Abstract
Background:
Retinoblastoma (Rb) is the most common primary intraocular tumor of infancy and childhood. Survivors’ ocular and visual problems and increased risk for subsequent malignancy are well documented, but data on long-term health status of Rb survivors are limited, this being particularly true for India.
Methodology:
Children who had completed treatment for Rb at least 2 years ago before and were under follow-up at the after cancer treatment clinic were evaluated.
Results:
In our series of 213 patients, the median age was 29 months, there was a male preponderance, and majority had unilateral disease. Enucleation was done in almost three-fourth and 3% underwent bilateral enucleation. Majority of the patients received chemotherapy, and few received radiation. Growth was affected in about one-third and majority were those who had received radiation. Diminished vision was noticed in about one-sixth. Orbital hypoplasia and contracted socket were seen in 14.1% cases. 2.7% were hearing impaired. About one-sixth had a global intelligence delay. Second neoplasms were seen in 0.01%. No other abnormalities were seen.
Conclusions:
Common late effects in our Rb survivors include diminished vision in the salvage eye, intellectual disability, and contracted socket; there is a need for timely institution of prosthesis to avoid late effects such as hypoplasia, contracted sockets, and better cosmesis and enhanced self-esteem. Second neoplasm is a concern. Lifelong follow-up and counseling of a healthy lifestyle are needed for Rb survivors.
Keywords: Children, long-term follow-up, retinoblastoma
Introduction
Retinoblastoma (Rb) is the most common primary intraocular tumor of infancy and childhood, originating in embryonic neural retina. When treated early and appropriately, it has a high-survival rate (>90%) with lesser morbidities, this being particularly true for developed countries where the tumor is most often diagnosed in intraocular stage.[1] The scenario in developing countries is different in that the burden of the disease is more and the survival rates are less; high disease burden has been seen in Latin America, Africa, and Asia including India.[2,3,4] It has an incidence of 1 in every 20,000 live births. India sees about 1500 new cases/year, i.e., 33% of the global burden. About 90% cases are diagnosed by age 3–4 years and 98% by 5 years. Bilateral disease is diagnosed earlier then unilateral disease. It contributes to 4% of all pediatric cancers.
With refinements in diagnostics and advances in therapeutics and supportive care, the overall survival of childhood malignancy has increased over the past few decades; this is true for Rb also.[5,6] This is, however, at the cost of increased morbidity in the form of various late effects of cancer treatment. Late effects appear months or years after completion of treatment (ACT) and occur secondary to the treatment that is given to a cancer/impact that the entire treatment process has on the child. For a child with Rb who would be treated with either surgery (enucleation), chemotherapy (vincristine, carboplatin, etoposide), or radiation alone or in combinations; the late effects could be varied. These vary from contracted socket/impaired orbital growth, cataract, midfacial hypoplasia, neurotoxicity, hearing impairment, deficits in intellect, growth impairment, visual compromise, psychosocial deficits, second neoplasm, or rarely myocardial dysfunction or impairments in fertility. These children need a systematic follow-up after the treatment is over to evaluate for these late effects. They need to be evaluated for primary disease, relapse, and eye care. Long-term follow-up focuses on supporting children to deal with life after cancer treatment and integrate them into society. Genetic counseling is an important integral component of not only acute but also long-term care. This aspect of cancer care after treatment care is provided ACT clinic at our center.
While survivors’ ocular and visual problems and increased risk for subsequent malignancy particularly in those with a genetic form of disease are well documented, data on long-term health/health status of Rb survivors are limited, this being particularly true for India.[6] We report data on long-term health status from one of the largest series (213) Rb survivors being followed at our center. Late treatment-related complications justify the long-term follow-up of childhood Rb survivors.
Methodology
Archive of patients who completed therapy and 2-year follow-up attending ACT clinic was explored for demographic and clinical details, imaging findings, histopathology, treatment, and long-term effects of Rb All information was entered in a predesigned pro forma. Patients were treated as per the institutional protocol with multimodal therapy.
Cumulative dose of chemotherapy and radiotherapy was calculated. Details of surgery were recorded. Growth was recorded systematically on growth charts. Evaluation for late effects of chemotherapy, surgery, and radiation was done with appropriate investigations and evaluation (contracted orbital socket, cataract, midfacial hypoplasia, hearing and intelligence assessment, examination for neurotoxicity, systemic examination for occurrence of second neoplasm). Genetic and counseling for healthy lifestyle are done at the ACT clinic. These patients and their families are supported to deal with life after cancer treatment as the child and the family need to be integrated into society. Patient education material is provided to them.
The grading of late toxicity was carried according to Radiation Therapy and Oncology Group late morbidity scoring criteria, respectively. Only those patients were included who had received at least one treatment modality (radical or palliative surgery, radiotherapy, chemotherapy alone, or in combination). Descriptive statistics was used for describing demographic and clinical characteristics.
Results
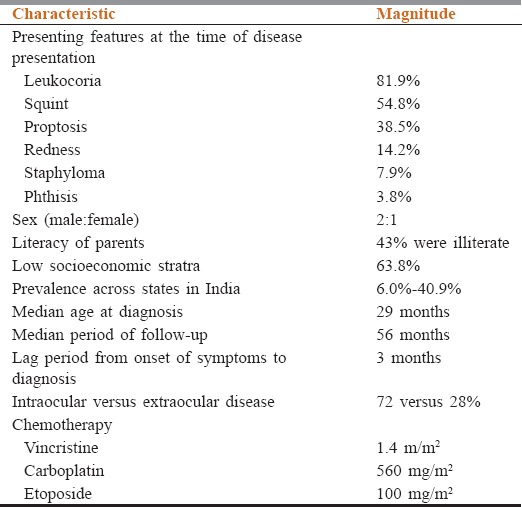
Two hundred and thirteen pediatric Rb survivor patients treated at our center for RB were evaluated for late treatment-related complications. Demographic details and presenting features were identified [Table 1].
Table 1.
Demographic and clinical features of retinoblastoma survivors
Treatment
87.2% patients received standard vincristine, etoposide, and cyclophosphamide chemotherapy (vincristine1.5 mg/sqm, carboplatin560 mg/sqm, and etoposide100 mg/sqm). The median dose of external beam radiation therapy used was 40–44 Gy in 20–22 fractions. Bilateral disease was observed in 24 patients.
Enucleation was done in 77.4% (n = 165). Prosthesis was used by only 41.4% (70/165) patients who underwent enucleation.
Problems in survivors
General
Growth was affected in 29% cases (50/193). The height in these children was less than third centile according to sex-matched Center for Disease Control growth charts. Hypoplasia/contracted socket/midfacial hypoplasia was seen in 14.1% of cases. These were children who received radiation after enucleation and chemotherapy.
Vision and hearing
Vision assessment was done in 143 Rb survivors. Diminished vision was seen in 15.8% cases (27/143). Of the children with diminished vision, 3% children with bilateral Rb underwent bilateral enucleation and are blind. Cataract was seen in 2 survivors. Hearing evaluation was done by pure tone audiometry alone or along with brainstem evoked response audiometry (brain stem evoked responses). Impairment was seen in 2.7% cases. Both children underwent enucleation and received chemotherapy. None of these children received radiation as part of treatment.
Intelligence/psychosocial deficits
Intelligence was assessed by Malin's Intelligence Scale for Indian Children and Vineland Social Maturity Scale. Global delay was found in 17% cases. 78.6% childhood Rb survivors are attending school. Psychosocial assessment was done using children apperception test. Self-esteem was found to be specially affected, particularly in female adolescents. They resorted to using dark glasses or change in hairstyle to hide the enucleated/deformed socket. Majority parents were well adjusted to society. Neurotoxicity attributed to vincristine use was not seen in our set of RB survivors.
Second neoplasms and relapse
Relapse requiring systemic chemotherapy was seen in 3 cases. Mortality was seen in two patients: one child died from progressive disease and one died from causes unrelated to RB, had hemihypertrophy, developmental delay, and failure to thrive. Second malignancy was seen in three of the 231 patients: one was a male child with bilateral Rb, underwent bilateral enucleation, and developed acute myeloid leukemia after 8 years of follow-up. The other child developed Hodgkin lymphoma as a second neoplasm. The third was a child with chondrosarcoma.
Other effects
Other late effects observed included dry eye and discharged from the enucleated eye. Cataract attributed to external beam radiotherapy was seen in two of our patients. Gonadal/renal/cardiac dysfunction was not seen in our set of patients. None of the patients reported any symptom of neurotoxicity attributable to vincristine exposure.
Discussion
Late treatment-related complications justify the long-term follow-up of childhood Rb survivors. Patients who receive external beam radiation are at increased risk for secondary tumors, optic neuropathy, and retinopathy. Dry eye or cataracts may occur secondary to radiation which may affect growing orbital bones producing facial hypoplasia and contracted socket. Pituitary dysfunction may occur.
In our series of 213 patients, the median age was 29 months, there was a male preponderance, and majority had unilateral disease. Majority of the patients received chemotherapy and few received radiation. Enucleation was done in almost three-fourth of patients. Growth was affected in about one-third of patients and majority were those who had received radiation. Hypoplasia and contracted socket were seen in 14.1% cases. Diminished vision was there in about one-sixth and 3% underwent bilateral enucleation. 2.7% were hearing impaired. About one-sixth had a global intelligence delay. Second neoplasms were seen in 0.01%. No other abnormalities seen.
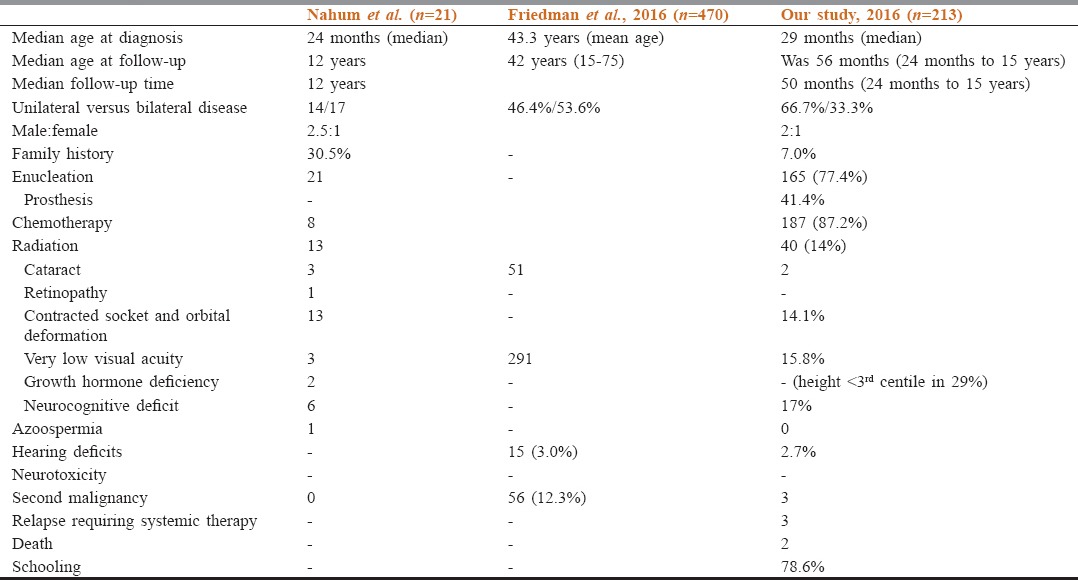
Friedman et al. evaluated 470 RB survivors through a comprehensive questionnaire adapted from Childhood Cancer Survivor Study Surveys. Chronic medical conditions were classified using the national cancer Institute's Common Terminology Criteria for adverse events. At a median follow-up of 42 years, 86.6% of survivors of RB had at least one condition and 71.1% had a severe/life-threatening (Grade 3–4) condition. Survivors of Rb had an increased risk of chronic condition compared to non-Rb controls. After excluding ocular conditions and second malignant neoplasms (SMNs), this excess risk was found in those with bilateral disease.[6]
Orbital growth is somewhat diminished after enucleation; however, the impact of enucleation on orbital volume may be less after placement of an orbital implant. It was surprising to note that prosthesis was used in 41.4% cases who underwent enucleation. Many families were not accepting the concept of prosthesis and often did not come at the appropriate time for insertion which is about 6 weeks’ postenucleation. It was also noticed that prosthesis is often ill-fitting, occasionally the implant gets dislodged requiring hospitalization for reinsertion. Customized prosthesis which is superior cosmetically is often not affordable by many families. In a study by Nahum et al., enucleation and postradiotherapy-contracted socket were seen in 1/21, radiation-induced cataract was seen in 3/21, and orbital deformation due to radiation bone atrophy was moderate severe in 12 patients.[7] Enucleation often causes psychosocial deficits: low self-esteem, limited social function, and limited educational attainment.
Patients with Rb demonstrate a variety of long-term visual field defects after treatment for their intraocular disease. These defects are related to tumor size, location, and treatment method.[8] One study of visual acuity after treatment with systemic chemotherapy and local ophthalmic therapy was conducted in 54 eyes in 40 children. After a mean follow-up of 68 months, 27 eyes (50%) had a final visual acuity of 20/40 or better, and 36 eyes (67%) had final visual acuity of 20/200 or better. In a study by Nahum et al., very low visual acuity postradiotherapy was seen in 3/21 patients.[7] In our study, diminished and absent vision was seen in 15.8% and 3.0% cases, respectively. The limitation in our study is that visual field defects and refractive error details are not available. Our assessment has been limited to assessment of gross vision. Cataract is a late effect seen in Rb patients and is most often attributed to radiotherapy. In a study by Nahum et al. and Freidman et al., cataract was seen in 3/21 and 51/470, respectively. Visual dysfunction was seen in 291/470 Rb survivors in a study by Friedman et al. where it was seen that 190 survivors were legally blind in 1 eye and 101 were legally blind in both eyes.[6,7]
Hearing deficit in our study population was seen in 2.7% cases because systemic carboplatin is now commonly used in the treatment of Rb; concern has been raised about hearing loss related to therapy. While an analysis of 164 children treated with six cycles of carboplatin-containing therapy (18.6 mg/kg per cycle) showed no loss of hearing among children who had a normal initial audiogram,[9] another series documented hearing loss in 17% of patients.[10] Age younger than 6 months at the time of treatment and higher carboplatin systemic exposures appear to correlate with an increased risk of otologic toxic effects.[10,11] Friedman et al. evaluated a cohort of 470 Rb survivors where hearing deficit was seen in 3% survivors (15/470).
Gonadal dysfunction is well described with many solid tumors, but not with Rb. Azoospermia was reported in one patient who received cyclophosphamide and vincristine as part of chemotherapy by Nahum et al.[7] Our patients did not receive cyclophosphamide as a chemotherapeutic drug for RB and gonadal dysfunction was not seen in our set of patients [Table 2].
Table 2.
Comparative data of long-term follow-up of retinoblastoma survivors
The occurrence of neuropsychological deficits is multifactorial being related to radiation at a young age, visual deficit, psychosocial issues directly related to treatment and survivorship, physical aspects due to growth retardation, orbital deformation, and enucleation. Nahum et al. reported psychosocial deficits in 6/21 pts.[7] In our patient cohort, psychosocial issues were occasionally seen more so in the girl child where self-esteem was affected consequent to enucleation, prosthesis use and orbital deformation.
Patients with heritable Rb have an increased incidence of second neoplasms. The most frequent secondary malignant occurring within the radiation field are osteosarcoma, fibrosarcoma, and other spindle cell sarcomas. Second neoplasm occurring outside the radiation field includes osteosarcoma and soft tissue sarcoma. In our study, 3 patients suffered from a malignant neoplasm as described.
In a study by Marees et al., long-term cause-specific mortality among 998 Dutch Rb survivors diagnosed from 1862 to 2005 was done. After a median follow-up of 30.8 years, only cause-specific mortality for second malignancies among hereditary Rb survivors was statistically significantly increased by 12.8-fold. Risk of death from second malignancies among nonhereditary survivors was not increased. Mortality rates of second malignancy among hereditary patients were nonsignificantly elevated with 1.6-fold for treated with radiotherapy, compared to those treated otherwise. Standardized mortality ratios for second malignancy among hereditary patients increased during the first three decades after Rb diagnosis. Whereas these risks decreased after three decades, the absolute excess risk increased significantly, up to 23.2 excess cases per 1000 patients/year after five decades of follow-up. Fifty years after Rb diagnosis, the cumulative mortality from any second malignancy was 17.3% for hereditary patients. Friedman et al. evaluated 470 Rb survivors for occurrence of SMN. The study reported that 58 RB survivors had at least 1 pathologically confirmed SMN (Grade 3–4) at a median follow-up of 40.5 years.[12]
Very long-term follow-up of Rb patients revealed an emerging excess risk of mortality in hereditary Rb survivors. This implies that lifelong follow-up is needed, whereas at the same time, patients and their physicians must be alerted to the increased second malignancy risks.
Conclusions
Rb is the most common intraocular childhood tumor. In the heritable form, tumors are often bilateral and survivors have a greatly increased risk both for a second malignancy and for having children with Rb. There is a need for early diagnosis and prompt treatment to salvage the globe and vision. This tumor has a potential for over 95% curable if treated in early stages. Long-term follow-up of RB survivors is important to identify late effects associated with treatment and occurrence of a second neoplasm. Common late effects in our RB survivors include diminished vision, intellectual disability, and contracted socket; there is a need for timely institution of prosthesis to avoid late effects such as hypoplasia, contracted sockets, and better cosmesis and enhanced self-esteem. Second neoplasm is a concern. Lifelong follow-up and counseling of a healthy lifestyle are needed for Rb survivors.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.MacCarthy A, Draper GJ, Steliarova-Foucher E, Kingston JE. Retinoblastoma incidence and survival in European children (1978-1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42:2092–102. doi: 10.1016/j.ejca.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 2.Bowman RJ, Mafwiri M, Luthert P, Luande J, Wood M. Outcome of retinoblastoma in east Africa. Pediatr Blood Cancer. 2008;50:160–2. doi: 10.1002/pbc.21080. [DOI] [PubMed] [Google Scholar]
- 3.Kao LY, Su WW, Lin YW. Retinoblastoma in Taiwan: Survival and clinical characteristics 1978-2000. Jpn J Ophthalmol. 2002;46:577–80. doi: 10.1016/s0021-5155(02)00546-4. [DOI] [PubMed] [Google Scholar]
- 4.Chawla B, Hasan F, Azad R, Seth R, Upadhyay AD, Pathy S, et al. Clinical presentation and survival of retinoblastoma in Indian children. Br J Ophthalmol. 2016;100:172–8. doi: 10.1136/bjophthalmol-2015-306672. [DOI] [PubMed] [Google Scholar]
- 5.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975-2009 (vintage 2009 Populations) [Last accessed on 2017 Sep 20]. Available from: http://www.seer.cancer.gov/csr/1975_2009_pops09)/
- 6.Friedman DN, Chou JF, Oeffinger KC, Kleinerman RA, Ford JS, Sklar CA, et al. Chronic medical conditions in adult survivors of retinoblastoma: Results of the Retinoblastoma Survivor Study. Cancer. 2016;122:773–81. doi: 10.1002/cncr.29704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nahum MP, Gdal-On M, Kuten A, Herzl G, Horovitz Y, Weyl Ben Arush M. Long-term follow-up of children with retinoblastoma. Pediatr Hematol Oncol. 2001;18:173–9. doi: 10.1080/08880010151114769. [DOI] [PubMed] [Google Scholar]
- 8.Abramson DH, Melson MR, Servodidio C. Visual fields in retinoblastoma survivors. Arch Ophthalmol. 2004;122:1324–30. doi: 10.1001/archopht.122.9.1324. [DOI] [PubMed] [Google Scholar]
- 9.Lambert MP, Shields C, Meadows AT. A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy. Pediatr Blood Cancer. 2008;50:223–6. doi: 10.1002/pbc.21155. [DOI] [PubMed] [Google Scholar]
- 10.Qaddoumi I, Bass JK, Wu J, Billups CA, Wozniak AW, Merchant TE, et al. Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol. 2012;30:1034–41. doi: 10.1200/JCO.2011.36.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leahey A. A cautionary tale: Dosing chemotherapy in infants with retinoblastoma. J Clin Oncol. 2012;30:1023–4. doi: 10.1200/JCO.2011.39.4254. [DOI] [PubMed] [Google Scholar]
- 12.Marees T, van Leeuwen FE, de Boer MR, Imhof SM, Ringens PJ, Moll AC. Eur J Cancer. 2009;45:3245–53. doi: 10.1016/j.ejca.2009.05.011. [DOI] [PubMed] [Google Scholar]


