Abstract
The field of tumor immunology has grown around the idea that one of the important roles of the immune system is to eliminate cancer. This idea was difficult to reconcile with the accepted notion that the immune system evolved to distinguish self from non-self and therefore tumors derived from self-tissues would not be recognized. Lack of appropriate animal models prevented experimental testing of cancer immunosurveillance. This changed with the realization that the immune system evolved to recognize danger and with the advent of mouse models deficient in one or more immune function, which showed predicted increases in susceptibility to cancer. Simultaneously, technical advances that enabled the study of the human immune system provided data for the existence of tumor-specific T cells and antibodies and led to molecular identification of tumor antigens, fully validating the cancer immunosurveillance hypothesis. Immunotherapy designed to strengthen cancer immunosurveillance has achieved unprecedented clinical successes.
Introduction
As this review is being written, the field of cancer immunotherapy is celebrating yet another milestone -- the US Food and Drug Administration took what they termed “a historic action” on August 30, 2017, by approving the drug Kymriah (Tisagenlecleucel), the first approved gene therapy in the United States, a T-cell based immunotherapy for B cell ALL (acute lymphoblastic leukemia). Children and young adults suffering from relapsing ALL refractory to standard therapies, who up to now have had no other treatment options and no hope for survival, when given this form of immunotherapy readily go into remission. The multicenter trial on 63 ALL patients that led to the approval of this new immunotherapy showed the overall remission rate of 83% within 3 months of treatment. The anti-tumor effect is caused by patient’s own T cells that are collected and genetically engineered ex vivo to express a chimeric antigen receptor (CAR) containing an antigen binding site of a humanized mouse antibody that recognizes the human molecule CD19 expressed on the leukemic cells.
From the time the idea of creating a CAR was first proposed (1) (2) (3), it took almost 30 years of research conducted in many laboratories around the world to arrive to this milestone. The road was even longer if one were to take into account all the previous basic research on antibody structure and genetics, T cell receptor biology, MHC restricted antigen recognition and elucidation of various co-stimulatory signaling pathways in T cells. The approval of this latest immunotherapy follows a series of recent approvals starting with the first therapeutic cancer vaccine Sipuleucel T for patients with metastatic, castration resistant prostate cancer (4), and Guardasil (5), human papilloma virus (HPV) vaccine for the prevention of cervical, vulvar and vaginal cancers. The first checkpoint inhibitor, anti-CTLA-4 antibody Ipilimumab, was approved in 2011 for the treatment of melanoma (6) and since then several other antibodies have been approved against additional negative regulators of T cell function, such as PD-1 and its ligand PD-L1. These immunotherapies cover numerous liquid and solid tumors. Unprecedented clinical successes of immunotherapy drugs that engage and support the patient’s immune system in the fight against cancer were made possible by advances in the field of tumor immunology that have contributed to a more complete understanding of the tumor cell/immune system interactions during tumor development.
Cancer immunotherapy, and the science behind it, was recognized in 2013 by journal Science as the 2013 Scientific Breakthrough of the year. For a generation of tumor immunologists who entered the field 50 or so years ago, and for decades worked hard to convince the skeptics in other fields of immunology that the immune system has an important role to play in tumor immunosurveillance, and for subsequent generations of believers that received and passed the torch often to the detriment of a more exalted career track, these are very exciting days. This review will reflect on the work that brought us to this point, what we now know about cancer immunosurveillance and how and why this important guardian of the body’s integrity sometimes fails.
After a period of skepticism, overwhelming evidence for cancer immunosurveillance
It has been 60 years since the first experimental evidence was published by Prehn and Main (7) that mice could generate immunity against autochtonous carcinogen induced tumors. The immune response was specific for each tumor suggesting the existence of unique molecules that are recognized as tumor antigens. Several of these molecules were later identified as products of carcinogen-induced mutations in various genes, including in the p53 tumor suppressor gene (8), which was later also found to be mutated in human tumors (9). George Klein and his team contemporaneously showed that even a progressing tumor could generate an immune response such that if it was removed, the mouse remained immune and could reject the challenge with the same tumor (10). Work with transplantable tumors in experimental mouse models (11), combined with rare but nevertheless remarkable observations of spontaneous tumor regressions in humans, raised the idea that surveillance of tumors was one of the highly important functions of the immune system. The main proponents were Sir Mcfarlane Burnet (12, 13) and Lewis Thomas (14), who independently proposed conceptually the same hypothesis that large complex organisms must poses a system that recognizes and destroys nascent tumors that likely arise frequently in tissues where cells undergo numerous proliferation cycles, each capable of giving rise to potentially carcinogenic genetic mutations.
The prevailing picture of the immune system at that time was that it evolved to distinguish “self” from “non-self.” (15) In addition to tissue allografts, the “non-self” would include viruses, bacteria and other pathogens. Opponents of the cancer immunosurveillance hypothesis held that tumors, being derived from self-tissues, would be invisible to the immune system. This narrow view of the immune system, which primarily applied to T and B cells and their antigen receptors, failed to explain and incorporate a number of findings that followed, such as evidence of the importance of innate immunity in initiating immune responses (16, 17), the need for co-stimulation (18) and the presence of Toll-like receptors (TLRs) (19) on antigen presenting cells (APCs). If only specific recognition of non-self by an antigen receptor was important, these other molecules and cells would be superfluous. Eventually the self-non-self discrimination hypothesis was replaced by the “danger” hypothesis championed by Polly Matzinger (20), which stated that to the immune system the “foreignness” of an entity is not as important as whether that entity causes damage to normal tissues. This helped rescue the cancer immunosurveillance hypothesis since clearly tumors could cause damage and that would be important to the immune system. The specific danger signals that would trigger an immune response against cancer were still to be identified.
While the conceptual barrier was partially crossed, a technical barrier to the acceptance of the cancer immunosurveillance hypothesis remained – the need for an appropriate animal model. If the immune system were responsible for eliminating nascent tumors, then in immunocompromised animals there would be a higher incidence of spontaneous or carcinogen induced tumors. Various methods of immunosuppression in mouse models were used, including neonatal thymectomy, steroids and anti-lymphocyte serum. The results were far from conclusive. Even when a state of immune deficiency could be achieved, and even when immunocompromised mice did show increased incidence of carcinogen-promoted tumors, there was usually an alternative explanation for the observed results that could be considered as likely as cancer immunosurveillance.
The discovery of the mutant mouse without a thymus (21), the “nude” mouse, that exhibited multiple effects of its particular genetic mutation, including lack of T cells and a profound deficiency in adaptive immunity (22), promised to provide a perfect mouse model for testing the cancer immunosurveillance hypothesis. It also eventually, but fortunately only temporarily, led to its demise. The most influential experiments that appeared to disprove the hypothesis where those of Osias Stutman (23, 24), confirmed by several other groups, that showed no difference in tumor incidence between CBA/H nude mice and wild type CBA/H littermates treated at birth with a chemical carcinogen methylcholanthrene (MCA). Ironically, as he and others were using these experiments to reject the existence of cancer immunosurveillance, Stutman was reporting on a new cell type with tumor-killing capacity, the natural killer (NK) cell, present also in nude mice, and proposing that it might have a role in cancer surveillance (25). It would take at least two decades to gain a better understanding of the interplay between the adaptive immune system and the innate immune system to which NK cells belong as well as the many cytokines and chemokines that help orchestrate anti-tumor immunity, before it was clear that experiments in nude mice were given too much credence considering the limited available information about its immune system.
In addition to the developments in immunology that brought better understanding of what it would take to generate immunity to cancer, development of new gene engineering technologies allowed the creation of new mouse strains that lacked diverse and very specific immune components or had deficiencies in signaling pathways important for immune effector functions. These included RAG−/− mice deficient in T, B and NKT cells; mice lacking an important immune cytokine interferon gamma (IFN-γ) or its receptor, or could not signal through the STAT-1 pathway used by interferon; Perforin−/− mice that lacked cytotoxic T and NK cell function; α/β T cell−/− or γ/δ T cell−/− mice and IL-12−/− mice. Each of these strains compared to their WT counterparts was found to be deficient in cancer immunosurveillance in one form or another (26–33). The cancer immunosurveillance hypothesis was back and quickly garnering support. Importantly, it was also being updated and modified to be consistent with all the new data.
Robert Schreiber and his group proposed a new and improved version of the cancer immunosurveillance hypothesis, “tumor immunoediting.” It incorporates three different potential outcomes: tumor elimination, equilibrium with the immune system, and escape from immune control (34). The immune system is alerted to the presence of the tumor as it begins to exert abnormal physiological and metabolic pressure on the surrounding normal tissues (as it becomes “dangerous”). The innate system is activated first and its activities at the nascent tumor site cause a certain amount of tumor cell death and initiate an inflammatory environment that recruits additional innate cells to amplify inflammation and attract T and B cells. The outcome is tumor elimination, the main tenet of the basic cancer immunosurveillance hypothesis. The second tenet is that If the immune system is compromised and these orderly processes are disturbed, the tumor will escape. Data from the new mouse models provided the first look into the equilibrium phase. This occurrence is different from what was previously known as tumor dormancy, in that the tumor is not really dormant but continues to proliferate and mutate against the immune pressure (immune editing) until it finally evolves into a less antigenic tumor capable of escaping immune destruction. This ability to survive in the face of an immune attack has now been recognized as one of the major hallmarks of cancer (35).
Immunosurveillance of human tumors
Results of experiments in immunocompromised mice provided mechanistic explanations for similar observations in humans, which were at first anecdotal and later confirmed with retrospective and prospective analyses. Spontaneous regressions of growing tumors were repeatedly observed but as in mice, there were many alternative explanations for those phenomena in addition to natural immune surveillance. The earliest experiments in humans that were intended to boost immune surveillance and affect cancer regression were those of William Coley in the late 19th century who noticed that occasional serious bacterial infections in cancer patients were associated with tumor regressions. He proceeded to infect patients intentionally and saw increased numbers of cases of cancer regression, which he credited to the immune defenses against the pathogens being able to strengthen in some manner the immune defenses against the cancer. These experiments are considered the first approach to cancer therapy that intended to stimulate cancer immunosurveillance, the basic principle of modern immunotherapy.
The advent of organ transplantation that led to the development of strong immunosuppressive drugs also created an opportunity to determine if immunocompromised transplant patients would be more susceptible to developing cancer. Indeed, numerous studies showed that life-long immunosuppression led to a highly significant increases in over 30 different cancer types (35–41). The emergence of HIV and the accompanying acquired immunodefficienty syndrome (AIDS) also resulted in higher cancer incidence in the affected population (42–44).
In addition to these acquired immunodeficiencies, there are inborn primary immunodeficiencies (PIDs). The ability to control infections in these populations and prolong life has allowed observations of increased incidence of cancer in these individuals as well (45). Conditions such as common variable immunodeficiency (CVID) and X-linked agammaglobulinemea are associated with defective humoral immunity and an increased incidence of cancer (46, 47). Patients with hyper-IgE syndrome (HIES) that carry a mutation in the STAT-3 gene have impaired B cell maturation into plasma cells as well as deficiency in Th17 T cells and IL-17 production, which causes drastically reduced immunosurveillance of both viruses and cancer. Immunodeficiencies that result from mutations in DNA repair genes also show increased susceptibility to cancer and reduced immunosurveillance due to deficiencies in multiple immune cell functions (48). The importance of innate immunity in cancer immunosurveillance was also revealed through various PIDs. For example, individuals with a mutation in the GATA2 gene (49), a transcription factor responsible for differentiation of hematopoietic cells, and those with the CSF3R mutation (50), suffer among other things from neuthropenia and other phagocytic disorders leading to disseminated bacterial and fungal infections and also multiple types of leukemias and lymphomas.
Identification of human tumor-specific antibodies and T cells as the indisputable proof of cancer immunosurveillance
Development of hybridoma technology (51) that enabled production of monoclonal antibodies, launched a large effort to discover molecules on cancer cells that are different from normal cells. Mice were immunized with every type of human tumor or tumor cell line and monoclonal antibodies from these immunizations were screened for the recognition of cancer cells and not their normal counterparts. These studies clearly showed that such molecules (a.k.a. tumor antigens) existed and, furthermore, yielded potentially immunotherapeutic antibodies that could be conjugated to toxins, drugs or radioisotopes for use in cancer imaging/diagnosis or therapy. This work, however, did not bring the field any closer to confirming cancer immunosurveillance or further elucidating human tumor immunity. The question still remained whether the human immune system would also be capable of seeing these tumor associated or tumor specific antigens and if both antibodies and T cells would be involved.
Efforts to answer these questions intensified in the mid eighties and early nineties spurred by numerous advances in basic immunology, genomics and proteomics and development of many useful technologies such as tandem mass spectrometry. Tumor-specific antibodies could be isolated from cancer patients and used to screen random peptide and protein libraries or tumor gene expression libraries to identify target antigens (52). T cells from cancer patients could be grown in vitro in the newly discovered T cell growth factor IL-2 and their tumor specificity maintained with tumor-loaded dendritic cells (DC) that had just been recognized as professional antigen-presenting cells and methods to grow them from blood monocytes had just been established (53, 54). This work generated numerous tumor-specific reagents with which human tumor antigens could for the first time be identified and fully characterized.
In 1989, epithelial mucin MUC1 was reported as the first human tumor antigen to be recognized by human cytotoxic T cells (CTL) that were grown from lymph nodes of patients with pancreatic cancer (55). MUC1 had previously been identified by a mouse monoclonal antibody DUPAN-2 (56) that detected its abnormal expression on all human adenocarcinomas, and by antibodies HMFG-1, HMFG-2, SM3 and DF-3 against breast cancer (57, 58). The gene for this antigen was cloned soon thereafter (59, 60) allowing transfection into MUC1− cells to confirm MUC1 as the target for tumor-specific CTL. Using melanoma specific CTL clones and transduction of melanoma genes into antigen-negative targets, the first melanoma tumor antigen was cloned and reported in 1991(61). Another highly productive method for tumor antigen discovery was elution of all peptides bound to HLA-Class I or Class II molecules from tumor cells, separating them by tandem mass spectrometry, loading them onto DC and presenting them to tumor-specific T cell clones (62, 63). Peptides that activated the T cells were identified as candidate tumor antigens that could then be synthesized and further characterized and confirmed. Within several years many human tumor antigens were identified belonging to several different categories, and on both viral and non-viral cancers (64, 65). Some were products of mutated oncogenes, such as K and H-ras and others were non-mutated antigens differentially expressed on tumor versus normal cells. Recent technical improvements that increased the ease of gene sequencing enabled sequencing of total tumor genomes and focused attention on many random mutations that could generate new mutated peptide epitopes unique to each patient’s tumor, similar to the unique mouse tumor antigens in the early models of MCA induced sarcomas (66). In several instances, T cells specific for the mutated epitopes predicted by the gene mutation have been found in the patient (67). Thus immune responses are spontaneously generated to both the non-mutated shared tumor antigens and the mutated unique tumor antigens as tumor develops, as would be predicted by the cancer immunosurveillance and tumor-editing hypothesis.
Timing of immunosurveillance
Because most of the information about human anti-tumor immune responses was acquired studying immunity in cancer patients, the best understood phase of cancer immunosurveillance is escape. The conundrum that arose when tumor antigens were identified and anti-tumor humoral and cellular immunity confirmed was why and how the tumor escapes and would an anti-tumor immune response ever be a tumor rejection response (68). One way to show the protective function of anti-tumor immunity even in the escape phase has been to evaluate tumor-specific immunity at the time of diagnosis and its effect on the disease outcome. Many such studies were done and results showed that patients with pre-existing anti-cancer immunity at diagnosis have longer disease free survival, slower tumor progression and extended overall survival. The best known are studies that evaluated tumor infiltrating T cells and their state of activation across different tumor types, which found that tumors that are more extensively infiltrated with activated T cells and other immune effector cells and show evidence of organized lymphoid structures within which these cells cooperate, recur much later and patients experience longer survival (69–71). It was also learned, however, that with advancing stages of tumor, the infiltrating cell composition changes. The effector cells become fewer and less activated while the tumor microenvironment becomes dominated by cells with regulatory and immunosuppressive activities, such as T regulatory cells, tumor-associated macrophages (TAMs) and myeloid derived suppressor cells (MDSC). These cells and their soluble mediators interfere with the ability of tumor-specific effector T cells, NKT cells and NK cells to kill tumor cells (72, 73).
There is still very little information available on when in cancer development the immune system becomes involved. The answer may be different for each tumor type or even for each tumor and its etiology or the initiating mutation. A lot of current emphasis is on using various imaging and other modern screening technologies to detect early tumors and their precursor lesions. The same type of research that has been performed on later stage tumors that yielded a very detailed picture of the tumor immune microenvironment, is beginning to be done on early tumor stages and on premalignant lesions. The limited information so far shows that premalignant lesions are under immune surveillance (74–76) and that their progression to cancer is also accompanied by changes that begin to shift the balance from effector immune cells to regulatory and suppressive cells, which likely promotes tumorigenesis.
Natural immunosurveillance as basis for immunotherapy
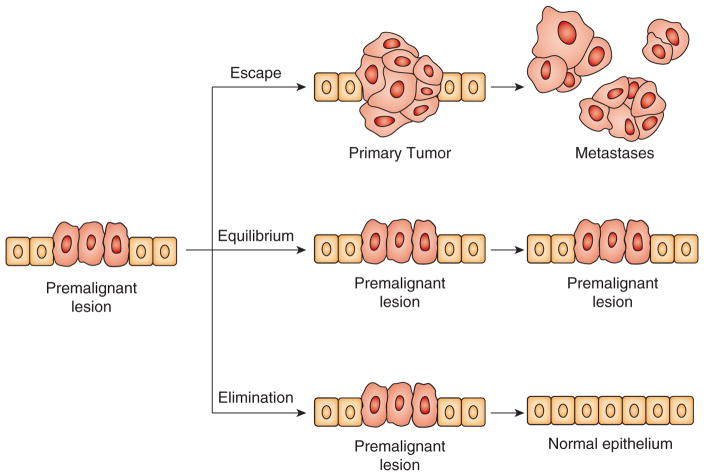
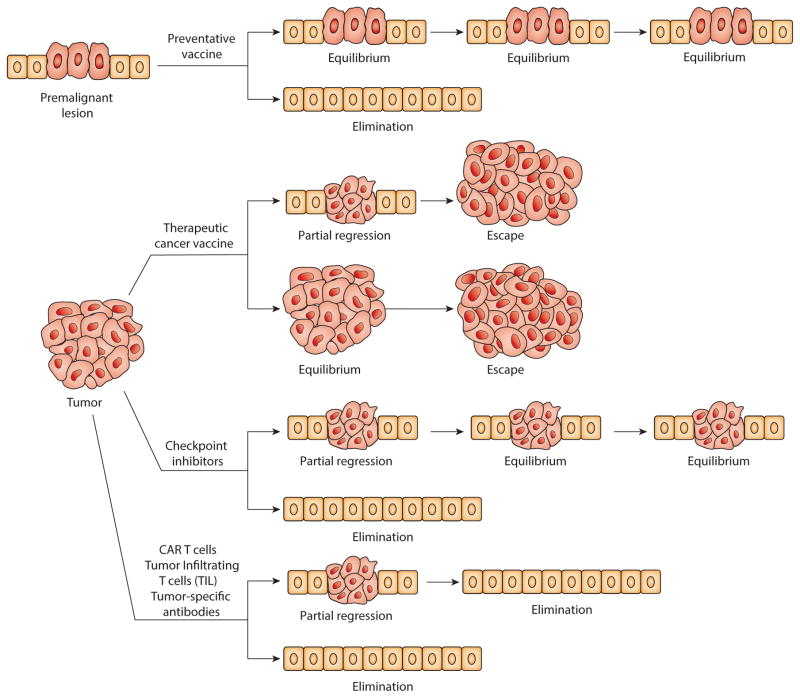
Figure 1 illustrates the three established major outcomes of natural immunosurveillance of cancer but applied here to a premalignant lesion. An optimally functional immune system would be expected to detect very early the disorder in the normal tissue morphology and physiology and the danger it presents to its integrity, and to recruit multiple innate and adaptive immune effector mechanisms to eliminate abnormal cells and restore tissue homeostasis (77). If the race for control between the immune effector mechanisms and their regulatory and suppressive counterparts is tied, the premalignant lesion could remain in an equilibrium with the immune system without further progression. If the balance shifts in favor of the regulatory and immunosuppressive mechanisms, premalignant lesion could escape immune control and progress to metastatic cancer. The odds of one outcome versus another depend on many variables unique to each individual and some common variables such as age (78). The goal of immunotherapy is to intervene in natural immunosurveillance and to change the odds in favor of elimination or at least equilibrium. As Figure 2 illustrates, a preventative vaccine based on tumor antigens expressed on both premalignant lesions and cancer could strengthen tumor-specific adaptive immunity and shift the balance at the site of a premalignant lesion in favor of elimination. This approach is the new frontier in immunotherapy (79). Other immunotherapies have been developed for tumors that have already escaped immune surveillance. Therapeutic vaccines and checkpoint inhibitors are designed to restore immunosurveillance. The experience with therapeutic vaccines is extensive but therapeutic efficacy has been limited to short-lived partial regressions or temporary disease stability (equilibrium) followed by tumor escape (80). This has resulted in the FDA approval of only one such vaccine, Sipuleucel-T for prostate cancer (4). So far the best therapeutic outcomes have been seen with checkpoint inhibitors such as anti-CTLA4, anti-PD1 and anti-PDL-1 (81), which have caused tumor regression and long-term equilibrium in a large percentage of treated patients and in some instances complete cancer elimination (82). There are almost monthly FDA approvals of one checkpoint inhibitor or another or their combinations for different cancer types. Adoptive T cell (83) and antibody (82) immunotherapy is designed for full elimination of cancer. The therapeutic effects have been impressive resulting in the FDA approval of several antibodies (e.g. anti-Her2/neu Trastuzumab for the treatment of breast cancer and anti-CD20 Rutuximab for the treatment of some leukemias and lymphomas) and the most recent approval of the first of many to come T cell therapies (anti-CD19 CAR Kymriah).
Figure 1.
Three established outcomes of natural immunosurveillance against cancer applied to the setting of a premalignant lesion.
Figure 2.
Immunotherapeutic interventions can change the outcome of natural immunosurveillance of a premalignant lesion or an advanced tumor.
Cancer immunosurveillance: variation on the premise
All portrayals of cancer immunosurveillance start with nascent tumors beginning to express danger signals that attract innate immunity, and with expression of new tumor antigens that elicit specific T cells and antibodies. Finding T cells specific for the unique mutated tumor antigens supports this picture. Yet, the majority of known tumor antigens are shared and not mutated but tumor associated due to their abnormal expression (e.g. overexpression, differential posttranslational modification and unscheduled expression) compared to healthy tissue. The change in expression of these molecules can be caused by many physiologic changes in the cell and its microenvironment and thus they could be transiently abnormally expressed by non-malignant tissues under other “dangerous” circumstances. Many molecules that we know as tumor antigens are expressed in their abnormal “tumor” form on acutely inflamed tissues during viral infections or in the setting of chronic inflammations. These abnormal forms of self-antigens can be encountered very early in life during strong febrile infections that characterize the common childhood diseases with which the immune system has evolved and which can serve to train the immune system during its development. It has been shown that healthy individuals often have stronger immune responses against certain tumor antigens than cancer patients. Moreover, these responses appear to lower lifetime risk of cancer. Thus immunosurveillance of cancer is part of the general immunosurveillance based on the immune memory for a family of self molecules abnormally expressed in many diseases including cancer and marking diseased cells for immune destruction. I proposed this modification of the immunosurveillance hypothesis in 2008 in my AAI Presidential address (84) based on experimental data derived from human studies (85, 86) that has been supplemented recently by additional studies in humans (87–89) and testing the concept in mice (90).
Zitvogel and Kroemer have also proposed the possibility that specific anti-tumor immunosurveillance is in place before tumor develops but they offer another possible explanation for the existence of tumor antigen specific immunity in the absence of tumor – the gut microbiome. In addition to many recently shown effects of the microbiota on the outcome of cancer immunotherapy (91), they propose that microbial proteins might be sufficiently similar to tumor antigens and are thus capable of eliciting tumor-specific T cells and antibodies that recognize future tumor cells via “antigenic mimicry.” These memory T cells primed as intra-epithelial or lamina propria T cells can then be seeded to all epithelial tissues where they can provide immunosurveillance against, in this case, epithelial tumors (92). Microbiota at other sites could prime and seed T cells to different sites for immunosurveillance of other types of tumors.
What these two proposals have in common is the idea that immune surveillance based on shared antigens is part of the immune preparedness program, which serves as the first responder, maintains the balance in favor of anti-tumor effector cells, and likely promotes generation of other tumor antigen-specific T cells and antibodies, including those against mutated tumor antigens, via the already known process of epitope spreading (93). If this is indeed the case, and the work is ongoing to test this, it would be possible to strengthen immunosurveillance much earlier in life with a vaccine based on a variety of these antigens, especially now that we have eliminated most childhood diseases and limited exposure to microorganisms through excessive hygiene.
Conclusions
The recent successes of cancer immunotherapies have re-energized the tumor immunology field and opened numerous opportunities for new research. Work is now ongoing to elucidate molecular vulnerabilities of immunosuppressive cells such as T regulatory cells, MDSCs, TAMs or their various products so that they can also be targeted to further improve native or elicited cancer immunosurveillance. The future of immunotherapy lies in various combinations of drugs that modulate tumor microenvironment and strengthen natural cancer immunosurveillance.
Acknowledgments
Funded by NIH grant R35 CA210039
References
- 1.Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, Nagase F, Kurosawa Y. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. 1987;149:960–968. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 2.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goverman J, Gomez SM, Segesman KD, Hunkapiller T, Laug WE, Hood L. Chimeric immunoglobulin-T cell receptor proteins form functional receptors: implications for T cell receptor complex formation and activation. Cell. 1990;60:929–939. doi: 10.1016/0092-8674(90)90341-b. [DOI] [PubMed] [Google Scholar]
- 4.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 5.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 6.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957;18:769–778. [PubMed] [Google Scholar]
- 8.DeLeo AB, Jay G, Appella E, Dubois GC, Law LW, Old LJ. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979;76:2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isobe M, Emanuel BS, Givol D, Oren M, Croce CM. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986;320:84–85. doi: 10.1038/320084a0. [DOI] [PubMed] [Google Scholar]
- 10.Klein G, Sjogren HO, Klein E, Hellstrom KE. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res. 1960;20:1561–1572. [PubMed] [Google Scholar]
- 11.Old LJ, Boyse EA. Immunology of Experimental Tumors. Annu Rev Med. 1964;15:167–186. doi: 10.1146/annurev.me.15.020164.001123. [DOI] [PubMed] [Google Scholar]
- 12.Burnet FM. Immunological aspects of malignant disease. Lancet. 1967;1:1171–1174. doi: 10.1016/s0140-6736(67)92837-1. [DOI] [PubMed] [Google Scholar]
- 13.Burnet FM. Immunological surveillance in neoplasia. Transplant Rev. 1971;7:3–25. doi: 10.1111/j.1600-065x.1971.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 14.Thomas L. On immunosurveillance in human cancer. Yale J Biol Med. 1982;55:329–333. [PMC free article] [PubMed] [Google Scholar]
- 15.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 16.Salazar-Mather TP, Ishikawa R, Biron CA. NK cell trafficking and cytokine expression in splenic compartments after IFN induction and viral infection. J Immunol. 1996;157:3054–3064. [PubMed] [Google Scholar]
- 17.Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell-produced interferon gamma in defense against murine cytomegalovirus infection and enhancement of this defense pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 19.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 20.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 21.Pantelouris EM. Absence of thymus in a mouse mutant. Nature. 1968;217:370–371. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- 22.Flanagan SP. ‘Nude’, a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966;8:295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 23.Stutman O. Tumor development after 3-methylcholanthrene in immunologically deficient athymic-nude mice. Science. 1974;183:534–536. doi: 10.1126/science.183.4124.534. [DOI] [PubMed] [Google Scholar]
- 24.Stutman O. Chemical carcinogenesis in nude mice: comparison between nude mice from homozygous matings and heterozygous matings and effect of age and carcinogen dose. J Natl Cancer Inst. 1979;62:353–358. [PubMed] [Google Scholar]
- 25.Stutman O, Paige CJ, Figarella EF. Natural cytotoxic cells against solid tumors in mice. I. Strain and age distribution and target cell susceptibility. J Immunol. 1978;121:1819–1826. [PubMed] [Google Scholar]
- 26.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1:447–456. doi: 10.1016/1074-7613(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 28.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 29.van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi Y, Jungbluth A, Richards EC, Old LJ. Effect of interleukin 12 on tumor induction by 3-methylcholanthrene. Proc Natl Acad Sci U S A. 1996;93:11798–11801. doi: 10.1073/pnas.93.21.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Birkeland SA, Storm HH, Lamm LU, Barlow L, Blohme I, Forsberg B, Eklund B, Fjeldborg O, Friedberg M, Frodin L, et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int J Cancer. 1995;60:183–189. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 37.Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 38.List AF, Greco FA, Vogler LB. Lymphoproliferative diseases in immunocompromised hosts: the role of Epstein-Barr virus. J Clin Oncol. 1987;5:1673–1689. doi: 10.1200/JCO.1987.5.10.1673. [DOI] [PubMed] [Google Scholar]
- 39.Leblond V, Davi F, Charlotte F, Dorent R, Bitker MO, Sutton L, Gandjbakhch I, Binet JL, Raphael M. Posttransplant lymphoproliferative disorders not associated with Epstein-Barr virus: a distinct entity? J Clin Oncol. 1998;16:2052–2059. doi: 10.1200/JCO.1998.16.6.2052. [DOI] [PubMed] [Google Scholar]
- 40.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiels MS, Pfeiffer RM, Hall HI, Li J, Goedert JJ, Morton LM, Hartge P, Engels EA. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980–2007. JAMA. 2011;305:1450–1459. doi: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, Rapiti E, Levi F, Jundt G, Fisch T, Bordoni A, De Weck D, Franceschi S. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 43.Shiels MS, Cole SR, Kirk GD, Poole C. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;52:611–622. doi: 10.1097/QAI.0b013e3181b327ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10:1152–1159. doi: 10.1016/S1470-2045(09)70282-7. [DOI] [PubMed] [Google Scholar]
- 45.Mortaz E, Tabarsi P, Mansouri D, Khosravi A, Garssen J, Velayati A, Adcock IM. Cancers Related to Immunodeficiencies: Update and Perspectives. Front Immunol. 2016;7:365. doi: 10.3389/fimmu.2016.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Salavoura K, Kolialexi A, Tsangaris G, Mavrou A. Development of cancer in patients with primary immunodeficiencies. Anticancer Res. 2008;28:1263–1269. [PubMed] [Google Scholar]
- 47.Kinlen LJ, Webster AD, Bird AG, Haile R, Peto J, Soothill JF, Thompson RA. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985;1:263–266. doi: 10.1016/s0140-6736(85)91037-2. [DOI] [PubMed] [Google Scholar]
- 48.de Miranda NF, Bjorkman A, Pan-Hammarstrom Q. DNA repair: the link between primary immunodeficiency and cancer. Ann N Y Acad Sci. 2011;1246:50–63. doi: 10.1111/j.1749-6632.2011.06322.x. [DOI] [PubMed] [Google Scholar]
- 49.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, Cowen EW, Freeman AF, Olivier KN, Uzel G, Zelazny AM, Daub JR, Spalding CD, Claypool RJ, Giri NK, Alter BP, Mace EM, Orange JS, Cuellar-Rodriguez J, Hickstein DD, Holland SM. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123:809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Germeshausen M, Ballmaier M, Welte K. Incidence of CSF3R mutations in severe congenital neutropenia and relevance for leukemogenesis: Results of a long-term survey. Blood. 2007;109:93–99. doi: 10.1182/blood-2006-02-004275. [DOI] [PubMed] [Google Scholar]
- 51.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 52.Tureci O, Sahin U, Pfreundschuh M. Serological analysis of human tumor antigens: molecular definition and implications. Mol Med Today. 1997;3:342–349. doi: 10.1016/s1357-4310(97)01081-2. [DOI] [PubMed] [Google Scholar]
- 53.Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant C, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kao H, Amoscato AA, Ciborowski P, Finn OJ. A new strategy for tumor antigen discovery based on in vitro priming of naive T cells with dendritic cells. Clin Cancer Res. 2001;7:773s–780s. [PubMed] [Google Scholar]
- 55.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86:7159–7163. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lan MS, Finn OJ, Fernsten PD, Metzgar RS. Isolation and properties of a human pancreatic adenocarcinoma-associated antigen, DU-PAN-2. Cancer Res. 1985;45:305–310. [PubMed] [Google Scholar]
- 57.Girling A, Bartkova J, Burchell J, Gendler S, Gillett C, Taylor-Papadimitriou J. A core protein epitope of the polymorphic epithelial mucin detected by the monoclonal antibody SM-3 is selectively exposed in a range of primary carcinomas. Int J Cancer. 1989;43:1072–1076. doi: 10.1002/ijc.2910430620. [DOI] [PubMed] [Google Scholar]
- 58.Hull SR, Bright A, Carraway KL, Abe M, Hayes DF, Kufe DW. Oligosaccharide differences in the DF3 sialomucin antigen from normal human milk and the BT-20 human breast carcinoma cell line. Cancer Commun. 1989;1:261–267. [PubMed] [Google Scholar]
- 59.Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, Pemberton L, Lalani EN, Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 60.Siddiqui J, Abe M, Hayes D, Shani E, Yunis E, Kufe D. Isolation and sequencing of a cDNA coding for the human DF3 breast carcinoma-associated antigen. Proc Natl Acad Sci U S A. 1988;85:2320–2323. doi: 10.1073/pnas.85.7.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 62.Hunt DF, Michel H, Dickinson TA, Shabanowitz J, Cox AL, Sakaguchi K, Appella E, Grey HM, Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992;256:1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- 63.Hunt DF, Henderson RA, Shabanowitz J, Sakaguchi K, Michel H, Sevilir N, Cox AL, Appella E, Engelhard VH. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science. 1992;255:1261–1263. doi: 10.1126/science.1546328. [DOI] [PubMed] [Google Scholar]
- 64.Finn OJ. Human Tumor Antigens Yesterday, Today, and Tomorrow. Cancer Immunol Res. 2017;5:347–354. doi: 10.1158/2326-6066.CIR-17-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finn OJ. Tumor-rejection antigens recognized by T lymphocytes. Curr Opin Immunol. 1993;5:701–708. doi: 10.1016/0952-7915(93)90124-b. [DOI] [PubMed] [Google Scholar]
- 66.Srivastava PK. Neoepitopes of Cancers: Looking Back, Looking Ahead. Cancer Immunol Res. 2015;3:969–977. doi: 10.1158/2326-6066.CIR-15-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, Trebska-McGowan K, Wunderlich JR, Yang JC, Rosenberg SA. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat Med. 2016;22:433–438. doi: 10.1038/nm.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 69.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 70.Fridman WH, Dieu-Nosjean MC, Pages F, Cremer I, Damotte D, Sautes-Fridman C, Galon J. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron. 2013;6:117–122. doi: 10.1007/s12307-012-0124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboue R, Frebourg T, Pages F, Valge-Archer V, Latouche JB, Galon J. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity. 2016;44:698–711. doi: 10.1016/j.immuni.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 72.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 73.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348:74–80. doi: 10.1126/science.aaa6204. [DOI] [PubMed] [Google Scholar]
- 74.Karlsson M, Lindberg K, Karlen P, Ost A, Thorn M, Winqvist O, Eberhardson M. Evidence for immunosurveillance in intestinal premalignant lesions. Scand J Immunol. 2010;71:362–368. doi: 10.1111/j.1365-3083.2010.02377.x. [DOI] [PubMed] [Google Scholar]
- 75.Ohman J, Magnusson B, Telemo E, Jontell M, Hasseus B. Langerhans cells and T cells sense cell dysplasia in oral leukoplakias and oral squamous cell carcinomas--evidence for immunosurveillance. Scand J Immunol. 2012;76:39–48. doi: 10.1111/j.1365-3083.2012.02701.x. [DOI] [PubMed] [Google Scholar]
- 76.Beatty PL, van der Geest R, Hashash JG, Kimura T, Gutkin D, Brand RE, Finn OJ. Immunobiology and immunosurveillance in patients with intraductal papillary mucinous neoplasms (IPMNs), premalignant precursors of pancreatic adenocarcinomas. Cancer Immunol Immunother. 2016;65:771–778. doi: 10.1007/s00262-016-1838-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Senovilla L, Galluzzi L, Zitvogel L, Kroemer G. Immunosurveillance as a regulator of tissue homeostasis. Trends Immunol. 2013;34:471–481. doi: 10.1016/j.it.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Pawelec G. Immunosenescence and cancer. Biogerontology. 2017;18:717–721. doi: 10.1007/s10522-017-9682-z. [DOI] [PubMed] [Google Scholar]
- 79.Finn OJ. Vaccines for cancer prevention: a practical and feasible approach to the cancer epidemic. Cancer Immunol Res. 2014;2:708–713. doi: 10.1158/2326-6066.CIR-14-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 81.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161:205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nghiem PT, Bhatia S, Lipson EJ, Kudchadkar RR, Miller NJ, Annamalai L, Berry S, Chartash EK, Daud A, Fling SP, Friedlander PA, Kluger HM, Kohrt HE, Lundgren L, Margolin K, Mitchell A, Olencki T, Pardoll DM, Reddy SA, Shantha EM, Sharfman WH, Sharon E, Shemanski LR, Shinohara MM, Sunshine JC, Taube JM, Thompson JA, Townson SM, Yearley JH, Topalian SL, Cheever MA. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N Engl J Med. 2016;374:2542–2552. doi: 10.1056/NEJMoa1603702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yeku O, Li X, Brentjens RJ. Adoptive T-Cell Therapy for Solid Tumors. Am Soc Clin Oncol Educ Book. 2017;37:193–204. doi: 10.14694/EDBK_180328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finn OJ. Immunological weapons acquired early in life win battles with cancer late in life. J Immunol. 2008;181:1589–1592. doi: 10.4049/jimmunol.181.3.1589. [DOI] [PubMed] [Google Scholar]
- 85.Terry KL, Titus-Ernstoff L, McKolanis JR, Welch WR, Finn OJ, Cramer DW. Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:30–35. doi: 10.1158/1055-9965.EPI-06-0688. [DOI] [PubMed] [Google Scholar]
- 86.Cramer DW, Titus-Ernstoff L, McKolanis JR, Welch WR, Vitonis AF, Berkowitz RS, Finn OJ. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1125–1131. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 87.Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–271. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cramer DW, Vitonis AF, Pinheiro SP, McKolanis JR, Fichorova RN, Brown KE, Hatchette TF, Finn OJ. Mumps and ovarian cancer: modern interpretation of an historic association. Cancer Causes Control. 2010;21:1193–1201. doi: 10.1007/s10552-010-9546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pinheiro SP, Hankinson SE, Tworoger SS, Rosner BA, McKolanis JR, Finn OJ, Cramer DW. Anti-MUC1 antibodies and ovarian cancer risk: prospective data from the Nurses’ Health Studies. Cancer Epidemiol Biomarkers Prev. 2010;19:1595–1601. doi: 10.1158/1055-9965.EPI-10-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iheagwara UK, Beatty PL, Van PT, Ross TM, Minden JS, Finn OJ. Influenza virus infection elicits protective antibodies and T cells specific for host cell antigens also expressed as tumor-associated antigens: a new view of cancer immunosurveillance. Cancer Immunol Res. 2014;2:263–273. doi: 10.1158/2326-6066.CIR-13-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17:271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
- 92.Zitvogel L, Ayyoub M, Routy B, Kroemer G. Microbiome and Anticancer Immunosurveillance. Cell. 2016;165:276–287. doi: 10.1016/j.cell.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 93.el-Shami K, Tirosh B, Bar-Haim E, Carmon L, Vadai E, Fridkin M, Feldman M, Eisenbach L. MHC class I-restricted epitope spreading in the context of tumor rejection following vaccination with a single immunodominant CTL epitope. Eur J Immunol. 1999;29:3295–3301. doi: 10.1002/(SICI)1521-4141(199910)29:10<3295::AID-IMMU3295>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]