Abstract
The surveillance of neurosyphilis, an uncommon but severe consequence of syphilis, is complex; surveillance classification of neurosyphilis requires a lumbar puncture and cerebrospinal fluid analysis. We examined the prevalence of reported neurosyphilis among primary, secondary, and early latent syphilis cases reported in the United States from 2009 to 2015. Overall, the prevalence of reported neurosyphilis from 2009 to 2015 was low (0.84%); however, this is likely an underestimate of the true burden in the United States.
BACKGROUND
In the United States, the rate of primary and secondary (P&S) syphilis has increased almost every year since 2001.1 As rates of P&S syphilis increase, a likely consequence will be a parallel increase in the prevalence of adverse health outcomes associated with syphilitic infection such as neurosyphilis. In recent years, several large urban areas in the United States have reported increases in neurosyphilis; however, it is unknown whether these reports are representative of a national trend in neurosyphilis or whether the incidence of neurosyphilis is proportional to what would be expected given the overall increase in early syphilis rates.2–6
Neurosyphilis is an uncommon but severe consequence of syphilis and can occur at any stage of infection.7 The diagnosis of neurosyphilis is complex. The clinical diagnosis of neurosyphilis depends on an evaluation of neurologic signs and symptoms, and results from serologic and cerebrospinal fluid (CSF) tests (including CSF cell count, protein, and CSF Venereal Disease Research Laboratory [CSF-VDRL]).7 Current Centers for Disease Control and Prevention (CDC) sexually transmitted disease treatment guidelines recommend performing a lumbar puncture and CSF analysis for patients infected with syphilis who display clinical signs of neurosyphilis (e.g., cranial nerve dysfunction, auditory or ophthalmic abnormalities, meningitis, stroke, acute or chronic altered mental status, and loss of vibration sense).7 Syphilis is a nationally notifiable condition, and neurosyphilis should be reported to CDC.1 In light of the limited availability of population-based data on neurosyphilis in the United States, we aimed to examine national case report data to describe the prevalence and epidemiology of reported neurosyphilis among primary, secondary, and early latent syphilis (“early syphilis”) cases reported in the United States from 2009 to 2015.
METHODS
We examined early syphilis case data reported to the National Notifiable Diseases Surveillance System at the CDC from 2009 to 2015, which include limited demographic and clinical information and whether neurosyphilis was present. Neurosyphilis is reported as confirmed, probable, no, or unknown. For surveillance purposes, “confirmed neurosyphilis” is defined as syphilis of any stage with a reactive CSF-VDRL test and either a reactive treponemal serologic test for syphilis or a reactive nontreponemal test for syphilis. “Probable neurosyphilis” is defined as syphilis of any stage with a negative CSF-VDRL test result and either a reactive treponemal serologic test for syphilis or a reactive nontreponemal serologic test for syphilis, an elevated CSF protein or leukocyte count, and clinical symptoms or signs consistent with neurosyphilis in the absence of other known causes for these abnormalities. We compared the prevalence of reported confirmed and probable neurosyphilis in 2015 among all early syphilis cases by sex, sex of sex partner, race/ethnicity (as defined by Office of Management and Budget standards), age group, and reported HIV status. When examining trends from 2009 to 2015, we limited our analysis to early syphilis cases reported from 10 states that, from 2009 to 2015, consistently reported data on neurosyphilis (confirmed, probable, or no) for at least 70% of total early syphilis cases reported. Data analyses were conducted using SAS version 9.3 (SAS Institute, Inc, Cary, NC).
RESULTS
In 2015, a total of 48,045 cases of early syphilis were reported to CDC from the 50 US states and the District of Columbia. Of these, there were 403 (0.8%) cases with neurosyphilis reported: 295 (0.6%) with confirmed neurosyphilis and 108 (0.2%) with probable neurosyphilis (Table 1). The prevalence of reported neurosyphilis was highest among those with secondary syphilis (1.1%) compared with those with primary (0.3%) or early latent syphilis (0.8%). Men with early syphilis had a higher prevalence (0.9%) compared with women with early syphilis (0.5%). Among men, the prevalence of reported neurosyphilis was highest among men who have sex with both men and women (1.4%) compared with men who have sex with men (MSM) only (0.9%), men with an unknown sex of sex partner (0.8%), and men who have sex with women only (0.7%). Whites with early syphilis had a higher prevalence of reported neurosyphilis (1.2%) compared with blacks (0.9%), Hispanics (0.5%), and persons reporting an “other” or multiple-race categories (0.4%). The prevalence of reported neurosyphilis among those 0 to 14 years old was 2.0%, with 1 case of neurosyphilis reported among 50 early syphilis cases, 1.2% (n = 225) among those 35 to 64 years old, 1.1% (n = 5) among those 65 years and older, and 0.6% (n = 172) among those 15 to 34 years old. The prevalence of reported neurosyphilis was higher among persons with early syphilis who were known to be living with diagnosed HIV (1.2%) compared with those who were HIV negative (0.7%).
TABLE 1.
Early Syphilis—Reported Cases of Neurosyphilis by Demographic and Behavioral Characteristics, United States, 2015
| Confirmed Neurosyphilis |
Probable Neurosyphilis |
Confirmed or Probable Neurosyphilis |
|||||
|---|---|---|---|---|---|---|---|
| n | % | 95% CI | % | 95% CI | % | 95% CI | |
| Total early syphilis cases | 48,045 | 0.6 | (0.5–0.7) | 0.2 | (0.2–0.3) | 0.8 | (0.8–0.9) |
| Stage | |||||||
| Primary | 7700 | 0.2 | (0.1–0.4) | 0.1 | (0.0–0.2) | 0.3 | (0.2–0.4) |
| Secondary | 16,172 | 0.8 | (0.6–0.9) | 0.3 | (0.3–0.4) | 1.1 | (0.9–1.3) |
| Early latent | 24,173 | 0.6 | (0.5–0.7) | 0.2 | (0.2–0.3) | 0.8 | (0.7–1.0) |
| Sex | |||||||
| Female | 5531 | 0.4 | (0.3–0.6) | 0.2 | (0.1–0.3) | 0.5 | (0.3–0.7) |
| Male | 42,448 | 0.6 | (0.6–0.7) | 0.2 | (0.2–0.3) | 0.9 | (0.8–1.0) |
| Sex of sex partner | |||||||
| MSM only | 24,385 | 0.7 | (0.6–0.8) | 0.2 | (0.2–0.3) | 0.9 | (0.8–1.0) |
| Men who have sex with both men and women | 2172 | 0.9 | (0.6–1.4) | 0.5 | (0.2–0.8) | 1.4 | (0.9–2.0) |
| Men who have sex with women only | 5275 | 0.4 | (0.3–0.6) | 0.3 | (0.2–0.5) | 0.7 | (0.5–1.0) |
| Men with unknown sex of sex partner | 10,616 | 0.7 | (0.5–0.8) | 0.2 | (0.1–0.3) | 0.8 | (0.7–1.0) |
| Race/ethnicity* | |||||||
| White | 15,081 | 0.8 | (0.7–0.9) | 0.4 | (0.3–0.5) | 1.2 | (1.0–1.4) |
| Black | 16,994 | 0.7 | (0.6–0.9) | 0.2 | (0.1–0.3) | 0.9 | (0.8–1.1) |
| Hispanic | 10,772 | 0.3 | (0.2–0.5) | 0.1 | (0.1–0.2) | 0.5 | (0.3–0.6) |
| Other/multiple | 4807 | 0.3 | (0.2–0.5) | 0.1 | (0.0–0.2) | 0.4 | (0.2–0.6) |
| Region | |||||||
| Midwest | 5721 | 0.7 | (0.5–0.9) | 0.3 | (0.2–0.5) | 1.0 | (0.8–1.3) |
| Northeast | 8505 | 0.4 | (0.3–0.5) | 0.2 | (0.1–0.3) | 0.6 | (0.4–0.8) |
| South | 20,440 | 0.8 | (0.6–0.9) | 0.3 | (0.2–0.4) | 1.0 | (0.9–1.2) |
| West | 13,379 | 0.5 | (0.4–0.7) | 0.1 | (0.1–0.2) | 0.6 | (0.5–0.8) |
| Age group, y | |||||||
| 0–14 | 50 | 2.0 | (0.1–11) | 0.0 | — | 2.0 | (0.0–5.9) |
| 15–34 | 28,109 | 0.5 | (0.4–0.6) | 0.1 | (0.1–0.2) | 0.6 | (0.5–0.7) |
| 35–64 | 19,437 | 0.8 | (0.7–1.0) | 0.3 | (0.3–0.4) | 1.2 | (1.0–1.3) |
| 65+ | 439 | 0.5 | (0.1–1.6) | 0.7 | (0.1–2.0) | 1.1 | (0.2–2.1) |
| HIV status | |||||||
| HIV negative | 16,629 | 0.5 | (0.4–0.6) | 0.2 | (0.1–0.3) | 0.7 | (1.0–1.4) |
| HIV positive | 14,996 | 0.9 | (0.8–1.1) | 0.3 | (0.2–0.4) | 1.2 | (0.5–0.8) |
| Other† | 16,420 | 0.5 | (0.4–0.6) | 0.2 | (0.1–0.3) | 0.7 | (0.6–0.8) |
Restricted to 49 jurisdictions that submitted data in the race and ethnicity categories according to Office of Management and Budget (OMB) standards.
Other includes “equivocal,” “refused to answer,” “did not ask,” “unknown,” and “missing.”
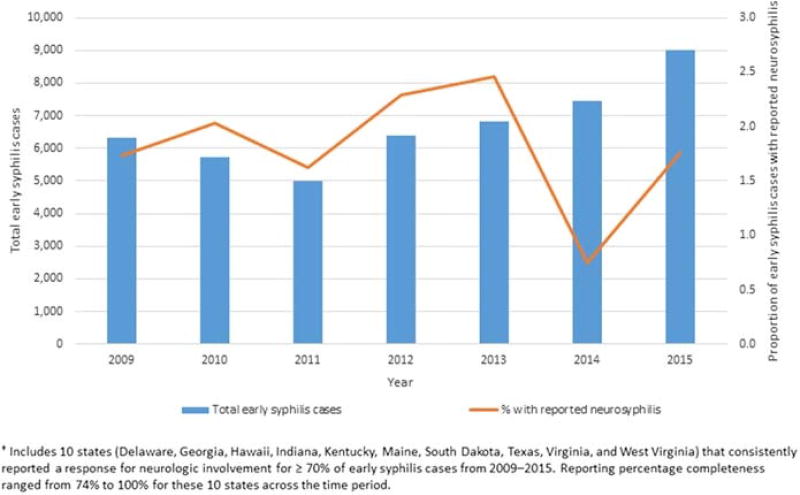
From 2009 to 2015, all 50 US states and the District of Columbia reported 1 or more cases of early syphilis. Ten states consistently reported data on neurosyphilis for at least 70% of early syphilis cases from 2009 to 2015 (Delaware, Georgia, Hawaii, Indiana, Kentucky, Maine, South Dakota, Texas, Virginia, and West Virginia). The percentage of total reported neurosyphilis cases accounted for by these 10 states varied from 2009 to 2015 but ranged from 19.3% in 2014 to 39.5% in 2015. Among these 10 states, the overall prevalence of reported neurosyphilis among reported cases of early syphilis from 2009 to 2015 was 1.8% (Fig. 1). Among persons reported with early syphilis, the annual prevalence of reported neurosyphilis varied over the study period (Fig. 1), with a relative decrease in the prevalence of reported neurosyphilis in 2014 (0.8%) and in 2015 (1.8%) when compared with 2013 (2.5%).
Figure 1.
Early syphilis—reported cases and prevalence of neurosyphilis by year, from 10 states†, 2009 to 2015.
DISCUSSION
Neurosyphilis is an uncommon but serious complication of syphilitic infection that can involve persistent disabilities for patients. As rates of syphilis increase in the United States,1 it is essential to continue to monitor trends in complicated syphilitic infections such as neurosyphilis. In summary, the results from our examination of national case report data indicate that the prevalence of reported neurosyphilis among early syphilis cases varied from 2009 to 2015, but that the overall prevalence remained low. Our prevalence estimate of 1.8% is comparable to earlier cohort prevalence estimates on the basis of retrospective health record reviews of MSM in the United States, which ranged from 1.2% to 1.7%.3,4 In 2015, compared with other groups, there was a higher prevalence of reported neurosyphilis among male and white patients with early syphilis, as well as among patients with early syphilis known to be living with diagnosed HIV.
Our use of national case report data to examine the prevalence of neurosyphilis is strengthened by the use of a standardized surveillance case definition for neurosyphilis. However, there are a number of challenges associated with the surveillance of neurosyphilis that can result in case reports underestimating the true burden of disease. First, variations in clinical practice, including variability in screening for neurologic signs and symptoms and variability in use of lumbar puncture to evaluate patients with neurologic signs or symptoms, likely lead to underascertainment of syphilis cases that meet the surveillance case definition of neurosyphilis. Because the surveillance case definition for both probable and confirmed neurosyphilis requires documentation of abnormal CSF results, clinical settings that have a higher clinical threshold for performing lumbar punctures or that treat a patient for neurosyphilis without performing a lumbar puncture will be less likely to have a case that meets the surveillance case definition of neurosyphilis. Second, variations in health department practices, namely, variability in the extent to which reported early syphilis cases are followed up with provider or patient interviews to identify cases that might meet the neurosyphilis surveillance case definition, might lead to underascertainment and underreporting of neurosyphilis cases.
This variation in successful contact of the patient or provider has implications for the interpretation of a “no” or “unknown” response when reporting neurosyphilis and limits our ability to disentangle patients who were not successfully contacted from patients whose neurosyphilis was ruled out. Because both “no” and “unknown” reports were included in our denominator when calculating prevalence, we are likely underestimating the true burden of disease. Changes in neurosyphilis screening and reporting practices over time could result in an increase or decrease in reported cases, which would not be reflective of a true change in incidence. Standardized monitoring of neurosyphilis screening practices by jurisdictions could help to better inform the interpretation of trends in surveillance data.
Our findings are subject to a few limitations. We found a higher prevalence of neurosyphilis among persons known to be living with diagnosed HIV compared with persons who were HIV negative or with an unknown HIV status. This finding may be subject to ascertainment bias and could be reflecting a higher likelihood of HIV-infected persons being screened for signs or symptoms of neurosyphilis. There have, however, been studies suggesting that immunocompromised persons may have a higher risk for developing neurosyphilis, even after appropriate treatment and declines in serum rapid plasma reagin.8,9 This is further complicated by the fact that almost 90% of reported early syphilis cases in 2015 were among MSM and reported cases of P&S syphilis continue to be characterized by a high rate of HIV coinfection.1 Furthermore, our trend analysis was limited to early syphilis cases reported from 10 states that, from 2009 to 2015, consistently reported data on neurosyphilis for at least 70% of total early syphilis cases. Early syphilis cases from these jurisdictions may not be representative of all persons who received a diagnosis of early syphilis in the United States from 2009 to 2015.
In conclusion, these are the first national prevalence estimates of reported neurosyphilis among early syphilis cases in the United States. These estimates are based on case report data and therefore subject to the inherent limitations of passive, case-based surveillance data. These estimates likely underestimate the true burden of neurosyphilis in the United States and are subject to variations in screening and diagnostic practices across jurisdictions. Possible ways to improve the surveillance of neurosyphilis could include the routinized clinical assessment of neurologic signs and symptoms among all diagnosed syphilis cases, which along with complete clinical data from reporting providers could help strengthen the ascertainment of neurosyphilis among reported syphilis cases by health departments. Because the prevalence of syphilis is increasing in the United States, we can expect to see an increase in the number of cases of complicated syphilis. Education and training of providers to be able to recognize signs and symptoms of neurosyphilis as early as possible and manage disease sequelae are critical.
Footnotes
Conflict of Interest and Sources of Funding: None declared.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2015. Atlanta: U.S. Department of Health and Human Services; 2016. Available at: https://www.cdc.gov/std/stats. [Google Scholar]
- 2.Dombrowski JC, Pedersen R, Marra CM, et al. Prevalence estimates of complicated syphilis. Sex Transm Dis. 2015;42:702–704. doi: 10.1097/OLQ.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MM, Aynalem G, Olea LM, et al. A consequence of the syphilis epidemic among men who have sex with men (MSM): Neurosyphilis in Los Angeles, 2001–2004. Sex Transm Dis. 2008;35:430–434. doi: 10.1097/OLQ.0b013e3181644b5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Symptomatic early neurosyphilis among HIV-positive men who have sex with men—Four cities, United States, January 2002–June 2004. MMWR Morb Mortal Wkly Rep. 2007;56:625–628. [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. [Accessed February 23, 2017];Clinical advisory: Ocular syphilis in the United States 2015. Available at: http://www.cdc.gov/std/syphilis/clinicaladvisoryos2015.htm.
- 6.Woolston S, Cohen SE, Fanfair RN, et al. A cluster of ocular syphilis cases—Seattle, Washington, and San Francisco, California, 2014–2015. MMWR Morb Mortal Wkly Rep. 2015;64:1150–1151. doi: 10.15585/mmwr.mm6440a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 8.Musher DM, Hamill RJ, Baughn RE. Effect of human immunodeficiency virus (HIV) infection on the course of syphilis and on the response to treatment. Ann Intern Med. 1990;113:872–881. doi: 10.7326/0003-4819-113-11-872. [DOI] [PubMed] [Google Scholar]
- 9.Marra CM, Maxwell CL, Tantalo L, et al. Normalization of cerebrospinal fluid abnormalities after neurosyphilis therapy: Does HIV status matter? Clin Infect Dis. 2004;38:1001–1006. doi: 10.1086/382532. [DOI] [PubMed] [Google Scholar]