Abstract
Objectives
Khat (Catha edulis), an amphetamine-like plant, is widely used in East Africa and the Arabian Peninsula and is becoming a growing problem in other parts of the world. The concurrent use of tobacco and khat is highly prevalent and represents a public health challenge. We examined for the first time associations of the concurrent use of tobacco and khat with psychophysiological responses to acute stress in two sites in Yemen.
Methods
Participants (N = 308; 135 women) included three groups: users of khat and tobacco, users of khat alone, and a control group (nonsmokers/nonusers of khat). These individuals completed a laboratory session in which blood pressures (BP), heart rate, and mood measures were assessed during rest and in response to acute stress.
Results
Concurrent use of khat and tobacco was associated with attenuated systolic BP, diastolic BP, and heart rate responses to laboratory stress (ps <0.05) and with increased negative affect relative to the control group (ps <0.05).
Conclusions
Results demonstrated blunted cardiovascular responses to stress and enhanced negative affect in concurrent khat and tobacco users. These findings extend previous studies with other substances and suggest that adverse effects of khat use may lie in its association with the use of tobacco.
Keywords: tobacco, khat, cardiovascular response, negative affect, psychopharmacology
INTRODUCTION
Chronic exposure to drugs of addiction can have profound effects on the brain. The impact of this exposure may be particularly devastating in poor communities and in low-income countries where effects of drugs are complicated by other environmental hardship, including malnutrition, poverty, and poor health care. Khat (Catha edulis) is a natural amphetamine that is widely used in East Africa and the Arabian Peninsula with a prevalence reaching 75% of the adult population (Krikorian, 1984; World-Bank, 2007; Wedegaertner et al., 2010; Reda et al., 2012). Its use has been growing in North America, Western Europe, and other parts of the world (Kassim and Croucher, 2006; Griffiths et al., 2010; Apps et al., 2011; UK-Home-Office, 2011), although accurate epidemiological estimates are not available. The harm to health caused by this drug has long been established (Cox and Ramsay, 2003; Al Motarreb et al., 2010; Ali et al., 2011), but there is virtually no research addressing its effects on behavioral functions either acutely or chronically. This knowledge is critical in understanding the biobehavioral influences of khat use, for guiding efforts to combat its escalating use, addressing its adverse consequences on health.
Chewing khat leaves is the most common mode of intake (Kennedy, 1988), although a small number of users use dried leaves to make drinks (Pantelis et al., 1989). Users fill their mouth with leaves and chew slowly and intermittently to release the active components of khat that are then swallowed (Nencini et al., 1986). Khat chewing has a social and cultural tradition, and it may occur while in the company of others or alone (Kennedy, 1988). One of the major pharmacologically active constituents of khat is cathinone, an amphetamine-like sympathomimetic amine (Kalix, 1996). Similar to amphetamines, khat ingestion produces several central nervous system effects, including increased motor stimulation, euphoria, and sense of excitement and energy (Kalix, 1996; Nencini et al., 1998), in addition to increased levels of alertness, ability to concentrate, confidence, friendliness, contentment, and flow of ideas (Kennedy, 1988; Brenneisen et al., 1990; Widler et al., 1994). It also results in decreased appetite and increased blood pressure (BP) and heart rate (HR) (Hassan et al., 2000). Within 2 h after ingestion, chewers report excessive tension, anxiety, emotional instability, irritability, and restlessness, which are followed by feelings of low mood, numbness, lack of concentration, sluggishness, and insomnia (Kennedy, 1988; Al-Motarreb et al., 2002). These effects suggest that khat acts through central mechanisms similar to amphetamine. Both cathinone and amphetamine increase the activity of the dopaminergic and noradrenergic transmission (Nielsen, 1985; Pehek and Schechter, 1990; Pehek et al., 1990; Patel, 2000). Although the nature of khat dependence remains under active debate, accumulating evidence suggests the existence of a withdrawal syndrome and a low-level tolerance (Cox and Ramsay, 2003; Manghi et al., 2009; Wedegaertner et al., 2010). Withdrawal symptoms usually include inertia, nightmares, trembling, depression, sedation, and hypotension.
Cigarettes and waterpipe tobacco smoking (shisha, nargile) are widely used during khat chewing sessions (Odenwald et al., 2007; Tesfaye et al., 2008; Kassim et al., 2011; Regassa and Kedir, 2011; Nakajima et al., 2012). Reports indicate high tobacco use reaching 65% among khat users (Bawazeer et al., 1999; Odenwald et al., 2007; Tesfaye et al., 2008). Although effects of tobacco and khat use have been examined separately in previous studies and have been found to activate similar physiological systems, no study has evaluated acute or chronic influences of the combination, and factors contributing to concurrent use have not been explored. Furthermore, the extent to which concurrent use of khat and tobacco interacts to modulate stress-related biobehavioral functions is not known. Studies examining effects of tobacco alone have shown that long-term smoking contributes to dysregulation of the neurobiological stress response (e.g., diminished reactions in cortisol and BP (Kirschbaum et al., 1993; al’Absi et al., 2002; al’Absi, 2006), and these alterations have potential impact on treatment outcome (al’Absi et al., 2005). Considering the complex interaction involved in the amphetamine-like effects of khat use and tobacco, it is possible that the use of both substances confers greater risk for impaired stress response regulation than the use of khat or tobacco alone. As such, this work is relevant to and could inform research focusing on concurrent addictive behaviors in other countries (Burns et al., 2000; Lai et al., 2000; John et al., 2003; Humfleet and Haas, 2004).
We report here data obtained from a program conducted through a partnership between the University of Minnesota and two Yemeni universities to examine effects of the concurrent use of khat and tobacco. Concurrent khat and tobacco users, those who use khat alone, and those who were not using either of these two substances were recruited into this study. Cardiovascular and mood measures were assessed during rest and in response to acute stress. On the basis of previous work on nicotine and other psychostimulants (Kirschbaum et al., 1993; al’Absi et al., 2005; al’Absi, 2006), and previous data from a smaller group of khat users (al’Absi et al., 2013), we predicted that khat use would be associated with blunted cardiovascular response and with exaggerated affective responses to stress. These response abnormalities would be more pronounced in khat users who also use tobacco than those who do not use tobacco.
METHODS
Participants
The current study used a cross-sectional design to examine the extent to which khat and tobacco use was associated with alterations with the stress response. Participants (concurrent users of khat and tobacco, users of khat alone, and a control group of nonsmokers/nonusers) were recruited between 2008 and 2010 on two university campuses and surrounding communities in two Yemeni cities, Sana’a and Taiz. Recruitment was conducted using flyers posted on both campuses and by word of mouth. Before enrolling in the study, participants attended a screening session to assess eligibility for the study and availability to participate in the protocol. The screening session included a brief medical history interview and assessment of current and past khat and tobacco use as well as the use of other substances. Participants were included if they were free from any medical or psychiatric conditions (e.g., high BP, cancer, heart disease, diabetes, major depression, anxiety disorders, and substance use disorders) and were not taking any medications. They also must have had a regular sleep/wake cycle. Khat users had to have been chewing khat on a daily basis. In addition to this, smokers had to have been smoking daily. We also assessed shisha smoking and used criteria for tobacco use status based on previously used methods (Maziak et al., 2004). Three hundred and eight participants completed the laboratory session. These included 104 (39 women) who were concurrent users of tobacco and khat, 85 (30 women) who reported using khat only, and 119 (66 women) who reported no use of khat or tobacco.
Participants signed a consent form approved by the local Institutional Review Boards from Sana’a University and Taiz University. After that, they completed forms related to behavioral health and stress.
Apparatus and measures
Cardiovascular measures
An automated BP monitor (MicroLife Automatic BP monitor 3AC1; MicroLife, Widnau, Switzerland) was used to measure BP and HR. Such automated units have the advantages of enhanced reliability and freedom from differences in auscultatory practices between operators. This oscillometric device has been validated by the European Society of Hypertension’s international protocol.
Self-report measures
We used the Subjective State measure to assess mood states during the laboratory session. This scale included 18 items that have been translated from previous adjective checklists used in lab experiments that is sensitive to acute mood changes (al’Absi et al., 1997) and covers both negative and positive affect (Lundberg and Frankenhaeuser, 1980; al’Absi et al., 1994a). Negative affect was assessed using items of anxiety, irritability, impatience, sadness, anger, and restlessness. Positive affect was assessed using items of cheerfulness, content, calmness, controllability, and interest. This scale has been previously validated and used in this population (Bongard et al., 2011). The Cronbach’s α values for negative affect and positive affect were 0.85 and 0.79, respectively (Bongard et al., 2011). Each item was referenced to an 8-point scale anchored by the end points ‘Not at All’ and ‘Very Strong’. Participants marked the scale at the point that best described how they felt during the previous 30 min. This measure was completed before and after performing an acute stress challenge.
Procedures
The laboratory session was conducted starting between 9:00 and 10:00 AM, and it lasted approximately 90 min. Prior to the laboratory session, a set of dietary guidelines and instructions about sleep were provided (e.g., participants were asked to have a light breakfast before the laboratory session). To minimize withdrawal symptoms, tobacco users continued to smoke at their regular rate until immediately before the laboratory session. Similarly, khat users continued their regular daily chewing habit (chewing the afternoon of the day before the session). At the beginning of the session, the participant was brought to a quiet room and sat in a comfortable chair. An inflatable BP cuff was attached to the subject’s left upper arm. Procedures included resting baseline for 15 min, the acute stressful challenge for 10 min, and a recovery rest period for 15 min. The acute challenge was a mental arithmetic task that involved subtraction of a number 7 from a four-digit number. Participants were asked to perform as fast as they could. When a mistake was made, the participant was asked to go back to the previous correct number. This task has been previously used in multiple stress reactivity studies and has been found to be effective in activating the sympathetic system (Sherwood et al., 1993; al’Absi et al., 1994b; al’Absi et al., 1997). BP and HR were measured every 3 min during resting baseline, the acute stressor, and during recovery. Participants also completed the Subjective State measure after baseline, the stressor, and after recovery.
Data analysis
The primary dependent variables in this study were HR (bpm), systolic BP (mmHg), diastolic BP (mmHg), and self-report measures of negative affect and positive affect. Rate pressure product (RPP, mmHg beat/min, calculated as HR × systolic BP) was included as an index of myocardial oxygen demand. Cardiovascular measures were averaged across three periods (baseline, stress, and recovery). Cardiovascular and self-report data were analyzed using 3 (groups: concurrent users of khat and tobacco, khat-only users, and nonusers) × 2 (gender: men, women) × 3 sampling times (baseline stress, and recovery) analysis of covariance with Greenhouse–Geisser corrections. Site variable was included as a covariate. Demographic variables such as age and body mass index [BMI; weight (kg)/height (m2)] were analyzed using 3 groups × 2 gender analysis of variance (ANOVA) as well as chi-square tests. Khat-user variables were analyzed using 2 groups (concurrent users and khat-only users) × 2 gender ANOVA and chi-square tests. Tobacco use among concurrent users was analyzed by one-way ANOVAs with gender as a factor and chi-square tests. For main effect comparison, we used the Bonferroni correction.
RESULTS
Participant characteristics
Concurrent and khat-only users were older than nonusers (group effect: F(2, 302) = 9.99, p <0.01; Table 1). Men were taller (F(1, 295) = 227, p <0.001) and heavier (F(1, 295) = 37.1, p <0.001) than women; however, BMI did not differ by groups or gender (ps >0.16). Concurrent users reported fewer average hours of sleep than nonusers (F(2, 105) = 5.13, p <0.01). Years of education did not differ by group or by gender (ps >0.25). Khat-only and concurrent users did not differ in patterns of khat use, with one exception that concurrent users reported longer hours of chewing per session than khat-alone users (F(1, 184) = 8.39, p <0.01). Reported age started was earlier, and hours and days of khat chewing were greater in men than in women (Fs(1, 181) = 16.4, ps <0.001). Reported number of cigarettes smoked per day was greater in men than in women (F(1, 101) = 94, p <0.001), whereas waterpipe use was greater in women than in men (F(1, 71) = 40, p <0.001). Because of observed differences in age across groups, age was included as a covariate where appropriate.
Table 1.
Sample characteristics
| Nonusers | Khat-only users | Concurrent (tobacco and khat) users | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Women | Men | Women | Men | Women | Men | |
|
|
|
|
||||
| (n = 66) | (n = 53) | (n = 30) | (n = 55) | (n = 39) | (n = 65) | |
| Demographic variables | ||||||
| Age (years)c | 22.1 (0.6) | 22.2 (0.7) | 25.2 (0.9) | 23.7 (0.7) | 25.5 (0.8) | 24.5 (0.6) |
| Height (cm)d | 153.2 (0.9) | 167.0 (1.0) | 155.1 (1.3) | 163.1 (0.9) | 150.2 (1.1) | 166.1 (0.9) |
| Weight (kg)d | 49.3 (1.2) | 59.6 (1.4) | 53.1 (1.8) | 56.5 (1.3) | 49.9 (1.6) | 57.4 (1.2) |
| Body mass index (kg/m2) | 21.1 (0.5) | 21.3 (0.5) | 22.1 (0.7) | 21.3 (0.5) | 22.1 (0.6) | 20.8 (0.5) |
| Education (years)a | 11.4 (0.7) | 13.1 (0.8) | 12.8 (1.4) | 12.7 (0.8) | 9.0 (3.2) | 11.5 (0.8) |
| Sleep (average hours per day)a | 8.0 (0.3) | 7.2 (0.3) | 6.8 (0.6) | 6.9 (0.3) | 5.9 (0.8) | 6.8 (0.4) |
| Khat use | ||||||
| Age started (years)d | n/a | n/a | 17.1 (0.7) | 15.6 (0.5) | 18.4 (0.6) | 15.3 (0.5) |
| Daily use (%)d | n/a | n/a | 56.7 | 80.0 | 48.7 | 92.3 |
| Duration (years) | n/a | n/a | 5.3 (0.9) | 7.0 (0.7) | 5.8 (0.8) | 6.5 (0.6) |
| Length of use (h/session) c | n/a | n/a | 3.7 (0.4) | 4.9 (0.3) | 4.5 (0.3) | 5.9 (0.3) |
| Times (per week)d | n/a | n/a | 5.0 (0.3) | 5.9 (0.3) | 4.4 (0.3) | 6.5 (0.2) |
| Tobacco use | ||||||
| Daily tobacco use (%)d | n/a | n/a | n/a | n/a | 33.3 | 67.7 |
| Duration (years) | n/a | n/a | n/a | n/a | 4.3 (0.7) | 5.4 (0.5) |
| Cigarettes (per day)d | n/a | n/a | n/a | n/a | 1.4 (1.0) | 13.5 (0.7) |
| Waterpipe (per day)b,d | n/a | n/a | n/a | n/a | 2.0 (0.2) | 0.2 (0.2) |
n/a, not applicable.
Data collected in Sana’a component only.
Data collected in Taiz component only.
Group effect was significant.
Gender effect was significant.
Cardiovascular and self-report measures
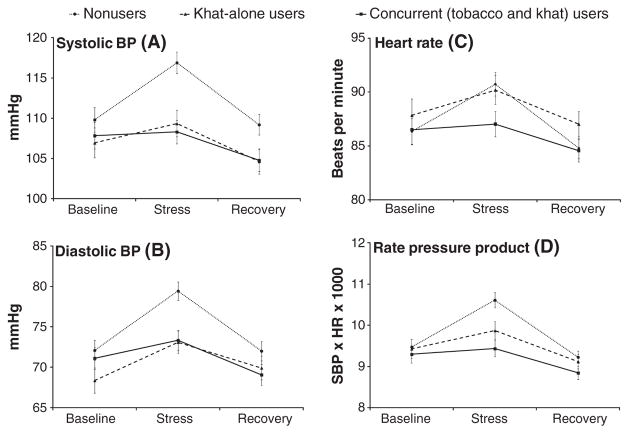
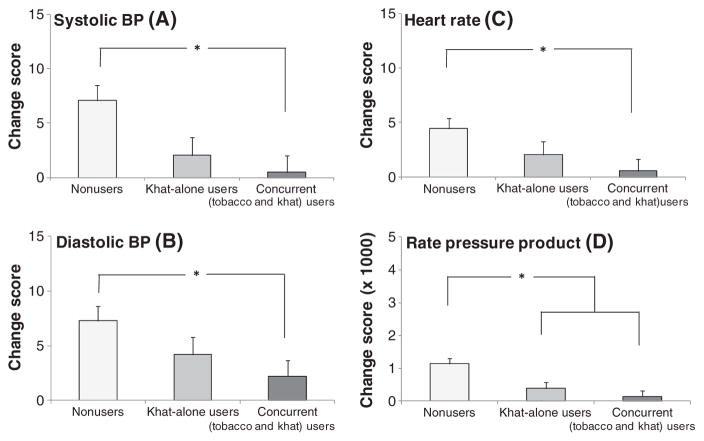
We found significant differences in the magnitude of cardiovascular responses to stress as demonstrated by significant group × sampling time interactions (Fs(4, 513) >2.57, ps <0.05) in systolic BP, diastolic BP, HR, and RPP. To further explore these interactions, change scores (the value during baseline period was subtracted from the value during stress period) were obtained. A series of 3 groups × 2 gender analyses of covariance including site as a covariate found significant group differences across all cardiovascular measures (Fs(2, 295) >3.28, ps <0.05) reflecting attenuated response among concurrent users relative to nonusers (ps <0.05; Figure 1). Khat-only users exhibited smaller RPP response to stress (p <0.01) and tended to show smaller systolic BP (p <0.07) than nonusers. Concurrent users of khat and tobacco did not differ from khat-only users (ps >0.99; Figure 2). Men showed greater systolic BP and RPP than women (Fs(1, 294) >4.13, ps <0.05).
Figure 1.
Mean cardiovascular measures during baseline, acute stress, and recovery. Note: Means and standard errors for systolic BP (A); diastolic BP (B); heart rate (C); and rate pressure product (D)
Figure 2.
Changes in cardiovascular measures in response to laboratory stress (differences between baseline and stress periods). Note: Means and standard errors for systolic BP (A); diastolic BP (B); heart rate (C); and rate pressure product (D). Asterisks reflect significant group differences
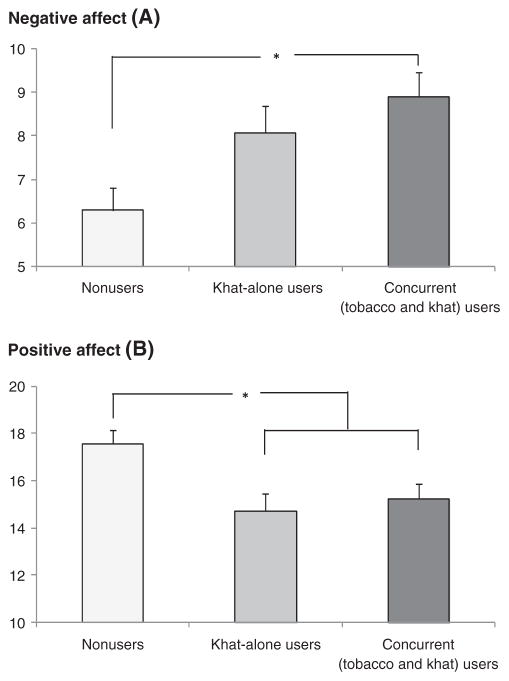
Exposure to the acute stressor was associated with increased negative affect and decreased positive affect (time effects: Fs(2, 574) >9.64, ps <0.001, specific comparisons: ps <0.001; Figure 3). A significant group effect was also found for these measures (Fs(2, 297) >5.68, ps <0.01). This was due to greater negative affect and lower positive affect in concurrent users than the nonusers (ps <0.05). In addition, lower positive affect was reported by khat-only users compared with the nonusers (p <0.01). Concurrent users of khat and tobacco did not differ from khat-only users (Fs <1). Women reported lower positive affect than men (F(1, 297) = 4.71, p <0.05).
Figure 3.
Negative affect (A) and positive affect (B) during the laboratory session. Note: Means and standard errors of reported negative affect and positive affect. Asterisks reflect significant group differences
Correlational analysis
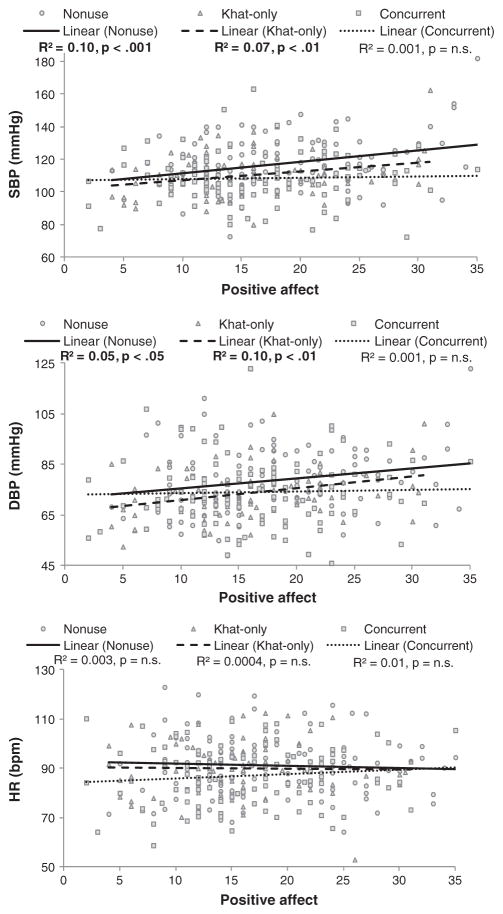
A series of correlational analyses were conducted to examine the extent to which patterns of khat use were associated with cardiovascular measures. These analyses included concurrent users and khat-only users. Daily khat use was positively related to systolic BP and RPP during all periods (rs 0.16–0.26, ps <0.05). The frequency of khat chewing sessions per week was positively linked with HR during baseline and recovery (rs 0.14–0.15, ps <0.05), and years of chewing were positively correlated with systolic BP during stress and recovery (rs 0.17–0.24, ps <0.05). Years of khat use were inversely associated with HR response (change score from baseline to stress; r = −0.15, p <0.05). Similarly, correlational analyses were conducted to test the relationships between tobacco use patterns and cardiovascular measures. The number of cigarettes per day was positively associated with systolic BP during stress and RPP during all periods (rs 0.19–0.26, ps <0.05). In contrast, the number of waterpipe heads was inversely related to HR during baseline (r = −0.30, p <0.01). Daily tobacco use was inversely associated with systolic BP, diastolic BP, and RPP responses (rs >−0.19, ps ≤ 0.05) (Figure 4).
Figure 4.
Associations between positive affect scores and cardiovascular measures during stress. Note: Positive affect is presented on the x-axis, and cardiovascular measures are presented on the y-axis
Correlational analyses were also conducted to examine whether self-report mood measures were linked with cardiovascular measures. When analyses were conducted including all participants, we found positive correlations between positive affect at all periods (baseline, stress, and recovery) and systolic BP and diastolic BP during stress (rs 0.11–0.24, ps ≤ 0.05). When the analyses were conducted in each group, similar patterns of results were obtained in nonusers and khat-only users. That is, there were correlations between positive affect at all periods and systolic BP during stress (rs 0.18–0.32, ps <0.05) and correlations between baseline positive affect and diastolic BP during stress (r = 0.22, p <0.05) in nonusers. Also, positive affect during baseline as well as recovery was related to systolic BP and diastolic BP during stress in khat-only users (rs 0.21–0.31, ps <0.05). In contrast, these findings were not found in concurrent users (ps >0.12; Figure 4).
DISCUSSION
The present study was prompted by the high prevalence of concurrent use of tobacco and khat in many countries around the world and by reports indicating that up to two thirds of khat users also smoke (Bawazeer et al., 1999; Odenwald et al., 2007; Tesfaye et al., 2008; al’Absi and Grabowski, 2012). The results showed that concurrent use of khat and tobacco was associated with attenuated cardiovascular responses to acute stress. Concurrent users also reported greater negative affect and less positive affect during the acute stress laboratory session relative to the control group. This is the first study to examine the influences of concurrent use of the psychostimulant khat and tobacco on physiological and mood responses to acute stress. Although increased reported negative affect by the concurrent khat and tobacco users may reflect a greater predisposition for using these substances, it is possible that these changes reflect long-term effects of chronic exposure to these substances. Previous research has shown that chronic tobacco use is associated with enhanced negative affect (al’Absi et al., 2003). Population studies in khat users have also indicated the association of chronic khat use with various psychological problems, including major psychiatric disorders such as psychotic disorders and mania (Yousef et al., 1995; Odenwald et al., 2007; Bhui and Warfa, 2010).
The patterns of associations between khat and tobacco use with cardiovascular measures implicate these substances in altering the stress response. Furthermore, results showing significant associations between subjective reports and cardiovascular responses in the nonusers and the khat-only users but not in the concurrent users demonstrate the disrupted connection of motivational states with stress physiological responding in the concurrent use group. Mechanisms responsible for these results in concurrent users likely involve dysregulation at multiple levels, including sympathetic nervous system, vagal tone, and baroreceptor-reflex sensitivity. Although little research has focused on the effects of chronic khat use, studies focusing on chronic tobacco use have shown the role of reduced beta-adrenergic receptor functions as a potential mediator of the effects of chronic tobacco use (Laustiola et al., 1988; Laustiola et al., 1991). It is possible that repeated responses of these systems to these substances would impose increased allostatic load leading to altered responses to the acute challenges and possibly contributing to the development of cardiovascular diseases in this population. Indeed, this possibility may explain the recent findings of increased risk for cardiovascular morbidity among khat users (Cox and Ramsay, 2003; Al Motarreb et al., 2010; Ali et al., 2011).
The established reactivity hypothesis indicates that repeated and exaggerated increases in cardiovascular activity in response to stress may increase or accelerate risk for cardiovascular diseases (Harris and Matthews, 2004; Chida and Steptoe, 2010). Similar to acute stress, both tobacco and khat acutely increase cardiovascular activity (Benowitz et al., 1984; Hassan et al., 2000). Ongoing and repeated exposure to these substances, however, may lead to long-term hemodynamic adjustment, contributing to poor responsiveness to acute stress. Consistent with this hypothesis, previous research has demonstrated that chronic use of addictive substances, such as alcohol, opiate, and amphetamine, is associated with blunted physiological responses to a wide range of psychological (e.g., mental arithmetic and public speaking) and physical stressors (e.g., cold pressor and isometric handgrip) (Bernardy et al., 1996; Gerra et al., 2003a; Gerra et al., 2003b). Research focusing on polysubstance use has also demonstrated pronounced dysregulation of the stress hormonal response (Lovallo, 2007). Findings of the current study demonstrate a similar pattern among concurrent users of khat and tobacco.
Both khat and tobacco are likely to exert their effects through common central and peripheral pathways, including the dopaminergic and adrenergic systems (Mereu et al., 1983; Kalix, 1984; Kalix and Braenden, 1985). These pathways are involved in various affective, motivational, and cognitive processes that directly and indirectly mediate the influence of drug use and participate in coordinating the stress response. Indeed, previous work has demonstrated the convergence of these processes during stress, showing clear connections between the sympathetic responses, hormonal activity, and negative affect during stress (Lovallo et al., 1990; Cacioppo, 1994; al’Absi et al., 1997). Frequent and repeated activation of these processes in response to chronic drug use may influence motivational processes during stress, possibly contributing to stress response dysfunction (al’Absi, 2007). It is also possible that this dysregulation process in the stress response precedes or increases risk for drug use. For example, there is evidence to suggest a connection between physiological (attenuated) response to stress and propensity to using drugs (al’Absi, 2007; Lovallo, 2007; Sinha, 2008). Studies on individuals with family history of drug use show a reduction in their physiological response to stress (Lovallo, 2007). The extent to which altered stress response predisposes these individuals to use drugs and/or draw greater benefit from drug use is not known, particularly in the context of tobacco and khat use.
We must note the limitations of this study. First, we measured only BPs and HR; a comprehensive assessment of hemodynamic measures would have informed this study to explain the attenuated BP response in concurrent users. Second, although we collected measures to assess subjective state after exposure to the task, it would have been useful to also collect data on subjects’ perception of the tasks per se to assess engagement and perception of task difficulty. Third, the directional sequence and causality cannot be inferred from this cross-sectional study. We note the challenge in recruiting smokers who do not chew khat, suggesting the possibility that khat is a significant cue for tobacco use (al’Absi and Grabowski, 2012; Nakajima et al., 2012). In light of this limitation, we cannot completely rule out the possibility that the observed attenuation in the cardiovascular response to stress may have been due to smoking. Nevertheless, patterns of changes in cardiovascular measures (Figure 1) were different between nonuser and the two khat using groups. Nonusers showed the expected increase in BP levels in response to stress, whereas this was not observed in the two khat groups: they exhibited blunted responses. These results indicate similarity in stress response patterns between concurrent users and khat-only users, suggesting that alterations in central stress regulatory systems may not be due to tobacco use alone. Although not statistically significant, results of changes score analyses suggest a dose–response relationship in cardiovascular measures. Replicating our protocol with larger and balanced sample may improve the results. We note that the study has several strengths including the inclusion of a large sample size, the novelty of the results in this unique population, and the use of a controlled setting to examine effect of acute stress. Future studies should address the impact of the acute effects of khat and tobacco use alone and in interaction with stress on emotion-regulation-related biobehavioral systems. In addition, it will be critical to examine the effects of abstinence from khat to determine intensity of withdrawal symptoms.
In conclusion, this study has demonstrated for the first time attenuated cardiovascular responses to stress and enhanced negative affect among khat users and those who also concurrently use tobacco relative to nonsmokers/ nonkhat users. The patterns of associations between khat and tobacco use with cardiovascular measures implicate these substances in altering the stress response. The results confirm and extend previous studies with other substances and indicate that adverse effects of khat use may be exacerbated by the use of other stimulants such as tobacco. To this end, successful efforts to combat the high prevalence of tobacco use in this population must take into consideration the concurrent use of khat and must address the dynamics of polysubstance use in sociocultural contexts.
Acknowledgments
Research reported in this publication was supported by the Fogarty International Center FIRCA grant award number R03TW007219 and by the Fogarty International Center and National Institute for Drug Abuse under award number R21 DA024626. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the following for their help with this project: Amal Alanisi, Tawfeek Alharazi, Ashrak Al-Awdari, Bakeer Dahmash, Essa Oumairi, Basma Ali Thabe, and Khaled Al-Sahmiry for assisting with data collection.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
References
- al’Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59:218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al’Absi M. Stress and Addiction: Biological and Psychological Mechanisms. Academic Press/Elsevier; London: 2007. [Google Scholar]
- al’Absi M, Grabowski J. Concurrent use of tobacco and khat: added burden on chronic disease epidemic. Addiction. 2012;107:451–452. doi: 10.1111/j.1360-0443.2011.03684.x. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Lovallo WR, McKey B, Pincomb G. Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosom Med. 1994a;56:245–250. doi: 10.1097/00006842-199405000-00011. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Lovallo WR, McKey BS, Pincomb GA. Borderline hypertensives produce exaggerated adrenocortical responses to mental stress. Psychosom Med. 1994b;56:245–250. doi: 10.1097/00006842-199405000-00011. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacol Biochem Behav. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Khalil NS, Al Habori M, Hoffman R, Fujiwara K, Wittmers L. Effects of chronic khat use on cardiovascular, adrenocortical, and psychological responses to stress in men and women. Am J Addict. 2013;22:99–107. doi: 10.1111/j.1521-0391.2013.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Motarreb A, Al Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: the current situation and directions for future research. J Ethnopharmacol. 2010;132:540–548. doi: 10.1016/j.jep.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Ali WM, Al Habib KF, Al-Motarreb A, et al. Acute coronary syndrome and khat herbal amphetamine use: an observational report. Circulation. 2011;124:2681–2689. doi: 10.1161/CIRCULATIONAHA.111.039768. [DOI] [PubMed] [Google Scholar]
- Al-Motarreb A, Baker K, Broadley KJ. Khat: pharmacological and medical aspects and its social use in Yemen. Phytother Res. 2002;16:403–413. doi: 10.1002/ptr.1106. [DOI] [PubMed] [Google Scholar]
- Apps A, Matloob S, Dahdal MT, Dubrey SW. Khat: an emerging threat to the heart in the UK. Postgrad Med J. 2011;87:387–388. doi: 10.1136/pgmj.2010.114603. [DOI] [PubMed] [Google Scholar]
- Bawazeer AA, Hattab AS, Morales E. First cigarette smoking experience among secondary-school students in Aden, Republic of Yemen. East Mediterr Health J. 1999;5:440–449. [PubMed] [Google Scholar]
- Benowitz NL, Kuyt F, Jacob P. Influence of nicotine on cardiovascular and hormonal effects of cigarette smoking. Clin Pharmacol Ther. 1984;36:74–81. doi: 10.1038/clpt.1984.142. [DOI] [PubMed] [Google Scholar]
- Bernardy NC, King AC, Parsons OA, Lovallo WR. Altered cortisol response in sober alcoholics: an examination of contributing factors. Alcohol. 1996;13:493–498. doi: 10.1016/0741-8329(96)00043-2. [DOI] [PubMed] [Google Scholar]
- Bhui K, Warfa N. Trauma, khat and common psychotic symptoms among Somali immigrants: a quantitative study. J Ethnopharmacol. 2010;132:549–553. doi: 10.1016/j.jep.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Bongard S, al’Absi M, Khalil NS, Al HM. Khat use and trait anger: effects on affect regulation during an acute stressful challenge. Eur Addict Res. 2011;17:285–291. doi: 10.1159/000330317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneisen R, Fisch HU, Koelbing U, Geisshusler S, Kalix P. Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol. 1990;30:825–828. doi: 10.1111/j.1365-2125.1990.tb05447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CB, Ivers RG, Lindorff KJ, Clough AR. Cannabis: a Trojan horse for nicotine? Aust NZJ Public Health. 2000;24:637. doi: 10.1111/j.1467-842x.2000.tb00533.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT. Social neuroscience: autonomic, neuroendocrine, and immune response to stress. Psychophysiology. 1994;31:113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Cox G, Ramsay L. Adverse effects of khat: a review. Adv Psychiatr Treat. 2003;9:456–463. [Google Scholar]
- Gerra G, Baldaro B, Zaimovic A, et al. Neuroendocrine responses to experimentally-induced emotions among abstinent opioid-dependent subjects. Drug Alcohol Depend. 2003a;71:25–35. doi: 10.1016/s0376-8716(03)00065-6. [DOI] [PubMed] [Google Scholar]
- Gerra G, Bassignana S, Zaimovic A, et al. Hypothalamic-pituitary-adrenal axis responses to stress in subjects with 3,4-methylenedioxy-methamphetamine (‘ecstasy’) use history: correlation with dopamine receptor sensitivity. Psychiatry Res. 2003b;120:115–124. doi: 10.1016/s0165-1781(03)00175-6. [DOI] [PubMed] [Google Scholar]
- Griffiths P, Lopez D, Sedefov R, et al. Khat use and monitoring drug use in Europe: the current situation and issues for the future. J Ethnopharmacol. 2010;132:578–583. doi: 10.1016/j.jep.2010.04.046. [DOI] [PubMed] [Google Scholar]
- Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med. 2004;66:153–164. doi: 10.1097/01.psy.0000116719.95524.e2. [DOI] [PubMed] [Google Scholar]
- Hassan NA, Gunaid AA, Abdo-Rabbo AA, et al. The effect of Qat chewing on blood pressure and heart rate in healthy volunteers. Trop Doct. 2000;30:107–108. doi: 10.1177/004947550003000219. [DOI] [PubMed] [Google Scholar]
- Humfleet GL, Haas AL. Is marijuana use becoming a ‘gateway’ to nicotine dependence? Addiction. 2004;99:5–6. doi: 10.1111/j.1360-0443.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003;38:606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- Kalix P. Recent advances in khat research. Alcohol Alcohol. 1984;19:319–323. [PubMed] [Google Scholar]
- Kalix P. Catha edulis, a plant that has amphetamine effects. Pharm World Sci. 1996;18:69–73. doi: 10.1007/BF00579708. [DOI] [PubMed] [Google Scholar]
- Kalix P, Braenden O. Pharmacological aspects of the chewing of khat leaves. Pharmacol Rev. 1985;37:149–164. [PubMed] [Google Scholar]
- Kassim S, Croucher R. Khat chewing amongst UK resident male Yemeni adults: an exploratory study. Int Dent J. 2006;56:97–101. doi: 10.1111/j.1875-595x.2006.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Kassim S, Islam S, Croucher RE. Correlates of nicotine dependence in U.K. resident Yemeni khat chewers: a cross-sectional study. Nicotine Tob Res. 2011;13:1240–1249. doi: 10.1093/ntr/ntr180. [DOI] [PubMed] [Google Scholar]
- Kennedy JG. The Institutionalized Use of the Drug Qat in North Yemen. D. Reidel Publishing Co; Dordrecht: 1988. The Flower of Paradise. [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993;44:527–531. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Krikorian AD. Kat and its use: an historical perspective. J Ethnopharmacol. 1984;12:115–178. doi: 10.1016/0378-8741(84)90047-3. [DOI] [PubMed] [Google Scholar]
- Lai S, Lai H, Page JB, McCoy CB. The association between cigarette smoking and drug abuse in the United States. J Addict Dis. 2000;19:11–24. doi: 10.1300/J069v19n04_02. [DOI] [PubMed] [Google Scholar]
- Laustiola KE, Lassila R, Kaprio J, Koskenvuo M. Decreased beta-adrenergic receptor density and catecholamine response in male cigarette smokers. A study of monozygotic twin pairs discordant for smoking. Circulation. 1988;78:1234–1240. doi: 10.1161/01.cir.78.5.1234. [DOI] [PubMed] [Google Scholar]
- Laustiola KE, Kotamaki M, Lassila R, Kallioniemi OP, Manninen V. Cigarette smoking alters sympathoadrenal regulation by decreasing the density of beta 2-adrenoceptors. A study of monitored smoking cessation. J Cardiovasc Pharmacol. 1991;17:923–928. doi: 10.1097/00005344-199106000-00010. [DOI] [PubMed] [Google Scholar]
- Lovallo W. Individual differences in response to stress and risk for addiction. In: al’Absi M, editor. Stress and Addiction: Biological and Psychological Mechanisms. Academic Press/Elsevier; London: 2007. pp. 265–284. [Google Scholar]
- Lovallo WR, Pincomb GA, Brackett DJ, Wilson MF. Heart rate reactivity as a predictor of neuroendocrine responses to aversive and appetitive challenges. Psychosom Med. 1990;52:17–26. doi: 10.1097/00006842-199001000-00002. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M. Pituitary-adrenal and sympathetic-adrenal correlates of distress and effort. J Psychosom Res. 1980;24:125–130. doi: 10.1016/0022-3999(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Manghi RA, Broers B, Khan R, Benguettat D, Khazaal Y, Zullino DF. Khat use: lifestyle or addiction? J Psychoactive Drugs. 2009;41:1–10. doi: 10.1080/02791072.2009.10400669. [DOI] [PubMed] [Google Scholar]
- Maziak W, Hammal F, Rastam S, et al. Characteristics of cigarette smoking and quitting among university students in Syria. Prev Med. 2004;39:330–336. doi: 10.1016/j.ypmed.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Mereu GP, Pacitti C, Argiolas A. Effect of (−)-cathinone, a khat leaf constituent, on dopaminergic firing and dopamine metabolism in the rat brain. Life Sci. 1983;32:1383–1389. doi: 10.1016/0024-3205(83)90814-7. [DOI] [PubMed] [Google Scholar]
- Nakajima M, al’Absi M, Dokam A, Alsoofi M, Khalil NS, Al Habori M. Gender differences in patterns and correlates of khat and tobacco use. Nicotine Tob Res. 2012;15:1130–1135. doi: 10.1093/ntr/nts257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencini P, Ahmed AM, Elmi AS. Subjective effects of khat chewing in humans. Drug Alcohol Depend. 1986;18:97–105. doi: 10.1016/0376-8716(86)90118-3. [DOI] [PubMed] [Google Scholar]
- Nencini P, Fraioli S, Pascucci T, Nucerito CV. (−)-Norpseudoephedrine, a metabolite of cathinone with amphetamine-like stimulus properties, enhances the analgesic and rate decreasing effects of morphine, but inhibits its discriminative properties. Behav Brain Res. 1998;92:11–20. doi: 10.1016/s0166-4328(97)00123-x. [DOI] [PubMed] [Google Scholar]
- Nielsen JA. Cathinone affects dopamine and 5-hydroxytryptamine neurons in vivo as measured by changes in metabolites and synthesis in four forebrain regions in the rat. Neuropharmacology. 1985;24:845–852. doi: 10.1016/0028-3908(85)90035-8. [DOI] [PubMed] [Google Scholar]
- Odenwald M, Hinkel H, Schauer E, et al. The consumption of khat and other drugs in Somali combatants: a cross-sectional study. PloS Med. 2007;4:e341. doi: 10.1371/journal.pmed.0040341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantelis C, Hindler CG, Taylor JC. Use and abuse of khat (Catha edulis): a review of the distribution, pharmacology, side effects and a description of psychosis attributed to khat chewing. Psychol Med. 1989;19:657–668. doi: 10.1017/s0033291700024259. [DOI] [PubMed] [Google Scholar]
- Patel NB. Mechanism of action of cathinone: the active ingredient of khat (Catha edulis) East Afr Med J. 2000;77:329–332. doi: 10.4314/eamj.v77i6.46651. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Schechter MD. Discriminative stimulus properties of (+) cathine, an alkaloid of the khat plant. Pharmacol Biochem Behav. 1990;36:267–271. doi: 10.1016/0091-3057(90)90402-4. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Schechter MD, Yamamoto BK. Effects of cathinone and amphetamine on the neurochemistry of dopamine in vivo. Neuropharmacology. 1990;29:1171–1176. doi: 10.1016/0028-3908(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Reda AA, Moges A, Biadgilign S, Wondmagegn BY. Prevalence and determinants of khat (Catha edulis) chewing among high school students in eastern Ethiopia: a cross-sectional study. PLoS ONE. 2012;7:e33946. doi: 10.1371/journal.pone.0033946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regassa N, Kedir S. Attitudes and practices on HIV preventions among students of higher education institutions in Ethiopia: the case of Addis Ababa University. East Afr J Public Health. 2011;8:141–154. [PubMed] [Google Scholar]
- Sherwood A, Royal SA, Light KC. Laboratory reactivity assessment: effects of casual blood pressure status and choice of task difficulty. Int J Psychophysiol. 1993;14:81–95. doi: 10.1016/0167-8760(93)90086-5. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann NY Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye F, Byass P, Wall S, Berhane Y, Bonita R. Association of smoking and khat (Catha edulis Forsk) use with high blood pressure among adults in Addis Ababa, Ethiopia, 2006. Prev Chronic Dis. 2008;5:A89. [PMC free article] [PubMed] [Google Scholar]
- UK-Home-Office. Khat: social harms and legislation: a literature review. 2011. [Google Scholar]
- Wedegaertner F, al-Warith H, Hillemacher T, et al. Motives for khat use and abstinence in Yemen—a gender perspective. BMC Public Health. 2010;10:735. doi: 10.1186/1471-2458-10-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widler P, Mathys K, Brenneisen R, Kalix P, Fisch HU. Pharmacodynamics and pharmacokinetics of khat: a controlled study. Clin Pharmacol Ther. 1994;55:556–562. doi: 10.1038/clpt.1994.69. [DOI] [PubMed] [Google Scholar]
- World-Bank; World B, editor. Yemen-towards qat demand reduction. Washington, DC: 2007. Report No. 39738-YE. [Google Scholar]
- Yousef G, Huq Z, Lambert T. Khat chewing as a cause of psychosis. Br J Hosp Med. 1995;54:322–326. [PubMed] [Google Scholar]