Abstract
The adipocyte hormone leptin regulates satiety and energy expenditure. Recent evidence suggests that leptin is associated with increased craving for alcohol and with shorter length of abstinence during alcohol treatment. This study examined leptin’s associations with craving for cigarettes and smoking relapse among smokers interested in cessation. Participants (32 smokers; 14 women) attended a laboratory session 24 h following their designated quit day where circulating leptin levels and craving for smoking were assessed. Other measures of withdrawal symptoms, affect, physical symptoms, as well as neuroendocrine and cardiovascular measures were collected before and after performing two stress tasks (public speaking and cognitive tasks). High circulating leptin levels were associated with increased craving, withdrawal symptoms, negative affect, physical symptoms, and reduced positive affect. Circulating leptin levels were not related to cardiovascular and neuroendocrine measures, responses to acute stressors, or to smoking relapse. These results indicate that circulating leptin is a promising biological marker of craving for smoking and warrant further investigation of the links between appetite regulation and nicotine dependence.
Keywords: Leptin, Smoking, Nicotine, Stress, Craving, Withdrawal symptoms, Relapse, Appetite, ACTH, Cortisol
1. Introduction
Craving to smoke is an important construct in understanding tobacco dependence, since it represents a strong motivational factor for smoking (Ferguson and Shiffman, 2009; West et al., 2007), and is a risk factor for relapse (al’Absi et al., 2004; Killen and Fortman, 1997). Intensity of craving to smoke experienced during the first few days of abstinence predicts who will relapse (al’Absi et al., 2007, 2004; Killen and Fortman, 1997; Shiffman et al., 1996). There is however little research to identify a specific biological marker of craving for cigarettes, although recent studies provide promising results. One study has shown that smokers who relapsed within the first week post quitting exhibited an exaggerated drop in morning cortisol concentrations during abstinence relative to their ad libitum smoking levels (al’Absi et al., 2004). Smokers who relapsed within four weeks after quitting showed attenuated adrenocorticotropin (ACTH) levels, diastolic blood pressure (BP), and smoking urges during acute stress (al’Absi et al., 2005). Similar results have also been reported in the context of treatment studies (Frederick et al., 1998; Ussher et al., 2006).
Leptin is a protein product of the ob gene that presents primarily in adipocytes (CampFIeld et al., 1995). It regulates hypothalamic centers involved in energy homeostasis, body weight, and gene expression of corticotropin-releasing hormone and pro-opiomelanocortin (Cheung et al., 1997; Enriori et al., 2006; Mantzoros, 1999). It also interacts with neuroendocrine systems that are involved in appetite regulation, such as neuropeptide Y and agouti-related peptide (AgRP; Cone, 2005; Mantzoros, 1999). Beyond its effects on hypothalamic centers and neuroendocrine pathways, leptin was shown to directly modulate activity of mesocorticolimbic reward pathways. Fulton et al. (2000) showed that the effectiveness of a rewarding electrical stimulation was attenuated by intra-cerebro-ventricular infusion of leptin. Leptin binds to specific receptors located on the dopamine neurons of the ventral tegmental area (VTA) and inhibits dopamine signalling in the nucleus accumbens (Palmiter, 2007). Given the influence of leptin on dopaminergic transmission and reward processing, a number of studies have been designed to examine whether leptin also affects the subjective reward value and the reinforcing properties of drugs of abuse (Kiefer et al., 2001a,b).
Leptin modulates the hypothalamic-pituitary-adrenocortical (HPA) axis by blunting the cortisol stress response, presumably acting at the level of the hypothalamus (Ahima et al., 1996; Heiman et al., 1997). Dysregulation in the neuroendocrine stress system, in particular the HPA axis, has been linked to a variety of psychiatric disorders, including addiction. Preclinical and clinical data suggest that impaired functioning of the HPA axis and blunted stress response are directly associated with increased alcohol intake (Junghanns et al., 2003; Sillaber et al., 2002) and smoking relapse (al’Absi et al., 2005; Shaw and al’Absi, 2008). Recent research has also shown that leptin concentrations predicted craving and relapse among alcoholics under treatment (Kiefer et al., 2005; Hillemacher et al., 2007). Taken together, these findings suggest direct or indirect effects of leptin on motivational, mesolimbic structures and neuroendocrine stress systems of the brain (Inui, 1999; Fulton et al., 2000). It is therefore possible that leptin would be a promising biological correlate for craving and mood changes during smoking withdrawal.
Previous studies have examined leptin levels in smokers and have shown an association between this hormone and smoking status, although this was not consistently found (Eliasson and Smith, 1999; Reseland et al., 2005; Koc et al., 2009), and studies have not found consistent effects of short-term smoking abstinence on leptin (Klein et al., 2004; Perkins and Fonte, 2002). These inconsistencies may be related to different sample selection criteria and variable length of abstinence. The goal of this study was to examine the extent to which circulating leptin levels are associated with nicotine craving measured after a 24-hour period of abstinence, and explore the extent to which these levels predict relapse during a 4 week follow-up period. Measures of craving and withdrawal symptoms as well as plasma leptin levels were collected from a subgroup of smokers who were participating in a larger study that examined the extent to which their responses to stress predicted smoking relapse (al’Absi et al., 2005).
2. Methods
2.1. Participants
This study included 32 participants (14 women) between the ages of 18 and 68 (mean±S.E.M.=34.1±14.6 years) who were dependent cigarette smokers and expressed a strong motivation to stop smoking. Inclusion criteria included absence of major physical illness or psychiatric disorders, weight within ±30% of Metropolitan Life Insurance norms, and consumption of two or fewer alcoholic drinks a day. Qualified participants reviewed and signed a written consent form approved by the Institutional Review Board of the University of Minnesota. Monetary compensation ($15/h) was provided. During the screening session, participants completed self-report measures to assess history and level of their nicotine dependence, including the Fagerström Test of Nicotine Dependence (FTND; Heatherton et al., 1991).
2.2. Procedures
Participants were asked to report to the laboratory between 12 and 2 pm for the experimental session after the first 24-hour period of abstinence. They were instructed to have a light lunch at least 2 h prior to the session. Those who reported hunger at the beginning of the session were provided two oatmeal granola bars. The session protocol included a 30-minute baseline rest period, followed by public-speaking challenges (al’Absi et al., 1997), and a 30-minute recovery period. Following the recovery participants performed the arithmetic task and the Paced Auditory Serial Addition Task. These were followed by a final recovery period of 30 min. Ratings of craving and withdrawal symptoms were collected during baseline rest, after completing the tasks, and during the recovery rest periods. Blood and saliva samples were collected at these times and cardiovascular measures were collected throughout the session.
Four weekly follow-up assessments were conducted after quit day to assess abstinence status. Participants provided expired carbon monoxide (CO) and saliva samples for cotinine assays to verify abstinence from smoking. A detailed description of the hormone analysis and cardiovascular measures has been published previously (al’Absi et al., 2005). The current paper includes data from assays conducted to measure leptin, and therefore only participants with adequate blood samples and volume to complete these assays are presented (N=32).
2.3. Measures
Leptin was assayed from a sample collected at the beginning of the laboratory session. Samples were collected using an indwelling catheter into an 8 ml EDTA Vacutainer tube. Additional blood samples were collected during the session and were assayed for ACTH and cortisol, and saliva samples were collected to assay for free cortisol and cotinine concentrations. Samples were centrifuged and stored at −70 °C until analysis. Plasma leptin was assayed using a direct sandwich ELISA (Linco, Missouri). Inter- and intra-assay coefficients of variance for these assays were below 8%. ACTH was assayed using an RIA kit and plasma cortisol was assayed using EIA (DSL, Sinsheim, Germany). Inter- and intra-assay coefficients of variance for these assays were below 10%. Salivary cortisol assays were conducted using a time-resolved immunoassay with fluorometric end point detection. Cotinine levels were assayed using enzyme immunoassay (EIA; DRG Diagnostics, Marburg, Germany). Inter- and intra-assay variations for cotinine measurements were below 12%. Measurement of expired CO was performed using MicroCO™ monitors (Micro Direct Inc., Auburn, Maine). We also measured systolic and diastolic BP as well as heart rate during the session using Dinamap BP monitors.
Withdrawal symptoms were measured using a modified version of the Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami, 1986, 1998) that excluded the ‘desire to smoke’ item. Due to evidence suggesting that craving patterns are distinct from other symptoms of withdrawal (Hughes and Hatsukami, 1998), the “desire to smoke” item was analyzed separately. In addition, we administered the abbreviated version of the Questionnaire of Smoking Urges (QSU-brief; Cox et al., 2001; Tiffany and Drobes, 1991). The QSU-brief includes two factors measuring appetitive urges to smoke (Factor 1: Intention/Desire to Smoke) and urges associated with relieving the aversive experience of withdrawal (Factor 2: Relief of Negative Affect; Cox et al., 2001). Measures were collected during rest, immediately after performing the acute stressful challenges, and during the recovery–rest period.
We also assessed positive and negative affect using the Revised Subjective States Questionnaire (SSQ), a measure developed specifically to assess feelings of global activation and subjective distress (Lundberg and Frankenhaeuser, 1980). Positive affect was assessed using items of cheerfulness, content, calmness, controllability, and interest. Distress was assessed using items of anxiety, irritability, impatience, and restlessness. These two factors were previously shown to be sensitive to acute stress and to have sound psychometric properties in smokers, including Cronbach’s alpha for positive affect and distress of 0.85 and 0.82 (al’Absi et al., 2003). Finally we assessed physical symptoms that have been shown to be associated with smoking abstinence, including headache, stomachache, drowsiness, sweating, tremor, fatigue, and coughing (American Psychiatric Association, 1994).
2.4. Data analysis
We conducted a series of simple linear regression models to examine the extent to which leptin levels predicted each of the psychological and physiological measures obtained during the lab session. Because of our focus on craving, we conducted a multivariate repeated analysis of variance (MANOVA) examining time effects during the session, and found no significant changes over time (Fs(5, 25)<1.9; ps>0.13). We therefore used an overall average of the craving scores obtained during the lab session. In order to use the same analysis model across all measures, we used the averaged measure for these variables in the regression models. We also examined gender differences on all demographic, psychological, and physiological measures using ANOVA. When measures differed between men and women, the leptin-by-gender term was included in the regression model. We conducted the residual analysis to check the validity of the regression assumptions (Shapiro-Wilk test: ps>0.06 and White test: ps>0.1). All available diagnostic statistics were investigated to detect outlying and influential observations. We also conducted logistic and Cox regression analysis using leptin levels as the independent variable to predict relapse status and time to relapse during each of the four week visits. Participants were classified as relapsed if they smoked at least one cigarette, and time to relapse was calculated starting from the cessation day. All analyses were conducted using SAS Software Version 9.2 (SAS Institute Inc., Cary, NC).
3. Results
3.1. Subject characteristics
Table 1 shows participant characteristics. Men and women did not differ significantly on age, education, alcohol intake, and average hours of nightly sleep (all Fs<1.28; ps>0.27). They also did not differ in number of cigarettes per day, scores on FTND, duration of smoking, motivation to quit, or number of previous quit attempts (all Fs<3.7; ps>0.07).
Table 1.
Subject characteristics and averaged measures obtained during the lab session.
| Female (N=14) | Male (N=18) | |
|---|---|---|
| Age (years) | 31.07 (3.66) | 36.39 (3.61) |
| BMI (kg/m2)* | 22.27 (0.85) | 25.74 (0.87) |
| Education (years) | 14.64 (0.39) | 13.29 (0.91) |
| Caffeine intake (servings/day)* | 3.08 (0.76) | 5.88 (0.78) |
| Alcohol intake (drinks/day) | 1 (0.11) | 1.83 (1.07) |
| Average sleep (hours/night) | 6.82 (0.35) | 6.82 (0.32) |
| Smoking Variables | ||
| Cigarettes per day | 21.21 (3.05) | 18.06 (0.89) |
| Fagerström Test of Nicotine Dependence | 5.43 (0.75) | 5.33 (0.32) |
| Duration of smoking (years) | 7.77 (1.28) | 15.94 (3.57) |
| Motivation to quit (rating) | 6.29 (0.27) | 5.89 (0.2) |
| Previous quit attempts | 4.08 (0.75) | 3.41 (0.58) |
| Psychological and Craving Measures | ||
| Craving (rating) | 3.82 (0.66) | 3.27 (0.57) |
| QSU-B F1: Desire to Smoke | 24.75 (3.72) | 21.19 (2.95) |
| QSU-B F2: Relief of Negative Affect | 16.5 (3.28) | 15.27 (3.03) |
| MNWS | 10.2 (2.2) | 10.07 (2.3) |
| Physical Symptoms | 5.49 (1.18) | 3.49 (0.86) |
| Positive Mood | 20.25 (2.23) | 19.44 (2.62) |
| Distress | 8.19 (1.78) | 7.89 (1.86) |
| Physiological Measures | ||
| Leptin (ng/mL)* | 6.39 (0.87) | 4.11 (0.57) |
| Salivary Cortisol (nmol/L) | 6.35 (0.92) | 8.94 (1.39) |
| Plasma Cortisol (ug/dL)* | 7.66 (0.91) | 10.17 (0.9) |
| ACTH (pg/mL) | 21.73 (3.57) | 25.69 (2.36) |
| Heart Rate (bpm) | 67 (2.53) | 64 (2.06) |
| Systolic BP (mmHg) *** | 112 (2.8) | 136 (3.89) |
| Diastolic BP (mmHg) * | 68 (1.77) | 75 (2.26) |
p<0.05,
p<0.01,
p<0.001.
Means (standard error of the mean); BMI=body mass index; QSU-B=Questionnaire of Smoking Urges-Brief; MNWS=Minnesota Nicotine Withdrawal Scale; ACTH= Adrenocorticotropin; BP=Blood Pressure.
Men and women did not differ in craving as measured by the ‘desire to smoke’ item from the MNWS and by the QSU-B, and they also reported similar levels of withdrawal, physical symptoms, distress, and positive affect during the lab session (all Fs<1.95; ps>0.17). Neuroendocrine and cardiovascular measures were also comparable, except that men had higher levels of plasma cortisol and systolic and diastolic BP than women (all Fs>4.45; ps<0.05), while women had greater leptin levels than men (p<0.05). There was no time effect within the session in craving, QSU-B F1, QSU-B F2, or physical symptoms (Fs<2.3; ps>0.1). Time effect was found in positive affect, distress, heart rate, systolic and diastolic BP, and cortisol measures (Fs>3.3; ps<0.05). This was due to changes in response to the acute stressors (Fs>4.4, ps<0.05), although these responses were independent of leptin levels (Fs<1.72; ps>0.13).
The cardiovascular measures also showed significant changes, reflecting the increases in blood pressure and heart rate responses to the acute stressors (Fs>9.87; ps <0.0001). These effects were comparable in men and women.
3.2. Regression analyses
3.2.1. Laboratory measures
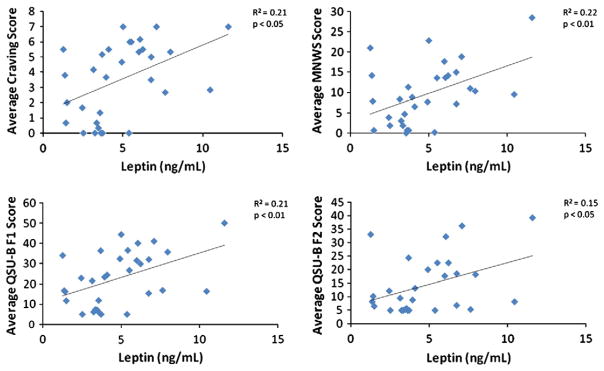
Table 2 shows the results of the regression analyses using leptin as the independent variable to predict craving, mood, and physiological measures collected during the laboratory session. The results show that leptin significantly predicted craving rating, Factor 1 of QSU-B (Desire to Smoke), Factor 2 of QSU-B (Relief of Negative Affect), and withdrawal symptoms (ps<0.05; see Fig. 1). Leptin also predicted other mood reports during the lab session, including positive affect, distress, physical symptoms, and heart rate (ps<0.05). Leptin did not predict any of the neuroendocrine measures (Table 2).
Table 2.
Summary of simple linear regression analyses.
| Dependent Variable | R2 | Beta | SE (B) | Standardized Beta |
|---|---|---|---|---|
| Craving (rating) | 0.21 | 0.44 | 0.16 | 0.45 * |
| QSU-B F1: Desire to Smoke | 0.21 | 2.41 | 0.87 | 0.46 ** |
| QSU-B F2: Relief of Negative Affect | 0.15 | 1.59 | 0.72 | 0.39 * |
| MNWS | 0.22 | 1.36 | 0.49 | 0.47 ** |
| Physical Symptoms | 0.32 | 0.71 | 0.20 | 0.56 ** |
| Positive Mood | 0.20 | −1.54 | 0.58 | −0.45 * |
| Distress | 0.34 | 1.38 | 0.37 | 0.58 *** |
| Salivary Cortisol (nmol/L) | 0.06 | −0.04 | 0.03 | −0.24 |
| Plasma Cortisol (ug/dL) | 0.02 | −0.02 | 0.02 | −0.15 |
| ACTH (pg/mL) | 0.00 | 0.00 | 0.03 | −0.04 |
| Heart Rate (bpm) | 0.18 | 1.04 | 0.43 | 0.42 * |
p<0.05,
p<0.01,
p<0.001.
Simple linear regression using leptin as the independent variable to predict the craving and mood measures. Note. QSU-B=Questionnaire of Smoking Urges-Brief; MNWS= Minnesota Nicotine Withdrawal Scale; ACTH=Adrenocorticotropin.
Fig. 1.
Scatterplot depicting the relationship between leptin (ng/mL) and craving rating, MNWS, QSU-B F1 (Desire to Smoke), and QSU-B F2 (Relief of Negative Affect) score. All measures were collected after 24 h of verified abstinence. Note. MNWS=Minnesota Nicotine Withdrawal Scale; QSU-B=Questionnaire of Smoking Urges-Brief.
Since there were gender differences in systolic BP, diastolic BP, and plasma cortisol, these regression models included a gender-by-leptin interaction term. This interaction effect was significant in the systolic BP and diastolic BP models (ps<0.01), but not for plasma cortisol (p=0.11). Specific analysis to identify the source of the interaction showed that for men there was positive association between leptin levels and systolic BP (r=0.50; p<0.05). This association was not significant in women (r=−0.24; p=0.41). Similarly, a marginal positive association was found between diastolic BP and leptin levels in men (r=0.43; p=0.07); however, no association was found in women (r=−0.29; p=0.31).
3.2.2. Follow-up prediction of relapse status
Cox regression analysis was conducted to predict the number of days until relapse over the four-week follow-up period. Leptin did not predict time to relapse (Chi-Square=0.07; p=0.80). In addition, logistic regression analysis was conducted to predict relapse status during each of the four weekly follow-up visits. Leptin did not predict relapse status in any week (Chi-Square’s<0.13).
3.3. Additional analyses
We also conducted a stepwise regression analysis to examine the extent to which leptin levels remain as a significant predictor of craving when combined with other demographic and smoking measures. The model included age, BMI, alcohol intake, cigarettes per day, FTND, levels of motivation to quit, and leptin. Only motivation to quit and leptin levels were significant, suggesting these two factors as independent predictors of craving. Consistent with earlier results, high leptin predicted enhanced craving (Beta=0.58; p<0.01), while high motivation for quitting predicted reduced craving (Beta=−0.4; p<0.05).
Finally, we conducted a series of correlation analyses to examine intercorrelations among the measures collected during the session. These results showed consistent association between the multiple measures of craving (ratings and QSU-B scores) and other mood measures (rs>0.67; ps<0.0001). In addition, plasma cortisol concentrations correlated negatively with craving rating, QSU-B F2 score (Relief of Negative Affect), and withdrawal symptoms (rs<−0.34; ps<0.05). Systolic and diastolic BP correlated with plasma and salivary cortisol levels (rs>0.41; ps<0.05).
4. Discussion
The primary finding of this study is the significant association between circulating leptin levels and craving for cigarettes after 24 h of abstinence. Increased circulating leptin levels were also associated with increased negative affect, physical symptoms, and reduced positive affect. While leptin was associated with abstinence-related measures, it was not associated with stress-related hormonal and cardiovascular changes. The current results are novel and rather striking considering the small sample size. They indicate the potential utility of leptin as a biological correlate for craving and mood changes during smoking withdrawal. They are also consistent with previous studies conducted with alcohol-dependent individuals showing that leptin levels predicted craving and relapse among alcoholics under treatment (Hillemacher et al., 2007; Kiefer et al., 2005).
Mechanisms mediating the association between circulating leptin level and craving for cigarettes are yet to be established. Several studies suggest a role of appetite-regulating peptides, including leptin, in the modulation of reward pathways that goes beyond their involvement in the hypothalamic regulation of energy homeostasis (Kalra and Kalra, 2004; Palmiter, 2007). In both nicotine and alcohol dependent individuals, a down-regulated dopaminergic mesolimbic system seems to be involved in the maintenance of addictive behaviors (Tan et al., 2009; Volkow et al., 2007). Both dopamine D1 (Dagher et al., 2001) and D2 receptors have been found to be decreased in the striatum of smokers compared to non-smokers (Fehr et al., 2008). Leptin binds to specific dopaminergic receptors located on neurons within the ventral tegmental area and inhibits dopamine signalling in the nucleus accumbens (Laviolette and van der Kooy, 2004; Palmiter, 2007), and one study showed that the effectiveness of a rewarding electrical stimulation was attenuated by intra-cerebro-ventricular infusion of leptin (Fulton et al., 2000). Given that leptin inhibits the dopaminergic transmission, the present results may be interpreted as a leptin-modulated reward deficit during nicotine withdrawal that resulted in increased craving.
While these findings are promising and consistent with results from other substance use populations, the current results should be considered preliminary, in light of the small sample size and the short follow-up period. It is possible that these factors may have contributed to the lack of associations between leptin levels and relapse (relapse status and the number of days until relapse). The lack of association between leptin and stress-related hormonal measures may also reflect the limited scope of assessment and the small sample size of the study. In addition, potential confounding factors were not directly addressed and still need to be examined in future studies. For example, the influence of sleep should be further considered given the significant changes in sleep patterns during smoking withdrawal (Colrain et al., 2004).
We note the strengths of the design of this study, including the use of multiple measures of craving, the well-controlled, repeated measure assessment after smoking abstinence, and the recruitment of smokers in the process of cessation. The latter feature increases the clinical relevance of the results. This work is also relevant to our understanding of the interaction of nicotine addictive processes and appetite regulation. The interaction between smoking and the regulation of body weight has been repeatedly reported (O’Hara et al., 1998; Williamson et al., 1991) and appetite-regulating neuropeptides, like leptin, have been suggested as a possible mediator (Li et al., 2000). Additionally, the results identify a promising direction for developing an objective biological marker of craving for smoking. Such a marker would be a more reliable and informative method to identify smokers at greatest risk of relapse than the exclusive reliance on self-report measures. Future studies should address the extent to which leptin and other appetite-regulating hormones predict smoking relapse and contribute to post-cessation changes in appetite and weight.
In summary, the results suggest that circulating leptin may be a useful marker of craving during smoking abstinence. Because intensity of craving and withdrawal symptoms are predictive of risk for smoking relapse, leptin may be a useful marker of risk for smoking relapse. Ongoing research is investigating these hypotheses.
Acknowledgments
This study was supported in part by National Institute of Health grants CA88272 and DA016351 to Dr. al’Absi.
References
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–75. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami DK, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–10. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend. 2004;73:267–78. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181:107–17. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Carr SB, Bongard S. Anger and psychobiological changes during smoking abstinence and in response to acute stress: prediction of smoking relapse. Int J Psychophysiol. 2007;66:109–15. doi: 10.1016/j.ijpsycho.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 1994. [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–9. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Cheung CC, Clifton DK, Steiner RA. Proopiomelanocortin neurons are direct targets for leptin in the hypothalamus. Endocrinology. 1997;138:4489–92. doi: 10.1210/endo.138.10.5570. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Trinder J, Swan GE. The impact of smoking cessation on objective and subjective markers of sleep: review, synthesis, and recommendations. Nicotine Tob Res. 2004;6:913–25. doi: 10.1080/14622200412331324938. [DOI] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–8. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JA, Gunn RN, Clarke PB, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42(1):48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- Eliasson B, Smith U. Leptin levels in smokers and long-term users of nicotine gum. Eur J Clin Invest. 1999;29(2):145–52. doi: 10.1046/j.1365-2362.1999.00420.x. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Cowley MA. Leptin resistance and obesity. Obesity. 2006;14:254S–8S. doi: 10.1038/oby.2006.319. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, et al. Association of low striatal dopamine d2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;165(4):507–14. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat. 2009;36:235–43. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry. 1998;43:525–30. doi: 10.1016/S0006-3223(97)00423-X. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287(5450):125–8. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS. Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress. Endocrinology. 1997;138:3859–63. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- Hillemacher T, Bleich S, Frieling H, Kraus T, Ramskogler K, Lesch O, et al. Evidence of an association of leptin serum levels and craving in alcohol dependence. Psychoneuroendocrinology. 2007;32:87–90. doi: 10.1016/j.psyneuen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7:92–3. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui A. Feeding and body-weight regulation by hypothalamic neuropeptides—mediation of the actions of leptin. Trends Neurosci. 1999;22:62–7. doi: 10.1016/s0166-2236(98)01292-2. [DOI] [PubMed] [Google Scholar]
- Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol. 2003;38:189–93. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Overlapping and interactive pathways regulating appetite and craving. J Addict Dis. 2004;23:5–21. doi: 10.1300/J069v23n03_02. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Jaschinski M, Holzbach R, Wolf K, Naber D, et al. Leptin: a modulator of alcohol craving? Biol Psychiatry. 2001a;49:782–7. doi: 10.1016/s0006-3223(01)01081-2. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Kellner M, Naber D, Wiedemann K. Leptin as a possible modulator of craving for alcohol. Arch Gen Psychiatry. 2001b;58(5):509–10. doi: 10.1001/archpsyc.58.5.509. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Holger J, Otte C, Demiralay C, Wolf K, Wiedemann K. Increasing leptin precedes craving and relapse during pharmacological abstinence maintenance treatment of alcoholism. J Psychiatr Res. 2005;39:545–51. doi: 10.1016/j.jpsychires.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortman SP. Craving is associated with smoking relapse: findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5:137–42. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Klein LC, Corwin EJ, Ceballos RM. Leptin, hunger, and body weight: influence of gender, tobacco smoking, and smoking abstinence. Addict Behav. 2004;29:921–7. doi: 10.1016/j.addbeh.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Koc B, Bulucu F, Karadurmus N, Sahin M. Lower leptin levels in young non-obese male smokers than non-smokers. Ups J Med Sci. 2009;114(3):165–9. doi: 10.1080/03009730902761631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci. 2004;5(1):55–65. doi: 10.1038/nrn1298. [DOI] [PubMed] [Google Scholar]
- Li MD, Parker SL, Kane JK. Regulation of feeding-associated peptides and receptors by nicotine. Mol Neurobiol. 2000;22(1–3):143–65. doi: 10.1385/MN:22:1-3:143. [DOI] [PubMed] [Google Scholar]
- Lundberg U, Frankenhaeuser M. Pituitary-adrenal and sypathetic-adrenal correlates of distress and effort. J Psychosom Res. 1980;24:125–30. doi: 10.1016/0022-3999(80)90033-1. [DOI] [PubMed] [Google Scholar]
- Mantzoros CS. Leptin and the hypothalamus: neuroendocrine regulation of food intake. Mol Psychiatry. 1999;4(8–12):6–7. doi: 10.1038/sj.mp.4000497. [DOI] [PubMed] [Google Scholar]
- O’Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol. 1998;148(9):821–30. doi: 10.1093/oxfordjournals.aje.a009706. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30(8):375–81. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C. Effects of smoking status and smoking cessation on leptin levels. Nicotine Tob Res. 2002;4(4):459–66. doi: 10.1080/1462220021000018434. [DOI] [PubMed] [Google Scholar]
- Reseland JE, Mundal HH, Hollung K, Haugen F, Zahid N, Anderssen SA, et al. Cigarette smoking may reduce plasma leptin concentration via catecholamines. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):43–9. doi: 10.1016/j.plefa.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Shaw D, al’Absi M. Attenuated beta endorphin response to acute stress is associated with smoking relapse. Pharmacol Biochem Behav. 2008;90:357–62. doi: 10.1016/j.pbb.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD. Tempatations to smoke after quitting: a comparison of lapsers and maintainers. Health Psychol. 1996;15:455–61. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, et al. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–3. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Tan H, Bishop SF, Lauzon NM, Sun N, Laviolette SR. Chronic nicotine exposure switches the functional role of mesolimbic dopamine transmission in the processing of nicotine’s rewarding and aversive effects. Neuropharmacology. 2009;56:741–51. doi: 10.1016/j.neuropharm.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–76. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Doshi R, Sampuran AK. Acute effect of isometric exercise on desire to smoke and tobacco withdrawal symptoms. Hum Psychopharmacol. 2006;21:39–46. doi: 10.1002/hup.744. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–6. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Baker CL, Capelleri JC, Bushmakin AG. Effect of varnicline and buproprion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl) 2007;197:371–7. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324(11):739–45. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]