica, C. sputigena, mouse E. coli (control), or medium alone and monitored for disease incidence and severity. P. histicola treated mice had reduced disease incidence, with only 5 of 20 mice (25%) developing EAE compared with 100% EAE incidence (20/20) in medium fed mice (P<0.005) (Figure 1A, 1B, and Table 1). Challenge with P. melaninogenica had a mild suppressive effect but the cumulative disease score was not different from control groups. Whereas all mice in the C. sputigena or E. coli treated group developed disease. Because only P. histicola challenged (13.6±18.9 vs 76.75±7.7; P<0.001) (Figure 1B) but not P. melaninogenica challenged mice had less cumulative disease, we focused on P. histicola for later experiments. Further, disease onset was delayed in P. histicola challenged mice compared with medium challenged mice (17.5±0.3 vs 10.6±0.2 days; P<0. 005) (Table 1). To address whether colonization with P. histicola alone can modulate the disease, we depleted microbial flora of mice using broad acting antibiotics for three weeks (Rakoff-Nahoum et al., 2004) and gavaged with medium or P. histicola. P. histicola challenged group developed milder EAE compared to medium-treated group (See Figure S1B). These data suggests that P. histicola alone can modulate EAE in HLA-DR3.DQ8 mice.
Figure 1. Effect of various human commensals on PLP91–110-induced EAE in HLA-DR3.DQ8 transgenic mice.
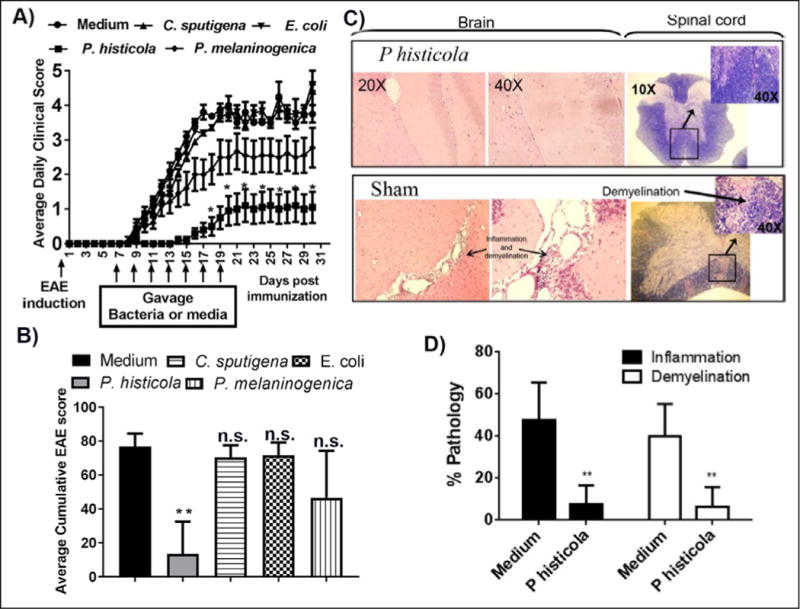
Animals were immunized with PLP91–110 peptide and treated with either bacteria or medium starting at day 7 post-immunization and every other day for a total of 7 doses. A) Only P. histicola treated mice had late disease onset and lower disease incidence. No significant effect was observed in the groups receiving P. melaninogenica, C. sputigena, or E. coli. Animals were scored daily for disease, and the daily mean disease score for each group is plotted. Error bars represent the standard error of mean. B) P. histicola treated animals had a lower average cumulative disease score compared with medium treated animals. The data in figures A and B represent average cumulative scores of 3 experiments performed at different times with n=10 mice per group. C) Mice were sacrificed on day 30 post-immunization and the brain and spinal cord were examined by histopathology. Representative photomicrographs show mild to severe inflammation and demyelination in medium treated mice compared with P. histicola treated mice. D) Quantitative analysis of spinal cord pathology similarly showed increased inflammation and demyelination in medium treated groups compared with the P. histicola treated group. The data in figures C and D represent 1 of 3 experiments performed at different time points (n=5 mice per group). A single asterisk indicates p≤0.05, double asterisks indicate p≤0.005, and ‘n.s.’ indicates not significant, when compared to medium treated group.
Table 1.
Effect of commensal bacteria on PLP91–110 induced EAE in HLA-DR3.DQ8 transgenic mice
| Treatment | Disease incidence (%) | Disease free, Mean ± SE, daysA | Hazard ratio (95% CI) | p-value |
|---|---|---|---|---|
| Medium fed | 20/20 (100%) | 10.6 ± 0.2 | 1 (ref) | |
| P. histicola | 5/20 (25%) | 17.5 ± 0.3 | 0.03 (0.01–0.09) | <0.0001 |
| C. sputigena | 20/20 (100%) | 11.4 ± 0.4 | 0.6 (0.3–1.2) | 0.15 |
| P. melaninogenica | 16/20 (80%) | 12.5 ± 0.4 | 0.2 (0.1 –0.5) | <0.0009 |
| E. coli | 20/20 (100%) | 11 ± 0.3 | 0.8 (0.4 – 0.6) | 0.47 |
Estimated by the log-rank test
P. histicola challenge did not cause any pathology in the upper gut (See Figure S1C). To investigate which part of the gut P. histicola colonize naïve mice (8–12 weeks old) were treated with either medium or P. histicola. Utilizing qPCR and Prevotella specific primers, we observed higher colonization in the stomach and jejunum/ileum (See Figure S1D). Thus our data suggest that P. histicola might colonize the upper gut of HLA-DR3.DQ8 transgenic mice.
Disease-suppressive effects of P. histicola required viable bacteria
To determine whether P. histicola suppression of disease required whole bacteria or bacteria-derived soluble factors, we investigated the ability of cell-free P. histicola culture supernatant (PH-CS) to suppress EAE. The PH-CS–treated group had a disease incidence of 67% (10/15) compared with a 27% incidence rate in the P. histicola – challenged group (See Table S1). Mice challenged with PH-CS had a greater risk of developing EAE than mice given live P. histicola (hazard ratio, 3.3 [95% CI, 1.03–10.5]; P=0.04), indicating that PH-CS had a modest effect on disease incidence (Supplemental Figure 2). The risk of EAE development was significantly lower in the live-bacterium group (hazard ratio, 7.7 [95% CI, 2.5–24]; P=0.005). We tested live P. histicola in doses that ranged from 1×107 to 1×109 CFU/ml and observed a dose-dependent effect with optimal suppression at 1×108 CFU/ml (See Table S2).
Treatment with P. histicola reduced inflammation and demyelination in the CNS
Analysis of CNS tissues from medium challenged HLA-DR3.DQ8 transgenic mice showed severe inflammation and demyelination in the brain and spinal cord compared to the P. histicola challenged groups (Figure 1C). Quantitative analysis of spinal cord inflammation and demyelination showed that P. histicola challenged animals had fewer regions with inflammation and demyelination (Figure 1D). Groups receiving other bacteria (E. coli and C. sputigena) had severe CNS inflammation and demyelination similar to the medium challenged group. Thus, treatment with P. histicola reduced CNS inflammation and demyelination compared to the medium only group.
P. histicola administration downregulated PLP91–110–specific T-cell and cytokine response
To determine the effect of P. histicola on antigen-specific T cell responses, we isolated splenocytes from mice given bacteria or medium and stimulated with the PLP91–110 peptide. P. histicola challenged mice had less antigen-specific T cell response compared with medium or E. coli challenged group (Figure 2A). Splenocytes from P. histicola challenged mice produced less proinflammatory cytokines IL-17 and IFN-γ after stimulation with PLP91–110, whereas anti-inflammatory cytokine IL-10 was increased (Figure 2B). Levels of tumor necrosis factor (TNF)-α was similar among different groups (Figure 2B). Animals given control bacteria (E. coli) showed cytokine levels similar to the medium only group.
Figure 2. P. histicola treated mice show decreased T cell recall response, CNS inflammation and cytokine levels.
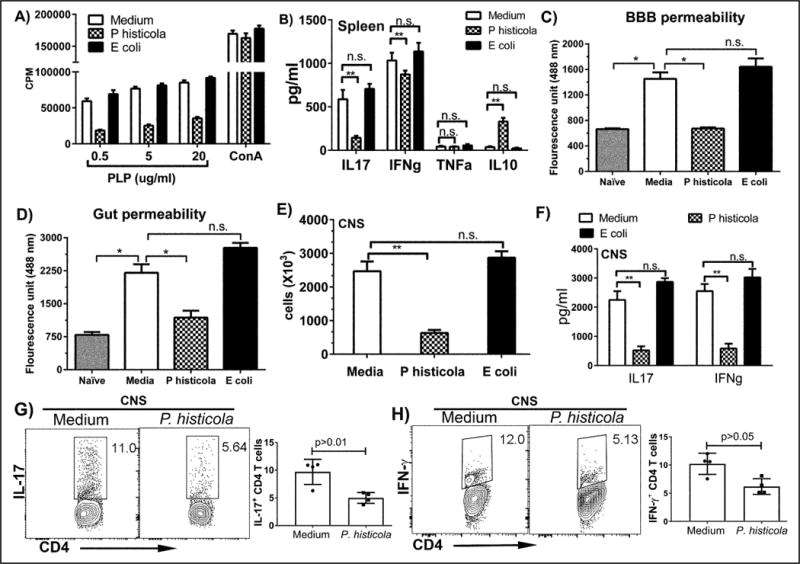
A) P. histicola treated animals had reduced T cell proliferative response to PLP91–110 peptide in vitro compared with the medium or E. coli treated group. Response to Concanavalin A was similar among three groups. The data presented are the mean±SD CPM. B) Splenocytes stimulated with antigen from P. histicola treated mice had reduced levels of inflammatory cytokines IL17 and IFN-γ, and increased levels of anti-inflammatory cytokine IL10 compared with medium treated mice. The data presented are the average of 2 independent experiments (n=4 mice per group). C) Medium treated HLA-DR3.DQ8 transgenic mice had compromised BBB permeability (increased FITC uptake in the brain). Challenge with P. histicola reduced BBB permeability and reduced gut permeability. E) Cellular infiltration into the CNS was reduced in P. histicola treated mice compared with medium or E. coli treated mice. F) CNS inflammatory cells from P. histicola treated group had reduced levels of inflammatory cytokines IFN-γ and IL-17 as measured by ELISA (F) and flow cytometry (G and H) compared with the medium treated group. The data represent 2 separate experiments performed in triplicate (n=5 mice per group). A single asterisk indicates p≤0.05; double asterisks indicate p≤0.005 and ‘n.s.’ indicates not significant, when compared to medium treated group.
P. histicola treatment reduced the blood brain barrier (BBB) and gut permeability and downregulated CNS inflammation
The P. histicola challenged group had reduced BBB permeability as measured by FITC-albumin compared with the medium only group (Figure 2C). Mice with EAE also had increased gut permeability, however, challenge with P. histicola restored (Figure 2D). Groups receiving E. coli had both increased BBB and gut permeability similar to the medium treated group (2C and D). CNS tissue from the P. histicola treated mice had reduced cellular infiltration compared with the control groups (Figure 2E) with lower levels of total and CD4 T cell specific IFN-γ and IL-17 expressing cells compared with the medium or E. coli groups (Figure 2F, G, and H). Thus, P. histicola decreased BBB permeability and reduced frequency of pro-inflammatory Th1 and Th17 cells in CNS.
P. histicola suppresses EAE through an increase in CD4+FoxP3+Tregs
To identify the cell population(s) responsible for the disease-protective effect, we characterized the cellular profile of bacterially challenged and untreated animals. Prevotella challenged mice had an increased frequency of CD4+FoxP3+ Tregs in the spleen and mesenteric lymph nodes (MLNs) (Figure 3 A–B). To test whether P. histicola could directly induce a Treg population, naïve HLA-DR3.DQ8 transgenic mice were challenged with 1×108 CFU/ml P. histicola or medium on alternate days for 7 doses. P. histicola treated animals had higher levels of CD4+CD25+FoxP3+ Tregs in splenocytes, MLNs, and cervical lymph nodes (CLNs) compared with the medium treated group (See Figure S3). CD4+CD25+ Treg cells isolated from P. histicola challenged animals also showed higher suppression of PLP91–110-specific CD4+ T effector cells compared with the Tregs isolated from naïve, medium, or E. coli treated mice (Figure 3C). Therefore, P. histicola increased regulatory CD4+ T cell numbers and also enhanced their suppressive function.
Figure 3. P. histicola challenge increased the frequency and activity of CD4+FoxP3+ regulatory T cells and tolerogenic DCs.
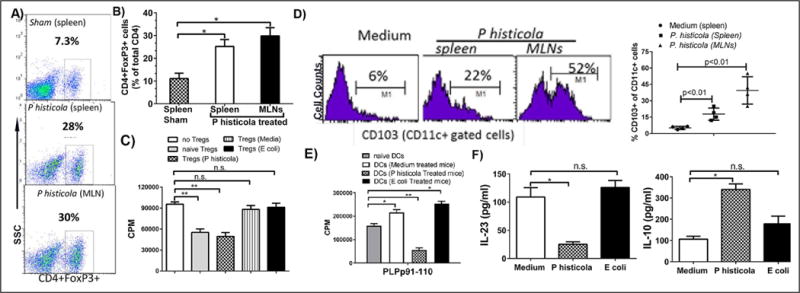
A) P. histicola treated mice had an increased frequency of CD4+FoxP3+ Tregs compared with the medium or E. coli treated group. Numbers in scatter plots indicate the percentage of positive cells. B) Histogram plot of Treg frequency in the P. histicola treated and untreated groups. The data presented are the average of 2 independent experiments (n=4 mice per group). C) CD4+CD25+ Tregs from P. histicola treated mice had increased suppressive activity compared with the medium or E. coli treated group. D) The P. histicola treated group had increased levels of CD103+CD11c+ DCs in the spleen and MLNs compared with the medium treated group. Numbers in the histogram indicate the percentage of CD103+ cells gated from the CD11c+ population. Data are from 1 of 3 experiments performed at different times. E) Co-culture of CD11c+ cells from the P. histicola treated group with PLP specific CD4 T cells resulted in reduced proliferation compared to medium treated or naïve group. The data are presented as the mean±SD CPM and are the average of 2 independent experiments with n=3 mice per group. F) CD11c+ DCs from the P. histicola treated group produced low IL-23 and high IL-10 compared with the medium treated group. The data are presented as the mean±SD CPM and are the average of 2 independent experiments (n=3 mice per group). A single asterisk indicates p≤0.05, double asterisks indicate p≤0.005 and ‘n.s.’ indicates not significant.
P. histicola challenge increased the frequency and activity of tolerogenic DCs
Next, we analyzed levels of CD103+CD11c+ tolerogenic DCs in MLNs, and splenocytes among different treatment groups. P. histicola treated animals had increased levels of CD103+CD11c+ DCs in the spleen and MLNs compared with medium treated group (Figure 3D). CD11c+ cells from P. histicola treated animals had lower antigen presentation capacity compared with CD11c+ DCs isolated from naïve, medium or E. coli treated mice (Figure 3E). Lipopolysaccharide-stimulated CD11c+ DCs from medium treated mice produced high levels of IL-23 and low levels of IL-10, whereas P. histicola treated animals produced low levels of IL-23 and high levels of IL-10 (Figure 3F). CD11c+ DCs isolated from E. coli treated group behaved similarly to medium treated group (Figure 3F). We also observed that P. histicola challenge led to induction of CD11b+Gr-1med macrophages (See Figure S4A) and CD11b+ cells from P. histicola challenge group had reduced antigen presentation capacity compared to medium group (See Figure S4B). Macrophages from P. histicola treated animals produced higher IL-10 and lower IL-12 (See Figure S4C) compared with medium or E.coli treated groups. Thus, P. histicola treated animals had an increased frequency of tolerogenic antigen presenting cells and decreased antigen presentation capacity compared with medium treated animals.
Splenocytes from P. histicola treated animals suppressed PLP91–110–induced EAE
Next, we tested whether adoptive transfer of immune cells from P. histicola treated mice would ameliorate EAE. We transferred splenocytes (1×107) from EAE-immunized animals treated with either P. histicola or medium into immunized mice 5 days after immunization (Mangalam et al., 2012). The group receiving splenocytes from P. histicola treated animals had lower disease incidence and severity compared with the untreated mice or those receiving splenocytes from medium treated animals (See Figure S5). Thus, our data indicated that the disease-suppressive effect of P. histicola was transferable.
P. histicola challenge caused shift in gut microbiota
Finally, to investigate whether treatment with P. histicola can alter gut microbiota composition, fecal samples were collected from pre (naïve) and post-immunized (EAE) mice receiving either medium or P. histicola. Mice with EAE had a distinct fecal microbiota compared to naïve mice (Figure 4A); however, challenge with P. histicola shifted gut microbiota composition closer to pre-immunized (naïve) mice. Naïve mice had a higher relative abundance of certain genera such as Prevotella, Lactobacillus, and Sutterella which were reduced in the EAE group receiving medium. However, mice induced for EAE and treated with P. histicola had increased abundance of Prevotella, Lactobacillus, and Sutterella (Figure 4B and C). Thus P. histicola challenge restored gut microbiota to pre-immunized state.
Figure 4. P. histicola challenge caused shift in gut microbiota.
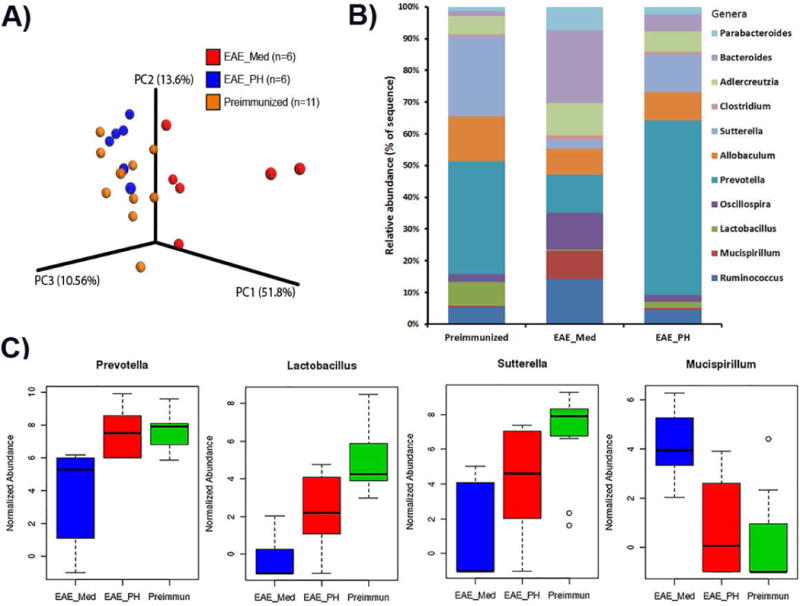
A) HLA-DR3.DQ8 transgenic mice with EAE had a distinct gut microbiome profile compared to those from pre-immunized (naïve mice). The P. histicola treated group had gut microbiota profile similar to pre-immunized (naïve mice) group. Weighted UniFrac-based 3D PCoA plot based on all OTUs from fecal samples of mice from, pre-immunized (n=6), medium treated EAE group (n=11), and P. histicola treated group (n=6). B) Relative abundance of gut microbiota at the genus levels in pre-immunized (naïve mice), EAE mice treated with medium, and EAE mice treated with P. histicola. C) Box plot showing normalized relative abundance of bacteria (genus-level profile) showing difference between groups. Mice with EAE had loss of certain genera especially Prevotella, Sutterella, and Lactobacillus compared to pre-immunized (naïve) mice. P. histicola challenge restored abundance of Prevotella, Sutterella, and Lactobacillus. The difference between groups were analyzed using one-way ANOVA (Kruskal-Wallis rank sum test) and FDR-adjusted p<0.05.
DISCUSSION
We have identified a commensal bacterium Prevotella histicola (P. histicola) from the human upper gastrointestinal tract that had a systemic suppressive effect distant from the small intestine. P. histicola inhibited the development of EAE in HLA-DR3.DQ8 transgenic mice, a preclinical model of MS. We observed that Prevotella histicola treatment markedly attenuated inflammation, demyelination, and reduced BBB permeability compared to medium or control bacteria. We demonstrated that Prevotella histicola suppressed disease by downregulating pro-inflammatory cytokines IFNγ and IL-17, inducing CD4+CD25+FoxP3+ Tregs, tolerogenic DCs, and suppressive macrophages. These observations suggest that Prevotella histicola monotherapy is effective when administered to HLA transgenic mice, a model of the chronic disease. Finally, that disease protection induced by Prevotella histicola was transferable into other animals; strengthening our hypothesis that Prevotella histicola protects the mice from EAE by modulating the systemic immune response. Our study shows that a human commensal from the upper gastrointestinal tract possesses potent disease-protective characteristics and provide proof of principle that human commensal gut bacteria protect against neuroinflammation.
Recent studies have indicated that human commensal bacteria (i.e., the microbiome) are a new frontier of biologic discovery with great therapeutic potential. Examples of bacterial therapeutics for ameliorating EAE include B. fragilis (Round and Mazmanian, 2010; Surana and Kasper, 2012) and mixtures of Lactobacillus strains (Lavasani et al., 2010). The disease-protective effects of B. fragilis have been attributed to its production of polysaccharide A (PSA) (Ochoa-Reparaz et al., 2010). However, live P. histicola was needed for maximal disease protection in our study, which suggests that the interaction of the living bacteria with the gut mucosal immune systems may be required. The ability of P. histicola to suppress EAE in mice with depleted gut flora suggests a direct role for P. histicola in disease suppression. However we cannot rule out potential additional indirect effects through modulation of other microbes because P. histicola treatment was also associated with a shift in gut microbiota to more closely resemble a healthy mouse with an increase in the relative abundance of Prevotella, Sutterella and Lactobacillus at the genus level. Although both Prevotella and Sutterella genera had been shown to be increased in RRMS patients on disease modifying treatments (Jangi et al., 2016), it is currently unclear whether the species of Prevotella (i.e. P. histicola) used in our study is part of the observed alteration of Prevotella at the genus levels in patients with MS. Previous study has shown that B. fragilis, a gut commensal can influence host behavior by modulation of gut microbes (Hsiao et al., 2013). Thus it is possible that P. histicola induced changes in gut microbiome might also contribute to its disease protective effect either directly, indirectly or both.
P. histicola challenge was associated with elevated levels of IL-10, CD4+FoxP3+ Tregs, tolerogenic DCs and suppressive macrophages. The disease-protective effect of other human commensals such as B. fragilis and a mixture of Lactobacillus species in EAE have been attributed to their ability to induce CD4+FoxP3+ Tregs and IL-10 (Lavasani et al., 2010; Ochoa-Reparaz et al., 2010). A role of tolerogenic DCs observed in our study is consistent with earlier studies (Ochoa-Reparaz et al., 2010) showing a role for CD11c+ DCs in suppressing disease in a PSA-treated EAE model. P. histicola increased levels of tolerogenic CD103+ DCs producing high levels of IL-10 and low levels of IL-23.
Both IL-17 and IFN-y are the major pro-inflammatory cytokines associated with the pathology of MS. P. histicola treatment suppressed myelin antigen–specific T cell recall response and reduced IL-17 and IFN-γ in the periphery as well as in CNS. Thus, P. histicola might mediate its effect through downregulation of pro-inflammatory cells of both Th1 and Th17 phenotypes. Once inflammatory cells are activated in the periphery, their movement across the BBB and into the CNS is an essential step for initiating inflammation and demyelination in the brain and spinal cord. Reduced BBB permeability and milder pathology in the brain and spinal cord in P. histicola treated mice suggests that P. histicola mediates its suppressive effect through modulation of inflammatory cells trafficking to CNS.
Our study demonstrates that P. histicola can suppress EAE in mice, however it is currently unclear whether P. histicola supplementation will be effective as a treatment for MS. Interestingly, genus Prevotella are reduced in relapsing remitting MS (RRMS) patients compared with healthy controls (Chen et al., 2016; Miyake et al., 2015) and increased in RRMS patients on disease modifying treatments (Jangi et al., 2016). Thus human MS studies suggest that individual or a combination of Prevotella strains might have immunomodulatory properties; however, it is unknown whether these changes at genus level correlate with changes in P. histicola or some other species of Prevotella.
In summary, we report the association of a human commensal bacterium with disease-protective abilities in an animal model of MS. Our data indicate that P. histicola, a human commensal, has immunomodulatory and anti-inflammatory capabilities that suppress PLP91–110-induced EAE in HLA-DR3.DQ8 transgenic mice. The disease protection was due to the ability of P. histicola to induce regulatory and suppressive immune subsets. Our future studies will study whether P. histicola modulates human immune cells directly or via its interaction with intestinal epithelial cells. This work demonstrates that P. histicola can manipulate the systemic immunity and organ-specific disease far away from its localization in the gut.
EXPERIMENTAL PROCEDURE
Mice
HLA-DR3.DQ8 double-transgenic [DQ8 (DQA1*0103, DQB1*0302)-DR3 (DRB1*0301] mice lacking MHC class II genes (AE−/−), were generated as previously described (Das et al., 2000; Mangalam et al., 2009b) and are referred as HLA-DR3.DQ8 transgenic mice in the text. Mice of both sexes were studied. All mice were bred and maintained in the pathogen-free Immunogenetics Mouse Colony of Mayo Clinic (Rochester, Minnesota) and University of Iowa in the accordance with NIH and institutional guidelines. All experiments were approved by the Institutional Animal Care and Use Committee at Mayo Clinic, Rochester, and University of Iowa, Iowa City.
Isolation and culture of bacteria
Prevotella and similar anaerobic gram-negative Bacteroidetes bacteria were isolated from the duodenum of treated coeliac disease patients, and their identity was confirmed using 16S rRNA specific PCR followed by sequencing as well as whole genome sequencing as described previously (Marietta et al., 2016).
Flow Cytometry
Expression of HLA-DR and HLA-DQ molecules on peripheral blood leukocytes, lymph node cells, and splenocytes were analyzed by flow cytometry. Additional details are provided in the supplemental methods.
Disease induction and scoring
For disease induction, 8–12 week-old transgenic mice were immunized subcutaneously in both flanks with 100 μg of PLP91–110. Disease severity was scored as described previously (Mangalam et al., 2009a) with additional details provided in the supplemental methods.
Treatment of animals with commensal bacteria
HLA-DR3.DQ8 transgenic mice were treated with P. histicola, P. melaninogenica, C. sputigena, and mouse-specific E. coli. or media by oral gavage, starting 7 days after immunization. Animals were gavage-fed on alternate days with 1×108 colony-forming units (CFU) in 100 μL of culture medium or medium alone for a total of 7 doses. Mice were evaluated for disease incidence, duration, and severity for 4 weeks after immunization.
T cell proliferation and cytokine assay
T-cell recall response was measured in splenocytes and lymph nodes from immunized mice using standard thymidine incorporation methods (Das et al., 2000).
Gut flora depletion and colonization with Prevotella
Gut flora was depleted by giving broad spectrum antibiotic cocktail (0.5 g/L vancomycin, 1g/L neomycin sulfate, 1g/L metronidazole, 1g/L ampicillin) in drinking water as described previously (Rakoff-Nahoum et al., 2004). After three weeks of antibiotic treatment, animals were placed on sterile water for 3 days before challenged with P. histicola (108 CFU) or medium on alternate days for the total of seven doses. One week after the last dose, EAE was induced and disease was monitored (Mangalam et al., 2009a).
Isolation of Tregs, dendritic cells, and macrophages
Tregs, dendritic cells (DCs), and macrophages were isolated from splenocytes using commercial cell isolation kits (Miltenyi Biotec Inc; San Diego, CA). Additional details are provided in SI methods.
In vitro functional analysis of Tregs, DCs, and macrophages
Suppressive abilities of CD4+CD25+ Tregs isolated from P. histicola or medium treated HLA-DR3.DQ8 transgenic mice were analyzed by coculturing Tregs (5×104 cells/well) with PLP91–110-specific CD4+ T cells (5×104 cells/well) in the presence of irradiated antigen-presenting cells (APCs) loaded with antigen. Additional details are provided in Sl methods. Lipopolysaccharides from E. Coli 026:B6 (Sigma-Aldrich, St. Louis, MO, USA) was used to stimulate macrophages and dendritic cells.
Adoptive transfer of splenocytes
The splenocytes were collected from EAE-immunized animals treated with either P. histicola or medium and transferred (1×107 cells/mouse) intravenously into PLP91–110-immunized HLA-DR3.DQ8 transgenic mice 5 days after immunization. The timing for adoptive transfer studies was based on our previous study (Mangalam et al., 2012).
Microbiome Analysis
Fecal pellets were collected from pre and post-immunized mice. Microbial DNA extraction, 16S amplicon preparation (V3-V5 region), and sequencing were done as described previously (Chen et al., 2016). R1 and R2 fastq reads were merged using Paired-End read merger (PEAR) (Zhang et al., 2014), merged reads were converted to fasta and merged fasta sequences were processed by Cloud Virtual Resource (CloVR) (Angiuoli et al., 2011) to form operational taxonomic units (OTUs) at 97% similarity and histograms were generated using METAGENassist (Arndt et al., 2012).
Pathology
Brain and spinal cord sections were analyzed for inflammation and demyelination after staining with modified hematoxylin and eosin as described previously (Mangalam et al., 2009a). Additional details are provided in Sl methods.
Statistical analysis
Differences in proliferation or cytokine levels between groups was assessed by a one-way analysis of variance with multiple comparisons of the means when more than two groups were analyzed, or by Student’s t-test when only two groups were analyzed if their data were normally distributed. The Kaplan-Meier method was used to estimate the probability of survival. The log-rank test was used to compare outcomes of different treatment groups. Hazard ratios (HR) for developing disease (and 95% confidence intervals [CI]) calculated from the estimated coefficients in Cox proportional hazards regression models. All statistical analyses were done with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA) or SAS version 9.3 (SAS Institute, Cary, NC). A significance level of 0.05 was used.
Supplementary Material
Acknowledgments
The work was supported by Department of Defense (DOD) grant (W81XWH-10-1-0254) and National Multiple Sclerosis Society (NMSS) research grant RG 5138A1/1T to Ashutosh Mangalam. We thank Drs. Legge, Waldschmidt, Karandikar, and Sinha for helpful discussion. We thank Julie Hanson, Michele Smart, and Transgenic mouse colony staff for HLA class II transgenic mice and Dr. Kevin Knudtson, Iowa Institute of Human Genomics for DNA sequencing. We also thank Louisa Papke, Laurie Zoecklein, Mabel Pierce for technical assistance and Lea Dacy for proofreading and editing the manuscript.
A.M. receives research support from National Multiple Sclerosis Society (RG 5138A1/1). S.K.S., D.L., M.K., N.L., R.S.C, J.J., R.S., K.G.C., and C.S.D. report no disclosures. A.M., E.M., V.T., and J.M. have a patent issued for the technology used in this study. R.P. reports grants from BioFire, Check-Points, Curetis, 3M, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, Allergan, and The Medicines Company, consults to Curetis, Roche, Qvella, and Diaxonhit (monies paid to Mayo Clinic), has patents on Bordetella pertussis/parapertussis PCR, anti-biofilm substance, and device/method for sonication (royalties paid by Samsung to Mayo Clinic). R.P. serves on an Actelion data monitoring board. Dr. Patel receives travel reimbursement and an editor’s stipend from ASM and IDSA, and honoraria from the USMLE, Up-to-Date and the Infectious Diseases Board Review Course. M.R. receives research support from NIH (GM092993, NS048357 and NS073684), NIH CTSA (RR024150 and TR000135), Novartis Pharmaceuticals, the European Regional Development Fund (FNUSA-ICRC CZ.1.05/1.1.00/02.0123), and the Applebaum, Hilton, Peterson, and McNeilus Foundations.
Footnotes
AUTHOR CONTRIBUTIONS TO THE MANUSCRIPT
A.M. conceptualized the study, designed and performed the experiments, wrote the manuscript, and gave final approval of the manuscript to be published; S.K.S, D.L, M.K., N.L., and J.J. performed the experiments; E.M. helped with experimental design and performed experiment; R.S.C. and R.S. performed statistical analysis. K.G-C. performed intestinal pathology. R.P. helped with experimental designs and manuscript writing; M.R. performed histological scoring, helped with experimental design, and manuscript editing. C.S. and V.T. helped with the study design and interpretation of the data; J.M. conceptualized the study, edited and approved final version of the manuscript. All authors commented on the manuscript.
References
- Angiuoli SV, Matalka M, Gussman A, Galens K, Vangala M, Riley DR, Arze C, White JR, White O, Fricke WF. CloVR: a virtual machine for automated and portable sequence analysis from the desktop using cloud computing. BMC Bioinformatics. 2011;12:356. doi: 10.1186/1471-2105-12-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt D, Xia J, Liu Y, Zhou Y, Guo AC, Cruz JA, Sinelnikov I, Budwill K, Nesbo CL, Wishart DS. METAGENassist: a comprehensive web server for comparative metagenomics. Nucleic Acids Res. 2012;40:W88–95. doi: 10.1093/nar/gks497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- Charbonneau MR, Blanton LV, DiGiulio DB, Relman DA, Lebrilla CB, Mills DA, Gordon JI. A microbial perspective of human developmental biology. Nature. 2016;535:48–55. doi: 10.1038/nature18845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Drescher KM, Geluk A, Bradley DS, Rodriguez M, David CS. Complementation between specific HLA-DR and HLA-DQ genes in transgenic mice determines susceptibility to experimental autoimmune encephalomyelitis. Hum Immunol. 2000;61:279–289. doi: 10.1016/s0198-8859(99)00135-4. [DOI] [PubMed] [Google Scholar]
- Dyment DA, Herrera BM, Cader MZ, Willer CJ, Lincoln MR, Sadovnick AD, Risch N, Ebers GC. Complex interactions among MHC haplotypes in multiple sclerosis: susceptibility and resistance. Hum Mol Genet. 2005;14:2019–2026. doi: 10.1093/hmg/ddi206. [DOI] [PubMed] [Google Scholar]
- Gold R, Hartung HP, Toyka KV. Animal models for autoimmune demyelinating disorders of the nervous system. Mol Med Today. 2000;6:88–91. doi: 10.1016/s1357-4310(99)01639-1. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jangi S, Gandhi R, Cox LM, Li N, von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavasani S, Dzhambazov B, Nouri M, Fak F, Buske S, Molin G, Thorlacius H, Alenfall J, Jeppsson B, Westrom B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PloS one. 2010;5:e9009. doi: 10.1371/journal.pone.0009009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckey D, Bastakoty D, Mangalam AK. Role of HLA class II genes in susceptibility and resistance to multiple sclerosis: Studies using HLA transgenic mice. Journal of autoimmunity. 37:122–128. doi: 10.1016/j.jaut.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam A, Luckey D, Basal E, Jackson M, Smart M, Rodriguez M, David C. HLA-DQ8 (DQB1*0302)-restricted Th17 cells exacerbate experimental autoimmune encephalomyelitis in HLA-DR3-transgenic mice. J Immunol. 2009a;182:5131–5139. doi: 10.4049/jimmunol.0803918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam A, Luckey D, Basal E, Jackson M, Smart M, Rodriguez M, David C. HLA-DQ8 (DQB1*0302)-restricted Th17 cells exacerbate experimental autoimmune encephalomyelitis in HLA-DR3-transgenic mice. J Immunol. 2009b;182:5131–5139. doi: 10.4049/jimmunol.0803918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam AK, Khare M, Krco C, Rodriguez M, David C. Identification of T cell epitopes on human proteolipid protein and induction of experimental autoimmune encephalomyelitis in HLA class II-transgenic mice. Eur J Immunol. 2004;34:280–290. doi: 10.1002/eji.200324597. [DOI] [PubMed] [Google Scholar]
- Mangalam AK, Luckey D, Giri S, Smart M, Pease LR, Rodriguez M, David CS. Two discreet subsets of CD8 T cells modulate PLP(91–110) induced experimental autoimmune encephalomyelitis in HLA-DR3 transgenic mice. J Autoimmun. 2012;38:344–353. doi: 10.1016/j.jaut.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangalam AK, Rajagopalan G, Taneja V, David CS. HLA class II transgenic mice mimic human inflammatory diseases. Adv Immunol. 2008;97:65–147. doi: 10.1016/S0065-2776(08)00002-3. [DOI] [PubMed] [Google Scholar]
- Marietta EV, Murray JA, Luckey DH, Jeraldo PR, Lamba A, Patel R, Luthra HS, Mangalam A, Taneja V. Human Gut-Derived Prevotella histicola Suppresses Inflammatory Arthritis in Humanized Mice. Arthritis Rheumatol. 2016 doi: 10.1002/art.39785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake S, Kim S, Suda W, Oshima K, Nakamura M, Matsuoka T, Chihara N, Tomita A, Sato W, Kim SW, et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 2015;10:e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal immunology. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- Okada H, Kuhn C, Feillet H, Bach JF. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clinical and experimental immunology. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:5–15. doi: 10.1007/s12016-011-8285-8. [DOI] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164:337–340. doi: 10.1016/j.cell.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Surana NK, Kasper DL. The yin yang of bacterial polysaccharides: lessons learned from B. fragilis PSA. Immunological reviews. 2012;245:13–26. doi: 10.1111/j.1600-065X.2011.01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2014;30:614–620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Uxa L, Bratina A, Bosco A, Srinivasaraghavan B, Minagar A, Ukmar M, Benedetto S, Zorzon M. HLA-DRB1*1501, -DQB1*0301, -DQB1*0302, -DQB1*0602, and -DQB1*0603 alleles are associated with more severe disease outcome on MRI in patients with multiple sclerosis. Int Rev Neurobiol. 2007;79:521–535. doi: 10.1016/S0074-7742(07)79023-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


