Abstract
Malignant brain tumors are known to utilize acetate as an alternate carbon source in the citric acid cycle for their bioenergetics. 13C NMR based isotopomer analysis has been used to measure turnover of 13C-acetate carbons into glutamate and glutamine pools in tumors. Plasma from the patients infused with [1,2-13C]acetate further revealed the presence of 13C isotopomers of glutamine, glucose and lactate in the circulation that were generated due to metabolism of [1,2-13C]acetate by peripheral organs. In the tumor cells, [4-13C] and [3,4-13C] glutamate and glutamine isotopomers were generated from blood-borne 13C labeled glucose and lactate which were formed due to [1,2-13C[acetate metabolism of peripheral tissues. [4,5-13C] and [3,4,5-13C] glutamate and glutamine isotopomers were produced from [1,2-13C]acetyl-CoA that were derived from direct oxidation of [1,2-13C]acetate in the tumor.
Keywords: [1,2-13C]acetate; acetyl-CoA; 13C isotopomer; glutamate and glutamine synthesis; peripheral metabolism
Introduction
13C NMR spectroscopy is a powerful method to investigate metabolic pathways in malignant human tumors in vivo using intravenous infusion of non-radioactive 13C enriched nutrients during the surgical removal of the tumor mass. Previously, it was shown that human glioblastoma (GBM) and metastatic brain tumors were able to oxidize glucose in the citric acid cycle and only a small fraction of glucose carbons was used to derive acetyl-CoA [1,2]. Recently, using 13C NMR spectroscopy of surgically resected brain tumor tissues from cancer patients who were infused with [1,2-13C]acetate, we have identified that acetate acts as a major bioenergetic substrate in GBM and metastatic brain tumors and contributes up to 48.0% ± 4.0 of the acetyl-CoA pool [3]. Acetate-derived acetyl-CoA enters citric acid cycle via condensation with oxaloacetate (OAA) to form citrate, which further metabolized to form α-ketoglutarate (α-KG) and glutamate [4]. Glutamine is a conditionally essential amino acid that has been shown as a cellular precursor for nucleotide synthesis. Higher glutamine utilization by tumor has been found to support its growth through nucleotide de novo synthesis [5, 6]. In PET imaging, using 18F-4F-glutamine (18F-FGln), it was demonstrated that gliomas showed higher uptake of glutamine than that of the normal surrounding brain [7]. Human brain tumor expresses glutamine synthetase (GS) which converts glutamate into glutamine [1–3]. Furthermore, acetate may be metabolized by skeletal muscle, liver and renal tissues to produce key glutamine [5–7]. Although no net synthesis of glucose from acetate carbons occurs in mammalian liver, [1,2-13C]acetate influences hepatic gluconeogenesis pathways that leads to 13C label incorporation into pyruvate, lactate and glucose in the liver citric acid cycle, which upon entering citric acid cycle in the tumor contributes (via α-ketoglutarate) to glutamate and glutamine pools [8]. Therefore, a comprehensive analysis is required to accurately quantify glutamate and glutamine synthesis in the tumors that includes contributions from circulating 13C labeled compounds that are derived from peripheral metabolic activities of [1,2-13C]acetate. The purpose of the article was to demonstrate a comprehensive analysis of 13C turnover of tumor glutamate and glutamine pools under the presence of blood borne 13C-labeled glutamine, 13C-lactate and 13C-glucose during the infusion of [1,2-13C]acetate. This method can be readily applicable to other pathologic and physiologic conditions.
Materials and Methods
Infusion of [1,2-13C]acetate in brain tumor patients
Two patients diagnosed with GBM and two patients with metastatic brain tumor (a breast cancer brain metastasis and a non-small-cell lung cancer brain metastasis patient) were enrolled for this study following a clinical protocol approved by University of Texas Southwestern Medical Center Institutional Review Board. As described previously, these patients were infused with sterile and pyrogen free [1,2-13C]acetate (Cambridge Isotope Laboratories, Inc. Tewksbury, MA) at 6.0 mg/kg/min for the first 5 min, followed by 3.0 mg/kg/min for 2 hr [3]. Tumor tissue sampling and processing for NMR spectroscopic analysis have been previously described [1–3].
NMR Spectroscopy
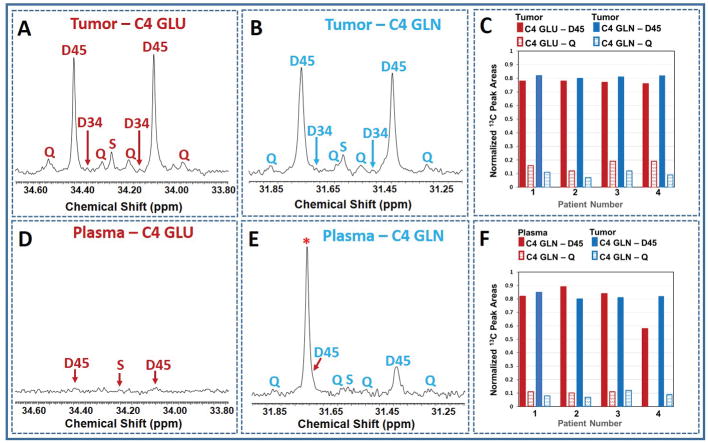
1H and 1H-decoupled carbon (13C) NMR spectra of tumor and plasma extracts were acquired at 600 MHz (1H frequency)/150 MHz (13C frequency) on a Bruker Avance NMR Spectrometer equipped with 10-mm broadband cryogenically-cooled probe (Bruker Biospin, Billerica, MA). The NMR spectra of tumor tissues and plasma used in this work have been published [3]. The lactate C3 carbon resonance at 20.8 ppm was used as an internal chemical shift reference. Peak areas of 13C signals of glutamate and glutamine were measured using ACD software (Advanced Chemistry Development, Toronto, Canada). Amounts of various 13C-13C multiplet (doublet, D45; quartet, Q) and singlet (S) peak areas are reported as a fraction normalized to its total carbon resonance area. 13C enrichment of plasma acetate at the end of the infusion period was obtained from 1H NMR spectra by measuring peak area of 13C satellites appearing due to heteronuclear 13C-1H spin-spin coupling (Figure 1A).
Figure 1. Plasma acetate 13C enrichment and carbon isotopomers of glutamate and glutamine.
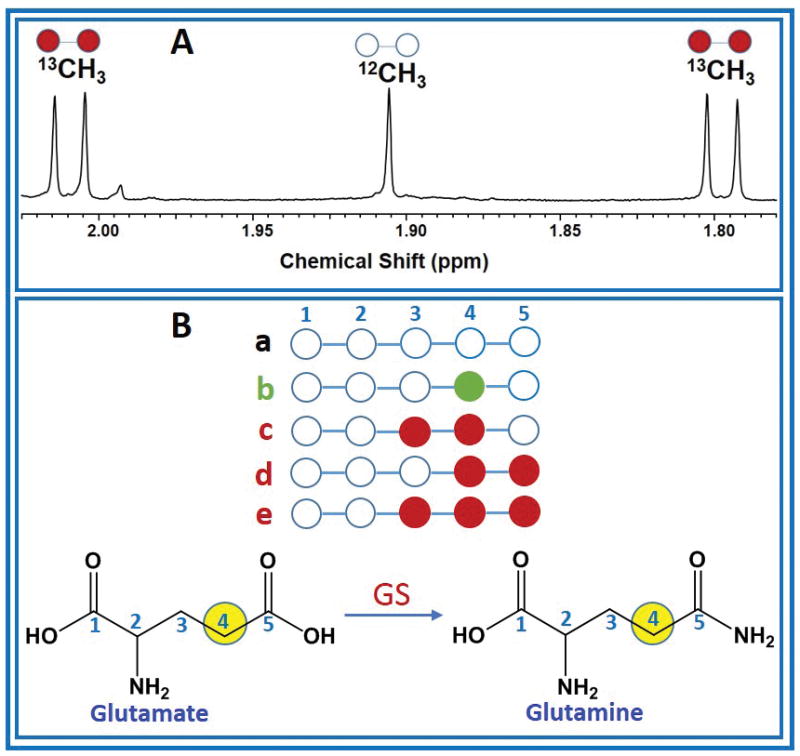
(A) 1H NMR spectrum of plasma from the breast cancer brain metastasis patient who was infused with [1,2-13C]acetate prior to the surgical removal of the tumor, showing methyl (CH3) protons of acetate, 12CH3 (open circle) refers to the methyl proton signals that are covalently bonded to 12C and 13CH3 (filled circle) refers to the methyl protons that are covalently bonded to 13C carbon which appear as two sets of doublets separated by 1JC-H = 127.10 Hz. The splitting observed in the doublet (5.94 Hz) is due to 2JC-H (B) illustration of multiple 13C isotopomers (a–e) of C4 carbon of glutamate or glutamine used in this analysis. GS, glutamine synthetase.
13C-isotopomer analysis
Various 13C isotopomers of C4 glutamate or glutamine have been pictorially represented in Figure 1B, and their fractional 13C enrichments were determined as described below:
a = the fraction of glutamine or glutamate that does not have 13C in C4 position
b = the fraction of glutamine or glutamate that has 13C only in C4 position
c = the fraction of glutamine or glutamate that has [3,4-13C] labeled isotopomer
d = the fraction of glutamine or glutamate that has [4,5-13C] labeled isotopomer
e = the fraction of glutamine or glutamate that has [3,4,5-13C] labeled isotopomer
By definition, (a + b + c + d + e) = 1, where the variables a–e, are defined as above.
[1,2-13C]acetyl-CoA generates the 13C-isotopomers, d and e in the citric acid cycle. Isotopomers b and c are derived from [2-13C]acetyl-CoA that can be generated from both [1-13C]glucose (C1S) and [3-13C]lactate (C3S). Multiple mechanisms are responsible for the generation [1-13C]glucose and [3-13C]lactate that produce [2-13C]acetyl-CoA in the tumor. [2-13C]acetyl-CoA (Fc2, fraction of acetyl-CoA enriched only in C2 methyl carbon) generates [4-13C] glutamate on the first turn and [3,4-13C]glutamate on the subsequent turns upon entering citric acid cycle. From the 13C NMR spectra of C4 glutamine or glutamate multiplets, the following 13C-13C spin-spin coupled doublets (D45), quartets (Q) and singlet 13C C4 carbon signal (S) areas, are determined. Consequently, the following four equations are derived from the experimental 13C NMR peak areas and are given below.
| Equation (1) |
| Equation (2) |
| Equation (3) |
| Equation (4) |
Solutions of the above equations yield fractional contributions of C4 glutamate and glutamine carbon isotopomers (a, b, c, d and e). In the absence of blood-borne 13C enriched nutrients due to peripheral metabolism of [1,2-13C]acetate, Fc2 should be at the level of natural abundance of 13C (1.1%).
Results and Discussion
Figure 1A shows the portion of 1H NMR spectrum of plasma from a GBM patient who was infused with [1,2-13C]acetate prior to the surgical removal of the tumor. In the spectrum, 12CH3 refers to the methyl proton signals of acetate that are covalently bonded to 12C carbon (resonating at 1.91 ppm) and 13CH3 refers to the methyl protons that are covalently bonded to 13C carbon which appear as two sets of doublets (at ~1.80 and ~2.10 ppm), separated by one-bond with a 13C-1H spin-spin coupling, 1JC-H = 127 Hz. The splitting observed within the doublet (5.94 Hz) is due to 2JC-H. This doublet patterns confirms the presence of [1,2-13C]acetate in the plasma. 13C-enrichment of plasma acetate was 88.0 ± 6.0% and its concentration at the end of the infusion period was 1.27 ± 0.22 mM. Figure 2 shows schematic diagram illustrating metabolism of infused [1,2-13C]acetate in a tumor cell. By the action of nucleocytosolic acetyl-CoA synthetase (ACSS2), [1,2-13C]acetate is converted to [1,2-13C]acetyl-CoA which enters the citric acid cycle yielding 13C-labeling at carbons 4 and 5 of α-ketoglutarate (α-KG), glutamate and glutamine during the first turn of the cycle. After multiple turns of the cycle, carbons 3, 4 and 5 of α-KG, glutamate and glutamine are labeled with 13C. Glutamine synthetase (GS), the key enzyme responsible for the conversion of glutamate to glutamine, is highly expressed in human brain tumors, supporting in situ glutamine synthesis from glutamate [1–3,11]. We have observed similar levels of doublets (D45) and quartets (Q) in glutamate and glutamine C4 carbon signals in 13C NMR spectra of tumor extracts (Figs. 3A and 3B) which provide evidence for de novo glutamine synthesis from glutamate in the tumor cells. 13C multiplet peak areas of C4 glutamate and glutamine in the tumor are given in Fig. 3C. These peak areas were used to determine fractional contributions of various C4 13C isotopomers of glutamate and glutamine as described in the methods and are summarized in the Tables 1A and B. Previously, we observed the presence of 13C-labeled glucose and lactate isotopomers in the circulation that were generated due to systemic metabolism of [1,2-13C]acetate [8]. Additionally, 13C labeled glutamine was also detected in the plasma of these tumor patients. Figures 3D and 3E show 13C NMR spectra of C4 carbon multiplet signals of plasma glutamate and glutamine of a GBM patient. Detection of prominent doubly labeled 4 and 5 carbons of glutamine 13C isotopomer (D45) in the plasma of a GBM patient (Fig. 3E) indicates that the presence of 13C labeled glutamine in the circulating blood generated due to [1,2-13C]acetate metabolism by peripheral organs. As illustrated in Fig 3D, C4 glutamate signals are very weak in the plasma. Plasma C4 glutamine (Fig. 3E) 13C-multiplet peak areas are given in Fig. 3F and are dominated by D45 with weaker quartet (Q) signals. The following are the summary of the results from these spectral data: (i) some of the blood-borne 13C labeled glutamine from the metabolism of [1,2-13C]acetate by peripheral tissues may have entered the tumor and both glutamine pools show very similar D45 and Q values, (Fig. 3F and supplementary Table S1). (ii) 13C fractional enrichment of C4 glutamate pool in the tumors was 35.90 ± 11.25% ((b+c+d+e) of tumor C4 GLU in Table 1A) whereas, this value in C4 glutamine was only 28.10 ± 6.44% ((b+c+d+e) of tumor C4 GLN in Table 1B). If the 13C-glutamine in the tumor was mainly synthesized from 13C-glutamate (Tables 1A and B), then the total 13C fractional enrichment of the C4 carbon of glutamine is expected to be less than or equal to that of its precursor, glutamate C4 carbon, assuming similar pool sizes. Our current results of lower 13C fractional enrichment of C4 glutamine (Table 1B) compared to C4 glutamate in the tumor suggests that 13C labeling from infused [1,2-13C]acetate entered glutamate pool in the tumor before it reached glutamine. ((d+e)/FE) given in Tables 1A and 1B corresponds to the portion of C4 13C fractional enrichment of glutamate and glutamine that was derived from direct utilization of [1,2-13C]acetate by tumor cells. As illustrated in Figure 2, amount of [2-13C]acetyl-CoA gives the readout of contributions from peripheral metabolism of infused [1,2-13C]acetate to the tumor. Through the following mechanisms, [2-13C]acetyl-CoA may have attributed to 13C labeling at C4 of glutamate and glutamine (C4S), in addition to background signal due to natural abundance of 13C(1.1%): (i) as we reported earlier, 13C label from [1,2-13C]acetate entering the liver citric acid cycle may enter gluconeogenesis pathways via oxaloacetate pool which led to the detection of multiple 13C labeled isotopomers including [1-13C]/[1,2-13C]glucose in the plasma [8]. [3-13C]lactate isotopomer was also detected in the plasma in these patients due to the systemic metabolism of infused acetate. When these 13C labeled isotopomers of glucose and lactate enter the tumor, subsequently they generate [2-13C]acetyl-CoA, which further yield [4-13C]glutamate/glutamine and [3,4-13C]glutamate/glutamine after first and multiple turns of the cycle respectively (Figure 2). (ii) increased flux through oxidative branch of pentose phosphate pathway (PPP) was reported in human orthotopic mouse models of GBM and renal cell carcinoma [11–13]. Here, peripherally-derived [1,2-13C]glucose in the plasma was used to measure PPP flux relative to glycolysis using the following approach: C3 lactate singlet (C3S) is produced through the combination of flux through oxidative branch of PPP and a minor contribution from natural abundance 13C signal of C3 lactate carbon (1.1%). The singlet (C3S) to doublet (D23) ratio of lactate C3 multiplet gives the measure of PPP flux relative to glycolysis (4.81 ± 0.62, Supplementary Table S2). This result indicates that activity of PPP may contribute to 13C labeling at C3 lactate which produces [2-13C]acetyl-CoA via [3-13C]pyruvate in these patient tumors. As described earlier, [2-13C]acetyl-CoA yields [4-13C]glutamate/glutamine and [3,4-13C]glutamate/glutamine after first and multiple turns of the cycle respectively. (iii) pyruvate recycling mechanism in the tumor cells generates [3-13C] and [1,2-13C]pyruvate isotopomers from [3,4-13C] and [1,2-13C]OAA isotopomers respectively, after the first turn of the citric acid cycle during the metabolism of [1,2-13C]acetate [3,14–16]. [3-13C]pyruvate further metabolized to generate [2-13C]acetyl-CoA via pyruvate dehydrogenase (PDH) which yielded [4-13C]glutamate/glutamine and [3,4-13C]glutamate/glutamine in the citric acid cycle. [1,2-13C]pyruvate generates [1-13C]acetyl-CoA, which upon entering citric acid cycle yield [5-13C]glutamate and glutamine isotopomer. Contributions from peripheral metabolism of acetate to overall C4 13C fractional enrichment of glutamate and glutamine were 6.60% ± 0.02% and 9.08 ± 0.03% respectively.
Figure 2. Metabolic model.
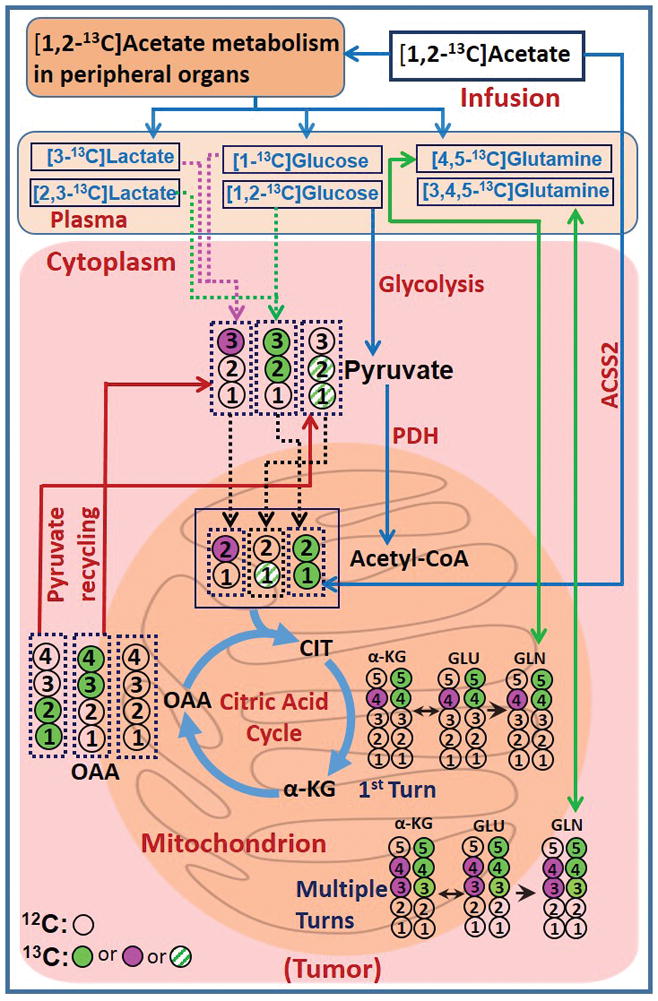
Schematic diagram illustrating the fate of the individual 13C carbon atoms from infused [1,2-13C]acetate. [4,5-13C] and [3,4,5-13C]GLU/GLN (green filled circles) were generated from acetate-derived [1,2-13C]acetyl-CoA. [4-13C] and [3,4-13C]GLU/GLN (purple filled circles) were mainly generated from blood-borne 13C labeled glucose/lactate that originated from [1,2-13C]acetate metabolism of peripheral organs. 13C labeling patterns in α-KG, GLU and GLN are shown along with the numbers representing the carbon atom positions. Abbreviations: α-KG, α-ketoglutarate; GLU, glutamate; GLN, glutamine; OAA, oxaloacetate; PDH, pyruvate dehydrogenase; ACSS2, nucleocytosolic acetyl-CoA synthetase 2. Filled circle (green or purple) refers to 13C and open circle refers to 12C carbon nuclei. Green pattern filled circles correspond to 13C isotopomers that generate 13C labeled C5 glutamate isotopomer and do not influence C4 13C fractional enrichment.
Figure 3. 13C NMR spectral data.
(A,B) and (D,E) show the 13C NMR spectra of C4 carbon of glutamate and glutamine of tumor and plasma from a GBM patient respectively. In E, * refers to an overlap of unknown carbon signal with one of the D45 GLN doublet peaks. The patient was infused with [1,2-13C]acetate prior to the resection of the tumor. Charts C and F show normalized 13C peak areas of C4 carbon multiplets of glutamine and glutamate from four brain tumor patients participated in the study. Chart C compares the peak areas between glutamate and glutamine C4 carbon multiplets in the tumor. Chart F shows the comparison of C4 GLN peak areas between plasma and tumor. S, singlet is due to both natural abundance of 13C and Fc2; D34, doublet due to 13C-13C J coupling between the carbons 3 and 4; D45, doublet arises due to 13C-13C J coupling between the carbons 4 and 5; Q, quartet is due to 13C-13C J couplings between the carbons 3,4 and 5.
Table 1A.
13C fractional enrichments of GLU C4 in the tumors. Calculation of a–e are from 13C NMR spectra of C4 GLU as described in the text (Materials and Methods). d and e correspond to the fractions that have [4,5-13C] and [3,4,5-13C] isotopomers respectively. a is the fraction that has no 13C. (b+c) together represents the contributions from the metabolism of [1,2-13C]acetate by peripheral organs to tumor GLU C4 13C pool. FE is equal to (b+c+d+e) and corresponds to fractional enrichment of GLU C4 in the tumor.
| Tumor type | a | b | c | d | e | FE | (b+c)/FE | (d+e)/FE |
|---|---|---|---|---|---|---|---|---|
| GBM | 0.758 | 0.014 | 0.002 | 0.188 | 0.038 | 0.242 | 0.066 | 0.934 |
| GBM | 0.717 | 0.024 | 0.003 | 0.222 | 0.035 | 0.283 | 0.094 | 0.906 |
| Breast metastasis | 0.537 | 0.017 | 0.006 | 0.354 | 0.087 | 0.464 | 0.050 | 0.950 |
| Lung metastasis | 0.554 | 0.019 | 0.006 | 0.339 | 0.083 | 0.446 | 0.056 | 0.944 |
Table 1B.
13C fractional enrichments of GLN C4 in the tumors. Calculation of a–e are from 13C NMR spectra of GLN C4 as described in the text (Materials and Methods). d and e correspond to the fractions that have [4,5-13C] and [3,4,5-13C] isotopomers respectively. a is the fraction that has no 13C. (b+c) together represents the contributions from the metabolism of [1,2-13C]acetate by peripheral organs to tumor GLN C4 13C pool. FE is equal to (b+c+d+e) and corresponds to fractional enrichment of GLN C4 in the tumor.
| Tumor type | a | b | c | d | e | FE | (b+c)/FE | (d+e)/FE |
|---|---|---|---|---|---|---|---|---|
| GBM | 0.762 | 0.014 | 0.002 | 0.196 | 0.027 | 0.238 | 0.065 | 0.935 |
| GBM | 0.787 | 0.026 | 0.002 | 0.171 | 0.015 | 0.213 | 0.131 | 0.869 |
| Breast metastasis | 0.659 | 0.021 | 0.003 | 0.276 | 0.041 | 0.341 | 0.071 | 0.929 |
| Lung metastasis | 0.669 | 0.023 | 0.009 | 0.271 | 0.028 | 0.331 | 0.096 | 0.904 |
Conclusion
In summary, we have illustrated an analysis to determine 13C turnovers in glutamate and glutamine pools in four brain tumor patients from the infusion of [1,2-13C]acetate prior to surgical removal of the tumor. This analysis included the contributions from 13C labeled nutrients detected in the plasma that originated from systemic metabolism of [1,2-13C]acetate. 13C-glutamine is also detected in the plasma and it was shown to have similar 13C isotopomer populations of the glutamine present in the tumor tissues. The current analysis revealed that major portion of C4 13C fractional enrichment of glutamate and glutamine (93.4 ± 0.02% in glutamate and 90.9 ± 0.03% in glutamine) was derived from [1,2-13C]acetate-derived acetyl-CoA. The analysis described here provides a simple method to quantity the relative contributions from direct utilization of acetate by tumor cells mediated via ACSS2 and peripheral metabolism of [1,2-13C]acetate through 13C labeled circulating nutrients to 13C fractional enrichment of glutamate and glutamine pool in the tumor. In addition, the presence of 13C isotopomers of glucose was utilized to measure relative flux of PPP. This current methodology can be easily extended to similar scenarios for investigating in situ metabolism of other citric acid cycle intermediates and also to other pathologic and physiologic conditions.
Supplementary Material
Acknowledgments
This study was supported by the grants from National Institute of Health (P41EB015908, RO1CA154843), Cancer Prevention Research Institute of Texas (RP140021-P2), the Donna and Kenneth R. Peak Foundation, The Kenneth R. Peak Brain and Pituitary Tumor Treatment Center at Houston Methodist Hospital, The Taub Foundation, The John S. Dunn Foundation, The Blanche Green Estate Fund of the Pauline Sterne Wolff Memorial Foundation, The Verelan Foundation, The Houston Methodist Hospital Foundation, The American Brain Tumor Association, and by many brave patients and families who have been impacted by the devastating effects of brain cancers and central nervous system disease.
References
- 1.Maher EA, Marin-Valencia I, Bachoo RM, Mashimo T, Raisane J, Hatanpaa KJ, Jindal A, Jeffrey FM, Choi C, Madden C, Mathews D, Pascual JM, Mickey BE, Malloy CR, DeBerardinis RJ. Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 2012;25:1234–1244. doi: 10.1002/nbm.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin-Valencia I, Yang C, Mashimo T, Cho S, Baek H, Yang XL, Rajagopalan KN, Maddie M, Vemireddy V, Zhao Z, Cai L, Good L, Tu BP, Hatanpaa KJ, Mickey BE, Mates JM, Pascual JM, Maher EA, Malloy CR, DeBerardinis RJ, Bachoo RM. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 2012;15:827–837. doi: 10.1016/j.cmet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mashimo T, Pichumani K, Vemireddy V, Hatanpaa KJ, Singh DK, Sirasanagadla S, Nannepaga S, Piccirillo SG, Kovacs Z, Foong C, Huang Z, Barnett S, Mickey BE, DeBerardinis RJ, Tu BP, Maher EA, Bachoo RM. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell. 2014;159:1603–1614. doi: 10.1016/j.cell.2014.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schug ZT, Voorde JV, Gottlieb E. The metabolic fate of acetate in cancer. Nat Rev Cancer. 2016;16:708–717. doi: 10.1038/nrc.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeBeradinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:345–350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tardito S, Oudin A, Ahmed SU, Fack F, Keunen O, Zheng L, Miletic H, Sakariassen PO, Weinstock A, Wagner A, Lindsay SL, Hock AK, Barnett SC, Ruppin E, Morkve SH, Johansen ML, Chalmers AJ, Bjerkvig R, Niclou SP, Gottlieb E. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat Chem Biol. 2015;17:1556–1568. doi: 10.1038/ncb3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, Carlin SD, La Rocca G, Lyashchenko S, Ploessl K, Rohle D, Omuro AM, Cross JR, Brennan CW, Weber WA, Holland EC, Mellinghoff IK, Kung HF, Lewis JS, Thompson CB. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. 2015;7:274ra17. doi: 10.1126/scitranslmed.aaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pichumani K, Mashimo T, Vemireddy V, Kovacs Z, Ratnakar J, Mickey BE, Malloy CR, DeBerardinis RJ, Bachoo RM, Maher EA. Neurochem Int. 2016;97:133–136. doi: 10.1016/j.neuint.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szczepaniak L, Babcock EE, Malloy CR, Sherry AD. Oxidation of acetate in rabbit skeletal muscle: Detection by 13C NMR Spectroscopy in vivo. Magn Reson Med. 1996;36:451–457. doi: 10.1002/mrm.1910360318. [DOI] [PubMed] [Google Scholar]
- 10.Befroy DE, Perry RJ, Jain N, Dufour S, Cline GW, Trimmer JK, Brosnan J, Rothman DL, Petersen KF, Shulman GI. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nat Med. 2014;20:98–102. doi: 10.1038/nm.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marin-Valencia I, Cho SK, Rakheja D, Hatanpaa KJ, Kapur P, Mashimo T, Jindal A, Vemireddy V, Good LB, Raisanen J, Sun X, Mickey B, Choi C, Takahashi M, Togao O, Pascual JM, DeBerardinis RJ, Maher EA, Malloy CR, Bachoo RM. Glucose metabolism via the pentose phosphate pathway, glycolysis and Krebs cycle in an orthotopic mouse model of human brain tumors. NMR Biomed. 2012;25:1234-1177–1186. doi: 10.1002/nbm.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patra KC, Hay N. The pentose phosphate pathway and cancer. Trends Biochem Sci. 2015;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buhmann AK, Schulte A, Weller J, Holz M, Mende CH, Glass R, Lamszus K. Glycolysis and the pentose phosphate pathway are differentially associated with the dichotomous regulation of glioblastoma cell migration versus proliferation. Neuro-oncol. 2016;18:1219–1229. doi: 10.1093/neuonc/now024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerdan S, Kunnecke B, Seelig J. Cerebral metabolism of [1,2-13C2]acetate as detected by in Vivo and in Vitro 13C NMR. 1990;265:12916–12926. [PubMed] [Google Scholar]
- 15.Cruz F, Scott SR, Barroso I, Santisteban P, Cerdan S. Ontogeny and cellular localization of the pyruvate recycling in rat brain. J Neurochem. 1998;70:2613–2619. doi: 10.1046/j.1471-4159.1998.70062613.x. [DOI] [PubMed] [Google Scholar]
- 16.Haberg A, Qu H, Haraldseth O, Unsgard G, Sonnewald U. In vivo injection of [1-13C]glucose and [1,2-13C]acetate combined with ex-vivo 13C nuclear magnetic resonance spectroscopy: a novel approach to the study of middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1998;18:1223–1232. doi: 10.1097/00004647-199811000-00008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.