Abstract
Abnormal DNA methylation patterns have been demonstrated to be associated with the pathogenesis of Alzheimer's disease (AD). The present study aimed to identify differential methylation in the superior temporal gyrus (STG) of patients with late-onset AD based on epigenome-wide DNA methylation data by bioinformatics analysis. The genome-wide DNA methylation data in the STG region of 34 patients with late-onset AD and 34 controls without dementia were recruited from the Gene Expression Omnibus database. Through systemic quality control, differentially methylated CpG sites were determined by the Student's t-test and mean methylation value differences between the two conditions. Hierarchical clustering analysis was applied to assess the classification performance of differentially methylated CpGs. Functional analysis was performed to investigate the biological functions of the genes associated with differentially methylated CpGs. A total of 17,895 differentially methylated CpG sites were initially identified, including 11,822 hypermethylated CpGs and 6,073 hypomethylated CpGs. Further analysis examined 2,211 differentially methylated CpGs (covering 1,991 genes). AD subjects demonstrated distinctive DNA methylation patterns when compared with the controls, with a classification accuracy value of 1. Hypermethylation was mainly detected for genes regulating the cell cycle progression, whereas hypomethylation was observed in genes involved in transcription factor binding. The present study demonstrated widespread and distinctive DNA methylation alterations in late-onset AD. Identification of AD-associated epigenetic biomarkers may allow for the development of novel diagnostic and therapeutic targets.
Keywords: Alzheimer's disease, DNA methylation, superior temporal gyrus, epigenetic biomarkers
Introduction
Alzheimer's disease (AD) is a chronic degenerative brain disorder and the leading cause of dementia in the elderly (1,2). AD is characterized by gradual neuronal death, memory loss and impaired cognitive ability, and contributes to 60–70% of dementia cases (2). Current advances are able to temporarily improve symptoms, while no effective treatment is available that can cure AD or reverse the progressive course (3). Understanding the risk factors for AD contributes to the investigations on reducing the disease occurrence. The vast majority of patients with AD have late-onset disease, and the susceptible risk is determined by complex mechanisms (2,4).
Previous studies have revealed the roles of genetic, environmental and epigenetic factors in AD risk (4–6). Epigenetics refers to DNA modifications that alter gene expression and phenotype without causing changes to the nucleotide sequences (7). As the best-studied epigenetic modification, DNA methylation is closely associated with several key cellular processes, including cell differentiation, gene regulation and genomic imprinting (8–10). Several lines of evidence has indicated the effect of DNA methylation on the pathogenesis of AD (11–13). Furthermore, the genome-wide methylation profiling technique has been applied for the characterization of methylation patterns across the genome, and has been considered as a reliable tool in identifying methylation differences associated with complex diseases (13,14).
Numerous studies have focused on the identification of specific brain regions that are particularly vulnerable throughout the progression of AD (15–17). Haroutunian et al (18) performed an extensive study on the transcriptional vulnerability involved in the progression of late-onset AD across 15 brain regions, and observed that the superior temporal gyrus (STG) region presented significant transcriptional abnormalities. Thus, the present study focused on the STG region of patients with late-onset AD. Recently, Watson et al (19) performed a genome-wide DNA methylation profiling in the STG region of patients with late-onset AD, and focused on the identification of differentially methylated regions. In the present study, the publicly available DNA methylation data of the study by Watson et al (19) were used to extract AD-associated methylated genes and identify the underlying biological processes impacted by hypomethylated and hypermethylated genes. Furthermore, epigenetic biomarkers in the STG region associated with AD risk were identified in the current study, and the findings may contribute to the development of novel diagnostic and therapeutic targets for late-onset AD.
Materials and methods
Genome-wide DNA methylation data
The genome-wide DNA methylation data of late-onset AD were obtained from the Gene Expression Omnibus database (GEO; https://www.ncbi.nlm.nih.gov/geo/), under the GSE76105 accession number (19). The current study was performed using the Illumina Infinium Human Methylation 450 array platform (Illumina, Inc., San Diego, CA, USA). The data analysed from the database included tissue samples obtained from the STG region of 34 patients with confirmed late-onset AD and 34 controls without dementia. Detailed information of these tissue samples is described in a previous study (19).
Prior to analysis, the raw DNA methylation data were subjected to a rigorous preprocessing procedure following the method developed by Huynh et al (20). Probes were removed from the data according to the following criteria: i) Single nucleotide polymorphisms within 5 bp upstream of the targeted CpG; ii) minimum allelic frequency of <0.05; iii) probes on X and Y chromosomes; and iv) cross-hybridising probes. Subsequent to applying the exclusion criteria, a total of 424,497 CpGs were selected and used for subsequent analysis. These remaining CpGs were quantile normalized using the lumi package in R software (21) and the beta-mixture quantile method (22).
Differential methylation analysis
In the dataset, the methylation values for individual CpGs in each sample were expressed as β-values. β-value is a quantitative measure of methylation for each CpG site, ranging between 0 (completely unmethylated site) to 1 (fully methylated site). Initially, the β-values of individual CpG sites were obtained in AD subjects and controls, respectively. Next, the mean β-value difference of each CpG between two conditions was calculated, and CpG sites with an absolute mean β-value difference of >0.05 were included into the study. For each probe, β-values in AD subjects were compared against the controls using the Student's t-test. The CpGs with an absolute mean β-value differences of >0.05, as well as P<0.05, were considered to be differentially methylated sites.
Previous studies indicated that methylation alterations rarely exist in genomic regions at the extreme ends of methylation values (<0.2 or >0.8), but are preferentially detected in genomic locations at intermediate methylation levels, which is a range associated with active distal regulatory regions, such as enhancers (20,23). Thus, in order to reduce the number of non-variable CpGs and improve the statistical power of subsequent analyses, the CpG sites with β-values of >0.8 and <0.2 in all samples were removed from the current study. In addition, it is reported that numerous differentially methylated CpGs with a β-value difference of <0.2 may be difficult to reproduce by alternative methodologies or in replication studies (24). Therefore, only CpGs that had a mean β-value difference of ≥0.2 were retained in the current study.
Hierarchical clustering analysis
Hierarchical clustering is a method of cluster analysis that seeks to build a hierarchy of clusters in data mining and statistics. Theoretically, samples with similar methylation profiles can be clustered together. To assess the classification performance of differentially methylated CpGs, a hierarchical clustering analysis was applied using the Euclidian distance and average linkage criteria (25). Ideally, the samples should be classified into two distinct clusters, namely the AD subjects and controls. In order to assess the classification efficiency, the accuracy was measured, which is a fraction of correctly classified samples over all samples, according to the following formula: Accuracy=(TP+TN)/(TP+FP+TN+FN). TP represents the number of positive samples correctly predicted as positive, TN is the number of negative samples correctly predicted as negative, FP is the number of negative samples incorrectly predicted as positive, and FN represents the number of positive samples incorrectly predicted as negative.
Functional enrichment analysis
To further investigate the biological functions of the genes associated with differentially methylated CpGs, functional enrichment analysis was performed based on Gene Ontology (GO; http://www.geneontology.org) and Kyoto Encyclopedia of Genes and Genomes database (KEGG; http://www.genome.jp/kegg/pathway.html). In addition, enrichment analysis was conducted using the online software Database for Annotation, Visualization and Integrated Discovery (DAVID 6.8; https://david.ncifcrf.gov). Fisher's test was utilized to measure the significance of GO terms and biological pathways. The P-values were adjusted using Benjamini-Hochberg false discovery rate (FDR). Statistically significantly different terms were determined under the criterion of P<0.01.
Results
Differential methylation analysis
The present study focused on comparing the DNA methylation in the STG region of 34 late-onset AD subjects with that in 34 controls without dementia. Following quality control processing of the DNA methylation data, probes were removed under the filtering criteria described earlier, and methylation data for 424,497 autosomal CpGs in each of the 68 individuals were used in subsequent differential methylation analysis. Under the criteria of absolute mean β-value differences between AD subjects and controls of >0.05, as well as P<0.05, a total of 17,895 differentially methylated CpG sites covering 8,678 genes were initially identified. Among these differentially methylated CpGs, a total of 11,822 CpGs were hypermethylated and 6,073 CpGs were hypomethylated. A volcano plot exhibiting the distribution of differentially methylated CpGs is shown in Fig. 1.
Figure 1.
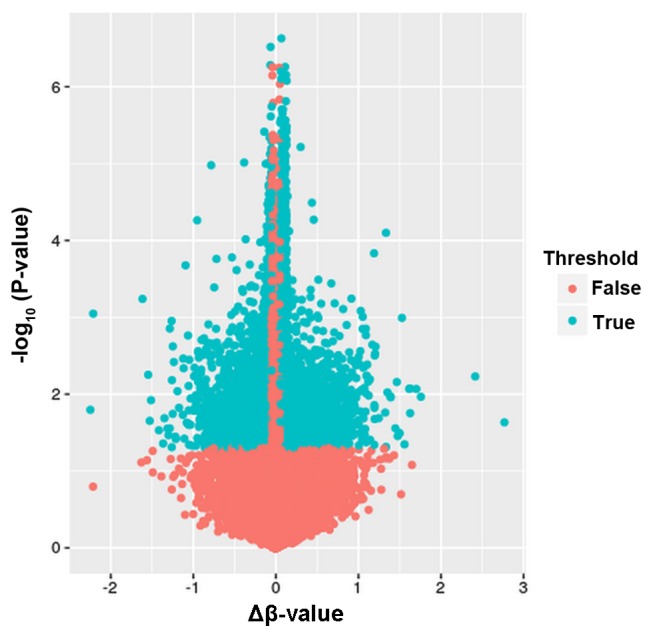
Volcano plot exhibiting the distribution of differentially methylated CpGs in patients with AD compared with those in control individuals. The x-axis represents the mean methylation differences between AD patients and controls, while the y-axis represents the log transformed P-values. AD, Alzheimer's disease.
Since methylation changes were preferentially detected in genomic regions at intermediate methylation levels, further selection was performed to reduce non-variable CpGs and improve the statistical power. By removing CpGs at the extreme ends of methylation values (β-values of >0.8 and <0.2), 2,225 differentially methylated CpGs covering 2,001 genes were identified. Furthermore, differentially methylated CpGs with a β-value difference of <0.2 were considered to be difficult to reproduce and excluded. Thus, only differentially methylated CpGs with a mean β-value difference of ≥0.2 were extracted, and 2,211 differentially methylated CpGs (covering 1,991 genes) were finally identified.
Hierarchical clustering analysis
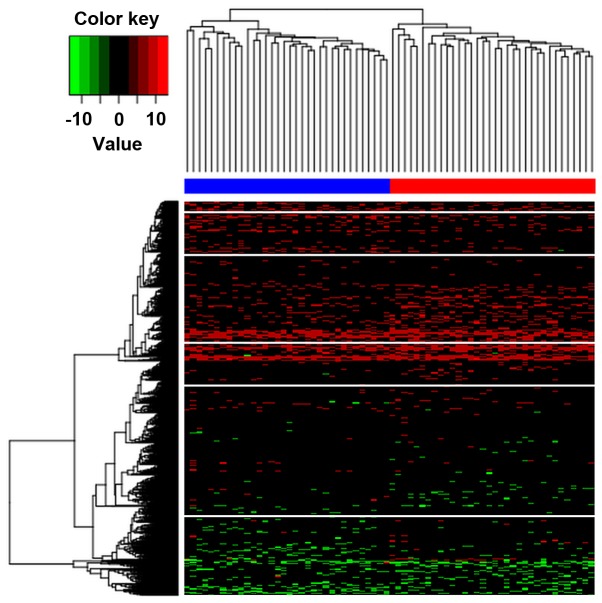
Hierarchical clustering was implemented to determine whether the identified differentially methylated CpGs can be applied to distinguish AD subjects from controls. The results illustrated that these two conditions in the AD and control groups exhibited distinctive DNA methylation patterns (Fig. 2). In addition, it was observed that the samples were classified into two distinct clusters by these differentially methylated CpGs with an accuracy of 1.
Figure 2.
Hierarchical clustering analysis of differentially methylated CpGs between Alzheimer's disease samples and controls. The differentially methylated CpGs were able to distinctly distinguish between the two types of samples.
Functional enrichment analysis
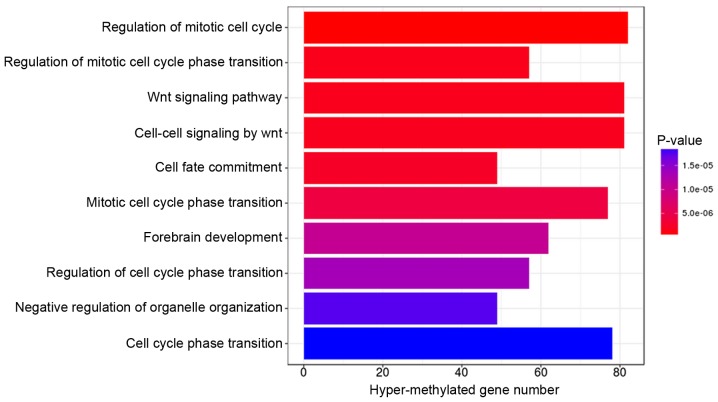
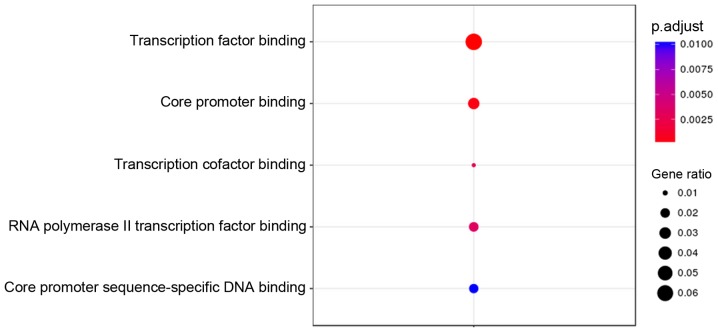
GO terms and KEGG pathway enrichment analysis for genes associated with the 2,211 differentially methylated CpGs were performed using the DAVID online tool. Under the criterion of P<0.01, hypermethylated genes were significantly enriched in 10 GO terms, with the top three (ranked according to their P-value) involved in the regulation of the mitotic cell cycle, regulation of the mitotic cell cycle phase transition, and the Wnt signaling pathway (Fig. 3). The hypomethylated genes were significantly enriched in 5 GO terms, with the top one associated with transcription factor binding (Fig. 4). However, KEGG pathway analysis demonstrated that no statistically significant pathways were enriched by both the hypermethylated and hypomethylated genes under an FDR-adjusted P-value of <0.01.
Figure 3.
Gene oncology analysis of genes associated with hypermethylated CpGs in Alzheimer's disease subjects.
Figure 4.
Gene oncology analysis of genes associated with hypomethylated CpGs in Alzheimer's disease subjects.
Discussion
Recently, epigenetic modification in diseases has attracted increasing attention from researchers. Late-onset AD results from the interactions of multiple genetic and environmental factors, along with the disruption of epigenetic mechanisms controlling gene expression (26). Abnormal DNA methylation patterns have been observed to be associated with the pathogenesis of AD. In the present study, the epigenome-wide DNA methylation patterns in the STG of patients with late-onset AD were assessed, and differentially methylated genes and biological functions were identified under the AD condition, which may contribute to the development of novel targets for late-onset AD diagnosis and therapy.
The present study revealed significant alterations to the DNA methylation profiles in AD, with 2,211 CpGs displaying significantly differential methylation levels. The hierarchical clustering results illustrated that AD subjects exhibited distinctive DNA methylation patterns compared with the controls, which was consistent with the observations of a previous study (19). Initially, the differential methylation identification demonstrated a strong bias to hypermethylated alterations in late-onset AD (11,822 hypermethylated and 6,073 hypomethylated CpGs). Given that the greatest risk factor for late-onset AD is older age (27), it may be hypothesized that CpG methylation increased with age. Previous studies also indicated that CpG methylation in AD was significantly associated with age (13,28).
To further examine the functions of genes associated with differentially methylated CpGs, GO and KEGG analysis were performed in the present study. The results revealed that genes associated with hypermethylated CpGs were enriched in 10 GO terms, 6 of which were cell cycle-associated biological process. The cell cycle involves a series of events that lead to cell division and DNA replication to produce two daughter cells. Cell cycle progression ensures a correct inheritance of epigenetic modifications. A previous study has indicated that epigenetic modifications arising within the cells of an individual are transmitted through multiple cell cycles, which is crucial in maintaining a given chromatin state (29). In the current study, it was identified that genes associated with hypermethylated CpGs participated in two Wnt-associated biological processes, including the Wnt signaling pathway and cell-cell signaling by Wnt. The Wnt signaling pathway is a vital signal transduction pathway involved in various biological activities. Increasing evidence suggested the protective effects of the Wnt pathway on synaptic modulation and cognitive processes in neurodegenerative diseases, implying the potential role of aberrant Wnt signaling in cognitive impairment (30,31). Riise et al (32) also confirmed an aberrant Wnt signaling pathway in medial temporal lobe structures of neurodegenerative AD samples. Recent studies have further affirmed the potential role of the Wnt signaling pathway as a therapeutic target for the treatment of AD (33,34).
In the present study, hypomethylation was detected in genes involved in transcription factor binding and core promoter binding. Numerous studies have revealed the effects of DNA methylation on the transcription factor binding (35–37). Transcription factors serve important roles in the epigenetic regulation of gene transcription, while DNA methylation prohibits the recruitment of transcription factors, resulting in transcription suppression. A protein microarray-based approach observed that methylation-dependent transcription factor binding was a widespread phenomenon in biological processes (38). Methylated CpG sites are typically considered to reduce gene expression by preventing transcription factors from binding to promoter regions (38). Promoter site-specific CpG methylation is associated with various biological processes. Cyclical methylation alterations in promoter CpGs represent a critical event in achieving transcription (39). Pieper et al (40) also identified a tendency for hypomethylation in the tumor necrosis factor α (TNF-α) core promoter region in neurodegenerative disorders, resulting in reduced binding of the transcription factors and suppressed TNF-α promoter activity.
The focus of the present study was the STG region of patients with late-onset AD. Increasing studies have already identified unique epigenetic signatures involved in the progression of AD in various brain regions. For instance, human AD case-cognitively normal control studies observed global hypomethylation in the entorhinal cortex (41) and in the temporal neocortex neuronal nuclei (42). Additionally, Bakulski et al (13) demonstrated widespread discordant DNA methylation in AD in the human frontal cortex. Chouliaras et al (43) identified a consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of AD subjects. Furthermore, the present study demonstrated a strong bias to hypermethylated alterations in the STG region in AD patients, implying different methylation changes across various brain regions.
In conclusion, the present study demonstrated significant and distinctive DNA methylation patterns in the STG region of patients with late-onset AD. Hypermethylation was mainly detected for genes regulating the cell cycle progression, while hypomethylation was identified in genes involved in transcription factor binding. These results suggested AD-associated epigenetic marks, potentially contributing to the understanding of underlying mechanisms involved in the epigenetic regulation of AD and the development of novel therapeutic targets for AD.
References
- 1.Wilson RS, Segawa E, Boyle PA, Anagnos SE, Hizel LP, Bennett DA. The natural history of cognitive decline in Alzheimer's disease. Psychol Aging. 2012;27:1008–1017. doi: 10.1037/a0029857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Association As: 2017 Alzheimer's disease facts and figures. Alzheimers Dement. 2017;13:325–37310. doi: 10.1016/j.jalz.2017.02.001. [DOI] [Google Scholar]
- 3.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399(6738 Suppl):A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 4.Migliore L, Coppedè F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat Res. 2009;667:82–97. doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Iatrou A, Kenis G, Rutten BP, Lunnon K, van den Hove DL. Epigenetic dysregulation of brainstem nuclei in the pathogenesis of Alzheimer's disease: Looking in the correct place at the right time? Cell Mol Life Sci. 2017;74:509–523. doi: 10.1007/s00018-016-2361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–174. doi: 10.1001/archpsyc.63.2.168. [DOI] [PubMed] [Google Scholar]
- 7.Bird A. Perceptions of epigenetics. Nature. 2007;447:396–398. doi: 10.1038/nature05913. [DOI] [PubMed] [Google Scholar]
- 8.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 9.Ziller MJ, Gu H, Müller F, Donaghey J, Tsai LT, Kohlbacher O, De Jager PL, Rosen ED, Bennett DA, Bernstein BE, et al. Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500:477–481. doi: 10.1038/nature12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith ZD, Meissner A. DNA methylation: Roles in mammalian development. Nat Rev Genet. 2013;14:204–220. doi: 10.1038/nrg3354. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Yu JT, Tan MS, Jiang T, Tan L. Epigenetic mechanisms in Alzheimer's disease: Implications for pathogenesis and therapy. Ageing Res Rev. 2013;12:1024–1041. doi: 10.1016/j.arr.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Bollati V, Galimberti D, Pergoli L, Valle E Dalla, Barretta F, Cortini F, Scarpini E, Bertazzi PA, Baccarelli A. DNA methylation in repetitive elements and Alzheimer disease. Brain Behav Immun. 2011;25:1078–1083. doi: 10.1016/j.bbi.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakulski KM, Dolinoy DC, Sartor MA, Paulson HL, Konen JR, Lieberman AP, Albin RL, Hu H, Rozek LS. Genome-wide DNA methylation differences between late-onset Alzheimer's disease and cognitively normal controls in human frontal cortex. J Alzheimers Dis. 2012;29:571–588. doi: 10.3233/JAD-2012-111223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–367. doi: 10.1016/j.molcel.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussière T, Gold G, Kövari E, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. Stereologic analysis of neurofibrillary tangle formation in prefrontal cortex area 9 in aging and Alzheimer's disease. Neuroscience. 2003;117:577–592. doi: 10.1016/S0306-4522(02)00942-9. [DOI] [PubMed] [Google Scholar]
- 16.von Gunten A, Kövari E, Rivara CB, Bouras C, Hof PR, Giannakopoulos P. Stereologic analysis of hippocampal Alzheimer's disease pathology in the oldest-old: Evidence for sparing of the entorhinal cortex and CA1 field. Exp Neurol. 2005;193:198–206. doi: 10.1016/j.expneurol.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Ginsberg SD, Hemby SE, Lee VM, Eberwine JH, Trojanowski JQ. Expression profile of transcripts in Alzheimer's disease tangle-bearing CA1 neurons. Ann Neurol. 2000;48:77–87. doi: 10.1002/1531-8249(200007)48:1<77::AID-ANA12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Haroutunian V, Katsel P, Schmeidler J. Transcriptional vulnerability of brain regions in Alzheimer's disease and dementia. Neurobiol Aging. 2009;30:561–573. doi: 10.1016/j.neurobiolaging.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson CT, Roussos P, Garg P, Ho DJ, Azam N, Katsel PL, Haroutunian V, Sharp AJ. Genome-wide DNA methylation profiling in the superior temporal gyrus reveals epigenetic signatures associated with Alzheimer's disease. Genome Med. 2016;8:5. doi: 10.1186/s13073-015-0258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huynh JL, Garg P, Thin TH, Yoo S, Dutta R, Trapp BD, Haroutunian V, Zhu J, Donovan MJ, Sharp AJ, Casaccia P. Epigenome-wide differences in pathology-free regions of multiple sclerosis-affected brains. Nat Neurosci. 2014;17:121–130. doi: 10.1038/nn.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du P, Kibbe WA, Lin SM. lumi: A pipeline for processing illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 22.Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29:189–196. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stadler MB, Murr R, Burger L, Ivanek R, Lienert F, Schöler A, van Nimwegen E, Wirbelauer C, Oakeley EJ, Gaidatzis D, et al. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature. 2011;480:490–495. doi: 10.1038/nature10716. [DOI] [PubMed] [Google Scholar]
- 24.Finer S, Mathews C, Lowe R, Smart M, Hillman S, Foo L, Sinha A, Williams D, Rakyan VK, Hitman GA. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum Mol Genet. 2015;24:3021–3029. doi: 10.1093/hmg/ddv013. [DOI] [PubMed] [Google Scholar]
- 25.Sturn A, Quackenbush J, Trajanoski Z. Genesis: Cluster analysis of microarray data. Bioinformatics. 2002;18:207–218. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 26.Millan MJ. The epigenetic dimension of Alzheimer's disease: Causal, consequence, or curiosity? Dialogues Clin Neurosci. 2014;16:373–393. doi: 10.31887/DCNS.2014.16.3/mmillan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hebert LE, Bienias JL, Aggarwal NT, Wilson RS, Bennett DA, Shah RC, Evans DA. Change in risk of Alzheimer disease over time. Neurology. 2010;75:786–791. doi: 10.1212/WNL.0b013e3181f0754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez DG, Nalls MA, Gibbs JR, Arepalli S, van der Brug M, Chong S, Moore M, Longo DL, Cookson MR, Traynor BJ, Singleton AB. Distinct DNA methylation changes highly correlated with chronological age in the human brain. Hum Mol Genet. 2011;20:1164–1172. doi: 10.1093/hmg/ddq561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol. 2009;10:192–206. doi: 10.1038/nrm2640. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz-Matamoros A, Salcedo-Tello P, Avila-Muñoz E, Zepeda A, Arias C. Role of Wnt signaling in the control of adult hippocampal functioning in health and disease: Therapeutic implications. Curr Neuropharmacol. 2013;11:465–476. doi: 10.2174/1570159X11311050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arrázola MS, Silva-Alvarez C, Inestrosa NC. How the Wnt signaling pathway protects from neurodegeneration: The mitochondrial scenario. Front Cell Neurosci. 2015;9:166. doi: 10.3389/fncel.2015.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riise J, Plath N, Pakkenberg B, Parachikova A. Aberrant Wnt signaling pathway in medial temporal lobe structures of Alzheimer's disease. J Neural Transm (Vienna) 2015;122:1303–1318. doi: 10.1007/s00702-015-1375-7. [DOI] [PubMed] [Google Scholar]
- 33.Sinha A, Tamboli RS, Seth B, Kanhed AM, Tiwari SK, Agarwal S, Nair S, Giridhar R, Chaturvedi RK, Yadav MR. Neuroprotective role of novel triazine derivatives by activating Wnt/β catenin signaling pathway in rodent models of alzheimer's disease. Mol Neurobiol. 2015;52:638–652. doi: 10.1007/s12035-014-8899-y. [DOI] [PubMed] [Google Scholar]
- 34.del Pino J, Ramos E, Aguilera OM, Marco-Contelles J, Romero A. Wnt signaling pathway, a potential target for Alzheimer's disease treatment, is activated by a novel multitarget compound ASS234. CNS Neurosci Ther. 2014;20:568–570. doi: 10.1111/cns.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tenayuca J, Cousins K, Yang S, Zhang L. Computational modeling approach in probing the effects of cytosine methylation on the transcription factor binding to DNA. Curr Top Med Chem. 2017;17:1778–1787. doi: 10.2174/1568026617666161116142031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banovich NE, Lan X, McVicker G, van de Geijn B, Degner JF, Blischak JD, Roux J, Pritchard JK, Gilad Y. Methylation QTLs are associated with coordinated changes in transcription factor binding, histone modifications and gene expression levels. PLoS Genet. 2014;10:e1004663. doi: 10.1371/journal.pgen.1004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medvedeva YA, Khamis AM, Kulakovskiy IV, Ba-Alawi W, Bhuyan MS, Kawaji H, Lassmann T, Harbers M, Forrest AR, Bajic VB. FANTOM consortium: Effects of cytosine methylation on transcription factor binding sites. BMC Genomics. 2014;15:119. doi: 10.1186/1471-2164-15-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu S, Wan J, Su Y, Song Q, Zeng Y, Nguyen HN, Shin J, Cox E, Rho HS, Woodard C, et al. DNA methylation presents distinct binding sites for human transcription factors. Elife. 2013;2:e00726. doi: 10.7554/eLife.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Métivier R, Gallais R, Tiffoche C, Le Péron C, Jurkowska RZ, Carmouche RP, Ibberson D, Barath P, Demay F, Reid G. Cyclical DNA methylation of a transcriptionally active promoter. Nature. 2008;452:45–50. doi: 10.1038/nature06544. [DOI] [PubMed] [Google Scholar]
- 40.Pieper HC, Evert BO, Kaut O, Riederer PF, Waha A, Wüllner U. Different methylation of the TNF-alpha promoter in cortex and substantia nigra: Implications for selective neuronal vulnerability. Neurobiol Dis. 2008;32:521–527. doi: 10.1016/j.nbd.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Mastroeni D, Grover A, Delvaux E, Whiteside C, Coleman PD, Rogers J. Epigenetic changes in Alzheimer's disease: Decrements in DNA methylation. Neurobiol Aging. 2010;31:2025–2037. doi: 10.1016/j.neurobiolaging.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mastroeni D, McKee A, Grover A, Rogers J, Coleman PD. Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer's disease. PLoS One. 2009;4:e6617. doi: 10.1371/journal.pone.0006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chouliaras L, Mastroeni D, Delvaux E, Grover A, Kenis G, Hof PR, Steinbusch HW, Coleman PD, Rutten BP, van den Hove DL. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer's disease patients. Neurobiol Aging. 2013;34:2091–2099. doi: 10.1016/j.neurobiolaging.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]