Abstract
Context:
Carcinoma cervix is a leading cause of cancer in Indian females where 15%–60% of the cases eventually metastasize. Bone only metastasis is rare, and data on its response and survival with systemic therapy as compared to other visceral metastasis are limited.
Settings and Design:
The study design was a retrospective analysis.
Materials and Methods:
We retrospectively analyzed our data between May 2013 and April 2015 to identify the cases of bone only metastasis and visceral metastasis and tried to analyze their outcomes with paclitaxel- and carboplatin-based chemotherapy and bisphosphonates (for bone metastasis only).
Results:
Totally, 12 cases with bone only metastasis (Group 1) and 43 cases with visceral metastasis (Group 2) were identified. Most common sites of bone metastasis were vertebrae (66.67%) and pelvis (25%) while that of visceral metastasis was liver (44.18%) and lung (34.88%). Only 33.33% and 34.88% of cases in Group 1 and Group 2, respectively, could complete all six cycles of chemotherapy. Overall, response rates were 41.67% and 30.32% in Group 1 and Group 2, respectively. Median progression-free survival and overall survival (OS) were 10 months and 14 months, respectively, in Group 1 as compared to 4 months and 9 months, respectively, in Group 2. The difference in survival was statistically significant.
Statistical Analysis Used:
It was carried out by SPSS software version 20.
Conclusion:
Bone only metastasis is a rare and distinct entity with favorable outcomes as compared to visceral metastasis. However, disease remains aggressive and poor OS emphasizing the need of further research.
Keywords: Bone metastasis, cervical carcinoma, visceral metastasis
Introduction
Carcinoma cervix is the leading malignancy in Indian females in rural areas. Indian females contribute for one-fifth of the global burden of the disease each year.[1] Up to 15%–60% of all cervical carcinomas develop metastatic disease during lifetime, which is responsible for significant mortality.
Incidence of bone metastasis is reported in various series ranging from 1.1% to 18.6% of metastatic cases.[2] However, bone only metastasis remains a rare entity, and there is no data comparing its behavior to visceral metastatic disease. We hypothesized that bone only disease in carcinoma cervix may also behave differently than its counterpart with visceral metastasis as it does in certain diseases such as breast cancer.
Materials and Methods
We retrospectively analyzed data of our hospital from May 2013 to April 2015 for cases of recurrent/metastatic carcinoma cervix. We analyzed two groups of metastatic disease including bone only metastasis designated as Group 1 and visceral metastasis designated as Group 2. Cases with nonregional lymph node involvement were excluded from the study. Patient profile and risk factors for the disease, histology, baseline stage, and prior treatment received in the two groups were recorded. We also tried to analyze overall response rates (ORR) and survival outcomes after palliative computed tomography (CT) in the two groups. Survival analysis was performed by Kaplan–Meier method using log-rank test. Statistical Package for the Social Sciences 20 (SPSS Inc., 233 South Wacker Drive, 11th floor, Chicago, IL, USA) was used for analysis.
Results
We identified 170 cases of recurrent/metastatic disease during the described time frame out, of which 55 cases were eligible for our intended analysis having bone only or visceral metastasis. Twelve cases had bone only metastasis (Group 1) while 43 had visceral metastasis (Group 2). Nearly 41.66% (n = 5) of cases in Group 1 and 37.2% (n = 16) of cases in Group 2 had upfront metastasis while the rest had recurrent metastasis.
Baseline disease characteristics are shown in Table 1. Sites of visceral metastasis in Group 2 are shown in Table 2. Bone involvement in addition to visceral involvement was seen in 9.3% (n = 4) of cases in Group 2. Among Group 1, 66.67% (n = 8) of cases with vertebral involvement, 25% (n = 3) of cases with isolated pelvic bone lesions, and one case with isolated femur lesion were found. All the lesions were picked on bone scan. Seventy-five percent (n = 9) of cases in Group 1 had pain as the presenting symptom while one patient with femoral bone involvement had presented with local swelling.
Table 1.
Baseline disease characteristics
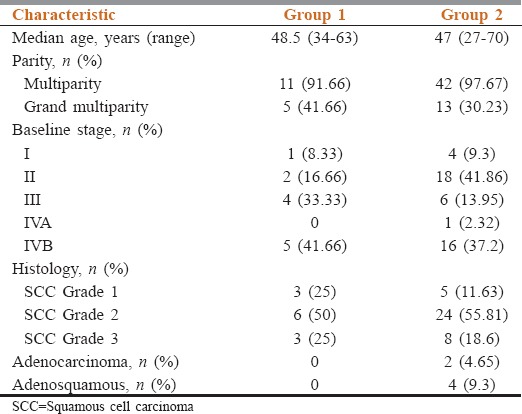
Table 2.
Sites of visceral metastasis in Group 2

Seven cases of recurrent metastatic disease in Group 1 and 27 cases in Group 2 were identified out of which, 57.1% (n = 4) in Group 1 and 70.4% (n = 19) in Group 2 had received prior CT- Median duration of relapse was 24 months for Group 1 while 18 months for Group 2.
All the patients were planned for paclitaxel 175 mg/m2 and carboplatin AUC 5 on day 1 every 21 days for six cycles. Zoledronic acid was added for bone metastatic disease at a dose of 4 mg given every 4 monthly till progression. Median numbers of CT cycles received in Group 1 were 4 while in Group 2 were 3.
ORR was 41.67% (n = 5) including 8.33% (n = 1) complete response (CR) and 33.33% (n = 4) partial response (PR) in Group 1. In Group 2, ORR was 30.32% (n = 13) with 6.98% (n = 3) CR and 23.26% (n = 10) PR. Difference in ORR was statistically not significant (P = 0.45). Stable disease was seen in 16.67% (n = 2) of Group 1 and 18.6% (n = 8) of Group 2 cases. Group 1 had superior survival outcomes. Median progression-free survival (PFS) in Group 1 was 10 months versus 4 months in Group 2 as shown in Figure 1 (P = 0.028). Median overall survival (OS) was 14 months in Group 1 versus 9 months in Group 2 as depicted in Figure 1 (P = 0.014).
Figure 1.
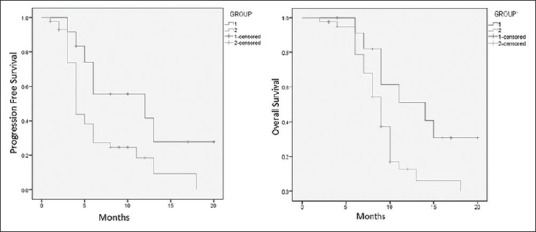
Progression-free survival and overall survival by Kaplan–Meier method
Discussion
Every year, 122,844 women are diagnosed with cervical cancer in India out of which 67,477 die from disease, recurrent/metastatic disease being a major contributor for the same.[3] Indian women are affected at an early age as evident by median age of about 48 years in our study. While analyzing histology, we identified that none of the Group 1 cases had adenocarcinoma histology whereas Group 2 also had adenocarcinoma and adenosquamous carcinoma in only 4.64% and 9.3% of cases, respectively, which are much less common than the Western literature. In a report from the National Cancer Institute, 69% of cases were squamous cell carcinoma while 25% being adenocarcinoma (including adenosquamous) histology.[4] However, Blythe et al. in their series of bone metastasis showed only 7% of the cases with adenocarcinoma.[5]
Cervical carcinoma spreads in a sequential manner first by direct invasion and later by lymphatic spread while hematogenous spread remains relatively uncommon. We found the most common sites of visceral metastasis being liver (44.18%) and lung (34.88%). Fulcher et al. in their report suggested frequency of metastasis to pelvic or para-aortic nodes (75% and 62%, respectively), lung (33%–38%), liver (33%), peritoneum (5%–27%), adrenal gland (14%–16%), intestines (12%), and skin (10%).[6]
Bone involvement in carcinoma cervix in cancer can be explained by three major modes of spread:[7] (1) direct extension from pelvic lesion, which remains the most common mode and can explain involvement of pelvic bones, (2) extension from a lymphatic focus, which can explain involvement of vertebrae from para-aortic lymph nodes and pelvic bone from external ileac lymph nodes, and (3) hematogenous spread, which can explain involvement of bones distant to primary lesion in pelvis as well as distant to lymphatic drainage of cervix.
While bone metastasis remains an uncommon occurrence, data on bone only metastasis are available only in form of a few case reports in literature. First report of bone metastasis in carcinoma cervix had come in 1938.[8] Later, Drescher reported the incidence of bone metastasis of 1.2% in his series.[9] On the other hand, Fagundes et al. have reported relatively higher incidence of bone metastasis of 16% in their series.[2]
Pain remains the most common presentation while some cases may present with local swelling or pathological fractures. Blythe et al. also found 80% of the cases presenting with pain while 5.4% of cases also had local swelling.[5] Most common site of bone metastasis in our series was a vertebra (66.67%) which was followed by pelvis. Blythe et al. also reported 52.7% vertebral involvement, followed by 30.9% pelvic bone, 18.18% long bones, and a small number of cases with metastasis to ribs, scapula, skull, and tarsal-metatarsal bones.[5]
Various CT regimens have been tried for the disease over the years ranging from single agents to combinations including platinum.[10] Role of bisphosphonates in solid tumor-related bone metastasis is proven, especially in breast cancer metastasis where it has shown to improve OS. Coleman et al. have described underlying mechanisms in their review.[11] All cases of bone metastasis in our series received bisphosphonates too.
We found no difference in ORR between the two groups with CT. However, there was significantly higher PFS and OS for Group 1. Blythe et al. have reported CR in 7.27% and PR in 43.64% in their series of bone metastasis.[5] Sixty-six percent of their 55 cases died within 6 months of diagnosis of bony metastases, 85% within 12 months, and 96% within 18 months. Matsuyama et al. also reported that 60% of their 48 cases died within 6 months.[12] However, rare cases of bone metastasis with prolonged survival beyond 5 years are also reported in literature.[13]
As the understanding of metastatic cascade has evolved, many molecular factors are identified which lead to homing of cancer cells in a specific site. This versatility probably reflects in outcomes of different subgroups of metastatic disease from the same primary. Our analysis suggests that site of metastasis is an additional prognostic factor. However, till now, major prognostic factors used in disease management are linked with histology, and there is little use of molecular markers in prognosticating this disease. Even the knowledge of molecular biology of cervical cancer largely revolves around human papillomavirus-related changes. Our outcomes confirm the hypothesis with visceral metastasis behaving more aggressively than bone only metastasis and highlights the need for further research to identify specific molecular markers which could identify and predict the possibility of bone metastasis or visceral metastasis may help to improve the understanding of biology of metastatic disease further and target therapies to improve outcomes of this aggressive disease.
Our study is the first attempt highlighting difference in biology of the different metastatic sites in carcinoma cervix. No direct comparisons in literature are available addressing this question. Although ours is a retrospective study with small number of patients, due to the rarity of bone only metastasis, larger prospective studies are unlikely to occur and systematic review of literature can help confirm these outcomes in a larger number of patients.
Conclusion
Bone only metastatic disease in carcinoma cervix is a distinct entity with favorable survival outcomes as compared to visceral disease. However, OS remains poor, emphasizing the need of further research to identify possible molecular targets to improve outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Cervical Cancer. International Agency for Research on Cancer, World Health Organization. 2015. [Last accessed on 2016 Apr 03]. Available from: http://www.globocan.iarc fr/old/FactSheets/cancers/cervix-new.asp .
- 2.Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992;24:197–204. doi: 10.1016/0360-3016(92)90671-4. [DOI] [PubMed] [Google Scholar]
- 3.ICO Information Centre on HPV and Cancer. Human Papillomavirus and Related Diseases in India (Summary Report 2014-08-22) 2014 [Google Scholar]
- 4.Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feurer EJ, et al. SEER Cancer Statistics Review, 1975-2004. Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- 5.Blythe JG, Cohen MH, Buchsbaum HJ, Latourette HB. Bony metastases from carcinoma of cervix. Occurrence, diagnosis, and treatment. Cancer. 1975;36:475–84. doi: 10.1002/1097-0142(197508)36:2<475::aid-cncr2820360226>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher AS, O’Sullivan SG, Segreti EM, Kavanagh BD. Recurrent cervical carcinoma: Typical and atypical manifestations. Radiographics. 1999;19:S103–16. doi: 10.1148/radiographics.19.suppl_1.g99oc19s103. [DOI] [PubMed] [Google Scholar]
- 7.Philipp E. Rontgenologic Dantellung of Tissue Disorders in the Uterus Cancer and its Course. Zentralbl. Zentralbl Gynaekol. 1932;56:45–58. [Google Scholar]
- 8.Albers-Schonberg H. Contributions to the Statistics of the Carcinoma Uteri. Jahrbwh der Hamburgische Staatskrankenunst. 1893-1894 [Google Scholar]
- 9.Drescher H. To the Clinic of Adenocarcinoma of the Cervix Uteri. Ceburtshilfe Frauenheilkd. 1949;9:31–7. [Google Scholar]
- 10.Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–55. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104:1059–67. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- 12.Matsuyama T, Tsukamoto N, Imachi M, Nakano H. Bone metastasis from cervix cancer. Gynecol Oncol. 1989;32:72–5. doi: 10.1016/0090-8258(89)90853-6. [DOI] [PubMed] [Google Scholar]
- 13.Tsubamoto H, Inoue K, Ukita Y, Ito Y, Kanazawa R. Long-term remission after multiple bone metastases following cervical cancer: A case report. Gynecol Oncol Case Rep. 2013;5:22–4. doi: 10.1016/j.gynor.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


