Abstract
Objective
To assess the epidemiology of rabies in rodents and lagomorphs and provide information that will enable public health officials to make recommendations regarding postexposure prophylaxis for humans after contact with these animals.
Design
Cross-sectional epidemiological analysis.
Sample
Rodents and lagomorphs submitted to state laboratories for rabies diagnosis from 1995 through 2010.
Procedures
Positive samples were identified by use of direct fluorescent antibody testing, typed by sequencing of viral genes, and quantified via titration in mice or cell culture.
Results
737 rabid rodents and lagomorphs were reported from 1995 through 2010, which represented a 62.3% increase, compared with the number of rabid rodents and lagomorphs reported from 1979 through 1994. The most commonly reported rodents or lagomorphs were groundhogs (Marmota monax). All animals submitted to the CDC for additional viral characterization were positive for the raccoon rabies virus variant. Infectious virus or viral RNA was detected in salivary glands or oral cavity tissues in 11 of 13 rabid rodents.
Conclusions and Clinical Relevance
The increase in reported rabid rodents, compared with results of previous studies, appeared to be associated with spillover infections from the raccoon rabies epizootic during the first half of the study period. Analysis supported the assumption that rabies remained rare in rodents and lagomorphs. However, transmission of rabies virus via exposure to a rabid rodent or lagomorph may be possible. Given the rarity of rabies in these species, diagnostic testing and consideration of postexposure prophylaxis for humans with potential exposures should be considered on a case-by-case basis.
Exposure to rodents and lagomorphs has never been implicated as the cause of infection for a case of rabies in humans in the United States, nor are these animals considered natural reservoirs of the disease.1 However, the number and reliability of such reports are limited, and there is concern about rabies in rodents and lagomorphs. Reliable reports of human exposure to rabid rodents or lagomorphs outside of the United States are limited.2 Many suspect rodents and lagomorphs are tested each year for rabies, and a small but increasing number are found to be rabid.3 Although they represent a low risk for rabies virus transmission, rodents and lagomorphs may contribute to possible rabies virus exposure in humans, domestic animals, and other wildlife.1 In addition, because of the close cohabitation of some rodent species with human populations and the high incidence of rodent bites, public health officials are frequently asked to evaluate the need for rabies PEP after human contact with these animals.3
Northeastern and mid-Atlantic states have reported the most rabies cases in rodents and lagomorphs as a result of spillover infections from the enzootic raccoon rabies virus variant circulating in this area.4,5 Among rodents and lagomorphs in the United States, groundhogs (Marmota monax) are the animals most commonly reported as rabid. This may be partially attributable to the comparatively larger body size of groundhogs than that of other rodent species. The small body size of most other rodent species likely results in higher mortality rates from injuries sustained during altercations with rabid mesocarnivores and may contribute to the rarity of smaller rodents reported as rabid. In addition, it may be more difficult to capture rodents of smaller size and submit them for diagnosis after potential exposure of humans. The purpose of the study reported here was to assess the epidemiology of rabies in rodents and lagomorphs and analyze spatial trends of rabies in groundhogs.
Materials and Methods
Passive animal rabies surveillance data submitted by health departments of the United States, the District of Columbia, and Puerto Rico to the CDC from 1995 through 2010 were analyzed. Laboratory analysis of rodent samples submitted to the CDC rabies laboratory was used to identify the presence of rabies virus in tissues (ie, salivary glands, tongue, tonsils, and buccal mucosa) that are likely to contribute to rabies exposure. Data consisted of animals submitted for rabies diagnosis by use of direct fluorescent antibody testing, as described elsewhere.6 Only animals of the orders Rodentia and Lagomorpha were included for analysis. Data reported included the state (or District of Columbia or Puerto Rico), county, year, month, and species of animal tested. In addition, many states provided information on all animals submitted for rabies diagnosis.7 Several states and the District of Columbia did not report the total number of animals submitted for diagnosis for all years of the 16-year study period: Alabama (2005), California (1995, 1996, 1998 through 2002, 2004, and 2005), Connecticut (1995 and 2002), District of Columbia (2001), Delaware (1995, 1997, and 1998), Florida (1995 through 2002), Georgia (1995 and 1997 through 2005), Indiana (2002), Iowa (1995 through 1997 and 2003 through 2005), Kentucky (1997 and 1998), Maryland (2002), Missouri (1997), New Mexico (1998 through 2001), Oklahoma (1997 through 1999 and 2001 through 2005), Pennsylvania (1995), South Carolina (1999, 2001, 2004, and 2005), Tennessee (1996), and Vermont (1998 through 2000). States and the District of Columbia and years for which data were incomplete were excluded from analysis when calculations of proportions were necessary. Characterization of rabies virus variants was not performed for most reported rabid rodents and lagomorphs.
To characterize the spatial distribution of rabid groundhogs, commercially available softwarea was used to create a map of reported rabid groundhogs by county from 2006 to 2010. The map represented the geographic range of groundhogs in the United States and the locations where most rabid rodents were reported.
Additional laboratory testing for rabies was performed on a convenience sample of rodents submitted to the CDC by state public health laboratories. These rodents were primarily groundhogs and beavers (Castor canadensis). The primary tissues that contribute to rabies virus transmission (ie, salivary glands, tongue, tonsils, and buccal mucosa) were examined to evaluate the potential for these species to transmit rabies virus via a bite. An RT-nPCR assay was performed on the nucleoprotein gene of rabies virus, as described elsewhere.8 All samples with positive results for the RT-nPCR assay were tested by use of intracranial inoculation of mice and propagated in mouse neuroblastoma cells for determination of infectivity and viral titers.
Results
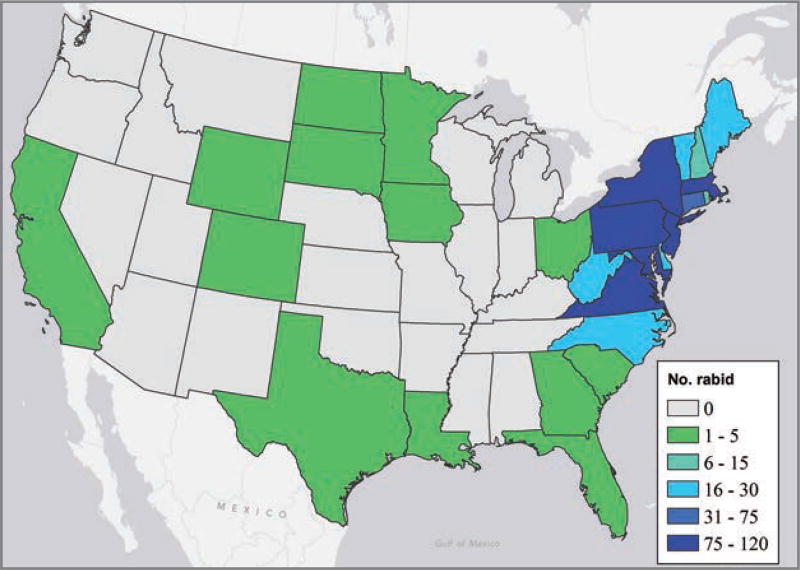
A total of 737 rabid rodents and lagomorphs were reported from 1995 through 2010 (Table 1). This represented an increase of 62.3% from the 454 reports of rabid rodents and lagomorphs for the previous 16-year period (1979 through 1994).3,9,10 Rabid rodents and lagomorphs were reported in 27 states and the District of Columbia; the majority (700/737 [95.0%]) were reported in the Northeastern and mid-Atlantic region (Figure 1).
Table 1.
Reported number of rabid rodents and lagomorphs in the United States, by year, from 1995 through 2010 and for 1979 through 1994.3,10
| Species | 1995 | 1996 | 1997 | 1998 | 1999 | 2000 | 2001 | 2002 | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Total (No. [%]) |
1979–1994 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beaver (Castor canadensis) | 2 | 0 | 5 | 3 | 3 | 0 | 3 | 2 | 2 | 1 | 3 | 0 | 4 | 1 | 2 | 0 | 31 (4.2) | 14 |
| Chinchilla (Chinchilla lanigera) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
| Chipmunk (Tamias striatus) | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (0.3) | 2 |
| Groundhog (Marmota monax) | 50 | 43 | 55 | 63 | 40 | 48 | 47 | 49 | 31 | 30 | 25 | 43 | 46 | 31 | 32 | 30 | 663 (90.0) | 379 |
| Guinea pig (Cavia porcellus) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 0 |
| Muskrat (Ondatra zibethicus) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 4 (0.5) | 7 |
| European rabbit (Oryctolagus cuniculus) | 1 | 2 | 3 | 2 | 1 | 2 | 5 | 1 | 2 | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 25 (3.4) | 24 |
| Brown rat (Rattus norvegicus) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.1) | 6 |
| Squirrel (Sciurus spp) | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 9 (1.2) | 22 |
| Total | 54 | 47 | 65 | 68 | 45 | 52 | 56 | 52 | 38 | 32 | 29 | 44 | 50 | 34 | 38 | 33 | 737 (100) | 454 |
Figure 1.
Number of reported cases of rabies in rodents or lagomorphs, by state, from 1995 through 2010.
The 737 rabid rodents and lagomorphs reported during 1995 through 2010 represented only 1.0% of the total number of animals submitted for rabies testing. For 48 rabid rodents, denominator data of the rodents and lagomorphs submitted for rabies testing in a given year were not reported. The rodents and lagomorphs most commonly tested were squirrels (Sciurus spp; 21,977/70,682 [31.1%]) and groundhogs (3,188/70,682 [4.5%]). However, only 9 of 21,977 (0.04%) squirrels tested were rabid.
Species comprising the majority of the 737 rabid rodents and lagomorphs were groundhogs (663 [90.0%]), beavers (31 [4.2%]), European rabbits (Oryctolagus cuniculus; 25 [3.4%]), and squirrels (9 [1.2%]). All other species (chinchilla [Chinchilla lanigera], chipmunk [Tamias striatus], guinea pig [Cavia porcellus], muskrat [Ondatra zibethicus], and brown rat [Rattus norvegicus]) with at least 1 reported rabies case during the 16-year study period each accounted for < 1% of the total number of cases reported (Table 1).
Groundhogs were the most frequently reported rabid rodent or lagomorph (663/737 [90.0%]). This represented an increase of 75% for the number of reported rabid groundhogs, compared with the number reported for the period from 1979 through 1994.3,10 The annual rate for reported rabid groundhogs remained relatively constant from 1995 through 2002, with a mean of approximately 50 cases/y. In 2003, the number of reported rabid groundhogs decreased to 31 cases/y. The rate in subsequent years remained consistent with that for 2003, with a mean of 34 cases/y from 2004 through 2010.
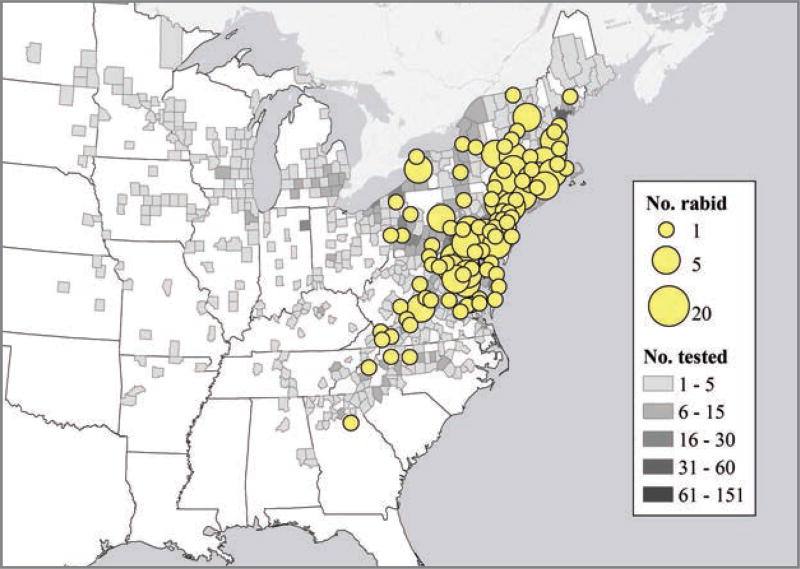
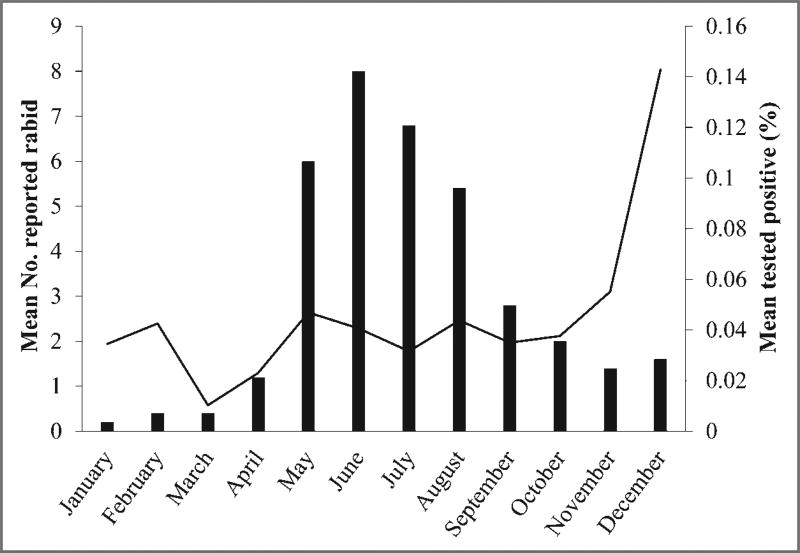
Spatial distribution for reported cases of rabies involving groundhogs, by county, in the United States from 2006 through 2010 was analyzed (Figure 2). Total number of reported rabid groundhogs was analyzed to determine the seasonal distribution for the period from 2006 through 2010 (Figure 3). June and July were the months with the largest number of reported rabid groundhogs. The reported number of groundhogs submitted for rabies testing differed for the first and last 8-year periods of the study. The approximate mean for 1995 through 2002 was 903 submissions/y, and the approximate mean for 2003 through 2010 was 898 submissions/y; these values excluded data for which denominator information was not available. Reported rabid groundhogs were clustered primarily in counties where the raccoon rabies virus variant was enzootic.
Figure 2.
Number of reported cases of rabies in groundhogs and the number of groundhogs submitted for rabies testing, by county, in the United States, from 2006 through 2010.
Figure 3.
Mean number of rabid groundhogs (black bars) and the percentage of groundhogs submitted for rabies testing that had positive test results (black line), by month, from 2006 through 2010.
Additional diagnostic testing for rabies was performed on 13 rabid groundhogs and beavers submitted to the CDC. Brain, salivary glands, tongue, tonsils, and buccal mucosa (ie, tissues primarily contributing to rabies virus transmission) were examined. Tests were not performed on all samples if the tissues had a previous negative test result or if tissue samples for each animal were not available. All animals were infected with the raccoon rabies virus variant. All RT-nPCR amplicons from the 13 brain samples yielded positive results; similarly, all 13 brain samples yielded positive results for inoculated tissue cultures. Rabies virus RNA was detected in 10 of 12 salivary gland samples tested. Ten of 12 salivary glands were examined for viral titers, and 8 had a detectable infectious rabies virus (mean titer, 3.8 log10 MICLD50 [95% confidence interval, 2.7 to 4.9 log10 MICLD50]). All 13 tongue samples were tested by use of RT-nPCR assay, but viral RNA was detected in only 5 tongue samples. Of these 5 samples, 1 had a rabies virus titer of 0.7 log10 MICLD50. Nine tonsil samples were tested by use of the RT-nPCR, and 5 had positive results. Testing of buccal mucosa samples (n = 10) by use of the RT-nPCR revealed 2 samples with rabies virus RNA. Of these 2 samples, 1 had a rabies virus titer of 0.7 log10 MICLD50.
Discussion
In the present study, groundhogs accounted for most of the cases of rabies in rodents and lagomorphs and were primarily responsible for most of the increase in reports of rabid rodents, compared with results of studies3,10 for a previous 16-year period (1979 through 1994). The increase in reported rabid groundhogs may have been attributable to the ongoing expansions of the raccoon rabies epizootic in the Northeast during the early 2000s.9 The decrease in rabid groundhogs reported in 2003 further coincided with the time when the raccoon rabies epizootic reached its maximum geographic expansion in the United States.
Because of their size, groundhogs may be more likely to survive the bite of a larger rabid animal (eg, raccoon or skunk) and therefore would have the potential to incubate the virus and become rabid.11 The size and aggressive behavior of rabid groundhogs may also make them more visible and likely to be captured and submitted for diagnostic testing following a potential exposure of humans or domestic animals.
Reports of rabies in other rodents or lagomorphs continue to be uncommon. Squirrels were the rodent most commonly submitted for rabies testing (21,977/70,682 [31.1%]). However, only 9 (0.04%) squirrels were found to be rabid during the study period. Increased risk assessments with a focus on submission of rodents with unusual behavior (eg, paralysis, ataxia, atypical aggression, or abnormal vocalization) involved in unprovoked bites may help reduce the number of submissions and increase the focus on animals with a higher likelihood of being rabid.
Given the high rate of spillover infection, groundhogs would represent the most likely rodent species within which there would be potential adaptation and independent circulation of a unique rabies virus variant. However, on the basis of results of genetic typing, spillover infection remained the most likely explanation for the number of rabid groundhogs, possibly because of interactions between raccoons and groundhogs at ground dens. All groundhogs tested were infected with the raccoon rabies virus variant. Furthermore, rabid groundhogs were clustered in counties where raccoon rabies is enzootic. On the basis of this evidence, it is unlikely that there is independent transmission of rabies virus from groundhog to groundhog. However, more submissions and genetic analyses of rabies virus isolates from rodents are needed to monitor potential adaptation of viruses in these species.
Excretion of rabies virus in saliva may result in transmission of infection via a bite. All mammals appear to be susceptible to rabies virus, but their ability to act as reservoirs are variable among and within species. 12 The shedding period for rabies virus, compared with the time of onset of clinical signs, is unknown in rodents and lagomorphs. However, analysis of a convenience sample of oral and salivary gland tissues from groundhogs and beavers suggested a potential risk for transmission of rabies virus from these animals.
Results of the analysis of national rabies surveillance data supported previous assumptions that there are no known rabies reservoirs in rodents or lagomorphs in the United States. However, national animal rabies surveillance programs are primarily passive and rely on potential exposures of humans or domestic animals and the capture and submission of suspect animals for diagnostic testing. Analysis of rabid rodents and exposures of humans could be enhanced by more complete laboratory data (ie, records of all animals submitted for testing and antigenic or molecular typing of rabies viruses) and routine data collection for human encounters involving potential rabies virus exposures from rodents, especially when PEP is administered.
It is important to consider the rabies risk for pet rabbits and rodents. Groundhogs account for most rabid rodents and lagomorphs; however, other species, including pet rabbits and rodents, have become infected with the rabies virus after contact with an infected animal. Infected pet animals may represent a high public health risk because of the amount of contact with humans. These pets are at an increased risk of exposure when they are allowed to roam outside or are housed in cages that are accessible to wildlife. To prevent infection of pet rodents and lagomorphs and therefore potential transmission to humans, pets that are housed outside should be protected from contact with wildlife. If contact with a rabid or suspect wild animal occurs, the most conservative approach would be to euthanize the pet immediately; alternatively, the pet could be placed under strict quarantine for 6 months. However, additional research is needed on the time frame for onset of clinical signs and shedding of rabies in these species, so euthanasia (with subsequent testing) or quarantine should be considered on an individual basis with guidance from public health authorities.13
Testing of rodents or lagomorphs for rabies should be considered on a case-by-case basis. As indicated by the present study, rabies is seldom reported in these animals and exposure rarely necessitates administration of PEP to humans. However, potential transmission of rabies virus via a bite or nonbite exposure from rabid rodents or lagomorphs is possible. Unprovoked bites by rodents or lagomorphs with unusual behavior or that appear sick should be reported to enable local health authorities to evaluate the circumstances and assess the need for administration of PEP to humans. When possible, these animals should be submitted for diagnostic testing to rule out the potential for exposure of humans and other animals to rabies virus.
Acknowledgments
The authors thank Lillian A. Orciari, Pamela A. Yager, and Dillon S. Hightower for assistance with diagnostic testing and viral typing.
Use of trade names and commercial sources is for identification only and does not imply endorsement by the US Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
Abbreviations
- MICLD50
Mouse intracerebral LD50
- PEP
Postexposure prophylaxis
- RT-nPCR
Reverse transcription–nested PCR
Footnotes
ArcGIS Desktop, release 10, Environmental Systems Research Institute, Redlands, Calif.
References
- 1.Winkler WG. Rodent rabies in the United States. J Infect Dis. 1972;126:565–567. doi: 10.1093/infdis/126.5.565. [DOI] [PubMed] [Google Scholar]
- 2.Kamoltham T, Tepsumethanon V, Wilde H. Rat rabies in Phetchabun Province, Thailand. J Travel Med. 2002;9:106–107. doi: 10.2310/7060.2002.21976. [DOI] [PubMed] [Google Scholar]
- 3.Childs JE, Colby L, Krebs JW, et al. Surveillance and spatiotemporal associations of rabies in rodents and lagomorphs in the United States, 1985–1994. J Wildl Dis. 1997;33:20–27. doi: 10.7589/0090-3558-33.1.20. [DOI] [PubMed] [Google Scholar]
- 4.Krebs JW, Strine TW, Smith JS, et al. Rabies surveillance in the United States during 1994. J Am Vet Med Assoc. 1995;207:1562–1575. [PubMed] [Google Scholar]
- 5.Jenkins SR, Winkler WG. Descriptive epidemiology from an epizootic of raccoon rabies in the Middle Atlantic States, 1982–1983. Am J Epidemiol. 1987;126:429–437. doi: 10.1093/oxfordjournals.aje.a114674. [DOI] [PubMed] [Google Scholar]
- 6.Dean D, Ableseth M, Atanasiu P. The fluorescent antibody test. Geneva: World Health Organisation; 1996. [Google Scholar]
- 7.Blanton JD, Palmer D, Rupprecht CE. Rabies surveillance in the United States during 2009. J Am Vet Med Assoc. 2010;237:646–657. doi: 10.2460/javma.237.6.646. [DOI] [PubMed] [Google Scholar]
- 8.Bourhy H, Kissi B, Tordo N. Molecular diversity of the Lyssavirus genus. Virology. 1993;194:70–81. doi: 10.1006/viro.1993.1236. [DOI] [PubMed] [Google Scholar]
- 9.Rupprecht CE, Hanlon CA, Hemachudha T. Rabies re-examined. Lancet Infect Dis. 2002;2:327–343. doi: 10.1016/s1473-3099(02)00287-6. [DOI] [PubMed] [Google Scholar]
- 10.Fishbein DB, Belotto AJ, Pacer RE, et al. Rabies in rodents and lagomorphs in the United States, 1971–1984: increased cases in the woodchuck (Marmota monax) in mid-Atlantic states. J Wildl Dis. 1986;22:151–155. doi: 10.7589/0090-3558-22.2.151. [DOI] [PubMed] [Google Scholar]
- 11.Butterfield R. Some raccoon and groundhog relationships. J Wildl Manage. 1954;18:433–437. [Google Scholar]
- 12.Nadin-Davis SA. Molecular epidemiology. In: Jackson AC, Wunner WH, editors. Rabies. San Diego: Elsevier; 2007. pp. 69–115. [Google Scholar]
- 13.Eidson M, Matthews SD, Wilset AL, et al. Rabies virus infection in a pet guinea pig and seven pet rabbits. J Am Vet Med Assoc. 2005;227:932–935. doi: 10.2460/javma.2005.227.932. [DOI] [PubMed] [Google Scholar]