Abstract
Background
Endoscopic radiofrequency ablation (RFA) appears a safe and effective treatment for flat-type non-invasive squamous neoplasia of the esophagus. However, if RFA is applied to lesions containing invasive cancer (ESCC) histological features associated with lymph node metastases may remain undetected. In addition, extension of neoplasia down the ducts of esophageal submucosal glands (SMGs) may create a sheltered ‘niche’ beyond the reach of ablation.
Objective
To determine the RFA eligibility of flat-type ESCC.
Design
Retrospective analysis of prospectively collected data of ESCC patients.
Setting
National Cancer Center Hospital, Tokyo, Japan.
Patients
Patients with flat-type ESCC >3cm removed by endoscopic submucosal dissection (ESD).
Interventions
Three endoscopists involved in RFA studies in China reviewed endoscopic images to select lesions eligible for RFA. Corresponding ESD resection specimens were histologically examined.
Main outcome measurements
Presence of poor histological features (i.e. ≥m3-invasion; poor tumor differentiation; or lymphovascular invasion) and the number of involved esophageal SMGs and ducts.
Results
65 lesions were included of which 17 (26%) qualified as RFA eligible by RFA endoscopists. Inter-observer agreement for this assessment was poor (κ 0.09). Six of the 17 specimens (35%) showed relevant disease: 4 lesions invaded into the muscularis mucosae of which one also showed lymphovascular invasion; two lesions showed extension of neoplasia into SMGs.
Limitations
Limited number of cases. RFA eligibility status was based on analysis of still images.
Conclusions
One third of flat-type ESCC, deemed eligible for RFA, demonstrated histological features that are considered (relative) contraindications for endoscopic treatment. As it appears difficult for endoscopists to identify low-risk ESCC, conservative use of RFA for flat-type ESCC is advocated until long-term follow-up data are available.
Keywords: Esophageal neoplasm, squamous epithelium, radiofrequency ablation
Introduction
Although the incidence of esophageal adenocarcinoma (EAC) is rising rapidly in the Western world, 80% of esophageal carcinomas occur in other parts of the world, where 90% of cases are esophageal squamous cell carcinoma (ESCC). Especially in South- and Eastern-Africa, central Asian countries, and parts of China, with regional incidences in excess of 100 per 100.000 person-years, ESCC is far more prevalent than in the West [1].
When diagnosed at a symptomatic stage the prognosis of esophageal squamous cell cancer is poor, as lymph node metastasis (LNM) and distant metastasis are frequently present. The prognosis is favorable when ESCC or its precursor lesion – intra-epithelial neoplasia (IEN) – is detected at an early, generally asymptomatic stage. This early stage can be identified at endoscopy and detection is enhanced by chromoendoscopy with Lugol’s iodine staining. In normal esophageal squamous cell epithelium, iodine reversibly binds to the intracellular glycogen causing a brown stained mucosa. In contrast, squamous neoplastic cells contain little glycogen and therefore appear as unstained lesions (USLs).
The risk of lymph node metastasis (LNM) in ESCC is the key factor determining treatment strategies and is linked to the depth of tumor invasion. In IEN (T1m1) and ESCC limited to the lamina propria (T1m2) the risk of LNM is <5%. This marginal risk is deemed acceptable for endoscopic therapy. Lesions invading into the muscularis mucosa (T1m3) or superficial submucosa (T1sm1) carry a higher risk of distant LNM and are considered “borderline lesions”: the choice between endoscopic treatment or surgery should be individualized and discussed in a multidisciplinary setting in centers with a tertiary referral function for esophageal cancer.. Lesions infiltrating into the deep submucosa (≥T1sm2) are not eligible for curative endoscopic treatment [2, 3]. On endoscopy, protruding and excavated lesions (Paris classification type 0-I and 0-III, respectively) harbor a >90% risk of submucosal invasion; in flat-type lesions (Paris type 0-IIa; 0-IIb or 0-IIc) this risk is estimated around 15% [4].
Endoscopic submucosal dissection (ESD) is currently considered the treatment of choice for those cases where curative endoscopic treatment is attempted. En-bloc resection of large neoplastic areas by ESD allows accurate histopathological staging and grading, but is technically demanding and has a long learning curve [5]. In Japan and Korea, en-bloc resection of large neoplastic areas with ESD is well established but the same level of expertise is not widely available elsewhere. Furthermore, esophageal ESD is associated with a significant risk of perforation, and stricture formation occurs in case of larger resections (i.e. >75% of esophageal circumference) [6]. Radiofrequency ablation (RFA) is a safe and effective treatment of Barrett’s esophagus with or without dysplasia [7, 8]. RFA is potentially a less demanding alternative for ESD in the treatment of flat squamous cell neoplasia. This is particularly attractive in those regions (such as Sub-Saharan Africa and the Far East) that have high incidences of early squamous neoplasia, but lack a high level of ESD expertise. Recent studies on RFA in these patients suggest that this technique is safe and effective for flat type squamous neoplasia, although studies are small-sized, have mainly enrolled T1m1-lesions and follow-up remains short [9, 10].
The main drawback of RFA is that it precludes histopathological examination of the ablated lesion. RFA appears therefore most efficacious in patients with low (i.e. <5 %) risk of LNM in whom the benefits of ESD do not outweigh its risks. At this point however, the exact rate of poor histological features lost to pathology work-up by ablative treatment of flat-type early ESCC is not known. In addition, extension of neoplastic disease along mucosal surfaces into gland orifices and ducts of esophageal submucosal glands has previously been reported in ESCC [11, 12]. However, this has only been done in esophagectomy specimens, and not for early type lesions. Extension of neoplastic epithelium along pre-existent ductal linings into the submucosa may create a ‘niche’ for neoplastic epithelium beyond the reach of ablative treatment.
In this study we therefore set out to define the occurrence of poor histological features in patients with extensive (> 3cm), flat-type ESCC deemed eligible for ablative treatment. Secondly, we recorded the pattern of neoplastic ductal extension in ESD specimens of lesions considered eligible for ablative therapy and related the extent of ductal involvement to stage of disease.
Methods
Patient selection
Study patients were identified from a prospectively collected database containing all consecutive patients with esophageal cancer discussed in the multidisciplinary meeting at the National Cancer Center Hospital (NCCH) in Tokyo, Japan. We selected all patients in the database that had undergone an ESD for flat or slightly depressed ESCC (Paris type 0-IIb or 0-IIc respectively, as scored by local endoscopists). Lesions showing partly elevated or excavated features were not included in the study. Only en bloc resections with a minimum diameter of 3 cm (as measured on the fixed specimen in the pathology department) were included in this analysis. All consecutive lesions meeting these inclusion criteria between January 2008 and December 2012 were included. The Internal Review Board granted exemption from approval for this study.
Endoscopic resection and histology processing
All ESD procedures and prior mapping endoscopies were performed according to local protocol with GIF-H260, GIF-H260Z or GIF-Q260J endoscopes (Olympus Medical Systems Co, Ltd, Tokyo, Japan) using a standard video endoscopy processor (EVIS Lucera; Olympus Medical Systems Co, Ltd, Tokyo, Japan). First, the lesion was demarcated by placing coagulation dots with the Dual-knife (Olympus Medical Systems Co, Ltd, Tokyo, Japan). The lesion was then lifted with 0.4% sodium hyaluronate (MucoUp; Johnson & Johnson Co, Ltd, Tokyo, Japan) diluted with normal saline and a minute amount of epinephrine and indigo carmine dye. An initial incision with the Dual-knife was made, followed by the circumferential mucosal incision around the coagulation markings with the IT-knife-2 or IT-knife nano (Olympus Medical Systems Co, Ltd, Tokyo, Japan). After additional submucosal injections, the submucosal layer was dissected with the IT-knife-2 or IT-knife nano.
All specimens retrieved were processed uniformly according to standard pathology protocols. In brief, ESD specimens were pinned down on corkboards and fixed overnight in 10% buffered formalin. Fixed specimens were photographed and cut at regular 2 mm intervals and embedded in paraffin. Slides and stainings were prepared using routine histology protocols.
Review of endoscopic images for the selection of RFA eligible lesions
All images acquired during the endoscopic treatment of the selected lesions were stored in a dedicated database. From the collection of images contained within this database, the team of endoscopists that had performed the ESD procedures in Japan selected a set of images in conventional white light imaging, with narrow-band imaging (NBI) and after Lugol spraying that best represented each individual lesion. A median of 3 images were selected for each modality. Two endoscopists, with extensive experience in RFA for ESCC [9,10], reviewed the set of representative images contained within each individual patient’s case record file and independently assessed eligibility for RFA treatment. Reviewing endoscopists were blinded to previous endoscopic assessment, mapping pathology, and final pathology work-up. ESCC lesions deemed eligible for RFA by both primary reviewers (BW and DF) were taken further without secondary review. All lesions selected by either of one of the primary endoscopists were presented to a third endoscopist with experience in RFA for ESCC (JB) for final RFA eligibility classification.
Histology review
The corresponding histology slides of all ESD specimens were retrieved from the pathology archives at the NCCH and reviewed by two GI-pathologists blinded to endoscopic classification. Deeper histological cuts were not prepared to ensure that every specimen was sampled uniformly for size. Specimens were assessed for maximum depth of invasion, lymphovascular invasion (LVI), and tumor differentiation grade. Depth of invasion was scored as follows: ‘m1’ for high-grade dysplasia or ‘in situ’ carcinoma; ‘m2’ for tumors invading the lamina propria; ‘m3’ for tumors in contact with or invading the muscularis mucosa; ‘sm1’ for tumors invading ≤200 μm into the submucosa; and ‘sm2’ for tumors invading >200 μm [13]. Tumor differentiation was assessed using the World Health Organization criteria as well-differentiated, moderately-differentiated, or poorly-differentiated.
For the assessment of ductal extension, all submucosal glands (SMGs) and ducts were counted. Only non-cauterized, adequately evaluable SMGs and ducts with overlying atypical epithelium were counted. Extension of neoplastic epithelium in at least one duct or SMG was scored as evidence of ductal extension. The number of ducts and SMGs involved by neoplasia were counted separately. The maximum observed depth of ductal extension per specimen (either mucosal, submucosal or reaching into SMGs) was recorded. Ductal extension was not included in the depth of tumor invasion (i.e. m-stage), unless bona fide stromal invasion arising from ducts or SMGs was found.
Outcome parameters
Primary outcomes were: 1) the number of lesions with poor histological features amongst those ESCC lesions considered eligible for ablative treatment after panel review. Poor histological features were defined as m3 (tumors in contact with or invading the muscularis mucosa) invasion or beyond, poor tumor differentiation, or the presence of LVI; 2) the proportion of lesions with submucosal extension of neoplastic epithelium into esophageal ducts or glands amongst those ESCC lesions considered eligible for ablative treatment after panel review.
Secondary outcomes were: 1) the number of lesions with poor histological features or submucosal extension of neoplastic epithelium into esophageal ducts or glands in the non-RFA cases; 2) the inter-observer agreement for flat ESCC among the panel of endoscopists with extensive experience in RFA.
Statistical analyses
The presence of histopathological findings was evaluated by frequency analysis; the Fisher’s exact probability test was used to calculate the P-values between the different groups for binary data, the X2 test (for trend) was used for ordinal data, and the independent T-test and Mann Whitney U test for continuous data. Inter-observer agreement was calculated using the Cohen’s kappa-value and interpreted according to the classification by Landis and Koch (0 ‘poor’ agreement; 0.00 – 0.20 ‘slight’ agreement; 0.21 – 0.40 ‘fair’ agreement; 0.41 – 0.60 ‘moderate’ agreement; 0.61 – 0.80 ‘substantial’ agreement; 0.81 – 1.00 ‘almost perfect’ agreement [14]. Data analysis was carried out by Statistical Software Package version 20.0.0.1 (SPSS IBM, Chicago, Illinois, USA); P < 0.05 was considered significant.
Results
Study population
Sixty-five patients with one lesion each met the initial selection criteria and were evaluated for eligibility for ablative treatment by the endoscopists panel. Table 1 shows the demographics and Table 2 shows the histological findings of the whole cohort.
Table 1. Demographic characteristics.
The demographic characteristics of all patients and lesions from the prospectively collected database containing all consecutive patients with esophageal squamous cell cancer discussed in the multidisciplinary meeting at the National Cancer Center Hospital (NCCH) in Tokyo (Japan) that met the inclusion criteria for this study. Demographic characteristics are shown separately for lesions deemed eligible for RFA by a panel of endoscopists with expertise in RFA for esophageal squamous cell neoplasia. IQR: interquartile range; RFA: radiofrequency ablation; SD: standard deviation.
| Lesions | p-value* | |||
|---|---|---|---|---|
| All | Not RFA eligible | RFA eligible | ||
| General | ||||
| Total, n | 65 | 48 | 17 | 0.17 |
| Age, mean (SD) | 68 (± 8.1) | 67 (± 8.4) | 70 (±6.9) | |
| Gender | ||||
| Male | 58 (89%) | 44 (92%) | 14 (82%) | 0.36 |
| Female | 7 (11%) | 4 (8%) | 3 (18%) | |
| Lesion | ||||
| Location within esophagus | ||||
| Upper thoracic | 1 (2%) | 1 (2%) | 0 (0%) | 0.35 |
| Mid thoracic | 39 (60%) | 30 (62%) | 9 (53%) | |
| Lower thoracic | 25 (38%) | 17 (26%) | 8 (47%) | |
| Macroscopic type (during initial endoscopy in Japan) | ||||
| 0-IIb | 4 (6%) | 4 (17%) | 0 (0%) | 0.34 |
| 0-Iic | 61 (94%) | 44 (83%) | 17 (100%) | |
| Specimen size, median (IQR) | 45 (40–54) | 45 (40–50) | 42 (35–50) | 0.51 |
Either Fisher’s Exact Probability Test, independent sample T-test or Mann Whitney U Test
Table 2. Histopathological findings of all included specimens.
All ESD-specimens were assessed for standard histopatholgical characteristics (depth of invasion, differentiation grade and lymphovascular invasion) and for the extension of neoplasia into esophageal ducts and submucosal glands. The histopathological findings are shown separately for lesions deemed eligible for RFA by a panel of endoscopists with expertise in RFA for esophageal squamous cell neoplasia. CI: confidence interval; LVI: lymphovascular invasion; RFA: radiofrequency ablation.
| Characteristics | Lesions | P-value* | ||
|---|---|---|---|---|
| All | Not RFA eligible | RFA eligible | ||
| N = 65 | N = 48 | N = 17 | ||
| N (%; [95% CI]) | N (%; [95% CI]) | N (%; [95% CI]) | ||
| Standard histopathology | ||||
| Depth of invasion | ||||
| M1 | 7 (11%; [5–20%]) | 3 (6%; [2–17%]) | 4 (24%; [10–47%]) | 0.02 |
| M2 | 31 (48%; [36–60%]) | 22 (46%; [33–60%]) | 9 (53%; [31–74%]) | |
| M3 | 22 (34%; [24–46%]) | 18 (38%; [25–52%]) | 4 (24%; [10–47%]) | |
| SM1/2 | 5 (8%; [3–16%]) | 5 (10%; [5–22%]) | 0 (0%; [0–18%]) | |
| Differentiation grade | ||||
| G1 | 10 (15%; [9–26%]) | 8 (17%; [9–30%]) | 2 (12%; [3–34%]) | 0.95 |
| G2 | 53 (82%; [69–88%]) | 38 (79%; [66–88%]) | 15 (88%; [66–97%]) | |
| G3 | 2 (3%; [1–11%]) | 2 (4%; [1–14%]) | 0 (0%; [0–18%]) | |
| Lymphovascular invasion | ||||
| Present | 6 (9%; [4–19%]) | 5 (10%; [5–22%]) | 1 (6%; [1–27%]) | 1 |
| Total incidence of relevant standard histopathology (>m2, G3 or LVI+) | 28 (43%; [32–55%]) | 24 (50%; [36–64%]) | 4 (24%; [10–47%]) | 0.09 |
| Ductal extension | ||||
| Presence of ≥ 1 duct | 49 (75%; [64–84%]) | 36 (75%; [61–85%]) | 13 (76%; [53–90%]) | 1 |
| Containing neoplasia in ≥ 1 duct | 39 (60%; [48–71%]) | 30 (63%; [48–75%]) | 9 (53%; [31–74%]) | 0.57 |
| Submucosal extension | 13 (20%; [12–31%]) | 11 (23%; [13–37%]) | 2 (12%; [3–34%]) | 0.49 |
| No duct containing neoplasia | 10 (15%; [9–26%]) | 6 (13%; [6–25%]) | 4 (24%; [10–47%]) | 0.43 |
| Glands present | 47 (72%; [60–82%]) | 35 (73%; [59–83%]) | 12 (71%; [47–87%]) | 1 |
| Containing neoplasia | 4 (6%; [2–15%]) | 2 (4%; [1–14%]) | 2 (12%; [3–34%]) | 0.57 |
| Not containing neoplasia | 43 (66%; [54–76%]) | 33 (69%; [55–80%]) | 10 (59%; [36–78%]) | 0.55 |
| Total incidence of submucosal (duct or gland) extension of neoplasia | 13 (20%; [12–31%]) | 11 (23%; [13–37%]) | 2 (12%; [3–34%]) | 0.49 |
| Combined standard histopathology and ductal extension | ||||
| Combined incidence of relevant standard histopathology and ductal extension (>m2, G3, LVI+, or submucosal duct extension) | 34 (52%; [40–64%]) | 28 (58%; [44–71%]) | 6 (35%; [17–59%]) | 0.16 |
Either Fisher’s Exact Probability Test or Chi-Square for Trend Test
Note: percentages may not total 100 because of rounding
Selection of lesions eligible for ablative treatment
Of the total study population of 65 ESCC lesions, 17 lesions were deemed eligible for RFA by the panel of endoscopists. There were no significant demographic differences between lesions selected and excluded as candidate lesions for ablative treatment (Table 1). The two primary endoscopy reviewers agreed upon inclusion of 7 cases and exclusion of 27 cases (n=34); disagreement was seen in 31 cases (κ 0.09 [95% CI: 0.00 – 0.26]). The third endoscopy reviewer scored 10 of these remaining 31 lesions as eligible for RFA, resulting in a total 17 cases deemed eligible for RFA by majority of vote; all other lesions were discarded as eligible candidates for ablative treatment (Table 3).
Table 3. Scoring of RFA-eligibility by panel of endoscopists.
Based on a set of the best endoscopic images, all lesions that met the inclusion criteria were assessed for RFA eligibility by a panel of endoscopists with expertise in RFA for esophageal squamous cell neoplasia. This table shows the agreement amongst the panel. RFA: radiofrequency ablation.
| Reviewer | Lesions | ||
|---|---|---|---|
|
| |||
| Total | Selected for RFA treatment | Discarded for RFA treatment | |
| I | 65 | 10 | 55 |
| II | 65 | 35 | 30 |
| Agreement I&II | 34 | 7 | 27 |
| Disagreement I&II (scored by III) | 31 | 10 | 21 |
| Agreement I & III | 20 | 1 | 19 |
| Agreement II & III | 11 | 9 | 2 |
Cohen’s kappa for endscopy-reviewers I & II: 0.09 [95% CI: 0.00 – 0.26] (poor agreement)
Histopathology in RFA eligible lesions
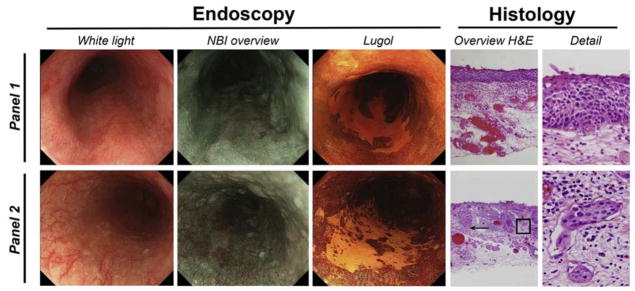
In four of 17 cases (24%) deemed eligible for RFA treatment poor histological features were found (Table 2). All four specimens contained T1m3 lesions. Additionally, in one of the specimens lymphovascular invasion was also present. Figure 1 illustrates an example of an extensive flat unstained lesion (USL) selected as a candidate for ablative treatment without the presence of poor histological features (top panel). The lower panel shows an example of an extensive flat USL considered eligible for ablative treatment; however histopathology of this lesion showed invasion into the muscularis mucosae (T1m3) as well as focal LVI.
Figure 1. Ductal extension in an ESD specimen.
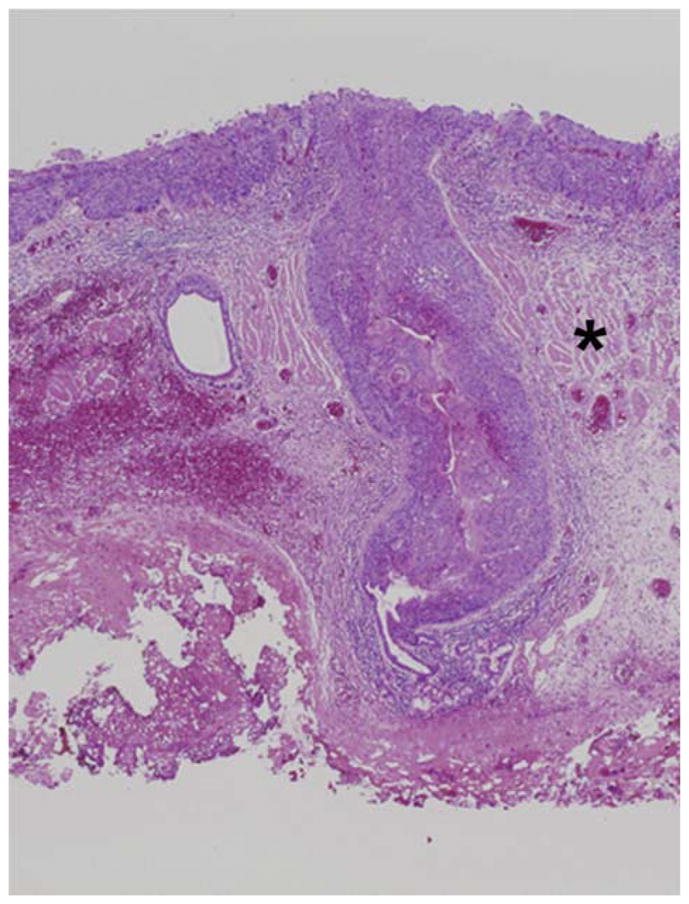
Representative example of a T1m2 lesion showing massive ductal extension. The superficial component of the lesion shows an undulating, expansive pattern of growth, consistent with T1m2. The muscularis mucosa is indicated by an asterisk. The asterisk indicates the muscularis mucosae. The lesion extends via the ductal system to the level of the submucosal gland.
In contrast, in 24 of 48 (50%) of the cases deemed ineligible for RFA poor histological features were identified. Although there was a trend towards a difference in the presence of poor histologic features between the cases considered eligible and ineligible as potential candidates for ablative treatment, these results did not reach statistical significance (p=0.09). Lesions deemed suitable for RFA did show less deep penetration of tumor compared to the ineligible cases (p=0.02), but no difference was found either in differentiation grade or in the presence of LVI.
Ductal extension
The ESD specimens showed a median number of 5 ducts (IQR 1 – 13) and 5 SMGs (IQR 0 – 19). No esophageal ducts or SMGs were detected in 16 (25%) and 18 (28%) lesions, respectively. On the other hand, 25% of resected lesions showed ≥ 15 ducts in their ESD specimen. Thus, esophageal duct numbers varied widely between individual lesions. We found no correlation between duct number, ESD specimen size or localization within the esophagus.
In specimens considered eligible for RFA at least 1 duct or at least 1 gland was identified in 13 (76%) and 12 (71%) cases, respectively. Of those lesions in which ≥1 duct was detected, extension of neoplastic epithelium into at least one duct was observed in 70% (9 of 13 cases). The median number of esophageal ducts with neoplastic extension was 2 (IQR 1 – 5). Overall, 53% of lesions considered RFA eligible (9 of 17 cases) showed extension of neoplastic epithelium into the ducts. Two of these cases (2 of 17; 12%) had extension of neoplastic epithelium into the ducts down to the level of the submucosa. Extension of neoplastic epithelium into SMGs was observed in two patients, the same two who also had ductal extension of neoplastic epithelium into the submucosa.
There were no significant differences between the lesions considered eligible and lesions considered not eligible for RFA in the number of lesions with ≥1 duct (76% vs 75% respectively), the number of lesions with any extension of neoplastic epithelium into ducts (53% vs 63% respectively) or extension into the submucosal level in the ducts (12% vs 23% respectively) [Table 2]. In addition, no difference was found in the number of SMGs (71% vs 73% respectively) or SMGs containing neoplasia (12% vs 4% respectively) between both groups.
Combining the poor histopathology parameters (m3 invasion, G3 differentiation, or LVI) and the extension of neoplastic epithelium into the ducts or glands at the submucosal level, we found that 6 of 17 (35%) of the cases deemed eligible for RFA had poor histological findings in their specimen.
Neoplastic ductal extension was not observed in any of the specimens showing T1m1 disease. Conversely, 91% of ≥m2-lesions and 95% of ≥m3 lesions showed extension of neoplastic epithelium into esophageal ducts (p<0.001).
Discussion
Although the standard of care for early ESCC is complete endoscopic resection by ESD, ablative treatment of flat-type pre-invasive and early ESCC may allow disease control in many regions where the disease is highly prevalent yet widespread availability of ESD is limited. Studies with RFA in high-incidence regions in China have proven this technique to be safe and effective for eradication of flat squamous neoplasia [9]. The main drawback of ablative treatment is the lack of conclusive histopathological examination. Ablative approaches are therefore predicated on careful endoscopy and mapping biopsies to exclude unsuitable lesions.
In the current study we evaluated whether endoscopic selection of flat-type ESCC by endoscopists experienced in RFA for ESCC is appropriate. We studied a consecutive cohort of patients with flat-type ESCC treated with ESD at a single institution. Multi-modality endoscopy images of lesions were distributed amongst a panel of RFA-experienced endoscopists. Corresponding ESD specimens were meticulously reviewed and scored for relevant pathology. We found that poor prognostic histological features placing a patient at risk for focal lymph node metastasis occur frequently in flat lesions considered eligible for RFA. Furthermore, we found that extension of neoplastic disease along pre-existent ductal linings into the submucosal space occurs commonly in ESCC. When combining the poor histopathological parameters with ductal extension into the submucosal space, 35% of lesions considered eligible for RFA (6 of 17) were positive. In these patients ablation would have resulted in either the loss of relevant histological information or potentially incomplete treatment of neoplasia in the submucosal space.
Poor histological features (≥m3 invasion, poor differentiation, and LVI) are associated with an increased risk for locoregional LNM [2]. LNM are reported in 6–18% of T1m3 cancers and in 8–53% of T1sm1 ESCC [2, 15–20]. The risk appears to be significantly lower for lesions without LVI or poor tumor differentiation. Well to moderately differentiated T1m3 and T1sm1 cancers without lymphovascular invasion are therefore often considered a relative indication for endoscopic management. The choice for additional treatment (surgical resection or chemoradiotherapy) is usually individualized also weighing factors such as co-morbidity and age. Three of the four lesions with T1m3 invasion fit these relative indication criteria, yet in one patient the T1m3 status was accompanied by LVI, a combination for which most guidelines strongly advocate additional treatment.
Our data suggest that neoplastic extension down the ducts of submucosal glands is a universal trait of ESCC. Although this neoplastic extension was not associated with local invasion (i.e. all neoplastic extension down the ducts remained at an intra-epithelial level) extension of neoplastic disease into the submucosal space may place a patient at risk of local failure if ablative therapy remains too superficial. It is currently unclear what the actual depth of ablation is using RFA for squamous neoplasia. To our knowledge, only two studies have assessed effects of RFA in humans at a histopathological level. In patients scheduled for esophagectomy Dunkin et al. observed no damage deeper than the muscularis mucosae after a single or double application with 12 J/cm2 directly preceding the surgical procedure [21]. In an identical patient group Ganz and co-workers also observed no damage deeper than the muscularis mucosae at 24–48 hours post-ablation after a single application with 12 J/cm2. RFA did not affect the submucosa as SMGs were still present [22]. True depth of ablation, however, may extend deeper, as shown in other studies of cryoablation in squamous mucosa which have found more profound damage after 4 days compared to an early time point [23]; similar studies have not yet been performed for this long after RFA. Therefore, the exact depth of treatment under current RFA protocols in squamous epithelium (single application of 12 J/cm2) – and whether SMGs are effectively ablated – remains unclear. This is obviously an important area of future research.
The most important limitation of this study is that the selection of RFA eligibility was based on still images of ESCC without knowledge of prior endoscopic mapping. In the prospective studies on the use of RFA for early squamous neoplasia in China, patients were selected by real-time endoscopic assessment by multiple endoscopists after extensive biopsy mapping. Mainly patients with non-invasive (i.e. MGIN and HGIN) were included and treated by RFA. Our data suggest that patients with non-invasive disease have a very low rate of ductal extension (0% in this study). Such extension mainly occurs in patients with invasive disease (≥T1m2). Although mapping biopsies may allow for a differentiation between intraepithelial neoplasia and invasive carcinoma, it is questionable whether this sampling method is able to reliably determine the depth of invasion, tumor grade, presence of lymphovascular invasion, or presence extension of neoplasia down the ducts. Magnification endoscopy may support the assessment of the depth of tumor invasion[24]. However, as magnifying endoscopy images were not available in all cases, these were excluded from assessment by the panel. Earlier RFA studies have also not used magnifying endoscopy to select cases and further studies into its potential value are warranted. In addition, predicting the presence of poor histological factors in squamous lesions appears to be difficult amongst endoscopists (even by those with extensive experience in RFA for ESCC), and frequent disagreement in the selection of lesions eligible for RFA was observed in the current study. Taken together, at this point selection of low-risk ESCC seems difficult and restraint in the use of ablative therapy for the treatment of flat ESCC seems appropriate.
In conclusion, poor prognostic histological indicators that are considered (relative) contraindications for endoscopic treatment occur frequently in flat-type ESCC deemed eligible for RFA. Moreover, ductal extension occurs commonly in ESCC. As it appears difficult for endoscopists to identify low-risk ESCC, conservative use of ablation therapy for flat-type ESCC is recommended until long-term follow-up data from trials are available.
Figure 2. Examples of radiofrequency ablation (RFA) eligible cases.
The upper panel shows an example of an extensive flat USL deemed eligible for RFA by the endoscopists panel. This lesion did not demonstrate poor prognostic histologic features. The corresponding histological image shows a non-expansive growth pattern, consistent with T1m1. The lower panel shows an example of an extensive flat USL deemed eligible for RFA by the endoscopists panel. Yet histopathology of the latter lesion showed invasion into the muscularis mucosae (T1m3, arrow) as well as focal LVI (boxed area shown in detail). H&E, hematoxylin and eosin; NBI, narrow-band imaging.
Acknowledgments
Grant support: no grant support was available
Reference list
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer JClin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Eguchi T, Nakanishi Y, Shimoda T, Iwasaki M, Igaki H, Tachimori Y, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. ModPathol. 2006;19:475–80. doi: 10.1038/modpathol.3800557. [DOI] [PubMed] [Google Scholar]
- 3.Katada C, Muto M, Momma K, Arima M, Tajiri H, Kanamaru C, et al. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy. 2007;39:779–83. doi: 10.1055/s-2007-966761. [DOI] [PubMed] [Google Scholar]
- 4.Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432–9. [PubMed] [Google Scholar]
- 5.Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Omata M. A learning curve for endoscopic submucosal dissection of gastric epithelial neoplasms. Endoscopy. 2006;38:991–5. doi: 10.1055/s-2006-944808. [DOI] [PubMed] [Google Scholar]
- 6.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–6. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. NEnglJMed. 2009;360:2277–88. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 8.Pouw RE, Wirths K, Eisendrath P, Sondermeijer CM, Ten Kate FJ, Fockens P, et al. Efficacy of radiofrequency ablation combined with endoscopic resection for barrett’s esophagus with early neoplasia. ClinGastroenterolHepatol. 2010;8:23–9. doi: 10.1016/j.cgh.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Bergman JJ, Zhang YM, He S, Weusten B, Xue L, Fleischer DE, et al. Outcomes from a prospective trial of endoscopic radiofrequency ablation of early squamous cell neoplasia of the esophagus. Gastrointest Endosc. 2011;74:1181–90. doi: 10.1016/j.gie.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Vilsteren FG, Alvarez HL, Pouw RE, Ten Kate FJ, Visser M, Seldenrijk CA, et al. Radiofrequency ablation for the endoscopic eradication of esophageal squamous high grade intraepithelial neoplasia and mucosal squamous cell carcinoma. Endoscopy. 2011;43:282–90. doi: 10.1055/s-0030-1256309. [DOI] [PubMed] [Google Scholar]
- 11.Takubo K, Takai A, Takayama S, Sasajima K, Yamashita K, Fujita K. Intraductal spread of esophageal squamous cell carcinoma. Cancer. 1987;59:1751–7. doi: 10.1002/1097-0142(19870515)59:10<1751::aid-cncr2820591013>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Tajima Y, Nakanishi Y, Tachimori Y, Kato H, Watanabe H, Yamaguchi H, et al. Significance of involvement by squamous cell carcinoma of the ducts of esophageal submucosal glands. Analysis of 201 surgically resected superficial squamous cell carcinomas. Cancer. 2000;89:248–54. doi: 10.1002/1097-0142(20000715)89:2<248::aid-cncr7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Takubo K, Aida J, Sawabe M, Kurosumi M, Arima M, Fujishiro M, et al. Early squamous cell carcinoma of the oesophagus: the Japanese viewpoint. Histopathology. 2007;51:733–42. doi: 10.1111/j.1365-2559.2007.02766.x. [DOI] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 15.Makuuchi H, Shimada H, Mizutani K, Chino O, Nishi T, Tanaka H, et al. Clinical pathological analysis of surgically resected superficial esophageal carcinoma to determine criteria for deciding on treatment strategy. DiagnTherEndosc. 1997;3:211–20. doi: 10.1155/DTE.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li B, Chen H, Xiang J, Zhang Y, Kong Y, Garfield DH, et al. Prevalence of lymph node metastases in superficial esophageal squamous cell carcinoma. JThoracCardiovascSurg. 2013;146:1198–203. doi: 10.1016/j.jtcvs.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Xue L, Ren L, Zou S, Shan L, Liu X, Xie Y, et al. Parameters predicting lymph node metastasis in patients with superficial esophageal squamous cell carcinoma. ModPathol. 2012;25:1364–77. doi: 10.1038/modpathol.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamashina T, Ishihara R, Nagai K, Matsuura N, Matsui F, Ito T, et al. Long-term outcome and metastatic risk after endoscopic resection of superficial esophageal squamous cell carcinoma. AmJGastroenterol. 2013;108:544–51. doi: 10.1038/ajg.2013.8. [DOI] [PubMed] [Google Scholar]
- 19.Akutsu Y, Uesato M, Shuto K, Kono T, Hoshino I, Horibe D, et al. The overall prevalence of metastasis in T1 esophageal squamous cell carcinoma: a retrospective analysis of 295 patients. AnnSurg. 2013;257:1032–8. doi: 10.1097/SLA.0b013e31827017fc. [DOI] [PubMed] [Google Scholar]
- 20.Shimada H, Nabeya Y, Matsubara H, Okazumi S, Shiratori T, Shimizu T, et al. Prediction of lymph node status in patients with superficial esophageal carcinoma: analysis of 160 surgically resected cancers. AmJSurg. 2006;191:250–4. doi: 10.1016/j.amjsurg.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Dunkin BJ, Martinez J, Bejarano PA, Smith CD, Chang K, Livingstone AS, et al. Thin-layer ablation of human esophageal epithelium using a bipolar radiofrequency balloon device. SurgEndosc. 2006;20:125–30. doi: 10.1007/s00464-005-8279-9. [DOI] [PubMed] [Google Scholar]
- 22.Ganz RA, Utley DS, Stern RA, Jackson J, Batts KP, Termin P. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointestinal endoscopy. 2004;60:1002–10. doi: 10.1016/s0016-5107(04)02220-5. [DOI] [PubMed] [Google Scholar]
- 23.DeMeester S, Awais O, Bergman J, Grant K, Jobe B, Niebisch S, et al. Initial Human Experience With a Novel Through-the-Scope Cryoballoon Device for Mucosal Ablation. Gastroenterology. 2012;142:S-1038. [Google Scholar]
- 24.Oyama T, Ishihara R, Takeuchi M, Hirasawa D, Arima M, Inoue H, et al. Usefulness of Japan Esophageal Society Classification of Magnified Endoscopy for the Diagnosis of Superficial Esophageal Squamous Cell Carcinoma. Gastrointestinal Endoscopy. 75:AB456. [Google Scholar]