Abstract
Background:
Diabetes mellitus is a metabolic disorder characterized by hyperglycemia along with biochemical alterations of glucose and lipid peroxidation. It produces free radicals that induce lipid peroxidation which acts as an indicator for oxidative stress in the body. The widely used assay for lipid peroxidation involves measurement of malondialdehyde (MDA). Defensive system in the body consists of antioxidant enzymes which help in scavenging free radicals. Two such antioxidant enzymes are reduced glutathione (GSH) and superoxide dismutase (SOD). In this study MDA, GSH and SOD are assessed in serum and saliva of age- and sex-matched 33 diabetics and nondiabetics.
Objective:
(1) To estimate the levels of MDA, GSH and SOD in saliva and serum of both diabetics and nondiabetics. (2) To correlate the levels of MDA, GSH and SOD in saliva and serum of both diabetics and nondiabetics. (3) To find if serum levels of MDA, GSH and SOD can be predicted from values of the same in saliva, in both groups.
Materials and Methods:
Whole unstimulated saliva and venous blood samples obtained after 12 h of overnight fast were transported to the designated laboratory chosen for the study. Supernatants of the centrifuged samples were used for the assays of MDA, GSH and SOD.
Results:
A significant correlation was obtained between serum and saliva values of MDA and GSH, hence the prediction of serum MDA and GSH was possible from their subsequent saliva values. Although the levels of serum and salivary SOD showed a weak positive correlation, prediction of SOD was not possible.
Conclusion:
Saliva can be used as a diagnostic tool for the estimation of MDA, GSH and SOD.
Keywords: Malondialdehyde, reduced glutathione, saliva, serum, superoxide dismutase
INTRODUCTION
Diabetes mellitus is a metabolic disorder whose prevalence is rising rapidly in an alarming rate. This disease has shown a change in its condition over the last 30 years from a mild disorder of the elderly to the major cause for morbidity and mortality affecting the youth and middle-aged people. India leads the world with the largest number of diabetic patients, earning the distinction, the “diabetes capital of the world.” Although there is an increase in the prevalence of type I diabetes, the most common form of diabetes is type II, which accounts for more than 90% of all diabetes cases.[1] Diabetes mellitus is characterized by hyperglycemia and insufficiency in the secretion and function of endogenous insulin.[2] Type II diabetes is a multicausal disease which develops slowly in a step-wise manner initially commencing with insulin resistance and progressing with time which results in failure of the body to maintain glucose hemostasis causing glucose intolerance.[3]
The most common factors which contribute to the development of type II diabetes mellitus include complex heritable genetic correlation, environmental and lifestyle factors such as obesity, physical inactivity and smoking and alcohol consumption along with other risk factors such as aging and pregnancy.[4]
The immune system of an individual works in a very well-organized manner for the sustenance of the normal equilibrium, thus helping in achieving a disease-free state. The immune system can respond overprotective at times leading to increased emission of free radicals, resulting in oxidative stress and lipid peroxidation.[5] The increase in the lipid peroxidation and decline in the antioxidant defense may appear early in noninsulin-dependent diabetes mellitus before the development of secondary complications and might play a role in the initiation and progression of acute and chronic diabetic complications such as diabetic ketoacidosis, nonketotic hyperosmolar state, nephropathy, neuropathy and dermatological, gastrointestinal, genitourinary and micro- and macro-vascular complications.[2]
Lipid peroxidation is formed as a result of reactive oxygen species (ROS) and causes considerable changes in the cell membrane. It has been related to the pathogenesis of many degenerative diseases such as atherosclerosis and carcinogenesis besides diabetes mellitus and aging. Lipid peroxidation forms a well-established mechanism of cellular injury.[6] It is initiated by the attack on a fatty acid or fatty acyl side chain of any chemical species which has sufficient reactivity to abstract a hydrogen atom from a methylene carbon in the side chain. The greater the number of double bonds in a fatty acid side chain, easier will be the removal of the hydrogen atom which is why polyunsaturated fatty acids are more susceptible to peroxidation.[7,8]
To control the influx of ROS, aerobic cells have developed their own defense system – the antioxidant protection system, which includes enzymatic and nonenzymatic components that function interactively and synergistically to neutralize free radicals. Antioxidants can neutralize free radicals by either donating one of their electrons or by ending the electron-stealing reaction of the free radicals. The antioxidant nutrients, however, do not become a free radical by donating an electron because they are stable in either form.[9] The endogenously produced antioxidant enzymes include superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase and reduced glutathione (GSH) and the exogenous free radical-scavenging substances including Vitamins E, C and carotenoids.[10]
SOD is an enzyme that removes the superoxide (O2-) radical, repairs cells and helps to reduce the damage done to them by superoxide, which is the most common free radical in the body. This antioxidant is found in both the dermis and the epidermis, and is the key to the production of healthy fibroblasts which are the skin-building cells.[11] SOD catalyzes the reduction of superoxide anions to hydrogen peroxide which can also be destroyed by catalase or GPx. SOD plays a critical role in the defence mechanism of cells against the toxic effects of oxygen radicals. It competes with nitric oxide (NO) for superoxide anion, which inactivates NO to form peroxynitrite. Therefore, by scavenging superoxide anions, SOD promotes the activity of NO.[12]
The antioxidant GSH is ubiquitous in aerobic tissues. It is synthesized from sulfhydryl-containing amino acids and is highly important in intermediary antioxidant metabolism. Glutathione is the principal intracellular nonprotein thiol. It is present in concentrations upto 10 mm in many cells and provides a primary defense mechanism against oxidative stress by its ability to scavenge free radicals or participate in the reduction of H2O2.[13]
This study intends to analyze the oxidative stress by measuring the lipid peroxidation by-product malondialdehyde (MDA) and the antioxidant status by measuring GSH and SOD in the serum and saliva of type II diabetic patients and nondiabetics, to correlate the levels of MDA, GSH and SOD in serum and saliva of both diabetic and nondiabetic individuals and to find if serum levels of MDA, GSH and SOD can be predicted from the values of the same in saliva, in both the groups.
MATERIALS AND METHODS
Study patients
The study included 33 diabetic and 33 nondiabetic individuals who were selected from the Outpatient Department of Oral Pathology and Microbiology, PMS College of Dental Science and Research, Thiruvananthapuram. All these individuals were briefly informed about the test procedure before sample collection and informed consent was obtained. The study was done in the morning between 8:00 am and 9:00 am. Blood and unstimulated saliva samples were obtained after 12 h of overnight fast. All the individuals included in the study were evaluated for their fasting blood glucose, erythrocyte sedimentation rate (ESR), body mass index (BMI) and postprandial blood sugar. The study individuals were grouped as well-controlled (70–130 mg/dl), moderately controlled (130–200 mg/dl) and poorly controlled (200–250 mg/dl) diabetics based on the fasting blood glucose levels.
Inclusion criteria
Diabetics: Patients with type II diabetes mellitus confirmed by fasting blood sugar, under medication (oral hypoglycemic drugs and insulin) in the age group of 30–60 years
Controls: Normal healthy nondiabetic individuals in the age group of 30–60 years.
Exclusion criteria
Diabetics: Patients with poorly controlled diabetes, habits (smoking, alcoholism), other systemic diseases such as coronary artery disease and renal diseases, patients under any medication other than oral hypoglycemic drugs and insulin and individuals with a history of any illness for the past 6 months
Controls: Individuals with any chronic systemic diseases, habits, patients under any medication other than oral hypoglycemic drugs and insulin and individuals with a history of any illness for the past 6 months.
Sample collection
All participants included in the study were asked to complete a pro forma with a questionnaire which elicited the demographic data (age, gender), medical status, deleterious habit history, prior or current exposure to medication, etc., Whole unstimulated saliva (3 ml) was taken during a period of 5 min and was collected by drooling the saliva into the vial. Blood samples were collected under aseptic conditions; 3 ml of venous blood was collected from each individual into a vial. Samples taken were transported in ice bags at a temperature range of 0°C–4°C to the laboratory designated for the study. Saliva and serum samples were cold centrifuged at 3000 rpm for 5 min. The supernatant was aspirated and was stored at −20°C until analyzed. The clear supernatant was used for the biochemical analysis of MDA, GSH and SOD using a “spectrophotometer.” A spectrophotometer is an instrument that measures the amount of photons (the intensity of light) reflected from a sample object or the amount of light that is absorbed by the same object.[14]
The study was reviewed and approved by the Institutional Ethics Committee, PMS College of Dental Science and Research, Thiruvananthapuram. Informed consent was obtained from all individuals enrolled for the study.
Spectrophotometry procedure
Materials required
Digital spectrometer
Cuvette.
Preparing the sample
Cuvettes were cleaned using deionized water
Proper volumes of the sample and control solution known as blank were added into the cuvette. The outside of the cuvette was wiped to avoid interference from dirt or dust particles.
Running the sample
First, the blank was placed into the cuvette holder and the lid of the instrument was shut
The blank was set to zero using the adjustment knobs
On removing the blank, the calibration on the digital reading continued to read zero
The blank was removed and the experimental samples were placed into the machine. The digital reading recorded the values of transmittance and/or absorbance on the reading panel.
Biochemical procedure
Estimation of lipid peroxidation product malondialdehyde by Hogberg et al's. (1974) method
Principle
MDA formed from the breakdown of polyunsaturated fatty acids serves as a convenient index for the determination of the extent of peroxidation reaction. MDA, a product of lipid peroxidation, reacts with thiobarbituric acid (TBA) to give a pink-colored product, having absorption maxima at 535 nm.[15]
Procedure
The reaction mixture contains 0.03 molar Tris-HCl buffer (pH 7.4), 0.2 millimolar sodium pyrophosphate and 0.2 ml of tissue extract in a total of volume of 2 ml
The reaction mixture was incubated at 37°C for 20 min
The reaction was stopped by the addition of 1 ml of 10% trichloroacetic acid
The reaction mixture was shaken well, and 1.5 ml of TBA was added and then heated
The pink-colored product formed was measured using spectrophotometer at an absorption maximum of 535 nm.
Estimation of enzymatic antioxidants
Reduced glutathione method of Moron et al. (1979)
Principle
GSH on reaction with 5,5’-dithiobis nitro benzoic acid (DTNB) produces a yellow-colored product that absorbs at 412 nm.[15]
Procedure
500 ml of the tissue extract was mixed with 125 μl of 25% of trichloroacetic acid and cooled for 5 min. The mixture was then further diluted with 600 μl of 5% of trichloroacetic acid
The mixture was centrifuged at 3000 rpm for 5 min to settle down the precipitate
150 ml of the supernatant was mixed with 350 μl of sodium phosphate buffer and 1.0 ml of DTNB
The yellow-colored product thus formed was measured using spectrophotometer at an absorption maximum at 412 nm.
Superoxide dismutase method of Kakkar et al. (1984)
Principle
The assay of SOD is based on the inhibition of the formation of Nicotinamide adenine dinucleotide (NADH)-phenazine methosulphate-nitroblue tetrazolium formazan. The color formed at the end of the reaction can be extracted with butanol and measured at 560 nm.[16]
Procedure
The reaction mixture consisted of tissue extract and the assay mixture contained 1.2 ml sodium pyrophosphate buffer, 0.1 ml 186 μM phenazine methosulfate, 0.3 ml of 300 μM nitroblue tetrazolium and 0.2 ml of NADH
The reaction mixture was incubated at 30°C for 90 s
The reaction mixture was shaken well with 4.0 ml of n-butanol and was allowed to stand for 10 min
The mixture was centrifuged, the butanol layer was extracted and the color intensity was measured using spectrophotometer at 560 nm.
RESULTS
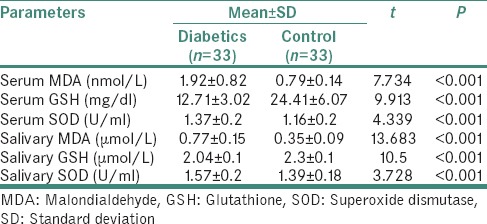
In the present study, the levels of serum and salivary MDA, GSH and SOD were assessed both in diabetic and control groups. MDA showed a statistically significant increase among the diabetic group. The antioxidant enzymes GSH and SOD assessed showed a statistically significant decrease and increase in their values among the diabetic groups, respectively [Table 1].
Table 1.
Distribution of various biochemical parameters in serum and saliva of diabetics and control group
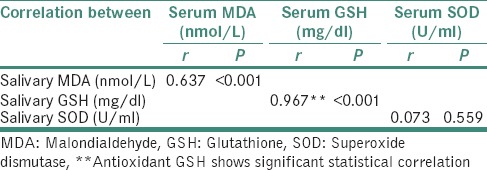
The correlation analysis between the salivary and the serum values of the biochemical parameters MDA, GSH and SOD was done using Pearson's correlation equation. On statistical evaluation, a strong significant positive correlation was observed for the antioxidant enzyme GSH and the lipid peroxidation by-product MDA in serum and saliva. While evaluating the correlation of SOD in serum and saliva, we could observe a positive correlation, but it was not statistically significant [Table 2 and Graphs 1–3].
Table 2.
Distribution of correlation coefficient (r) between malondialdehyde, glutathione and superoxide dismutase in serum and saliva of both diabetics and nondiabetics
Graph 1.
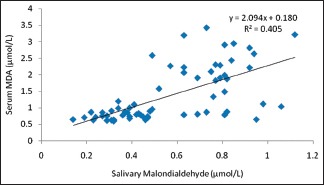
Correlation between salivary malondialdehyde and serum malondialdehyde in both diabetics and nondiabetics
Graph 3.
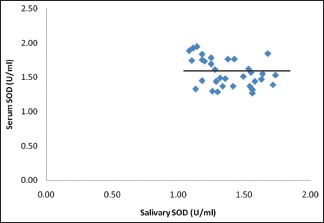
Correlation between salivary superoxide dismutase and serum superoxide dismutase in both diabetics and nondiabetics
Graph 2.
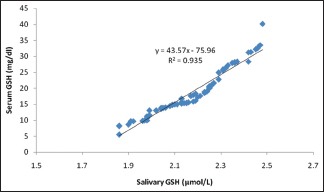
Correlation between salivary reduced glutathione and serum reduced glutathione in both diabetics and nondiabetics
In the present study as can be appreciated from Table 2, we were able to predict the serum values of MDA and GSH from their corresponding salivary values using the significant correlation observed from the Pearson's analysis. The principle used for interpretation in this table is that, for every 1 unit increase in the salivary MDA, there will be 2.094 unit increase in the average serum MDA [Table 3]. Similarly, for every 1 unit increase in the salivary GSH, there will be 43.579 unit increase in the average serum GSH [Table 4].
Table 3.
Prediction of serum malondialdehyde value (linear regression analysis) in both diabetics and nondiabetics
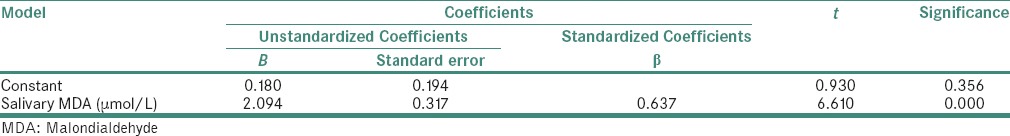
Table 4.
Prediction of serum glutathione value (linear regression analysis) in both diabetics and nondiabetics

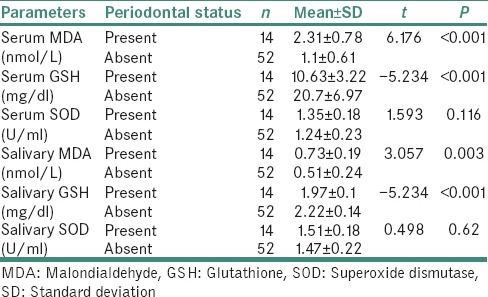
Table 5 shows the distribution of MDA, GSH and SOD in the serum and saliva of diabetic patients with periodontitis and diabetics and nondiabetics without periodontitis. The lipid peroxidation status of MDA value in patients having periodontitis was seen higher than that of the patients and controls without periodontitis. While assessing the antioxidant status, we could observe an increase in the average SOD value and a decrease in the average GSH value in patients with periodontitis compared with the patients and controls without periodontitis.
Table 5.
Distribution of biochemical parameters in serum and saliva of diabetics with periodontitis and diabetics and control without periodontitis
DISCUSSION
Type II diabetes mellitus is associated with multiple metabolic derangements which can cause secondary pathophysiological changes in multiple organ systems. This in turn can impose a heavy burden of morbidity and mortality from micro- and macro-vascular complications.[17] Diabetes can cause persistent hyperglycemia often accompanied with other features such as glucosuria, polydipsia and polyuria which can cause increased production of free radicals in all tissues from glucose auto-oxidation and protein glycosylation.[18]
Free radicals are generated as a by-product of normal cellular metabolism and the increase in the levels of ROS may be caused either due to increased production or decreased destruction of these formed free radicals by the enzymatic and nonenzymatic antioxidants. These altered levels of antioxidant enzymes can critically influence the susceptibility of various tissues to oxidative stress and therefore are associated with the development of complications in diabetes. This mechanism of increased free radical formation is relevant and is dangerous for the beta-islet cells of pancreas as this tissue has low levels of intrinsic antioxidant defenses.[18,19]
In our study, we analyzed the lipid peroxidation by-product MDA and the antioxidant enzymes GSH and SOD in the serum and saliva of 33 individuals with type II diabetes mellitus and 33 normal individuals and correlated the serum values with the saliva values. Among the 33 cases of type II diabetes mellitus, 14 were males and 19 were females, whereas in the control group, 9 were males and 24 were females. The mean age of the patients was 48.97 ± 6.30 years and that of the control group was 42.27 ± 7.94 years. Both these groups were comparable with regard to age and gender.
The parameters measured were BMI, ESR, fasting blood sugar, postprandial blood sugar, MDA, GSH and SOD. Individuals included in the study were well nourished with moderate physical activity. Routine oral examination for dental caries, periodontitis and missing teeth was done. Among these individuals, 21% showed periodontitis, 35% showed missing teeth and 91% showed dental caries. In each individual with periodontitis, <6 teeth were involved with a pocket depth of not more than 4 mm. Dental caries was found involving only enamel and dentin and was devoid of pulp exposure. When we correlated the presence of dental caries, periodontitis and missing teeth with the parameters of lipid peroxidation by-product MDA and the antioxidant enzymes GSH and SOD, statistical significance was observed only for periodontitis.
We observed that, in diabetic individuals, the levels of MDA were significantly elevated in both serum and saliva when compared with that of the nondiabetic individuals [Table 1]. This was in accordance with the studies done by Al-Rawi, Kumawat et al., Padalkar et al., Bikkad et al., Mahadevan and Velavan, Al-Koofee and Goodarzi et al.[6,20,21,22,23,24,25] Al-Rawi evaluated the MDA levels in saliva and serum while Kumawat et al., Padalkar et al., Bikkad et al., Al-Koofee and Goodarzi et al. evaluated only the serum of diabetic patients. Mahadevan and Velavan estimated the MDA levels in the saliva of the diabetic individuals. Bikkad et al. proposed that the increase in the MDA levels may be due to the increased activity of the free radical formation and prolonged exposure to hyperglycemia which in turn leads to increased oxidative stress.[22] A review by Arora et al. on the relationship between lipid peroxidation and diabetes mellitus showed that lipid peroxidation was increased in diabetes patients. Thus, they concluded that lipid peroxidation can be considered as an early marker for diabetes mellitus. Hypoinsulinemia in diabetes increases the activity of the enzyme fatty acyl coenzyme A oxidase, which initiates β-oxidation of fatty acids, resulting in lipid peroxidation. Increased lipid peroxidation impairs membrane function by decreasing membrane fluidity and changing the activity of membrane-bound enzymes and receptors. The products of lipid peroxidation are harmful to most cells in the body and are associated with a variety of diseases, such as atherosclerosis and brain damage.[5,25,26,27]
All the type II diabetic patients in our study showed a decrease in antioxidant GSH both in the serum and saliva when compared with the serum and saliva of nondiabetic individuals [Table 1]. This finding was in accordance with the study done by Al-Rawi. He explained that oxidative stress can consume some naturally occurring local antioxidants such as GSH which may manifest as decreased levels in the serum and saliva of diabetic patients.[6] Similar results were observed by Al-Koofee and Kumawat et al. (in serum) and Gümüş et al. and Arana et al. (in saliva) of type II diabetic individuals.[20,24,28,29] It has been suggested that reduction in GSH levels forms a risk factor for cerebrovascular diseases, especially for cerebral small vessel disease.[30]
In our study, the antioxidant SOD in the serum and saliva of diabetic individuals showed an increase in value when compared with the serum and saliva of nondiabetic individuals [Table 1]. Studies done by Al-Rawi on saliva and serum and Padalkar et al. on serum also showed an increase in the levels of SOD in diabetic group when compared with that of the control group. They proposed that this increase in the antioxidant SOD may be due to the body's mechanism to enhance the antioxidant defense so as to counterbalance the increasing oxidative stress.[6,21]
Contrary results were observed by Kumawat et al. and Taheri et al. Taheri et al. explained that, since SOD forms the first line of defense against free radicals, the activities of this enzyme may be affected first by oxidative stress before any other antioxidant enzymes. There is evidence to show that hyperglycemia is accompanied by the loss of copper, which forms an essential cofactor in SOD activity, and SOD is inactivated by glycosylation in erythrocytes.[31]
In our study, a novel attempt to correlate and predict the serum parameters of lipid peroxidation product MDA and antioxidants GSH and SOD with their corresponding salivary values was performed. We noted that there was a strong significant positive correlation between serum MDA and salivary MDA in diabetic as well as in control group. Equivalent results were also acquired between serum GSH and salivary GSH. Even though we could get a weak positive correlation between serum SOD and salivary SOD, it was not statistically significant [Table 2 and Graph 3]. The linear regression analysis showed that the prediction of serum MDA and serum GSH was possible from salivary MDA and salivary GSH levels, respectively, in both diabetic and control groups. Therefore, using linear regression analysis, we were able to find that 40% of the variability in serum MDA was explained by salivary MDA, thus the prediction of serum MDA from salivary MDA was also possible in both diabetic and nondiabetic individuals [Table 3]. Similar was the case with GSH, we were able to deduce that 93% of variability in serum GSH was explained by salivary GSH, thus by making use of this regression model, we were able to predict the serum GSH values from that of the salivary GSH values in both the groups [Table 4].
Evaluation of the levels of MDA, GSH and SOD in diabetic individuals with periodontitis was not our primary objective, but during the course of our study, we came across 14 (21%) diabetics with periodontitis. When analysis was done on them, a significant increase in serum MDA was observed in diabetic patients with periodontitis compared with both diabetics and controls without periodontitis. Similar results were obtained in the study done by Trivedi et al.[32] Serum GSH and salivary GSH values were found to be decreased in diabetic patients with periodontitis compared with diabetics and controls without periodontitis. In the study done by Thomas et al., they got a significantly lower serum GSH value for diabetic patients with periodontitis compared to the control group.[33] We also observed an increase in the serum SOD and salivary SOD in diabetics with periodontitis when compared to the diabetics and control without periodontitis [Table 5], which was in accordance with the results of Trivedi et al.[32] Further detailed analysis is beyond the scope of this study.
The increase in the lipid peroxidation status and the decline in the antioxidant defense mechanism are the prime factors in the development of complications in diabetics. These biochemical parameters can be evaluated from the serum samples. Saliva values of the same are much less; nevertheless, it can be estimated from this body fluid too. In our study, we estimated the oxidative stress status by measuring MDA and the antioxidant status by measuring GSH and SOD in the serum and saliva of age- and sex-matched diabetics and nondiabetics. Our result could not only substantiate the diagnostic potential of saliva as a body fluid, but also predict the serum values of MDA and GSH from the corresponding saliva values in diabetics and nondiabetics.
CONCLUSION
The use of saliva as a “diagnostic tool” is an upcoming area of research. It offers the advantage over serum as the collection process of saliva is noninvasive. It can be performed easily and cost effectively in a number of clinically challenging situations such as obtaining samples from children, disabled or anxious patients, etc., in whom blood sampling could be a difficult act to perform.
The results of our study helped us to arrive at the conclusion that saliva can indeed be used as an excellent medium for biochemical analysis. Further studies on larger samples with more sensitive devices for more accurate detection of biochemical changes in saliva would probably elevate saliva as a diagnostic tool that is in par with the gold standard serum. If that becomes true, only saliva needs to be investigated for estimating the various biochemical parameters that indicate the development and progression of many systemic diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 2.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: A review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 3.Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, et al., editors. Harrison's Principles of Internal Medicine. 17th ed. Vol. 2. New York: McGraw-Hill; 2008. [Google Scholar]
- 4.Colledge NR, Walker BR, Ralston S, Davidson S, editors. Davidson's Principles and Practice of Medicine. 18th ed. Edinburgh: Churchill Livingstone/Elsevier; 2010. [Google Scholar]
- 5.Arora R, Vig PA, Arora S. Lipid peroxidation: A possible marker for diabetes. J Diabetes Metab. 2013;S11:1–6. [Google Scholar]
- 6.Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diab Vasc Dis Res. 2011;8:22–8. doi: 10.1177/1479164110390243. [DOI] [PubMed] [Google Scholar]
- 7.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–36. [PMC free article] [PubMed] [Google Scholar]
- 8.Halliwell B, Chirico S. Lipid peroxidation: Its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–24S. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 9.Halliwell B. Free radicals and other reactive species in disease. Nature Encyclopaedia of Life Sciences. 2001:1–7. [Google Scholar]
- 10.Percival M. Antioxidants. Clin Nutr Insights. 1998;31:1–3. [Google Scholar]
- 11.Diplock AT, Charleux JL, Crozier-Willi G, Kok FJ, Rice-Evans C, Roberfroid M, et al. Functional food science and defence against reactive oxidative species. Br J Nutr. 1998;80(Suppl 1):S77–112. doi: 10.1079/bjn19980106. [DOI] [PubMed] [Google Scholar]
- 12.Keller JN, Kindy MS, Holtsberg FW, St. Clair DK, Yen HC, Germeyer A, et al. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: Suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–97. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pasupathi P, Chandrasekar V, Senthil KU. Evaluation of oxidative stress, antioxidant and thyroid hormone status in patients with diabetes mellitus. J Med. 2009;10:60–6. [Google Scholar]
- 14.Ninfa AJ, Ballon DP, Benore M, editors. Fundamental Laboratory Approaches for Biochemistry and Biotechnology. 2nd ed. United Kingdom: John Wiley and sons Ltd; 2009. [Google Scholar]
- 15.Thangaraj P. Pharmacological Assays of Plant Based Natural Products. Switzerland: Springer International Publishing; 2016. pp. 94–6. [Google Scholar]
- 16.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 17.Moussa SA, Youssef AA. Oxidative stress in diabetes mellitus. Rom J Biophys. 2008;18:225–36. [Google Scholar]
- 18.Grodsky GM, Anderson CE, Coleman DL, Craighead JE, Gerritsen GC, Hansen CT, et al. Metabolic and underlying causes of diabetes mellitus. Diabetes. 1982;31:45–53. doi: 10.2337/diab.31.1.s45. [DOI] [PubMed] [Google Scholar]
- 19.Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J Biol Chem. 2004;279:42351–4. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- 20.Kumawat M, Singh I, Singh N, Singh V, Kharb S. Lipid peroxidation and lipid profile in type 2 diabetes mellitus. WebmedCentral Biochem. 2012;3:2–9. [Google Scholar]
- 21.Padalkar RK, Shinde AV, Patil SM. Lipid profile, serum malondialdehyde, superoxide dismutase in chronic kidney diseases and type 2 diabetes mellitus. Biomed Res. 2012;23:207–10. [Google Scholar]
- 22.Bikkad MD, Somwanshi SD, Ghuge SH, Nagane NS. Oxidative stress in type II diabetes mellitus. Biomed Res. 2014;25:84–7. [Google Scholar]
- 23.Mahadevan K, Velavan S. Assessment of salivary lipid peroxidation and protein oxidation status in patients with diabetic and oral cancer. Int J Med Biosci. 2012;1:66–8. [Google Scholar]
- 24.Al-Koofee DA. Study of malondialdehyde, reduced glutathione, and peroxy nitrite levels in type 2 diabetics patients. J Appl Chem. 2013;2:1581–8. [Google Scholar]
- 25.Goodarzi MT, Varmaziar L, Navidi AA, Parivar K. Study of oxidative stress in type 2 diabetic patients and its relationship with glycated hemoglobin. Saudi Med J. 2008;29:503–6. [PubMed] [Google Scholar]
- 26.Horie S, Ishii H, Suga T. Changes in peroxisomal fatty acid oxidation in diabetic rat liver. J Biochem. 1981;90:1691–6. doi: 10.1093/oxfordjournals.jbchem.a133645. [DOI] [PubMed] [Google Scholar]
- 27.Acworth IN, McCabe DR, Maher T. The analysis of free radicals, their reaction products, and antioxidants. In: Baskin SI, Salem H, editors. Oxidants, Antioxidants and Free Radicals. Ch. 2. Washington, DC: Taylor and Francis; 1997. [Google Scholar]
- 28.Gümüş P, Buduneli N, Cetinkalp S, Hawkins SI, Renaud D, Kinane DF, et al. Salivary antioxidants in patients with type 1 or 2 diabetes and inflammatory periodontal disease: A case-control study. J Periodontol. 2009;80:1440–6. doi: 10.1902/jop.2009.090159. [DOI] [PubMed] [Google Scholar]
- 29.Arana C, Cutando A, Ferrera MJ, Gómez-Moreno G, Worf CV, Bolaños MJ, et al. Parameters of oxidative stress in saliva from diabetic and parenteral drug addict patients. J Oral Pathol Med. 2006;35:554–9. doi: 10.1111/j.1600-0714.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu H, Kiyohara Y, Kato I, Kitazono T, Tanizaki Y, Kubo M, et al. Relationship between plasma glutathione levels and cardiovascular disease in a defined population: The Hisayama study. Stroke. 2004;35:2072–7. doi: 10.1161/01.STR.0000138022.86509.2d. [DOI] [PubMed] [Google Scholar]
- 31.Taheri E, Djalali M, Saedisomeolia A, Moghadam MA, Djazayeri A, Qorbani M. The relationship between the activities of antioxidant enzymes in red blood cells and body mass index in Iranian type 2 diabetes and healthy subjects. J Diabetes Metab Disord. 2012;11:2–5. doi: 10.1186/2251-6581-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi S, Lal N, Mahdi AA, Mittal M, Singh B, Pandey S. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus. J Periodontol. 2014;85:713–20. doi: 10.1902/jop.2013.130066. [DOI] [PubMed] [Google Scholar]
- 33.Thomas B, Ramesh A, Suresh S, Prasad BR. A comparative evaluation of antioxidant enzymes and selenium in the serum of periodontitis patients with diabetes mellitus type 2. Contemp Clin Dent. 2013;4:176–80. doi: 10.4103/0976-237X.114867. [DOI] [PMC free article] [PubMed] [Google Scholar]


