Abstract
Drugs of abuse increase the frequency and magnitude of brief (1–3 s), high concentration (phasic) dopamine release events in terminal regions. These are thought to be a critical part of drug reinforcement and ultimately the development of addiction. Recently, metabolic regulatory peptides, including the satiety signal glucagon-like peptide-1 (GLP-1), have been shown to modulate cocaine reward-driven behavior and sustained dopamine levels after cocaine administration. Here, we use fast-scan cyclic voltammetry (FSCV) to explore GLP-1 receptor (GLP-1R) modulation of dynamic dopamine release in the nucleus accumbens (NAc) during cocaine administration. We analyzed dopamine release events in both the NAc shell and core, as these two subregions are differentially affected by cocaine and uniquely contribute to motivated behavior. We found that central delivery of the GLP-1R agonist Exendin-4 suppressed the induction of phasic dopamine release events by intravenous cocaine. This effect was selective for dopamine signaling in the NAc core. Suppression of phasic signaling in the core by Exendin-4 could not be attributed to interference with cocaine binding to one of its major substrates, the dopamine transporter, as cocaine-induced increases in reuptake were unaffected. The results suggest that GLP-1R activation, instead, exerts its suppressive effects by altering dopamine release – possibly by suppressing the excitability of dopamine neurons. Given the role of NAc core dopamine in the generation of conditioned responses based on associative learning, suppression of cocaine-induced dopamine signaling in this subregion by GLP-1R agonism may decrease the reinforcing properties of cocaine. Thus, GLP-1Rs remain viable targets for the treatment and prevention of cocaine seeking, taking and relapse.
Keywords: Glucagon-like peptide-1, cocaine, reward, dopamine, drug addiction, nucleus accumbens
1. Introduction
Drug addiction and dependence are major public health issues. There are currently few efficacious treatments for drug addiction due, in part, to an incomplete understanding of the neurobiology of the disease. While the mechanisms by which drugs of abuse drive behavior are complex and involve a diverse network of brain circuits, it is clear that increased release of the neurotransmitter dopamine plays a critical role in the reinforcing properties of abused drugs [1,2]. Synchronous burst firing of ventral tegmental area (VTA) dopamine-containing neurons results in brief, high concentration (phasic) increases in nucleus accumbens (NAc) dopamine concentration [3–5]. These transient increases have been shown to be associated with reward based learning, motivation and reinforcement [6–9] – all of which are central to the neurobiology of addiction. Cocaine, a highly abused drug with a well-investigated mechanism of action, augments NAc dopamine concentration by binding to and blocking dopamine reuptake by the dopamine transporter [10,11]. However, cocaine also increases the frequency of dopamine release events [12], a key property for the development of addiction [13]. The increased frequency of dopamine release events would not be predicted by reuptake blockade alone but rather suggests increased burst firing of dopamine neurons which has gained empirical support [14]. Suppression of these cocaine-evoked transients represents a potential therapeutic approach to treating cocaine addiction.
Metabolic state and its proxies have been shown to bidirectionally modulate reward-driven behavior, whether directed at natural (e.g. food; see [15] for review) or artificial (e.g. cocaine; see [16] for review) rewards. The ability of metabolic state to potentiate or suppress drug seeking behavior has been demonstrated with multiple gut-derived peptides and across many classes of drugs of abuse [17–22]. For example, exogenous administration of the orexigenic peptide ghrelin augments conditioned place preference for cocaine [23], while blockade of ghrelin signaling attenuates conditioned place preference for cocaine, amphetamine, and nicotine [21,24]. Conversely, anorectic signals like the adiposity hormone leptin reduces food-deprivation induced heroin seeking [19]. Hormonal communicators of hunger and satiety that defend energy balance are therefore sufficient to alter drug reward-driven behavior.
A powerful modulator of food and drug reward is the incretin hormone glucagon-like peptide-1 (GLP-1), which suppresses food intake and body weight in rodents and humans by its actions on central and peripheral GLP-1Rs [25–27]. Much of the existing work on the anorectic effects of GLP-1 is heavily focused on GLP-1 signaling through metabolic control nuclei including nuclei of the hypothalamus and the nucleus of the solitary tract [28–30]. Recent work, however, has demonstrated that that GLP-1 also decreases food intake by reducing the rewarding value of food [31–34], implicating a physiological role for the peptide beyond energy balance regulation. Importantly, neurons in the NAc and VTA of the mesolimbic circuit express GLP-1Rs and receive direct projections from central GLP-1 producing neurons [32,33]. Intra-VTA or NAc infusion of the GLP-1R agonist, Exendin-4, reduces palatable food intake [32,33] and goal-directed behavior for food [31]. Interestingly GLP-1R agonists also suppress goal-directed behavior for cocaine reward [35–39], likely by attenuating dopamine signaling in the NAc [35,39]. However, suppression of cocaine-evoked NAc dopamine release was demonstrated utilizing microdialysis, which lacks the spatiotemporal resolution to allow for investigation of phasic dopamine release patterns as well as potential regional differences. Importantly, the two subregions of the NAc (shell and core) respond differently to cocaine [40,41] and are postulated to contribute in unique ways to the neurobiological basis of reward- directed behavior and drug addiction [42–48].
The studies here use fast-scan cyclic voltammetry (FSCV) to measure the pharmacological effects of Exendin-4 (a GLP-1R agonist) on intravenous cocaine-evoked phasic dopamine signaling in the NAc core and shell of rats. We replicate findings that non-contingent cocaine increases dopamine signaling in both the NAc core and shell [49,50]. We show, for the first time, that central GLP-1R activation suppresses cocaine-evoked dopamine signaling but only within the NAc core. We also demonstrate that in cocaine-treated animals, Exendin-4 has no effect on the magnitude of electrically stimulated dopamine release or the rate of dopamine reuptake. This work suggests that GLP-1R agonism could curb cocaine-directed behavior, in part, by suppressing dopamine signaling in the NAc core. While future studies are needed to establish a causal relationship, GLP-1R targeted pharmacotherapies remain a promising approach for decreasing the reinforcing value of abused drugs like cocaine.
2. Materials and Methods
2.1. Subjects
Male Sprague-Dawley rats (n=39; 350–425 g at the time of testing) were housed individually and maintained on a 12:12-h light-dark cycle (on at 7:00 AM). Rats were provided with ad libitum laboratory chow (LabDiet 5012) until post-operative recovery of body weight. Following recovery from the second surgery, animals were fed enough chow to maintain 85% of their ad libitum body weight. Experimental sessions were conducted after 4 days of restricted food access. Animals were treated in accordance with the guidelines put forth by the National Institutes of Health and under the approval of the Animal Care Committee of the University of Illinois at Chicago.
2.2. Surgery
All rats received two surgeries separated by 5–7 days of post-operative recovery. Both surgeries were conducted under anesthesia with ketamine hydrochloride (100 mg/kg. i.p.) and xylazine hydrochloride (10 mg/kg, i.p.).
2.2.1. Jugular vein catheterization
All animals were implanted with back-mounted jugular vein catheters (Access Technologies) as described in detail elsewhere [51,52]. Briefly, a cannula port was implanted subcutaneously on the back of the animal and the length of the catheter (0.31 mm ID, 0.64 mm OD) was subcutaneously routed over the animal’s shoulder. The catheter was threaded 3.5 cm into the jugular vein. To maintain patency, rats were flushed daily with heparinized saline (33.33 units/ml).
2.2.2. Craniotomy
The rat brain atlas of Paxinos and Watson [53] was used as a reference to implant all rats with an infusion guide cannula (Plastics One, 22 gauge, 11 mm) directed at the lateral ventricle (LV), a voltammetry guide cannula (Bioanalytical Systems) dorsal to the NAc core (n=25) or shell (n=14), and a bipolar stimulating electrode (Plastics One) dorsal to the VTA. All implant measurements were made relative to bregma (LV:−0.8 mm AP,−2.1 mm ML,−3.7 mm DV, angled 10° away from midline; NAc core: +1.3 mm AP, −1.5 mm ML,−2.5 mm DV; NAc shell: +1.7 mm AP, −0.9 mm ML,−2.5 mm DV; VTA: −5.2 mm AP, −0.8 mm ML, −8.3 mm DV). A chlorinated silver reference electrode was placed in the left forebrain. All implants were secured using stainless steel screws and dental cement.
2.3. Experimental protocol
All experiments involved intravenous cocaine infusions paired with concurrent FSCV recordings to sample dopamine signaling patterns in real time. All experiments took place in a standard operant chamber (Med-Associates, Inc.) housed within a sound-attenuating box. Animals were tethered by a FSCV headstage (University of Washington EME Shop) which also applied voltage changes and measured resultant current changes from the oxidation and reduction of dopamine. FSCV in awake and behaving animals has been described previously [54]. Briefly, a glass insulated carbon fiber electrode was loaded into a micromanipulator. The micromanipulator was used to lower the electrode through the NAc guide cannula and into the NAc core (n=25) or NAc shell (n=14). The voltage of the electrode was held at −0.4 V, relative to the Ag/AgCl reference electrode, and intermittently (10 Hz) ramped in a triangular fashion (−0.4 to +1.3 to −0.4V; 400 V/s). Current at each voltage of the electrode was continuously monitored throughout the experimental session. Dopamine signaling was determined by comparing sampled current changes across electrode potentials (cyclic voltammogram) to a template cyclic voltammogram for dopamine acquired after electrical stimulation of the VTA. Stimulations (24 monophasic pulses, 4 ms/pulse, 60 Hz, 120 μA) were conducted at the beginning of the experimental session.
In addition to a headstage, animals were connected to two infusion lines which both passed through a commutator (Crist Instruments) above the operant chamber. Back-mounted catheter ports were connected to an infusion line which terminated in a 20 ml syringe in an infusion pump. The pump was controlled by software developed by Med-Associates, Inc. (MedPC) for automated delivery of 30 cocaine infusions (0.25 mg/0.1 ml, 3 second infusion, 4 min inter-infusion interval). An injector (28 gauge, 1 mm projection) connected to an infusion line was inserted into the LV guide cannula. The infusion line terminated in a 10 μl Hamilton syringe in an infusion pump which was used to deliver LV Ex-4 (0.15 μg in 1 μl; American Peptide) or artificial cerebrospinal fluid [aCSF (1 μl)] at a rate of 2 μl/min. This dose of Exendin-4 is supra-threshold for food-intake and body-weight regulatory effects [55]. For analysis of Exendin-4 on dopamine signaling in the NAc (n=13 or n=14 for NAc core or shell, respectively), LV infusions of vehicle (n=7 or n=7 for NAc core or shell, respectively) or Exendin-4 (n=6 or n=7 for NAc core or shell, respectively) were made immediately following the 10th cocaine infusion. For analysis of Exendin-4 on stimulated dopamine release and reuptake in the NAc core, electrical stimulations (n=30) were delivered to the VTA (24 monophasic pulses, 60 Hz, 120 μA for each stimulation) once every 4 minutes, 1 minute after the onset of cocaine infusion. Vehicle (n=6) or Exendin-4 (n=6) LV infusions were made immediately following the 10th electrical stimulation of the VTA.
2.4. Data Analysis
2.4.1. Phasic dopamine signaling: cyclic voltammogram match
Dopamine signaling was measured using a ‘sliding background’ subtraction method [50]. Dopamine transients were chemically identified by subtracting each cyclic voltammogram (current by voltage plot) in a 2-minute file from the average of the five cyclic voltammograms collected 1.0–0.5 seconds earlier. Linear regression was used to compare background subtracted cyclic voltammograms to an in vivo cyclic voltammogram template for dopamine (average background subtracted cyclic voltammogram taken from electrically evoked dopamine release at the beginning of the experimental session). Voltammograms with an r2 ≥ 0.75 were considered dopamine (“CV Matches”) and counted across the 4 minutes separating cocaine infusions [56].
To compare the effect of cocaine alone on dopamine signaling in the NAc core and shell, vehicle and Exendin-4 treatment groups were combined within NAc subregion. Pre-cocaine (infusion 0) and post-cocaine (sum of infusion bins 1–10) CV Matches were compared with a two-way [epoch (infusion bin) x region (core, shell)] repeated measures ANOVA with Bonferroni post hoc tests when appropriate. All statistical analyses were performed using GraphPad 5.0 (Prism).
To determine the effect of GLP-1R activation on dopamine transients in both the NAc core and shell, CV Matches were first binned by averaging the number of matches across five cocaine infusions. The resulting bins were compared with a two-way [epoch (infusion bin) x treatment (vehicle, Exendin-4)] repeated measures ANOVA with Bonferroni post hoc tests when appropriate.
2.4.2. Magnitude of stimulated dopamine release
Peak oxidative current for dopamine evoked in the NAc core by electrical stimulation was measured (see [54] for details). Current was converted into concentration after each electrode was calibrated with 1 μM of dopamine in a flow injection system [57]. Electrically evoked dopamine concentration changes were compared by first averaging dopamine concentration within drug treatment across bins of 5 stimulations. Stimulation bins were compared between vehicle and Exendin-4 treated animals by a two-way [epoch (stim bin) x treatment (vehicle, Exendin-4)] repeated measures ANOVA with Bonferroni post hoc tests when appropriate.
2.4.3. Rate of dopamine reuptake
The rate of dopamine reuptake was calculated as the time required for the dopamine concentration evoked by each stimulation to decay to 36.8% of the peak value (Tau). A measure of Tau has been shown to be positively correlated with the Michaelis-Menten kinetic model and is an accurate measure of dopamine reuptake [58,59]. Dopamine reuptake differences were compared by first averaging reuptake time values within drug treatment across bins of 5 stimulations. Stimulation bins were compared between vehicle and Exendin-4 treated animals by a two-way [epoch (stim bin) x treatment (vehicle, Exendin-4)] repeated measures ANOVA with Bonferroni post hoc tests when appropriate.
2.5. Verification of recording sites and cannula placements
After experimental testing, animals were deeply anesthetized with sodium pentobarbital (100 mg/kg). To verify the recording sites, a polyimide- insulated stainless steel electrode housed in a micromanipulator was lowered to the dorsal-ventral depth of the recording site. A small current was passed over the electrode to create an electrolytic lesion. To verify lateral LV cannula placement, guide cannula were infused with 1 μl of India ink (AMTS, Inc) at a rate of 2 μl/min. Brains were removed, stored in formalin for 24 h and then transferred to 30% sucrose in 0.1 M phosphate buffer. A cryostat was used to section brains (40 μm) through the lateral ventricle and NAc. Sections were mounted on slides and a light microscope was used to confirm the presence of an electrolytic lesion in the NAc core or shell and ink exclusively within the lateral ventricle with guidance from a rat stereotaxic atlas [53].
3. Results
3.1. Intravenous cocaine evokes phasic dopamine release in the NAc
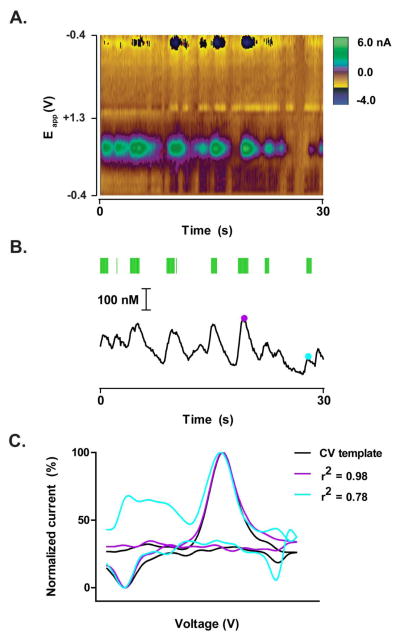
Figure 1 illustrates representative dopamine transients evoked by intravenous cocaine infusion. The color plot (Figure 1A) and associated concentration trace (Figure 1B) were acquired from the NAc core of a rat with 15 previous cocaine infusions. Experiments utilizing CV Matches as an index of dopamine signaling used linear regression to compare cyclic voltammograms taken across the recording session to a cyclic voltammogram template for dopamine (Figure 1C). Cyclic voltammogram templates were generated by electrical stimulation of the VTA prior to the onset of the recording session. Figure 1C shows examples of the range of acceptable cyclic voltammograms that were counted as CV Matches (r2 ≥ 0.75, Figure 1B green ticks above concentration trace).
Figure 1.
Cocaine evokes phasic dopamine release in the NAc. (A) Representative example of electrochemical data collected from the NAc core. The 30 second example shown here was taken from an intravenous non-contingent cocaine session after 15 infusions. Colorplot depicts changes in current (color) as a function of applied potential (Eapp; y-axis) and time (s; x-axis). Dopamine can be observed based on its oxidation (green feature at ~0.65 V) and reduction (dark blue feature at −0.2 V) currents. (B) Dopamine concentration over time extracted from the colorplot in (A). Conversion of current from the oxidation of dopamine to concentration was made based on a post-recording calibration of the electrode used for data collection. Cyclic voltammograms were considered “CV Matches” for dopamine if they met criteria, r2 value ≥ 0.75 (see data analysis). CV Matches are indicated by the green ticks above the concentration trace. Purple and blue dots are representative times at which the cyclic voltammogram met criteria and therefore considered a CV Match (r2 = 0.98 and 0.78, respectively). (C) Comparison of two cyclic voltammograms taken from the electrochemical data above (purple and blue dots in B) to a cyclic voltammogram template for dopamine. The purple and blue cyclic voltammograms capture the range of cyclic voltammograms that met criteria (CV Matches) in which r2 ≥ 0.75. The black cyclic voltammogram was taken from the dopamine response following electrical stimulation of the VTA before the recording session. Current magnitude of the three cyclic voltammograms were normalized to allow for visual comparison of cyclic voltammogram shape.
3.2. Cocaine evokes more frequent phasic dopamine events in the NAc shell than NAc core
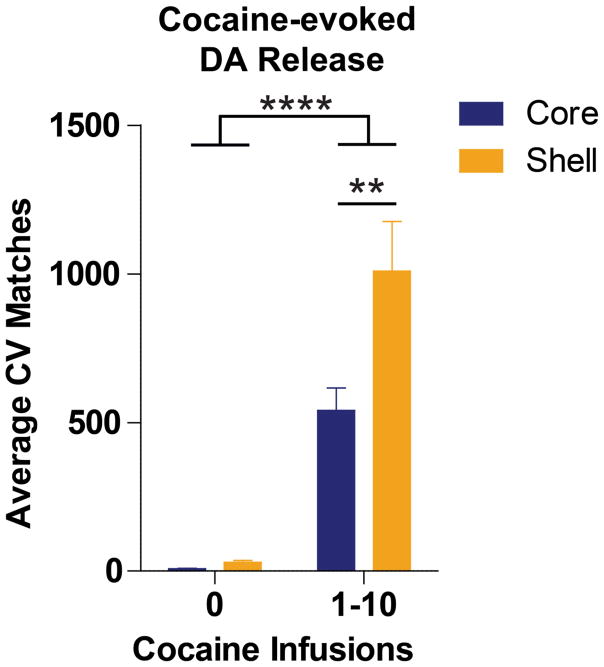
Intravenous cocaine infusion evoked increases in dopamine signaling in both the NAc core (n=13) and shell (n=14). There was a significant main effect of cocaine infusion bin [0 vs 1–10; F(1,25)=64.06, p<0.0001]. There were also differential responses of NAc subregions to cocaine, as there was a significant main effect of NAc subregion [core vs shell; F(1,25)= 6.73, p<0.05]. A significant infusion bin x NAc subregion interaction of these effects [F(1,25)=5.61; p<0.05] was further explored with Bonferroni tests, which revealed significantly more (p<0.01) NAc transients in the NAc shell (1008.36 ± 167.48 CV Matches) than the core (539.23 ± 76.25 CV Matches) after cocaine infusion (infusion bin 1–10) (Figure 2).
Figure 2.
Cocaine evokes increases in NAc dopamine signaling. While 10 cocaine infusions significantly (***p<0.0001) increases dopamine signaling (expressed as Average CV Matches) in both the NAc core (n=13, blue bars) and shell (n=14, gold bars), the NAc shell is more responsive to the dopamine-evoking effects of cocaine (**p<0.001). Bars represent group means for the 4 minutes preceding cocaine infusion (cocaine infusion 0) or binned (1–10) cocaine infusion periods. Error bars are ± 1 standard error of the mean (SEM).
3.3. Cocaine-evoked dopamine signaling in the NAc core is attenuated by central GLP-1R agonism
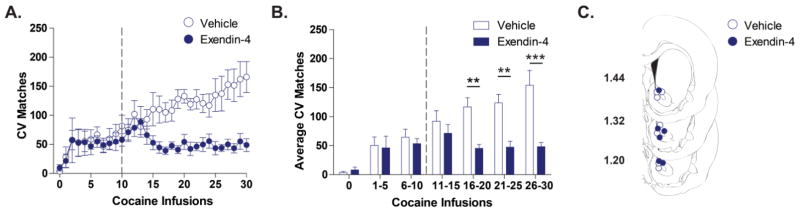
Intravenous cocaine infusions were non-contingently delivered to rats once every 4 minutes. CV Matches between cocaine infusions were plotted across the 30 infusions for animals that received LV vehicle (n=7) or Exendin-4 (n=6) after 10 infusions (Figure 3A). To analyze the effect of drug treatment on dopamine signaling, we grouped the 30 cocaine infusions into bins of 5 infusions and averaged the number of CV Matches within each bin (Figure 3B). There were significant main effects of both infusion bin [F(1,11)=15.14; p<0.0001] and drug treatment [vehicle vs Exendin-4; F(1,11)=7.98; p<0.05]. A significant infusion bin x drug treatment interaction of these effects [F(1,11)=7.14; p<0.0001) was further explored with Bonferroni tests, which revealed a significant attenuation of average CV Matches in the Exendin-4 treated group relative to the vehicle treated group during the 16–20 (45.23 ± 6.68 vs 116.83 ± 15.77), 21–25 (47.40 ± 10.21 vs 123.71 ± 14.67) and 25–30 (48.13 ± 7.44 vs 153.97 ± 25.77) cocaine infusion bins.
Figure 3.
LV Exendin-4 suppresses cocaine-evoked dopamine signaling in the NAc core. (A) Time-dependent effects of vehicle (open blue circles) and Exendin-4 (closed blue circles) on dopamine signaling (CV Matches) across a session of 30 intravenous cocaine infusions. Vehicle (n=7) or Exendin-4 (n=6) was administered after 10 cocaine infusions (denoted by the broken vertical line). (B) Statistical comparison of the Average CV Matches across bins of 5 cocaine infusions. Exendin-4 treated rats (blue bars) have significantly suppressed Average CV Matches for the 16–20, 21–25, and 26–30 cocaine infusion bins relative to vehicle treated rats (white bars, **p<0.001, ***p<0.0001). Bars represent group means for the 4 minutes preceding cocaine infusion (cocaine infusion 0) or binned cocaine infusions periods. Error bars are ± 1 standard error of the mean (SEM). (C) NAc core recording sites depicted as circles (blue open= vehicle, blue filled= Exendin-4) on coronal sections modified from Paxinos and Watson (2007). Numbers on the left indicate approximate distance of the section from bregma.
3.4. Cocaine-evoked dopamine signaling in the NAc shell is not affected by central GLP-1R agonism
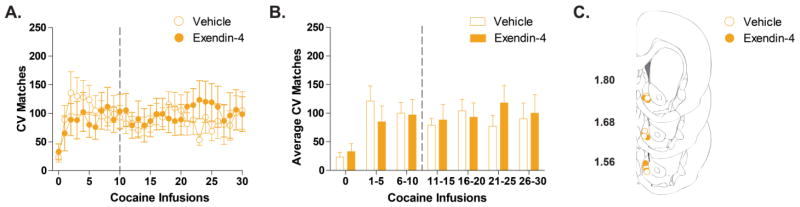
In the NAc shell, dopamine signaling was similar for LV vehicle (n=7) and Exendin-4 (n=7) treated animals (Figure 4B). While there was a significant main effect of infusion bin [F(1,12)=5.79; p<0.0001], there was no main effect of drug treatment [vehicle vs Exendin-4; F(1,12)=0.01; p>0.05] and no interaction of these effects [F(1,12)= 1.15; p>0.05].
Figure 4.
LV Exendin-4 does not alter cocaine-evoked dopamine signaling in the NAc shell. (A) Time-dependent effects of vehicle (open gold circles) and Exendin-4 (closed gold circles) on dopamine signaling (CV Matches) across a session of 30 intravenous cocaine infusions. Vehicle (n=7) or Exendin-4 (n=7) was administered after 10 cocaine infusions (denoted by the broken vertical line). (B) Statistical comparison of the Average CV Matches across bins of 5 cocaine infusions. There is no statistical difference (p>0.05) between Vehicle (white bars) and Exendin-4 treated rats (gold bars) over the course of the session. Bars represent group means for the 4 minutes preceding cocaine infusion (cocaine infusion 0) or binned cocaine infusions periods. Error bars are ± 1 standard error of the mean (SEM). (C) NAc shell recording sites depicted as circles (gold open= vehicle, gold filled= Exendin-4) on coronal sections modified from Paxinos and Watson (2007). Numbers on the left indicate approximate distance of the section from bregma.
3.5. Magnitude of stimulated dopamine release is not affected by central GLP-1R agonism
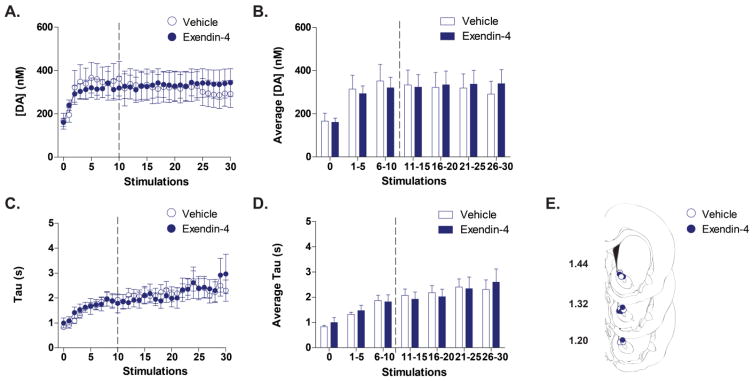
To determine if Exendin-4 modulated dopamine signaling in the NAc core by altering the ability of cocaine to affect dopamine reuptake, electrical stimulations of the VTA were performed once every 4 minutes over the course of a non-contingent cocaine infusion session (1 minute following cocaine infusion). We first examined the magnitude of dopamine evoked by each stimulation, a measurement that is influenced by opposing actions of both dopamine release and reuptake, by plotting peak evoked dopamine concentration across the session for animals receiving either LV vehicle (n=6) or Exendin-4 (n=6) after 10 stimulations (Figure 5A). To analyze the effect of drug treatment on maximum evoked dopamine concentration, we grouped the 30 stimulations into bins of 5 stimulations and averaged the maximum concentration of evoked dopamine within each bin. The magnitude of stimulated dopamine release evoked was statistically the same for vehicle and Exendin-4 treated groups over the course of a non-contingent cocaine session (Figure 5B). There was a significant main effect of stimulation bin [F(1,10)= 10.45; p<0.0001] but no main effect of drug treatment (vehicle vs Exendin-4; F(1,10)=0.001; p>0.05] and no interaction of these effects [F(1,10)=0.50; p>0.05].
Figure 5.
LV Exendin-4 does not alter indices of electrically-evoked dopamine in the NAc core of rats receiving non-contingent cocaine infusions. (A) Time-dependent effects of vehicle (open blue circles) and Exendin-4 (closed blue circles) on maximum concentration of stimulated dopamine release ([DA] (nm)) across a session of 30 stimulations (each stimulation occurred 1 minute after a cocaine infusion). Vehicle (n=6) or Exendin-4 (n=6) was administered after 10 cocaine infusions (denoted by the broken vertical line). (B) Statistical comparison of the Average [DA] across bins of 5 stimulations. There is no statistical difference (p>0.05) between Vehicle (white bars) and Exendin-4 treated rats (blue bars) over the course of the session. Bars represent group means for the 4 minutes preceding the first stimulation (stimulation 0) or binned stimulations. Error bars are ± 1 standard error of the mean (SEM). (C) Time-dependent effects of vehicle (open blue circles) and Exendin-4 (closed blue circles) on reuptake time [Tau (s)] of stimulated dopamine release a session of 30 stimulations. Vehicle (n=6) or Exendin-4 (n=6) was administered after 10 cocaine infusions (denoted by the broken vertical line). (D) Statistical comparison of the Average reuptake time across bins of 5 stimulations. There is no statistical difference (p>0.05) between Vehicle (white bars) and Exendin-4 treated rats (blue bars) over the course of the session. Bars represent group means for the 4 minutes preceding the first stimulation (stimulation 0) or binned stimulations. Error bars are ± 1 standard error of the mean (SEM). (E) NAc core recording sites depicted as circles (blue open= vehicle, blue filled= Exendin-4) on coronal sections modified from Paxinos and Watson (2007). Numbers on the left indicate approximate distance of the section from bregma.
3.6. Rate of dopamine reuptake is not affected by central GLP-1R agonism
To analyze reuptake rate of stimulated dopamine release over the course of a non-contingent cocaine session, the time for reuptake [Tau (s)] was plotted across the session for animals receiving either LV vehicle (n=6) or Exendin-4 (n=6) after 10 stimulations (Figure 5C). The time for dopamine reuptake was statistically the same for vehicle and Exendin-4 treated groups over the course of a non-contingent cocaine session (Figure 5D). There was a significant main effect of stimulation bin [F(1,10)= 28.19; p<0.0001] but no main effect of drug treatment (vehicle vs Exendin-4; F(1,10)=0.005; p>0.05] and no interaction of these effects [F(1,10)=0.70; p>0.05].
4. Discussion
Cocaine is highly reinforcing, in part, because it induces phasic dopamine signaling in the NAc. We captured these phasic events using FSCV and examined the hypothesis that central GLP-1R agonism would suppress them. Indeed, the GLP1R agonist Exendin-4 suppressed dopamine signaling but only in the NAc core and not in the NAc shell. The central effects of Exendin-4 on cocaine-induced phasic dopamine signaling and its regional sensitivity is novel. Given the role of the NAc core dopamine in the generation of conditioned responses following associative learning, our findings suggest the potential for GLP-1Rs as a novel therapeutic target for interfering with the progression of drug addiction.
Prior to manipulating GLP-1Rs, we found a regional difference in cocaine-induced dopamine signaling – with enhanced signaling within the NAc shell (Figure 2) similar to other reports [49,50]. Preferential enhancement of NAc shell extracellular dopamine by cocaine [46,60] has been attributed to mechanisms including increased firing of dopaminergic neurons projecting to the shell [49,61], slower dopamine reuptake [62], and more frequently occurring dopamine transients [49] relative to the NAc core prior to cocaine administration. It is possible that we failed to observe an effect of GLP-1R agonism on NAc shell dopamine signaling because of these differences in dopamine signaling dynamics. At the time of Exendin-4 delivery, dopamine transient frequency in the core was still increasing (Figure 3) whereas it had already stabilized in the shell (Figure 4). It is possible that the critical temporal window for affecting dopamine signaling with GLP-1R agonism in the shell may therefore have been missed. The temporal sensitivity of the NAc shell to GLP-1R modulation should continue to be explored, as the NAc shell is highly implicated in the initial reinforcing properties of drugs. The NAc shell is likely more responsive to the immediate rewarding effects of drugs of abuse, as rats will learn to self-administer psychostimulants (e.g. amphetamine and cocaine) as well as dopamine receptor agonists and reuptake inhibitors into the NAc shell, but not core [61,63–65]. These studies have been supported by others in which blockade of dopamine signaling in the NAc shell disrupts conditioned place preference for drugs of abuse [66–70]. Activation of VTA to NAc shell-projecting neurons are therefore activated by drugs of abuse and this activation is key for the reinforcing properties of addictive drugs.
The progression from drug use to addiction is complex and requires more than NAc shell processing of the primary reinforcing value of a drug. A hallmark of the pathophysiology of addiction is the ability of drug-associated cues to acquire the ability to drive drug-seeking behavior, often times resulting in relapse [71]. An accumulation of evidence has favored the role of the NAc core in the generation of conditioned responses based on stimulus-outcome associative learning [2,42,43,48,72]. Interfering with NAc core signaling disrupts previously learned responses to conditioned stimuli that support continued responding for cocaine [45,73] or reinstatement of cocaine seeking [74]. NAc core signaling processes that underlie habit learning likely involve dopamine. Dopamine signaling within the NAc core encodes reward-predictive sensory cues [44,75,76] that drive behavior during acquisition [41,77,78] and maintenance [50,78–80] of learned associations. Interestingly, the increase in NAc dopamine to reward predictive cues comes online as animals develop conditioned approach behavior towards cues [41,81]. Interfering with the associative tracking of dopamine that occurs during habit learning is a potential means to treat drug addiction [82]. Our work provides evidence that GLP-1R agonism suppresses the pharmacological induction of NAc core dopamine signaling by cocaine (Figure 3) and therefore may provide a means to intercept the development of addiction. It is important to note that our studies explored mesolimbic responses to acute non-contingent cocaine exposure and therefore capture a smaller subset of phasic dopamine signaling that may arise from prolonged cocaine self-administration. Indeed, it remains unclear if central GLP-1R agonism would influence the conditioned phasic dopamine reponses that are associated with lever presses and cocaine-associated cues [76]. Future studies should be extended to include examination of central GLP-1R agonism on phasic NAc dopamine signaling during cocaine self-administration. Like non-contingent cocaine-evoked dopamine release in the NAc core, we hypothesize that dopamine responses to these discrete events would also be suppressed by GLP-1R activation.
GLP-1R agonists suppress reward-directed behavior towards both food and drugs of abuse [38,83]. With respect to cocaine, Exendin-4 decreases cocaine-induced locomotion, conditioned place preference and self-administration [35–37,39]. GLP-1R agonism also suppresses hyperactivity induced by the non-selective dopamine agonist apomorphine [84]. The ability of the GLP-1 system to modulate dopamine signaling, likely underlying reward-driven behavioral alterations, is supported by studies employing microdialysis. Using this technique, which samples fluctuations in extracellular dopamine over minutes, studies have shown suppression of cocaine-induced increases in NAc dopamine concentration by Exendin-4 [35,39]. Our data suggest that this suppression is limited to the NAc core (Figures 3 and 4) and that terminal mechanisms for shaping extracellular dopamine concentration (e.g. reuptake) remain intact (Figure 5; see also [85]) and do not contribute to the GLP-1 mediated suppression. We captured phasic dopamine signaling by measuring by CV Matches, which serve as a proxy for dopamine transients. While this method is unbiased in that files are analyzed with fixed intervals between the time point of interest and the background to be subtracted, it does not perfectly correspond to individual dopamine release events (see Figure 1 for example in which single transients are represented by multiple CV Matches). Other methods for the counting of dopamine transients have been developed (see [86] for example). Extracellular phasic dopamine concentration fluctuations can be characterized by multiple components. These components include transient amplitude (an index of dopamine exocytosed per release event) and half width (duration of release event and affected by dopamine reuptake) [49,87–89] and could be differentially altered by GLP-1. To partially address these components, we examined the effects of GLP-1R agonism on electrically-evoked dopamine. Our findings suggest that Exendin-4 does not interfere with alterations in dopamine release potential or dopamine transporter function (Figure 5). However, a more in depth analysis is required, as electrical stimulation may prohibit subtle changes in these transmission components.
Our studies are the first to demonstrate modulation of NAc cocaine-evoked dopamine signaling by central GLP-1R agonism. Previous work demonstrating suppression of dopamine by Exendin-4 used systemic injection [35,39] which would target both peripheral and central GLP-1Rs, as Exendin-4 does cross the blood brain barrier [90]. While our data suggest a central site of action, the central population of GLP-1Rs that are targeted to exert effects on dopamine signaling remains unknown. GLP-1Rs are located throughout the brain [91]. Importantly, they are found in the VTA, which receives direct projections for GLP-1 producing neurons of the nucleus of the solitary tract [32], and can be targeted selectively to reduce motivated behavior for food and drug reward (see [38,83,92] for review). In support of this hypothesis, a recent study has demonstrated a reduction in cocaine self-administration behavior by intra-VTA agonism of GLP-1Rs [37]. There is evidence to suggest that VTA receptor activation may differentially modulate dopamine signaling depending on the NAc subregion targeted by the dopamine projection – with effects predominantly in the NAc core [32,33]. While the VTA represents a candidate nucleus, on which multiple peptides that signal metabolic state has been shown to directly act [92] to regulate dopamine signaling, it represents one of many potential populations of GLP-1Rs. Previous research has demonstrated GLP-1 action in other sites that regulate reward responding including the nucleus of the solitary tract, amygdala, NAc and lateral septum [34,93]. A recent investigation has shown a role for dorsal lateral septum GABAergic GLP- 1R positive cells in cocaine-induced locomotor responses and conditioned place preference to cocaine [94]. It is hypothesized that GLP-1 regulates motivated behavior through these neurons by their inhibitory connections with GABAergic interneurons of the VTA [95].
The suppression of reward driven behavior by action of energy balance-regulating peptides on mesolimbic circuitry has become a well explored approach [96–99] towards treating reward-related disorders (obesity, addiction). We propose that NAc core dopamine signaling is central to the development of addiction and demonstrate the ability of central GLP-1R activation to attenuate components of this signaling in the presence of cocaine. While GLP-1R agonists like Exendin-4, which is already approved by the Food and Drug Administration for the treatment of metabolic disorders, are promising candidates for addiction treatment [100], further studies are needed to fully reveal their therapeutic potential. Full dose response experiments with necessary controls for off-target consequences (i.e. nausea/visceral malaise [55,101], the suppression of body weight [25]) are necessary [38]. These studies are undoubtedly time consuming and expensive. However, the demonstrated ability of the GLP-1 system to regulate phasic mesolimbic dopamine signaling is promising for the future of drug addiction treatment.
Highlights.
Cocaine-evoked increases in dopamine signaling are greater in the NAc shell vs core
Central GLP-1R activation suppresses phasic dopamine signaling in the NAc core
GLP-1R effects are not due to alterations in dopamine reuptake
Acknowledgments
The authors gratefully acknowledge the support of The National Institutes of Health (DA025634 to MFR) and the University of Illinois at Chicago (University Fellowship to SMF). The authors would like to thank Dr. Robert Wheeler and Mykel Robbie for their assistance with jugular vein catheterization.
Footnotes
Conflict of interest
The authors have no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 2.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 3.Gonon FG, Buda MJ. Regulation of dopamine release by impulse flow and by autoreceptors as studied by in vivo voltammetry in the rat striatum. Neuroscience. 1985;14:765–74. doi: 10.1016/0306-4522(85)90141-1. http://www.ncbi.nlm.nih.gov/pubmed/2986044. [DOI] [PubMed] [Google Scholar]
- 4.Garris PA, Christensen JR, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem. 1997;68:152–61. doi: 10.1046/j.1471-4159.1997.68010152.x. http://www.ncbi.nlm.nih.gov/pubmed/8978721. [DOI] [PubMed] [Google Scholar]
- 5.Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–42. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD. Mesolimbic dopamine signals the value of work. Nat Neurosci. 2016;19:117–26. doi: 10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH. A causal link between prediction errors, dopamine neurons and learning. Nat Neurosci. 2013;16:966–73. doi: 10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker NF, Cameron CM, Taliaferro JP, Lee J, Choi JY, Davidson TJ, Daw ND, Witten IB. Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat Neurosci. 2016;19:845–54. doi: 10.1038/nn.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.du Hoffmann J, Nicola SM. Dopamine Invigorates Reward Seeking by Promoting Cue-Evoked Excitation in the Nucleus Accumbens. J Neurosci. 2014;34:14349–14364. doi: 10.1523/JNEUROSCI.3492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritz M, Lamb R, Goldberg, Kuhar M. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science (80-) 1987;237 doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 11.Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci U S A. 2006;103:9333–8. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–9. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Covey DP, Roitman MF, Garris PA. Illicit dopamine transients: reconciling actions of abused drugs. Trends Neurosci. 2014;37:200–10. doi: 10.1016/j.tins.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koulchitsky S, De Backer B, Quertemont E, Charlier C, Seutin V. Differential effects of cocaine on dopamine neuron firing in awake and anesthetized rats. Neuropsychopharmacology. 2012;37:1559–71. doi: 10.1038/npp.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulton S. Appetite and reward. Front Neuroendocrinol. 2010;31:85–103. doi: 10.1016/j.yfrne.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Engel JA, Jerlhag E. Role of Appetite-Regulating Peptides in the Pathophysiology of Addiction: Implications for Pharmacotherapy. CNS Drugs. 2014;28:875–886. doi: 10.1007/s40263-014-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–64. doi: 10.1016/s0031-9384(02)00759-x. http://www.ncbi.nlm.nih.gov/pubmed/12117572. [DOI] [PubMed] [Google Scholar]
- 18.Shen M, Jiang C, Liu P, Wang F, Ma L. Mesolimbic leptin signaling negatively regulates cocaine-conditioned reward. Transl Psychiatry. 2016;6:e972. doi: 10.1038/tp.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalev U, Yap J, Shaham Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. http://www.ncbi.nlm.nih.gov/pubmed/11160414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wellman PJ, Clifford PS, Rodriguez JA. Ghrelin and ghrelin receptor modulation of psychostimulant action. Front Neurosci. 2013;7:171. doi: 10.3389/fnins.2013.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jerlhag E, Egecioglu E, Dickson SL, Engel JA. Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl) 2010;211:415–22. doi: 10.1007/s00213-010-1907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340:80–7. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Davis KW, Wellman PJ, Clifford PS. Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept. 2007;140:148–52. doi: 10.1016/j.regpep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jerlhag E, Engel JA. Ghrelin receptor antagonism attenuates nicotine-induced locomotor stimulation, accumbal dopamine release and conditioned place preference in mice. Drug Alcohol Depend. 2011;117:126–31. doi: 10.1016/j.drugalcdep.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 2011;152:3103–12. doi: 10.1210/en.2011-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes MR, Kanoski SE, De Jonghe BC, Leichner TM, Alhadeff AL, Fortin SM, Arnold M, Langhans W, Grill HJ. The common hepatic branch of the vagus is not required to mediate the glycemic and food intake suppressive effects of glucagon-like-peptide-1. AJP Regul Integr Comp Physiol. 2011;301:R1479–R1485. doi: 10.1152/ajpregu.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Näslund E, Barkeling B, King N, Gutniak M, Blundell JE, Holst JJ, Rössner S, Hellström PM. Energy intake and appetite are suppressed by glucagon-like peptide-1 (GLP-1) in obese men. Int J Obes Relat Metab Disord. 1999;23:304–11. doi: 10.1038/sj.ijo.0800818. http://www.ncbi.nlm.nih.gov/pubmed/10193877. [DOI] [PubMed] [Google Scholar]
- 28.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–68. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Peptides that regulate food intake: glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1427–35. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 30.Alhadeff AL, Mergler BD, Zimmer DJ, Turner CA, Reiner DJ, Schmidt HD, Grill HJ, Hayes MR. Endogenous Glucagon-Like Peptide-1 Receptor Signaling in the Nucleus Tractus Solitarius is Required for Food Intake Control. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci. 2012;32:4812–20. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alhadeff AL, Rupprecht LE, Hayes MR. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–58. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dossat AM, Lilly N, Kay K, Williams DL. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci. 2011;31:14453–7. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard JE, Anderberg RH, Göteson A, Gribble FM, Reimann F, Skibicka KP. Activation of the GLP-1 Receptors in the Nucleus of the Solitary Tract Reduces Food Reward Behavior and Targets the Mesolimbic System. PLoS One. 2015;10:e0119034. doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Egecioglu E, Engel JA, Jerlhag E. The glucagon-like peptide 1 analogue, exendin-4, attenuates the rewarding properties of psychostimulant drugs in mice. PLoS One. 2013;8:e69010. doi: 10.1371/journal.pone.0069010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham DL, Erreger K, Galli A, Stanwood GD. GLP-1 analog attenuates cocaine reward. Mol Psychiatry. 2013;18:961–2. doi: 10.1038/mp.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology. 2016;41:1917–1928. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayes MR, Schmidt HD. GLP-1 influences food and drug reward. Curr Opin Behav Sci. 2016;9:66–70. doi: 10.1016/j.cobeha.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sørensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G, Galli A, Fink-Jensen A. The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav. 2015;149:262–8. doi: 10.1016/j.physbeh.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owesson-White CA, Ariansen J, Stuber GD, Cleaveland NA, Cheer JF, Wightman RM, Carelli RM. Neural encoding of cocaine-seeking behavior is coincident with phasic dopamine release in the accumbens core and shell. Eur J Neurosci. 2009;30:1117–27. doi: 10.1111/j.1460-9568.2009.06916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aragona BJ, Day JJ, Roitman MF, Cleaveland NA, Wightman RM, Carelli RM. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur J Neurosci. 2009;30:1889–99. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambroggi F, Ghazizadeh A, Nicola SM, Fields HL. Roles of nucleus accumbens core and shell in incentive-cue responding and behavioral inhibition. J Neurosci. 2011;31:6820–30. doi: 10.1523/JNEUROSCI.6491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–63. doi: 10.1523/JNEUROSCI.21-21-08655.2001. http://www.ncbi.nlm.nih.gov/pubmed/11606653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–95. doi: 10.1523/JNEUROSCI.20-19-07489.2000. http://www.ncbi.nlm.nih.gov/pubmed/11007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- 46.Lecca D, Cacciapaglia F, Valentini V, Acquas E, Di Chiara G. Differential neurochemical and behavioral adaptation to cocaine after response contingent and noncontingent exposure in the rat. Psychopharmacology (Berl) 2007;191:653–667. doi: 10.1007/s00213-006-0496-y. [DOI] [PubMed] [Google Scholar]
- 47.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr Opin Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 48.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. http://www.ncbi.nlm.nih.gov/pubmed/12445717. [DOI] [PubMed] [Google Scholar]
- 49.Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–31. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stuber GD, Roitman MF, Phillips PEM, Carelli RM, Wightman RM. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–63. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- 51.Nasim K, B Indwelling jugular vein catheterization in the unrestrained conscious rat. 2008;2:527–536. [Google Scholar]
- 52.Carelli RM, Deadwyler SA. A Comparison of Nucleus Accumbens Neuronal Firing Patterns during Cocaine Self-Administration and Water Reinforcement in Rats. J Neurosci. 1994;74:7735–7746. doi: 10.1523/JNEUROSCI.14-12-07735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2007 doi: 10.1016/0165-0270(80)90021-7. http://searchworks.stanford.edu/view/5790940. [DOI] [PubMed]
- 54.Fortin SM, Cone JJ, Ng-Evans S, McCutcheon JE, Roitman MF. Sampling phasic dopamine signaling with fast-scan cyclic voltammetry in awake, behaving rats. Curr Protoc Neurosci. 2015;70:7.25.1–7.25.20. doi: 10.1002/0471142301.ns0725s70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanoski SE, Rupprecht LE, Fortin SM, De Jonghe BC, Hayes MR. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology. 2012;62:1916–27. doi: 10.1016/j.neuropharm.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heien MLAV, Johnson MA, Wightman RM. Resolving neurotransmitters detected by fast-scan cyclic voltammetry. Anal Chem. 2004;76:5697–704. doi: 10.1021/ac0491509. [DOI] [PubMed] [Google Scholar]
- 57.Sinkala E, McCutcheon JE, Schuck MJ, Schmidt E, Roitman MF, Eddington DT. Electrode calibration with a microfluidic flow cell for fast-scan cyclic voltammetry. Lab Chip. 2012;12:2403–8. doi: 10.1039/c2lc40168a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yorgason JT, España RA, Jones SR. Demon Voltammetry and Analysis software: Analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cone JJ, Chartoff EH, Potter DN, Ebner SR, Roitman MF. Prolonged high fat diet reduces dopamine reuptake without altering DAT gene expression. PLoS One. 2013;8:e58251. doi: 10.1371/journal.pone.0058251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–8. doi: 10.1073/pnas.92.26.12304. http://www.ncbi.nlm.nih.gov/pubmed/8618890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jones SR, Lee TH, Wightman RM, Ellinwood EH. Effects of intermittent and continuous cocaine administration on dopamine release and uptake regulation in the striatum: in vitro voltammetric assessment. Psychopharmacology (Berl) 1996;126:331–8. doi: 10.1007/BF02247384. http://www.ncbi.nlm.nih.gov/pubmed/8878349. [DOI] [PubMed] [Google Scholar]
- 63.Carlezon WA, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacology (Berl) 1995;122:194–197. doi: 10.1007/BF02246095. [DOI] [PubMed] [Google Scholar]
- 64.Ikemoto S, Qin M, Liu ZH. The functional divide for primary reinforcement of D-amphetamine lies between the medial and lateral ventral striatum: is the division of the accumbens core, shell, and olfactory tubercle. J Neurosci. 2005;25:5061–5. doi: 10.1523/JNEUROSCI.0892-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, McBride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303:1216–26. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- 66.Fenu S, Spina L, Rivas E, Longoni R, Di Chiara G. Morphine-conditioned single-trial place preference: role of nucleus accumbens shell dopamine receptors in acquisition, but not expression. Psychopharmacology (Berl) 2006;187:143–153. doi: 10.1007/s00213-006-0415-2. [DOI] [PubMed] [Google Scholar]
- 67.Spina L, Fenu S, Longoni R, Rivas E, Di Chiara G. Nicotine-conditioned single-trial place preference: selective role of nucleus accumbens shell dopamine D1 receptors in acquisition. Psychopharmacology (Berl) 2006;184:447–455. doi: 10.1007/s00213-005-0211-4. [DOI] [PubMed] [Google Scholar]
- 68.Sellings LHL, McQuade LE, Clarke PBS. Evidence for Multiple Sites within Rat Ventral Striatum Mediating Cocaine-Conditioned Place Preference and Locomotor Activation. J Pharmacol Exp Ther. 2006;317 doi: 10.1124/jpet.105.100339. [DOI] [PubMed] [Google Scholar]
- 69.Sellings LHL, McQuade LE, Clarke PBS. Characterization of dopamine-dependent rewarding and locomotor stimulant effects of intravenously-administered methylphenidate in rats. Neuroscience. 2006;141:1457–68. doi: 10.1016/j.neuroscience.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 70.Sellings LHL, Clarke PBS. Segregation of amphetamine reward and locomotor stimulation between nucleus accumbens medial shell and core. J Neurosci. 2003;23:6295–303. doi: 10.1523/JNEUROSCI.23-15-06295.2003. http://www.ncbi.nlm.nih.gov/pubmed/12867514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine. Behav Brain Res. 2006;169:320–4. doi: 10.1016/j.bbr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 73.Bari AA, Pierce RC. D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience. 2005;135:959–968. doi: 10.1016/j.neuroscience.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 74.Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- 75.Roitman MF, Stuber GD, Phillips PEM, Wightman RM, Carelli RM. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–71. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–8. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 77.Smith-Roe SL, Kelley AE. Coincident Activation of NMDA and Dopamine D1Receptors within the Nucleus Accumbens Core Is Required for Appetitive Instrumental Learning. J Neurosci. 2000;20 doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ. Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci. 2001;21:9471–7. doi: 10.1523/JNEUROSCI.21-23-09471.2001. http://www.ncbi.nlm.nih.gov/pubmed/11717381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–32. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30:16585–600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-Predictive Cues Enhance Excitatory Synaptic Strength onto Midbrain Dopamine Neurons. Science (80-) 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Willuhn I, Burgeno LM, Everitt BJ, Phillips PEM. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci U S A. 2012;109:20703–8. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Skibicka KP. The central GLP-1: implications for food and drug reward. Front Neurosci. 2013;7:181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dixit TS, Sharma AN, Lucot JB, Elased KM. Antipsychotic-like effect of GLP-1 agonist liraglutide but not DPP-IV inhibitor sitagliptin in mouse model for psychosis. Physiol Behav. 2013;114–115:38–41. doi: 10.1016/j.physbeh.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 85.Mietlicki-Baase EG, Ortinski PI, Reiner DJ, Sinon CG, McCutcheon JE, Pierce RC, Roitman MF, Hayes MR. Glucagon-like peptide-1 receptor activation in the nucleus accumbens core suppresses feeding by increasing glutamatergic AMPA/kainate signaling. J Neurosci. 2014;34:6985–92. doi: 10.1523/JNEUROSCI.0115-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vander Weele CM, Porter-Stransky KA, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, Aragona BJ. Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. Eur J Neurosci. 2014;40:3041–54. doi: 10.1111/ejn.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- 88.Schmitz Y, Schmauss C, Sulzer D. Altered Dopamine Release and Uptake Kinetics in Mice Lacking D2 Receptors. J Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-18-08002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine Distorts Stimulation-Dependent Dopamine Overflow: Effects on D2 Autoreceptors, Transporters, and Synaptic Vesicle Stores. J Neurosci. 2001;21 doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kastin AJ, Akerstrom V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int J Obes. 2003;27:313–318. doi: 10.1038/sj.ijo.0802206. [DOI] [PubMed] [Google Scholar]
- 91.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. http://www.ncbi.nlm.nih.gov/pubmed/9886047. [DOI] [PubMed] [Google Scholar]
- 92.Liu S, Borgland SL. Regulation of the mesolimbic dopamine circuit by feeding peptides. Neuroscience. 2015;289:19–42. doi: 10.1016/j.neuroscience.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 93.Göke R, Larsen PJ, Mikkelsen JD, Sheikh SP. Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci. 1995;7:2294–300. doi: 10.1111/j.1460-9568.1995.tb00650.x. http://www.ncbi.nlm.nih.gov/pubmed/8563978. [DOI] [PubMed] [Google Scholar]
- 94.Harasta AE, Power JM, von Jonquieres G, Karl T, Drucker DJ, Housley GD, Schneider M, Klugmann M. Septal Glucagon-Like Peptide 1 Receptor Expression Determines Suppression of Cocaine-Induced Behavior. Neuropsychopharmacology. 2015;40:1969–78. doi: 10.1038/npp.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–7. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abizaid A, Liu ZW, Andrews ZB, Shanabrough M, Borok E, Elsworth JD, Roth RH, Sleeman MW, Picciotto MR, Tschöp MH, Gao XB, Horvath TL. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–8. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- 98.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–10. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 99.Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–37. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 100.van Bloemendaal L, IJzerman RG, ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, Veltman DJ, Diamant M. GLP-1 Receptor Activation Modulates Appetite- and Reward-Related Brain Areas in Humans. Diabetes. 2014;63 doi: 10.2337/db14-0849. [DOI] [PubMed] [Google Scholar]
- 101.Kinzig KP, D’Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci. 2002;22:10470–6. doi: 10.1523/JNEUROSCI.22-23-10470.2002. http://www.ncbi.nlm.nih.gov/pubmed/12451146. [DOI] [PMC free article] [PubMed] [Google Scholar]