Abstract
Maternal addiction constitutes a major public health problem affecting children, with high rates of abuse, neglect, and foster care placement. However, little is known about the ways in which substance addiction alters brain function related to maternal behavior. Prior studies have shown that infant face cues activate similar dopamine‐associated brain reward regions to substances of abuse. Here, we report on a functional MRI study documenting that mothers with addictions demonstrate reduced activation of reward regions when shown reward‐related cues of their own infants. Thirty‐six mothers receiving inpatient treatment for substance addiction were scanned at 6 months postpartum, while viewing happy and sad face images of their own infant compared to those of a matched unknown infant. When viewing happy face images of their own infant, mothers with addictions showed a striking pattern of decreased activation in dopamine‐ and oxytocin‐innervated brain regions, including the hypothalamus, ventral striatum, and ventromedial prefrontal cortex—regions in which increased activation has previously been observed in mothers without addictions. Our results are the first to demonstrate that mothers with addictions show reduced activation in key reward regions of the brain in response to their own infant's face cues. Hum Brain Mapp 38:5421–5439, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: maternal, addiction, reward, dopamine, oxytocin, infant
INTRODUCTION
Most mothers find engaging with their infants to be a uniquely rewarding and gratifying experience. With the birth of the infant, many facets of a woman's life that used to take precedence recede, and the infant becomes the center of the mother's attention and affection. Our previous work has shown that an infant's face, particularly when smiling, has unique salience and reward value to new mothers [Strathearn et al., 2008]. This reward experience may underlie and promote mother–infant attachment, motivating mothers to continue to care for their infants in the face of extreme fatigue or other competing needs. However, two decades of animal and human research have shown that this critical bond between the mother and her infant may be compromised as a result of maternal substance‐use behaviors, especially substance addictions. Even during intermittent periods of sobriety, mothers with addictions are observed to be less attentive and responsive, while more intrusive and hostile, to their infants [Strathearn and Mayes, 2010]. Compared to mothers without addictions, they find infant cues to be less gratifying and more stressful, which places their infants at increased risk for abuse or neglect [Rutherford and Mayes, 2017; Rutherford et al., 2011].
According to the most recent national survey, 24.6 million Americans aged 12 years or older (9.4% of the population) were active illicit substance users [Substance Abuse and Mental Health Services Administration, 2014]. Binge alcohol use was reported by 60.1 million (22.9%) and 21.6 million (8.2%) qualified for a substance‐use disorder as defined by the fourth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR) [American Psychiatric Association, 2000]. Among women who are substance users, approximately 90% are of reproductive age [Kuczkowski, 2007]. Annually, an estimated 212,000 pregnancies involve illicit drugs, 370,000 involve alcohol, and 606,000 involve tobacco [Martin et al., 2015; Substance Abuse and Mental Health Services Administration, 2014]. While many women abstain from substance use during pregnancy, substance use is often rapidly resumed after child birth, with 30% of mothers drinking, 20% smoking, and 10% binge‐drinking within 3 months of their child's birth [Substance Abuse and Mental Health Services Administration, 2009]. Substance use increases the odds of child neglect fourfold and is involved in up to 80% of child maltreatment cases [Barth, 2009] and 60% of infant out‐of‐home placements [Child Welfare Information Gateway, 2014; Wulczyn et al., 2011]. The annual public health burden related to substance misuse is estimated to be half a trillion dollars [Centers for Disease Control and Prevention, 2014; National Drug Intelligence Center, 2014; U.S. Department of Health and Human Services, 2014], although adverse consequences associated with maternal substance use extend far beyond this, taking a major toll on the well‐being and development of the young children who are affected.
Ultimately, offspring growth and development hinge on maternal neurobiological adaptation during the prepartum and postpartum period (Fig. 1). Rodent models have been instrumental in this line of work and have elucidated the following. Estrogen and progesterone rise steadily during pregnancy until progesterone drops sharply prior to parturition [Brunton and Russell, 2010]. As parturition approaches, there is heightened sensitivity to oxytocin (OT) through increased OT receptor production [Numan and Woodside, 2010; Rilling and Young, 2014]. The medial preoptic area (MPOA) of the hypothalamus, a region rich in OT receptors [Champagne et al., 2001], is thought to monitor changes in hormonal levels over the course of pregnancy and stimulate the onset of maternal behavior at parturition via interaction with the mesolimbic motivational circuitry [Rilling and Young, 2014; Stolzenberg and Numan, 2011]. The MPOA directly projects to the ventral tegmental area (VTA), ventral striatum (VS), and medial prefrontal cortex (mPFC) and induces dopamine (DA) release into the mesocorticolimbic DA pathway [Afonso et al., 2009; Pereira and Morrell, 2011; Stolzenberg and Numan, 2011; Vertes, 2004]. As a key modulatory site of maternal circuitry, the VS integrates corticolimbic inputs and projects to the ventral pallidum [Groenewegen et al., 1999; Pereira and Morrell, 2011; Sesack and Grace, 2010] to direct the mother's responsiveness toward infant cues. The mPFC, with its interconnections with the hypothalamic and mesolimbic structures and its established role in attention allocation, organization, and sequencing [Pereira and Morrell, 2011; Vertes, 2004], is central to the orchestration of a complex array of well‐timed and organized maternal behaviors.
Figure 1.
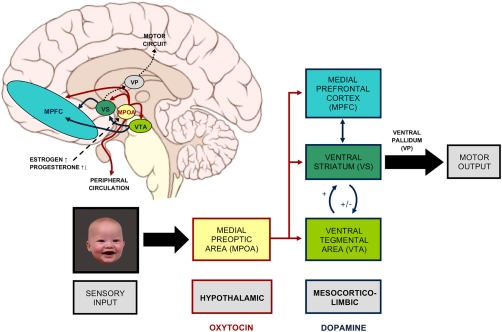
Hypothalamic and mesocorticolimbic regulation of maternal brain response to infant cues. These OT‐ and DA‐innervated brain regions are critical for the occurrence of maternal behavior. Brain schematic by P. J. Lynch [2006] (CC BY 2.5). [Color figure can be viewed at http://wileyonlinelibrary.com]
The involvement of these regions in the expression of maternal behavior has been studied extensively in rodents. Estrogen, OT, or DA release into the MPOA elicits maternal behavior in virgin rats, which are normally aversive toward rat pups, while lesions of the MPOA abolish many aspects of maternal behavior in postpartum mothers [Bridges et al., 1997; Numan et al., 1977; Pedersen et al., 1994; Stolzenberg et al., 2007]. VS neurons show increased Fos expression during mother–pup interactions [Stack et al., 2002], and the magnitude of DA release in the VS measured in vivo corresponds with immediately observed levels of maternal behavior [Champagne et al., 2004]. Increased DA input into the VS facilitates maternal responses [Champagne et al., 2004; Stolzenberg et al., 2007], whereas depletion of DA in the VS or lesions of the VS lead to impaired maternal behavior [Li and Fleming, 2003; Numan et al., 2005]. The role of cortical systems has garnered relatively little attention in rodent models of maternal behavior. However, lesions [Afonso et al., 2007] or localized inhibitions [Febo et al., 2010] of the medial aspects of the cortex, including the mPFC, or blockade of OT receptors within the mPFC [Sabihi et al., 2014] have been shown to disrupt the organization and sequential execution of maternal behavior.
Although our understanding of the corresponding neurobiology in humans is limited to broad conclusions drawn from neuroimaging data and may lack the specificity and sophistication of rodent models, recent data suggest that maternal behavior is similarly regulated by a core set of neural circuits and physiological processes across mammals, from rodents to humans [Numan and Woodside, 2010; Rilling and Young, 2014]. A steadily growing body of human neuroimaging studies converges with decades of rodent research pointing to the hypothalamic, mesolimbic, and cortical contributions to maternal behavior. Our previous work has demonstrated activations of OT‐ and DA‐innervated brain regions, including the hypothalamus, VS, and mPFC, in first‐time mothers when they viewed face images, particularly smiling images, of their own infant compared to those of a demographically matched unknown infant [Strathearn and Kim, 2013; Strathearn et al., 2008]. Differential activations of these regions reflected individual differences in maternal attachment and corresponded with the magnitude of maternal peripheral OT response during periods of mother–infant contact [Strathearn et al., 2009]. Although infant cues, particularly smiling cues from one's own infant, are powerful stimuli evoking widespread activation of the maternal brain, accumulating evidence demonstrates that maternal care is most importantly implicated in two key neural networks in humans: the limbic circuits regulating motivation/salience (e.g., hypothalamus, VTA, VS, amygdala, anterior cingulate cortex) and the cortical circuits subserving social cognition/empathy (e.g., mPFC, insula, orbitofrontal cortex, superior temporal gyrus [STG], inferior parietal lobule [IPL], inferior frontal gyrus) [Atzil et al., 2012; Barrett et al., 2012; Strathearn et al., 2008; Swain et al., 2014]. Increasing attention has been directed to how central components of the maternal circuitry go awry in at‐risk mothering and what prevention and intervention efforts may help reverse these abnormalities.
With maternal substance misuse, the DA mesocorticolimbic pathway, which is integral to the establishment of maternal behavior, may be co‐opted by addictive behaviors [Strathearn and Mayes, 2010]. DA‐regulated reward pathways that are involved in addiction overlap with those that are key to maternal caregiving, including the hypothalamus, VTA, VS, and mPFC [Grant et al., 2006; Koob and Volkow, 2010; Luscher and Malenka, 2011]. Addictive substances, such as cocaine and amphetamine, induce a surge of DA in the VTA and VS, which may positively reinforce subsequent substance use, and over time may result in multiple neuroadaptations in the mesolimbic DA system [Luscher and Malenka, 2011; Wolf, 2002]. With extended substance use, the DA reward threshold may be recalibrated to a level achieved through use of addictive substances, and a shift is made within the reward system. When such a shift occurs, the reward system may then function to modulate gratification and relief associated with addictive substances rather than to orient the individual to salient natural rewards [Koob and Volkow, 2010; Rutherford et al., 2011].
Strides have been made in empirically delineating this co‐opting of the DA mesocorticolimbic pathway in rodent models. These studies have primarily relied on cocaine administration and have documented cocaine‐induced deficits in maternal behavior [see Nephew and Febo, 2012 for review], likely via targeted actions on the MPOA, VS, and mPFC [Febo and Ferris, 2007; Mattson and Morrell, 2005; Vernotica et al., 1999]. These actions may be mediated in part by disruptions in the OT system, as cocaine administration suppresses levels of OT, OT neurons, and OT receptors in these regions, particularly in the MPOA where these actions have been studied most [Johns et al., 1997, 2004]. The study of corresponding mechanisms in humans is still in a nascent stage, with only two studies appearing on this topic to date. The first demonstrated reduced plasma OT levels in postpartum mothers reporting gestational cocaine use [Light et al., 2004]. The other is the only existing functional magnetic resonance imaging (fMRI) study of maternal brain responses in substance‐using mothers [Landi et al., 2011], documenting reduced prefrontal and limbic activations in response to unfamiliar face images and cries of infants. Perhaps due to the use of generic images of infants rather than images of the mothers' own infants, no findings emerged in this study with respect to the mothers' hypothalamic‐mesolimbic activations.
Here, we report on the first human study examining how the mother's brain response to her own infant is modified in the presence of substance addiction. We employed a well‐validated fMRI paradigm [Kim et al., 2014a; Strathearn and Kim, 2013; Strathearn et al., 2008, 2009] in which mothers with addictions viewed face images of their own infants in the scanner, interspersed with those of unknown infants. While both happy and sad faces were utilized, the aim of the present study was to examine activations of OT‐ and DA‐innervated brain regions in mothers with addictions in response to what is typically a robust rewarding cue from their own infant—the infant's smiling face. These cues typically form the basis of mother–infant attachment through the intrinsic reward value they hold for mothers and have previously been shown to evoke robust activations of the hypothalamic and mesocorticolimbic brain regions, in comparison to smiling faces of unknown infants, in mothers without addictions [Strathearn and Kim, 2013; Strathearn et al., 2009]. We predicted that mothers with addictions will demonstrate compromised brain response to their own infant's faces, particularly happy faces, and that this will be most prominently reflected in patterns of activations in OT‐ and DA‐innervated brain regions.
MATERIALS AND METHODS
Participants
Thirty‐six postpartum mothers ranging in age from 21 to 47 (M = 27.8 ± 5.6) years were recruited from an inpatient treatment facility1 for substance‐use disorders. All participants were English speaking, met criteria for one or more substance‐use disorder within the past year as assessed by the Mini International Neuropsychiatric Interview 6.0 (MINI) [Sheehan et al., 1998], and reported a history of substance use during the most recent pregnancy. Potential participants were excluded if any of the following criteria were met: (a) severe psychiatric symptoms requiring inpatient hospitalization; (b) past or present diagnosis of schizophrenia or other psychotic disorders; (c) pending legal cases (e.g., outstanding arrest warrants or parental rights hearings); (d) insufficient English fluency precluding interview assessments; (e) contraindications for MRI scanning; (f) out‐of‐home placement of infant for the past month or > 50% of infant's life; or (g) premature birth of infants, clinical evidence of in‐utero drug effects, or other infant medical complications. Of the 40 participants who completed the scanning visit, one participant was excluded due to the use of a narcotic drug that compromised her alertness during the visit; the scans of three additional participants were discarded due to motion artifacts and technical problems. Ethics approval for this study was granted by the institutional review board at Baylor College of Medicine. All participants provided written informed consent in accordance with the Declaration of Helsinki.
Study Design
Mothers were screened for study enrollment by licensed chemical dependency counselors and trained research assistants upon admission to the treatment facility. Eligible and enrolled mothers were transported to the Attachment and Neurodevelopment Laboratory for study visits. Infants were approximately 5‐month old (M = 5.0 ± 2.9) at the time of the first study visit, at which time infant face images were recorded, and mothers underwent fMRI scanning2 at approximately 6 months (M = 6.0 ± 3.4) postpartum, viewing face images of their own and a matched unknown infant. Upon completion of the scan, mothers provided ratings on how they perceived the infant to be feeling in each image (i.e., happy faces: “how happy do you think the baby was feeling?”; sad faces: “how sad do you think the baby was feeling?” and “how distressed do you think the baby was feeling?”) as well as their affective response to viewing each infant face (i.e., happy faces: “how happy did this picture make you feel?”; sad faces: “how sad did this picture make you feel?” and “how distressed did this picture make you feel?”).
Assessment of Substance Use
We used the MINI [Sheehan et al., 1998] to assess the DSM‐IV diagnoses of substance‐use disorders and the Addiction Severity Index (ASI‐Lite) [McLellan et al., 1992] to evaluate the nature and extent of past and present substance use. The Alcohol Use Disorders Identification Test (AUDIT) [Babor et al., 1992] and the Fagerstrom Test for Nicotine Dependence (FTND) [Heatherton et al., 1991] were used to measure the severity of alcohol and tobacco use, respectively. Urine toxicology screens and alcohol breathalyzer tests were performed at the outset of each scanning session.
Stimuli and fMRI Paradigm
Experimental stimuli consisted of 40 infant‐face images from four experimental conditions: 10 own‐happy (OH), 10 unknown‐happy (UH), 10 own‐sad (OS), and 10 unknown‐sad (US) infant faces (Fig. 2). For each mother, still face images were captured from the videos recorded of her own infant and of a single unknown infant matched on age, race, and affect intensity (and sex, if distinguishable). The video‐recording was undertaken at the laboratory approximately 5 months postpartum, while eliciting happy and sad expressions from each infant. Two trained raters with established reliability (kappa = 0.954, P < 0.001) classified still face images into one of four affect groups based on valence and intensity [see Cole et al., 1992], which provided the basis for the matching between own and unknown infant faces. The adequacy of classification and matching was confirmed by three independent female raters, who demonstrated that own and unknown infant face images did not differ in terms of affect intensity (M OH = 4.20 ± 0.03, M UH = 4.25 ± 0.02, P = 0.11; M OS = 1.50 ± 0.04, M US = 1.55 ± 0.04, P = 0.48).
Figure 2.
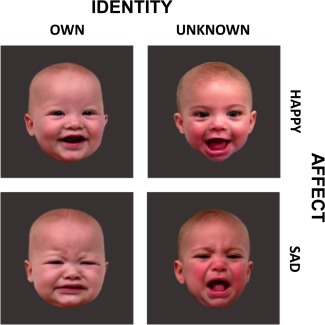
Infant face images used in the study. [Color figure can be viewed at http://wileyonlinelibrary.com]
Of the 40 total images, 12 own (i.e., 6 OH and 6 OS) and 12 unknown (i.e., 6 UH and 6 US) infant face images were selected for use in each of the two functional runs. Cry stimuli were also randomly interspersed with the face images, but were not the subject of the current paper. The face images were standardized for size (4.5 inches in height), adjusted for luminosity and contrast, displayed against a gray background, and projected onto an overhead mirror display for presentation to mothers during scanning. All images appeared in a pseudorandom sequence with a stimulus duration of 2 s and a random interstimulus interval of 2 to 11 s.
fMRI Data Acquisition
Imaging data were acquired using a 3‐Tesla Siemens Trio MRI system (Erlangen, Germany) with a standard 12‐channel head coil. Localizer images were acquired for prescribing the functional image volumes parallel to the intercommissural line. Functional images were obtained using a gradient‐echo echo‐planar imaging (EPI) sequence (32 axial‐oblique slices; repetition time [TR], 2,000 ms; echo time [TE], 30 ms; flip angle [FA], 80°; field of view [FOV], 220 mm × 220 mm; matrix, 64 × 64; in‐plane resolution, 3.4 mm × 3.4 mm; slice thickness, 4 mm), resulting in 121 images collected per 4:06 min functional run. Two types of anatomical images were acquired: T1 weighted anatomic scan using a fast low‐angle shot (FLASH) sequence (40 sagittal slices; TR, 20 ms; TE, 6.86 ms; FA, 25°; FOV, 240 mm × 240 mm; matrix, 256 × 241; in‐plane resolution, 1.0 mm × 0.9 mm; slice thickness, 4 mm) and high‐resolution anatomic scan using a magnetization‐prepared rapid gradient echo (MPRAGE) sequence (176 sagittal slices; TR, 2,530 ms; TE, 2.43 ms; FA, 7°; FOV, 256 mm × 256 mm; matrix, 256 × 246; in‐plane resolution, 1.0 mm × 1.0 mm; slice thickness, 1 mm) for 3D reconstruction.
Data Analysis
fMRI analysis
Preprocessing and analyses of the fMRI data were performed using BrainVoyager QX, version 2.3.1 (Brain Innovation, Maastricht, The Netherlands). Prior to analysis, blood oxygenation level‐dependent (BOLD) images were corrected for slice timing and realigned to the first volume for head motion correction. Head motion of < 2.0 mm translation or 2.0° rotation was deemed acceptable. Spatial smoothing was performed with a 4 mm full width at half maximum (FWHM) Gaussian kernel, followed by high‐pass filtering using a Fourier basis set of two cycles per run to remove low frequency drifts. Functional data were then coregistered with the anatomical data, transformed into 3 × 3 × 3 mm isotropic voxels, and normalized into the Talairach space.
For each run of each subject data, a general linear model (GLM) was specified, modeling the BOLD signal change for each experimental condition (OH, UH, OS, and US) using a double‐gamma hemodynamic response function. To adjust for signal magnitude variability and facilitate comparisons with other research [e.g., Atzil et al., 2011; Landi et al., 2011], the resulting regression weights (β) were then percent normalized to reflect percent signal change [Chen et al., 2017; Pernet, 2014; Poldrack et al., 2008] and concatenated across the entire group of 36 subjects for second‐level random‐effects analyses. Group t‐maps were generated representing percent BOLD contrasts between own and unknown infant conditions: OH versus UH and OS versus US. Analyses were performed at two levels: first using a whole‐brain voxel‐wise analysis, followed by a region‐of‐interest analysis of the bilateral striatum to evaluate within‐striatal differences in percent BOLD signals to our contrast of interest. The striatum was selected structurally, based on a mask derived from the subcortical atlas of the Brain Tutor HD, version 2.2 (Brain Innovation, Maastricht, The Netherlands), and comprised 289 and 302 voxels for the left and right, respectively. Post hoc examination of whole‐brain analysis results was conducted by extracting percent signal change values for OH and UH from cubes centered at local peaks within the cluster of (de)activation and extending up to ± 10 voxels per dimension, unless limited by the spatial extent of (de)activation. This allowed us to further contrast and visually display patterns of percent BOLD signals for our contrast of interest in our key hypothesized OT‐ and DA‐innervated regions. In all analyses, statistical threshold of false discovery rate (FDR) corrected q < 0.05 and a cluster threshold of ≥ 300 mm3 were used to determine clusters of significant activation. Supra‐threshold clusters of active voxels were labeled using a published atlas of the human brain [Mai et al., 2004] and the Talairach Client (Research Imaging Center, TX).
Postscan ratings analysis
Postscan ratings were analyzed with the STATA/SE, version 13.1 XTMIXED procedure. Mothers' ratings of affect, both how they perceived the infant to be feeling in each image and of their own affective responses to the image, were compared for own and unknown infant faces using mixed‐effects linear regression analysis that included a subject‐level random intercept. Mixed‐effects models were also used to probe for the association between mothers' two ratings to examine the degree to which mothers' own affective responses tracked with how they perceived the infant to be feeling. Model building was carried out as follows: (a) the initial model included the fixed main effects of infant identity (i.e., own vs. unknown) and mothers' perceived ratings of infant's affect; (b) a subject‐level random intercept was added to model systematic interindividual variability; and (c) interaction terms were added sequentially and retained in the model if they improved model fit. The models were fitted by maximum likelihood estimation, and nested models were contrasted using likelihood‐ratio chi‐squares.
RESULTS
Participant Characteristics
Participants' sociodemographic and substance‐use characteristics are shown in Tables 1 and 2, respectively. Participants were generally of low socioeconomic status, with only 33 percent completing any education beyond high school and 64 percent reporting an annual family income of less than $15,000. Twenty‐two percent of women had been court‐mandated to receive substance‐use treatment, and 58% had child protective services (CPS) involvement. Sixty‐seven percent (n = 24) were taking psychotropic medications at the time of the present study, including antipsychotic medications (n = 8), mood stabilizers (n = 2), medications for depression or anxiety (n = 13), and stimulant medication (n = 1). Because all women were recruited from an inpatient substance‐use treatment facility and the majority remained in treatment at the time of the study, most were not using substances at the time of the scanning visit. All except four women tested negative on a urine toxicology screen on the day of the scan, and none had a positive alcohol breathalyzer test. Results remained unchanged when the four subjects were excluded from the analyses; hence, data reported below include all subjects.
Table 1.
Sociodemographic characteristics of mothers and infants (N = 36)
| Maternal age, mean ± SD | 27.8 ± 5.6 |
| Infant sex, n (%) | |
| Male | 18 (50.0) |
| Female | 18 (50.0) |
| Maternal parity, n (%) | |
| Primiparous | 15 (41.7) |
| Multiparous | 21 (58.3) |
| Maternal ethnicitya, n (%) | |
| Hispanic or Latino | 12 (33.3) |
| Not Hispanic or Latino | 23 (63.9) |
| Maternal racea, n (%) | |
| White | 17 (47.2) |
| Black or African American | 11 (30.6) |
| Other | 4 (11.1) |
| Marital statusa, n (%) | |
| Single/never married | 17 (47.2) |
| Living together | 11 (30.6) |
| Separated/divorced/widowed | 6 (16.7) |
| Maternal educationa, n (%) | |
| Junior high school (7 to 9th grade) | 7 (19.4) |
| Some high school | 9 (25.0) |
| High school graduate | 7 (19.4) |
| Some college or higher | 12 (33.4) |
| Annual family incomea, n (%) | |
| < $15,000 | 23 (63.9) |
| $15,000 to 30,000 | 8 (22.2) |
| $30,001 to 45,000 | 3 (8.3) |
| > $100,000 | 1 (2.8) |
| Breastfeeding status, n (%) | |
| Still breastfeeding | 1 (2.8) |
| Not breastfeeding | 35 (97.2) |
Percentages do not total 100% due to missing data.
Table 2.
Substance‐use characteristics of mothers (N = 36)
| DSM‐IV substance‐use disordersa, n (%) | |
| Substance dependence | 33 (91.7) |
| Cocaine dependence | 16 (44.4) |
| Opioid dependence | 6 (16.7) |
| Amphetamine dependence | 5 (13.9) |
| Sedative, hypnotic, or anxiolytic dependence | 3 (8.3) |
| Cannabis dependence | 3 (8.3) |
| Substance abuse | 3 (8.3) |
| Cannabis abuse | 3 (8.3) |
| Substance of choice, n (%) | |
| Cocaine | 16 (44.4) |
| Narcotic | 6 (16.7) |
| Cannabis | 6 (16.7) |
| Stimulants | 5 (13.9) |
| Tranquilizers | 3 (8.3) |
| Substance use during pregnancy, n (%) | |
| Nicotine | 30 (83.3) |
| Alcohol | 21 (58.3) |
| Cocaine | 21 (58.3) |
| Cannabis | 18 (50.0) |
| Sedatives | 13 (36.1) |
| Opioids | 12 (33.3) |
| Amphetamines | 7 (19.4) |
| Others (Hallucinogens, Inhalants) | 3 (8.3) |
| Alcohol‐use severityb, n (%) | |
| Low level | 17 (47.2) |
| Medium level | 8 (22.2) |
| High level/possible dependency | 4 (11.2) |
| Tobacco‐use severityc, n (%) | |
| Very low dependence | 12 (33.3) |
| Low dependence | 8 (22.2) |
| High/very high dependence | 9 (25.0) |
| Extent of substance used, mean ± SD (range) | |
| Last 30 days in controlled environmente (days used) | |
| Illicit substance of choice | 0.14 ± 0.68 (0 to 4) |
| Cocaine | 0.07 ± 0.26 (0 to 1) |
| Narcotic | 0.80 ± 1.79 (0 to 4) |
| Cannabis | 0.00 ± 0.00 (0 to 0) |
| Stimulants | 0.00 ± 0.00 (0 to 0) |
| Tranquilizers | 0.00 ± 0.00 (0 to 0) |
| Any other illicit substancef | 0.19 ± 1.01 (0 to 6) |
| Last 30 days prior to admission to controlled environment (days used) | |
| Illicit substance of choice | 15.39 ± 12.73 (0 to 30) |
| Cocaine | 11.33 ± 11.35 (0 to 30) |
| Narcotic | 29.0 ± 2.24 (25 to 30) |
| Cannabis | 19.4 ± 14.72 (0 to 30) |
| Stimulants | 4.67 ± 8.08 (0 to 14) |
| Tranquilizers | 17 ± 14.73 (1 to 30) |
| Any other illicit substancef | 9.71 ± 11.08 (0 to 30) |
| Pregnancy (days used of 270 total days) | |
| Illicit substance of choice | 110.92 ± 107.55 (0 to 270) |
| Cocaine | 97.44 ± 102.90 (0 to 270) |
| Narcotic | 158.33 ± 130.24 (0 to 270) |
| Cannabis | 112.92 ± 106.64 (7.5 to 270) |
| Stimulants | 50.9 ± 48.84 (36 to 270) |
| Tranquilizers | 184.0 ± 148.96 (12 to 270) |
| Any other illicit substancef | 73.53 ± 103.85 (0 to 270) |
| Alcohol | 22.21 ± 35.83 (0 to 114) |
| Tobacco | 160.92 ± 114.59 (0 to 270) |
| Lifetime (years used) | |
| Illicit substance of choice | 7.94 ± 5.58 (0 to 29) |
| Cocaine | 9.27 ± 6.85 (1 to 29) |
| Narcotic | 7.0 ± 1.22 (5 to 8) |
| Cannabis | 10.8 ± 11.54 (0 to 30) |
| Stimulants | 6.67 ± 4.93 (1 to 10) |
| Tranquilizers | 6.0 ± 1.0 (5 to 7) |
| Total illicit substanceg | 10.83 ± 5.75 (0 to 29) |
| Alcohol | 9.31 ± 7.75 (0 to 36) |
DSM‐IV‐TR diagnoses of substance use disorders were given using the MINI. No mother received the diagnosis of substance dependence or abuse solely based on alcohol or tobacco use.
Severity of alcohol problems was determined by the AUDIT. An AUDIT score of ≤ 7 represented a low level, 8 to 15 represented a medium level, and 16 ≤ represented a high level of alcohol problems. The mean AUDIT score was 7.97 (SD = 7.24) for the current group of mothers.
Severity of tobacco use was assessed by the FTND. An FTND score of 0 to 2 indicated very low dependence, 3 to 4 indicated low dependence, 5 indicated medium dependence, 6 to 7 indicated high dependence, and 8 to 10 indicated very high dependence on nicotine. The mean FTND score was 3.10 (SD = 2.88) for the current group.
Extent (frequency) of substance use was assessed using the ASI‐Lite. The reported values refer to the number of days or years used during the respective period.
Controlled environment refers to residential treatment or incarceration.
Any other illicit substance refers to any illicit substances other than substance of choice (excluding alcohol and tobacco).
Total illicit substance refers to any combination of illicit substances including substance of choice (excluding alcohol and tobacco).
Note. MINI = Mini International Neuropsychiatric Interview 6.0; ASI = Addiction Severity Index‐Lite; AUDIT = Alcohol Use Disorders Identification Test; FTND = Fagerstrom Test for Nicotine Dependence.
fMRI Findings
Happy faces
As compared to viewing unknown happy infant faces, viewing the happy faces of their own infants induced significant activation in multiple brain regions of mothers with addictions, including the amygdala, hippocampus, thalamus, cingulate, and brainstem structures including the VTA and substantia nigra (Table 3), overlapping regions previously reported in normative groups of mothers [Strathearn and Kim, 2013; Strathearn et al., 2008; Swain et al., 2014]. Consistent with the extant literature on maternal brain responses, activations were also seen in the inferior frontal gyrus, ventral premotor cortex, insula, STG, visual association areas, and cerebellum.
Table 3.
Brain activation in mothers with addictions to the contrast of own happy (OH) versus unknown happy (UH)
| Hemisphere | Talairach coordinatesa | Peak t value | P | Volumeb (mm3) | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Frontal lobe | |||||||
| Superior Frontal Gyrus | Right | 2 | −5 | 60 | 7.99 | <0.00000001 | 507 |
| Precentral Gyrus | Right | 41 | −2 | 27 | 7.21 | 0.00000002 | 357 |
| Precentral Gyrus/Postcentral Gyrus | Left | −43 | −17 | 45 | 6.19 | 0.00000043 | 469 |
| Parietal lobe | |||||||
| Precentral Gyrus/Postcentral Gyrus | Right | 50 | −17 | 42 | 9.24 | <0.00000001 | 1,853 |
| Superior Parietal Lobule | Right | 23 | −59 | 30 | 6.14 | 0.00000051 | 360 |
| Temporal/Occipital lobe | |||||||
| Fusiform Gyrus/Inferior Temporal Gyrus/Occipital Gyri | Left | −28 | −47 | −18 | 9.37 | <0.00000001 | 8,044 |
| Occipital Gyri | Right | 29 | −77 | 0 | 7.51 | 0.00000001 | 2,275 |
| Fusiform Gyrus | Right | 32 | −44 | −15 | 7.40 | 0.00000001 | 2,631 |
| Sublobar regions | |||||||
| Middle Cingulate Gyrus | Right/Left | −1 | −5 | 33 | 10.10 | <0.00000001 | 1,395 |
| Limbicc | 2,869 | ||||||
| Dorsal Putamen/Globus Pallidus | Left | −22 | −2 | −6 | 8.77 | <0.00000001 | |
| Parahippocampal Gyrus | Left | −28 | −5 | −30 | 6.59 | 0.00000013 | |
| Insula/Inferior Frontal Gyrus | Left | −40 | 4 | 12 | 8.04 | <0.00000001 | 946 |
| Parahippocampal Gyrus/Lateral Amygdala | Right | 23 | −5 | −27 | 8.02 | <0.00000001 | 1,531 |
| Midbrain/limbicc | 8,895 | ||||||
| Thalamus | Left | −7 | −23 | 6 | 7.98 | <0.00000001 | |
| Substantia Nigra | Right | 4 | −26 | −9 | 7.26 | 0.00000002 | |
| Thalamus | Right | 2 | −14 | 8 | 7.01 | 0.00000004 | |
| Ventral Tegmental Area | Right/Left | −1 | −16 | −9 | 6.90 | 0.00000005 | |
| Hippocampus | Right | 23 | −23 | −6 | 6.75 | 0.00000008 | |
| Substantia Nigra | Left | −4 | −17 | −9 | 6.48 | 0.00000018 | |
| Globus Pallidus | Right | 17 | −5 | −6 | 6.59 | 0.00000013 | 324 |
| Posterior Cingulate Gyrus | Left | −4 | −53 | 9 | 6.36 | 0.00000026 | 720 |
| Cerebellum | |||||||
| Anterior Lobe, Culmen | Right | 29 | −50 | −21 | 9.38 | <0.00000001 | 4,395 |
| Anterior Lobe, Cerebellar Lingual | Left | −4 | −44 | −9 | 7.76 | <0.00000001 | 345 |
| Anterior Lobe, Culmen | Right | 14 | −59 | −18 | 6.25 | 0.00000036 | 602 |
| Posterior Lobe, Cerebellar Tonsil | Left | −7 | −50 | −30 | 6.21 | 0.00000041 | 494 |
Note. Data shown are from the activation map for the OH > UH contrast, thresholded at FDR‐corrected q < 0.001 and a minimum cluster size of 300 mm3. Results are presented at a more stringent threshold of q < 0.001 to better delineate specific neuroanatomical locations of activated clusters. Within each lobe, clusters are ordered by decreasing level of significance.
Talairach coordinates in millimeters with origin at the anterior commissure, representing peak voxels in each cluster. x, y, z coordinates refer to right (+) to left (−), anterior (+) to posterior(−), and superior (+) to inferior(−), respectively.
Number of contiguously activated voxels within a cluster.
Note that where no volume is given for an anatomical region, it forms part of a larger cluster that spans anatomically distinct regions.
However, as hypothesized, maternal brain response was significantly reduced in several key OT‐ and DA‐ innervated brain regions in own happy compared to unknown happy infant faces. Our whole‐brain analysis yielded a single cluster of deactivation amid clusters of highly significant activations (Fig. 3). The deactivated cluster encompassed the hypothalamus, bilateral VS, and bilateral ventromedial prefrontal regions spanning 258 voxels (Table 4 and Fig. 3a). Figure 3b displays a post hoc examination of local peaks within the deactivated cluster, depicting a negative percent BOLD signal change observed in key OT‐ and DA‐innervated regions in mothers with addictions while viewing their own infant's happy faces, in contrast to the positive change in percent BOLD signals seen in response to an unknown infant's face. Analysis within the striatum region‐of‐interest revealed that the observed deactivation was specific to the ventral portion of the striatum (Fig. 4).
Figure 3.
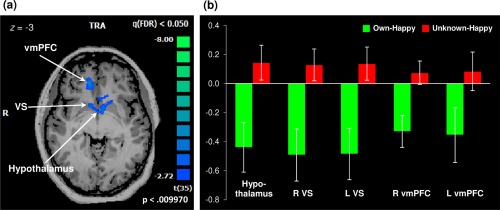
Reduced maternal brain response in mothers with addictions evidenced by a single cluster of deactivation observed for the own‐happy versus unknown‐happy contrast, encompassing the hypothalamic, bilateral VS, and bilateral vmPFC regions (258 voxels, P < 0.01, FDR‐corrected q < 0.05; Panel A). Striking deactivation is observed when mothers with addictions view their own infant's smiling faces, compared with unknown infant faces (Panel B). For a post hoc examination of local peaks within the deactivated cluster, percent BOLD signal values were extracted from cubes centered at the peak voxels of each anatomical region and extending up to ± 10 voxels per dimension, unless limited by the spatial extent of activation. Peak Talairach coordinates: hypothalamus, (–2, 1, −3); R VS, (8, 10, −3); L VS, (–13, 19, 3); R vmPFC, (11, 34, 6), L vmPFC, (–16, 37, 3). Error bars represent the standard error of the mean. VS = ventral striatum; vmPFC = ventromedial prefrontal cortex; R: right; L: left. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 4.
Brain deactivation in mothers with addictions to the contrast of own happy (OH) versus unknown happy (UH)
| Hemisphere | Talairach coordinatesa | Peak t value | P | Volumeb (mm3) | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Limbic lobe/Sublobar regions | 6970 | ||||||
| Ventromedial PFC | Right | 11 | 34 | 6 | −5.78 | 0.000002 | |
| Ventral Striatum | Left | −13 | 19 | 3 | −5.41 | 0.000005 | |
| Ventromedial PFC | Left | −16 | 37 | 3 | −4.60 | 0.000054 | |
| Ventral Striatum | Right | 8 | 10 | −3 | −4.38 | 0.000104 | |
| Hypothalamus | Right/Left | −2 | 1 | −3 | −3.32 | 0.002102 | |
Note. Data shown are from the activation map for the OH > UH contrast, thresholded at FDR‐corrected q < 0.05 and a minimum cluster size of 300 mm3.
Talairach coordinates in millimeters with origin at the anterior commissure, representing peak voxels in each cluster. x, y, z coordinates refer to right (+) to left (−), anterior (+) to posterior(−), and superior (+) to inferior(−), respectively.
Number of contiguously activated voxels within a cluster. Volume is not reported for each anatomical region, as each region forms part of a larger cluster that spans anatomically distinct regions.
Figure 4.
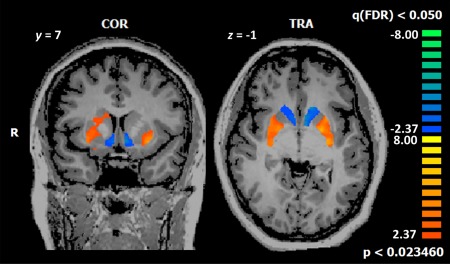
Striatal activation limited to the dorsal striatum, in response to own‐happy versus unknown‐happy infant faces (P < 0.02, FDR‐corrected q < 0.05). A contrasting pattern of deactivation is seen in the ventral striatum. [Color figure can be viewed at http://wileyonlinelibrary.com]
Sad faces
In our group of mothers with addictions, viewing the sad faces of their own infants relative to unknown infants evoked significant activation in brain regions that largely overlapped with those seen for the happy faces, including the cingulate, thalamus, substantia nigra, parahippocampal gyrus, inferior frontal gyrus, superior frontal gyrus, and cerebellum (Table 5). Activations were also present, and more widespread than those observed for happy faces, in the dorsomedial prefrontal cortex, the opercular part of the inferior frontal gyrus, and several parietal‐occipital regions, including the precuneus/paracentral lobule and occipital gyri. Our analysis yielded a single cluster of deactivation in the right IPL extending to the STG, spanning 12 voxels (peak Talairach coordinates: 50, −59, 36; t = −4.62; P = 0.000051). There was no activation or deactivation seen in the hypothalamus, VS, or ventromedial prefrontal regions in either whole‐brain or region‐of‐interest analysis of the striatum.
Table 5.
Brain activation in mothers with addictions to the contrast of own sad (OS) versus unknown sad (US)
| Hemisphere | Talairach coordinatesa | Peak t value | P | Volumeb (mm3) | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Frontal lobe | |||||||
| Precentral Gyrus | Right | 50 | −17 | 42 | 9.22 | <0.00000001 | 3,791 |
| Superior Frontal Gyrus | Right/Left | 2 | −5 | 60 | 8.30 | <0.00000001 | 1,525 |
| Precentral Gyrus/Postcentral Gyrus | Left | −46 | −17 | 39 | 7.20 | 0.00000002 | 899 |
| Inferior Frontal Gyrus | Right | 53 | 7 | 30 | 6.72 | 0.00000009 | 1,120 |
| Parietal lobe | |||||||
| Precuneus | Right | 17 | −71 | 33 | 7.96 | <0.00000001 | 1,018 |
| Temporal/Occipital lobe | |||||||
| Fusiform Gyrus | Left | −28 | −47 | −18 | 8.43 | <0.00000001 | 595 |
| Inferior Temporal Gyrus/Middle Temporal Gyrus | Left | −43 | −56 | −12 | 7.62 | 0.00000001 | 724 |
| Inferior Temporal Gyrus/Middle Temporal Gyrus | Right | 41 | −47 | 3 | 7.26 | 0.00000002 | 1,895 |
| Occipital Gyri | Right | 41 | −77 | −3 | 7.24 | 0.00000002 | 404 |
| Middle Temporal Gyrus/Occipital Gyri | Left | −37 | −74 | 18 | 6.66 | 0.00000011 | 1,128 |
| Cuneus | Left | −10 | −68 | 12 | 6.38 | 0.00000025 | 479 |
| Limbic/Sublobar regions | |||||||
| Substantia Nigra | Right/Left | −4 | −23 | −21 | 8.36 | <0.00000001 | 823 |
| Parahippocampal Gyrus/Thalamus | Right | 8 | −35 | 3 | 7.79 | <0.00000001 | 478 |
| Thalamus | Right/Left | −1 | −17 | 6 | 7.78 | <0.00000001 | 1,017 |
| Posterior Cingulate Gyrus | Left | −16 | −53 | 12 | 7.65 | 0.00000001 | 570 |
| Middle Cingulate Gyrus | Right/Left | −4 | 7 | 33 | 6.81 | 0.00000007 | 701 |
| Parahippocampal Gyrus/Thalamus | Left | −19 | −38 | −3 | 6.68 | 0.00000010 | 434 |
| Anterior Cingulate Gyrus | Left | −4 | 34 | 27 | 6.16 | 0.00000048 | 504 |
| Cerebellum | |||||||
| Anterior Lobe, Culmen/Posterior Lobe, Declive | Right | 32 | −50 | −21 | 9.08 | <0.00000001 | 1,844 |
| Anterior Lobe, Culmen/Posterior Lobe, Declive | Left | −28 | −56 | −21 | 7.96 | <0.00000001 | 1,878 |
| Anterior Lobe, Cerebellar Lingual | Right/Left | −1 | −44 | −9 | 7.32 | 0.00000002 | 461 |
| Anterior Lobe, Fastigium | Left | −7 | −56 | −18 | 6.09 | 0.00000059 | 322 |
Note. Data shown are from the activation map for the OS > US contrast, thresholded at FDR‐corrected q < 0.001 and a minimum cluster size of 300 mm3. Results are presented at a more stringent threshold of q < 0.001 to better delineate specific neuroanatomical locations of activated clusters. Within each lobe, clusters are ordered by decreasing level of significance.
Talairach coordinates in millimeters with origin at the anterior commissure, representing peak voxels in each cluster. x, y, z coordinates refer to right (+) to left (−), anterior (+) to posterior(−), and superior (+) to inferior(−), respectively.
Number of contiguously activated voxels within a cluster.
Postscan Rating Findings
Happy faces
Mothers with addictions reported perceiving happy faces of their own infants as happier than those of unknown infants (β identity = 0.84, 95% CI = 0.62 to 1.06, z = 7.39, P < 0.001; Table 6). Mothers' self‐reported affect was also more positive in response to their own infants compared to unknown infants (β identity = 2.45, 95% CI = 2.21 to 2.69, z = 19.69, P < 0.001; Table 6). Mothers' own report of positive affect correlated significantly with how happy they perceived infants to be feeling (β infant affect = 0.61, 95% CI = 0.51 to 0.70, z = 12.47, P < 0.001; Table 7). However, the strength of this association varied as a function of infant identity (β identity × infant affect = −0.33, 95% CI = −0.50 to −0.16, z = −3.76, P < 0.001; Table 7). Post hoc probing of the moderation revealed that this association was significantly stronger for unknown infants (β unknown = 0.61, 95% CI = 0.51 to 0.70, z = 12.47, P < 0.001) than for own infants (β own = 0.28, 95% CI = 0.13 to 0.42, z = 3.71, P < 0.001). This indicated that mothers' self‐reported positive affect increased more closely in line with their perceived increase in an unknown infant's positive affect than that of their own infant. In other words, mothers' own report of happiness increased to a greater degree when perceiving increased happiness in an unknown infant rather than in their own infant.
Table 6.
Mothers' postscan affect ratings of own and unknown infant face images
| Own infant | Unknown infant | Difference (z) a | |
|---|---|---|---|
| Mothers' perceived ratings of infants' affectb | |||
| Happy affect | 8.22 ± 0.12 | 7.39 ± 0.21 | 7.39** |
| Sad affect | 7.31 ± 0.24 | 6.70 ± 0.24 | 4.72** |
| Distressed affect | 7.13 ± 0.25 | 6.53 ± 0.28 | 4.26** |
| Mothers' own affective responses to infants' affectc | |||
| Happy affect | 8.54 ± 0.11 | 6.07 ± 0.36 | 19.69** |
| Sad affect | 6.97 ± 0.28 | 4.69 ± 0.32 | 13.95** |
| Distressed affect | 6.56 ± 0.35 | 4.51 ± 0.31 | 12.59** |
Note. Numbers shown (M ± SE) are mothers' self‐reported ratings of affect provided on a Likert scale of 1 to 9. For each given affect, 1 represented “not at all,” 5 represented “somewhat,” and 9 represented “very.”
z‐Statistic comparing own and unknown infant faces for each given affect within the final mixed‐effects linear regression model including a subject‐level random intercept.
Mothers' ratings of how they perceived the infant to be feeling in the image. For happy faces, mothers were asked, “how happy do you think the baby was feeling?” For sad faces, mothers were asked to respond to both, “how sad do you think the baby was feeling?” and, “how distressed do you think the baby was feeling?”
Mothers' ratings of their own affective responses to viewing the infant face image. For happy faces, mothers were asked, “how happy did this picture make you feel?” For sad faces, mothers were asked both, “how sad did this picture make you feel?” and, “how distressed did this picture make you feel?”
**P < 0.001.
Table 7.
Mixed‐effects regression models for mothers' postscan affect ratings
| Walda | Infant affect effecta | Identity effect | Infant affecta × Identity | |||
|---|---|---|---|---|---|---|
|
Mothers' affective responses to infants' affectb |
χ2 (df = 3) |
Coefficientc (95% CI) |
Coefficientc (95% CI) |
(95% CI) |
Simple slope own infant |
Simple slope unknown infant |
| Happy affect | 664.63** |
0.61** (0.51 to 0.70) |
4.64** (3.25 to 6.03) |
–0.33** (–0.50 to −0.16) |
0.28** (0.13 to 0.42) |
0.61** (0.51 to 0.70) |
| Sad affect | 664.53** |
0.62** (0.52 to 0.71) |
0.10 (–0.87 to 1.08) |
0.23** (0.10 to 0.37) |
0.85** (0.74 to 0.96) |
0.62** (0.52 to 0.71) |
| Distressed affect | 596.22** |
0.56** (0.47 to 0.65) |
–0.27 (–1.18 to 0.64) |
0.27** (0.14 to 0.39) |
0.82** (0.72 to 0.93) |
0.56** (0.47 to 0.65) |
Wald χ 2 values are those obtained for the best‐fitting mixed‐effects models for the respective outcome variables.
Mothers' perceived ratings of infants' affect. For happy faces, ratings were obtained from mothers' responses to the question, “how happy do you think the baby was feeling?” For sad faces, ratings were obtained from the questions, “how sad do you think the baby was feeling?” and, “how distressed do you think the baby was feeling?”
Mothers' own affective response to infant face images. For happy faces, ratings were obtained from mothers' responses to the question, “how happy did this picture make you feel?” For sad faces, ratings were obtained from the questions, “how sad did this picture make you feel?” and, “how distressed did this picture make you feel?”
Coefficients shown are beta weights (i.e., slopes) for the main and interaction effects of infant affect and identity derived from the best‐fitting multilevel mixed‐effects regression models.
Significance reflects differences in the strengths of associations between infant affect and maternal affective response as a function of infant identity.
**P < 0.001.
Sad faces
Consistent with the ratings for happy faces, mothers with addictions reported perceiving their own infants' sad faces as sadder and more distressed than those of unknown infants (sad: β identity = 0.64, 95% CI = 0.37 to 0.90, z = 4.72, P < 0.001; distressed: β identity = 0.61, 95% CI = 0.33 to 0.89, z = 4.26, P < 0.001; Table 6). Mothers' self‐reported negative affect was also stronger in response to their own infants compared to unknown infants (sad: β identity = 2.21, 95% CI = 1.90 to 2.52, z = 13.95, P < 0.001; distressed: β identity = 1.99, 95% CI = 1.68 to 2.30, z = 12.59, P < 0.001; Table 6). Mothers' own report of negative affect correlated significantly with how sad and distressed they perceived infants to be feeling (sad: β infant affect = 0.62, 95% CI = 0.52 to 0.71, z = 12.69, P < 0.001; distressed: β infant affect = 0.56, 95% CI = 0.47 to 0.65, z = 12.04, P < 0.001; Table 7). The strength of this association varied as a function of infant identity (sad: β identity × infant affect = 0.23, 95% CI = 0.10 to 0.37, z = 3.43, P < 0.001; distressed: β identity × infant affect = 0.27, 95% CI = 0.14 to 0.39, z = 4.15, P < 0.001). However, in contrast to the ratings for happy faces, post hoc probing of the moderation revealed that mothers' self‐reported negative affect increased more closely in line with their perceived increase in their own infant's negative affect (sad: β own = 0.85, 95% CI = 0.74 to 0.96, z = 15.40, P < 0.001; distressed: β own = 0.82, 95% CI = 0.72 to 0.93, z = 15.66, P < 0.001) rather than that of an unknown infant (sad: β unknown = 0.62, 95% CI = 0.52 to 0.71, z = 12.69, P < 0.001; distressed: β unknown = 0.56, 95% CI = 0.47 to 0.65, z = 12.04, P < 0.001). In other words, mothers' own report of sadness and distress increased to a greater extent when perceiving an increase in sadness and distress in their own infant rather than in an unknown infant.
DISCUSSION
When mothers are involved in substance addiction, the repercussions extend to their children. Until now, little work has elucidated the underlying neurobiology of this phenomenon. Our results are the first, to our knowledge, to show that mothers with addictions demonstrate reduced hypothalamic and mesocorticolimbic responses to reward‐related cues from their own infants. When viewing smiling face images of their own infants as compared to unknown infants, these mothers showed a striking pattern of decreased activation in key OT‐ and DA‐innervated brain regions, including the hypothalamus, VS, and mPFC. Notably, in mothers without addictions, prior research has indicated a robust pattern of activation in these regions [Strathearn and Kim, 2013; Strathearn et al., 2009] (see Fig. 5). As expected, in mothers with addictions, these compromised hypothalamic and mesocorticolimbic activations were seen in response to happy, but not sad, faces of their own infants. Sad faces of own infants instead elicited a decreased activation in a small region of the right IPL and STG in these mothers. These regions form part of the neural network subserving social cognition/empathy [Decety and Lamm, 2007], support maternal responsiveness to an array of affectively nuanced expressions from infants [Lenzi et al., 2009], and show increased activation in response to own compared to unknown, and sad compared to happy, infant cues in mothers without addictions [Lenzi et al., 2009; Noriuchi et al., 2008]. The dysregulated maternal response of mothers with addictions was also evident in their behavioral ratings, which demonstrated that their positive affect tracked more closely with how positive they perceived unknown infants to be feeling compared to their own infants. Of note, this pattern of dysregulation emerged only for happy, but not sad, faces, indicating that reward‐related dysregulations may be particularly relevant for mothers with addictions, manifesting especially in reward‐related attachment contexts. Our findings are consistent with the literature from the general population that has underscored the role of diminished salience of natural rewards in addictions [Koob and Volkow, 2010; Lubman et al., 2009; May et al., 2013], while demonstrating for the first time in human mothers how such disruptions may undermine a critical aspect of their responsiveness to their infants.
Figure 5.
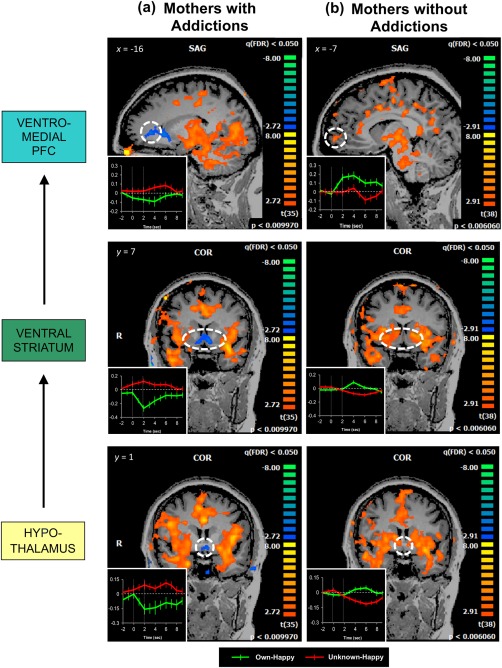
Mothers with addictions (Panel A; N = 36) show decreased activation in the hypothalamus, VS, and vmPFC, which appear different from activations previously observed in mothers without addictions (Panel B; N = 39) in corresponding regions. Activation maps for mothers without addictions were generated from our previously published data [Strathearn and Kim, 2013] and are reprinted here for comparison. Both maps are thresholded at FDR‐corrected q < 0.05. Insets of averaged hemodynamic brain responses to own‐happy and unknown‐happy infant faces were obtained from cubes centered at the peak voxels of each anatomical region and extending up to ± 10 voxels per dimension, unless limited by the spatial extent of activation; y axis indicates % BOLD signal change shown with standard error bars; the two vertical lines represent the onset (0 sec) and offset (2 sec) times of the infant‐face‐stimulus presentation. VS = ventral striatum; vmPFC = ventromedial prefrontal cortex. [Color figure can be viewed at http://wileyonlinelibrary.com]
Rodent models have shown that pup cues are highly reinforcing to postpartum mothers, sufficient to compete with the hedonic properties of cocaine [Pereira and Morrell, 2011]. Virgin females show activation of the mesocorticolimbic DA pathway upon cocaine administration, while the same pathway is deactivated to cocaine and activated to pup cues in postpartum lactating mothers [Ferris et al., 2005]. This difference preparturition and postparturition reflects a series of neuroadaptations primarily involving, but not limited to, the OT system [Kim and Strathearn, 2016], which occur during the transition to motherhood and ensure the reward salience of pup cues over other hedonic stimuli [Ferris et al., 2005; Numan and Woodside, 2010; Rilling and Young, 2014]. It is in this respect that our findings in mothers with addictions are particularly noteworthy. In contrast to the adaptive shifts that help promote mother–infant attachment during the postpartum period, postpartum mothers with addictions in our study showed a pattern of decreased hypothalamic and mesocorticolimbic activation to reward cues from their infants. This is consistent with the notion that substance use compromises maternal behavior by co‐opting the mesocorticolimbic DA system and disrupting naturally occurring OT‐related neuroadaptations [Rutherford et al., 2011; Strathearn and Mayes, 2010]. Alternatively, dysregulations of the OT‐ and DA‐systems may have already been present in susceptible mothers even prior to the onset of addiction [Buisman‐Pijlman et al., 2014; Kim et al., 2016]. In this case, the same neurobiological mechanisms that increase the mother's susceptibility to addiction may also undermine her reward response to her infant and may have contributed to the pattern of findings reported here. These hypotheses should be examined and substantiated in future longitudinal research.
Previously, the only other fMRI study of mothers with addictions reported a general pattern of reduced neural responsiveness to unknown infant face and cry stimuli. Using unknown faces and cries, this prior study observed reduced activations throughout prefrontal, limbic, and occipital regions for happy faces and similarly reduced prefrontal, limbic, posterior parietal, and occipital activations, including the IPL and STG, for sad faces and cries [Landi et al., 2011]. Here, we have compared mothers' neural responsiveness to own and unknown infant faces, documenting markedly reduced activations precisely in key OT‐ and DA‐regions in response to happy faces of own infants, along with reduced activations in a small region of the IPL and STG in response to sad faces of own infants. The observed deactivations, or robust negative percent BOLD signals, seen in the OT‐ and DA‐innervated regions are particularly noteworthy in two respects: first, they were specific to cues from the mothers' own infants; and second, they were in response to what could arguably be considered the most rewarding cues from infants—their smiling faces. Taken together, these studies indicate that the reward salience of infant cues may be generally reduced in mothers with addictions, but that this pattern may be most pronounced in the case of their own infants.
Why does reward‐related disruption become more pronounced with the mother's own infant? Growing evidence suggests that neurobiological systems that underlie reward and attachment functions undergo significant alteration over the course of development via interaction with one's early environment [see Kim et al., 2016 for review]. Consistent with this, some mothers may have developed altered brain reward sensitivities over the course of their upbringing, which may have contributed to both reduced response to natural reward and increased susceptibility to addictive substances. A key contribution of our work lies in demonstrating how these altered brain reward sensitivities, which have received intense scrutiny in the broader addiction literature [Balodis and Potenza, 2015; Kalivas and Volkow, 2005; Lubman et al., 2009; May et al., 2013], may intersect with and undermine the mother's experience of attachment with her own infant, an experience which we essentially conceptualize as that of reward. Furthermore, the broader literature on addictions proposes that, once developed, substance addiction may be maintained via complex interactions between reward and stress neurocircuitries and their dysregulation [Koob et al., 2014; Rutherford et al., 2011]. As addiction progresses, shifts may occur within the reward circuitry, which may promote addictive behaviors aimed at alleviating stress and discomfort experienced during periods of abstinence [Koob and Volkow, 2010; Koob et al., 2014]. In the face of such shifts, attachment cues (i.e., cues from one's own infant) and the demands for care they signify may no longer be perceived as rewarding but instead evoke stress in mothers with addictions [Rutherford et al., 2011; Suchman et al., 2010]. The negative percent BOLD signals observed in our study specifically for the happy faces of own infants, as well as the affective ratings that were observed to be less ‘in‐sync’ with own infant's happy faces, may be reflections of such dysregulation. Rather than providing a sense of reward and facilitating a mother's approach behavior toward her infant, infant signals may potentially evoke stress and/or intensify cravings for addictive substances that have previously provided mothers with relief from stress. This may be further compounded by heightened stress that these mothers encounter in their roles as mothers, exacerbated by feelings of shame and guilt associated with the perceived repercussions of their addictions on their behavior and competence as mothers. In this manner, in the case of maternal substance addiction, both substance use and disrupted maternal behavior may form a self‐perpetuating feedback loop sustained by dysregulated reward and stress systems.
While the present study focused on reward‐system‐related dysregulation, it should be noted that stress‐related dysregulation is as central to maternal addiction as reward‐related dysregulation [Rutherford et al., 2011]. In our sample, sad faces of the mothers' own infants did not yield significant activations or deactivations in key OT‐ and DA‐innervated brain regions, including the hypothalamus, VS, and mPFC. This is consistent with prior research, including our own [Strathearn and Kim, 2013; Strathearn et al., 2008], that similarly documented no activations of these regions in mothers without addictions in response to sad faces of their own infants, although a few studies have observed activations [e.g., Barrett et al., 2012]. While the lack of a control group precludes more conclusive inferences as to whether the absence of responses seen in these regions in mothers with addictions represents deviations from those observed in mothers without addictions, our data are consistent with the notion of the co‐optation of the reward circuitry discussed above, which appears to uniquely perturb the processing of happy faces as opposed to sad faces. Despite our findings, we acknowledge that disruptions in maternal responses to an infant's negative affective state are importantly linked to the infant's socioemotional outcomes [Kim et al., 2014a, 2014b] and may uniquely contribute to compromised maternal behavior seen in maternal addiction. It may be that regions that subserve social cognition/empathy—such as the IPL and STG—which were observed to be deactivated in our sample in response to sad faces, may contribute more to disrupted maternal attunement to infants' negative affective states [Noriuchi et al., 2008] than the reward‐related circuitry which has been the focus of this study. This is in line with the recent data in nulliparous women documenting that happy cues of infants tap into an individual's tendency to approach motivationally salient stimuli and activate regions associated with reward, while sad cues of infants tap into an individual's tendency to withdraw from such stimuli and activate regions associated with empathy [Montoya et al., 2012]. Additional research including a control group is warranted for a fuller and more systematic understanding of the neurocircuitry involved in stress‐related dysregulation.
Our findings presented here suggest a neurobiological account of why mothers with addictions may find it difficult to contend with the demands of caring for their infants. The transition to motherhood, while rewarding, can also be an inherently stressful period. It is the enhanced perceived appetitive value of infant cues, coupled with the sense of reward and pleasure experienced by the mother, that often help to sustain a mother's attention and responsiveness to her infant during a critical developmental period. When the functions of the OT‐ and DA‐innervated maternal circuitry go awry, as the data has suggested here in the case of substance addictions, mothers may be compromised in their abilities to care for their infants, and the risk for abuse and neglect may rise.
Several limitations of the study should be recognized. First, we did not include a matched control group of mothers without addictions and were therefore not able to directly examine between‐group differences. However, comparisons with the hypothalamic and mesocorticolimbic activations shown by mothers without addictions in previous studies, both from our group [Strathearn and Kim, 2013; Strathearn et al., 2009] and others [Barrett and Fleming, 2011; Noriuchi et al., 2008; Swain et al., 2014], suggest that the observed deactivations are noteworthy abnormalities that may be characteristic of this group. At the same time, without a direct comparison with a control group, we lack information as to whether the size of this effect would translate to a significant group difference. This remains a critical area for future research. Future studies should also examine in further detail differential patterns of (de)activations in the neurocircuitries involved in social cognition/empathy in mothers with and without addictions. Second, our sample showed variability in substance‐use characteristics, including the duration and extent of substance use, as well as specific classes of substances used. Our study focused on examining maternal brain responses in the context of a general process of substance addiction, and our small sample size precluded the investigation of potential effects specifically associated with individual substances or substance‐use chronicity. Given substantial preclinical and human data underscoring the role of altered reward sensitivities [Balodis and Potenza, 2015; Kalivas and Volkow, 2005], particularly the diminished salience of natural rewards [Koob and Volkow, 2010; Lubman et al., 2009; May et al., 2013], in the process of addiction, we anticipate that abnormalities reported here may be represented to a greater or lesser degree across a range of substances. However, the specific nature of pre‐existing OT‐ and DA‐related dysregulations in mothers may have led to differential susceptibility to distinct classes of substances, which, in turn, may have produced unique patterns of neuroadaptations, including those affecting the maternal circuitry, over the course of repeated substance use [Koob and Volkow, 2010]. In future studies with larger samples, it would be of interest to investigate unique and shared effects of individual substances as well as effects associated with the severity and chronicity of substance use. Third, the present study does not examine which aspects of this sample—addiction severity, associated sociodemographic characteristics (e.g., low SES), attachment disturbance, or unresolved trauma prevalent in this population—may account for the findings reported here. Although these factors often co‐occur and it is important to document the features associated with this constellation of maternal characteristics, it would be of interest to tease apart associations and interactions amongst these factors. Fourth, we did not collect systematic information on co‐occurring psychiatric disorders. Further studies are warranted to investigate and clarify possible contributions of co‐occurring disorders to the findings reported here. Fifth, we did not directly measure the quality of infant‐directed maternal behavior and hence acknowledge that the association proposed here between compromised reward‐related maternal brain response and disrupted maternal care is only speculative. A larger study with concurrent brain and behavioral measures is necessary to further corroborate this link. In a sufficiently large sample, it would be fruitful to examine whether compromised DA‐ and OT‐related activations relate to aberrant behavioral characteristics in mothers. Finally, additional limitations include a small sample size as well as variability seen in maternal age (27.8 ± 5.6 years), infant age (5.0 ± 2.9 months at the time face images were recorded), and the time elapsed since delivery (6.0 ± 3.4 months at the time of the scan). The maternal brain is understood to undergo structural and functional changes over the course of the postpartum period [Kim et al., 2010, 2016a; Swain et al., 2007] in interaction with the amount of exposure and experience the mother has with her infant. While there may have been developmental variability between younger and older infants (e.g., older infants may have been more expressive in their facial affect), all own and unknown infant face images were matched on infant age and affect intensity, allowing us to reasonably speculate that differences observed in maternal brain responses to own and unknown infant faces were likely a function of infant identity. Furthermore, while maternal age and the amount of maternal contact with the infant may have added to possible interindividual variations in maternal neurobiology among our participants, our findings of striking deactivations in key OT‐ and DA‐innervated brain regions were only seen in response to happy, but not sad, faces of the mothers' own infants. We believe that this corroborates our understanding that findings observed here in mothers with addictions are less likely to be a function of maternal age or postpartum exposure, but can reasonably be interpreted as a reflection of reward‐related dysregulations manifesting specifically in reward‐related attachment contexts. However, accounting for the role of the amount of maternal exposure, contact, and experience with the infant is critical in the study of maternal postpartum neuroadaptation and would be important to address in future research relating to mothers with addictions.
The present study marks one of the first attempts to delineate neurobiological correlates of disrupted mothering in mothers with addictions. The data suggest that mothers with substance addictions, even during intermittent periods of sobriety, demonstrate markedly reduced response to naturally rewarding cues from their infants. The deactivations observed here may help to explain the link between maternal substance use and impaired maternal caregiving. Our findings point to the mother–infant relationship as an important element and context for intervention for mothers with addictions, who are often treated in isolation from their infants. Understanding the neurobiological relationship between substance addictions and impaired maternal responses may facilitate earlier and more refined interventions to help support mothers with substance addictions and the infants in their care.
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutes or the National Institutes of Health. The authors report no conflicts of interest with respect to the content of this manuscript. Dr. Potenza has received financial support or compensation for the following: Dr. Potenza has consulted for Ironwood, Lundbeck, Shire, INSYS, RiverMend Health, Lakelight Therapeutics/Opiant and Jazz Pharmacetuicals; has received research support to Yale from Mohegan Sun Casino, the National Center for Responsible Gaming, and Pfizer; and has consulted for legal entities on issues related to addictive disorders.
Footnotes
The focus of treatment at this inpatient facility centered around co‐habitation of mothers and infants, which was integrated into the mothers' treatment program. All mothers cohabitated with at least one child, typically the child from their most recent pregnancy. Additional children, of up to the age of 13, were also encouraged to accompany and reside with mothers during treatment. Mothers placed infants in on‐campus childcare for 35 h per week. Infants spent the remainder of the week with their mother in treatment. The standard treatment program was 90 days inpatient, with the option of completing up to 70 additional days of outpatient, which was co‐located on the same campus. Mothers returned home at the conclusion of treatment.
Participants' average length of stay at the treatment facility prior to the scanning visit was approximately 110 (M = 109.6 ± 59.5) days. Besides the time spent in on‐campus childcare (35 h per week), no other mother–infant separation took place prior to the mother's scanning visit.
Contributor Information
Sohye Kim, Email: sohyek@bcm.edu.
Lane Strathearn, Email: lane-strathearn@uiowa.edu.
REFERENCES
- Afonso VM, Sison M, Lovic V, Fleming AS (2007): Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav. Neurosci 121:515–526. [DOI] [PubMed] [Google Scholar]
- Afonso VM, King S, Chatterjee D, Fleming AS (2009): Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup‐ and food‐stimuli in the female rat. Horm Behav 56:11–23. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000): Diagnostic and Statistical Manual of Mental Disorders, 4th ed., text rev. Washington, DC: American Psychiatric Association. [Google Scholar]
- Atzil S, Hendler T, Feldman R (2011): Specifying the neurobiological basis of human attachment: Brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology 36:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Zagoory‐Sharon O, Winetraub Y, Feldman R (2012): Synchrony and specificity in the maternal and the paternal brain: Relations to oxytocin and vasopressin. J Am Acad Child Adolesc Psychiatry 51:798–811. [DOI] [PubMed] [Google Scholar]
- Babor TF, de la Fuente JR, Saunders J Grant M (1992): The Alcohol Use Disorders Identification Test: Guidelines for use in primary care. World Health Organization; Geneva, Switzerland.
- Balodis IM, Potenza MN (2015): Anticipatory reward processing in addicted populations: A focus on the monetary incentive delay task. Biol Psychiatry 77:434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fleming AS (2011): Annual Research Review: All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry 52:368–397. [DOI] [PubMed] [Google Scholar]
- Barrett J, Wonch K, Gonzalez A, Ali N, Steiner M, Hall G, Fleming A (2012): Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc Neurosci 7:252–268. [DOI] [PubMed] [Google Scholar]
- Barth RP (2009): Preventing child abuse and neglect with parent training: Evidence and opportunities. Future Child 19:95–118. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE (1997): Central lactogenic regulation of maternal behavior in rats: Steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology 138:756–763. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA (2010): Endocrine induced changes in brain function during pregnancy. Brain Res 1364:198–215. [DOI] [PubMed] [Google Scholar]
- Buisman‐Pijlman FT, Sumracki NM, Gordon JJ, Hull PR, Carter CS, Tops M (2014): Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacol Biochem Behav 119:22–38. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2014): Excessive drinking costs U.S. $223.5 billion. In: U.S. Department of Health and Human Services, editor. Atlanta, GA.
- Champagne F, Diorio J, Sharma S, Meaney MJ (2001): Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen‐inducible central oxytocin receptors. Proc Natl Acad Sci USA 98:12736–12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ (2004): Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci 24:4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Taylor PA, Cox RW (2017): Is the statistic value all we should care about in neuroimaging? NeuroImage 147:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Child Welfare Information Gateway (2014): Parental substance use and the child welfare system. In: U.S. Department of Health and Human Services, Children's Bureau, editors. Washington, DC.
- Cole PM, Barrett KC, Zahn‐Waxler C (1992): Emotion displays in two‐year‐olds during mishaps. Child Dev 63:314–324. [PubMed] [Google Scholar]
- Decety J, Lamm C (2007): The role of the right temporoparietal junction in social interaction: How low‐level computational processes contribute to meta‐cognition. Neuroscientist 13:580–593. [DOI] [PubMed] [Google Scholar]
- Febo M, Felix‐Ortiz AC, Johnson TR (2010): Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats. Brain Res 1325:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Ferris CF (2007): Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience 148:400–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM Jr, Harder JA, Messenger TL, Febo M (2005): Pup suckling is more rewarding than cocaine: Evidence from functional magnetic resonance imaging and three‐dimensional computational analysis. J Neurosci 25:149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JE, Brewer JA, Potenza MN (2006): The neurobiology of substance and behavioral addictions. CNS Spectr 11:924–930. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P (1999): Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877:49–63. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991): The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction 86:1119–1127. [DOI] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Meter KE, Mason GA (1997): Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague‐Dawley rats. Neuropeptides 31:439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns JM, Lubin DA, Walker CH, Joyner P, Middleton C, Hofler V, McMurray M (2004): Gestational treatment with cocaine and fluoxetine alters oxytocin receptor number and binding affinity in lactating rat dams. Int J Dev Neurosci 22:321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND (2005): The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry 162:1403–1413. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE (2010): The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behav Neurosci 124:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, Swain JE (2016a): The maternal brain and its plasticity in humans. Horm Behav 77:113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Allen J, Strathearn L (2014a): Mothers' unresolved trauma blunts amygdala response to infant distress. Soc Neurosci 9:352–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Koos O, Dorsett K, Strathearn L (2014b): Maternal oxytocin response predicts mother‐to‐infant gaze. Brain Res 1580:133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kwok S, Mayes LC, Potenza MN, Rutherford HJ, Strathearn L (2016): Early adverse experience and substance addiction: Dopamine, oxytocin, and glucocorticoid pathways. Ann N Y Acad Sci 1394:74–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Strathearn L (2016): Oxytocin and maternal brain plasticity. New Dir Child Adolesc Dev 153:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010): Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr, George O (2014): Addiction as a stress surfeit disorder. Neuropharmacology 76 Pt B:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczkowski KM (2007): The effects of drug abuse on pregnancy. Curr Opin Obstet Gynecol 19:578–585. [DOI] [PubMed] [Google Scholar]
- Landi N, Montoya J, Kober H, Rutherford HJ, Mencl WE, Worhunsky PD, Potenza MN, Mayes LC (2011): Maternal neural responses to infant cries and faces: Relationships with substance use. Front Psychiatry 2:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, Ammaniti M (2009): Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cereb Cortex 19:1124–1133. [DOI] [PubMed] [Google Scholar]
- Li M, Fleming AS (2003): The nucleus accumbens shell is critical for normal expression of pup‐retrieval in postpartum female rats. Behav Brain Res 145:99–111. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM (2004): Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav 29:1541–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB (2009): Responsiveness to drug cues and natural rewards in opiate addiction: Associations with later heroin use. Arch Gen Psychiatry 66:205–212. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC (2011): Drug‐evoked synaptic plasticity in addiction: From molecular changes to circuit remodeling. Neuron 69:650–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G (2004): Atlas of the Human Brain. San Diego, CA: Elsevier Academic Press. [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ (2015): Births: Final data for 2013. Natl Vital Stat Rep 64. [PubMed] [Google Scholar]
- Mattson BJ, Morrell JI (2005): Preference for cocaine‐ versus pup‐associated cues differentially activates neurons expressing either Fos or cocaine‐ and amphetamine‐regulated transcript in lactating, maternal rodents. Neuroscience 135:315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May AC, Stewart JL, Migliorini R, Tapert SF, Paulus MP (2013): Methamphetamine dependent individuals show attenuated brain response to pleasant interoceptive stimuli. Drug Alcohol Depend 131:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M (1992): The fifth edition of the addiction severity index. J Subst Abuse Treat 9:199–213. [DOI] [PubMed] [Google Scholar]
- Montoya JL, Landi N, Kober H, Worhunsky PD, Rutherford HJ, Mencl WE, Mayes LC, Potenza MN (2012): Regional brain responses in nulliparous women to emotional infant stimuli. PLoS One 7:e36270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Drug Intelligence Center (2014): National drug threat assessment. In U.S. Department of Justice, editor. Washington, DC.
- Nephew BC, Febo M (2012): Effects of cocaine on maternal behavior and neurochemistry. Curr Neuropharmacol 10:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A (2008): The functional neuroanatomy of maternal love: Mother's response to infant's attachment behaviors. Biol Psychiatry 63:415–423. [DOI] [PubMed] [Google Scholar]
- Numan M, Woodside B (2010): Maternity: Neural mechanisms, motivational processes, and physiological adaptations. Behav Neurosci 124:715–756. [DOI] [PubMed] [Google Scholar]
- Numan M, Rosenblatt JS, Komisaruk BR (1977): Medial preoptic area and onset of maternal behavior in the rat. J Comp Physiol Psychol 91:146–164. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD (2005): The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci 119:1588–1604. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA (1994): Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci 108:1163–1171. [DOI] [PubMed] [Google Scholar]
- Pereira M, Morrell JI (2011): Functional mapping of the neural circuitry of rat maternal motivation: Effects of site‐specific transient neural inactivation. J Neuroendocrinol 23:1020–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet CR (2014): Misconceptions in the use of the General Linear Model applied to functional MRI: A tutorial for junior neuro‐imagers. Front Neurosci 8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Fletcher PC, Henson RN, Worsley KJ, Brett M, Nichols TE (2008): Guidelines for reporting an fMRI study. NeuroImage 40:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Young LJ (2014): The biology of mammalian parenting and its effect on offspring social development. Science 345:771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJ, Williams SK, Moy S, Mayes LC, Johns JM (2011): Disruption of maternal parenting circuitry by addictive process: Rewiring of reward and stress systems. Front Psychiatry 2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford HJV, Mayes LC (2017): Parenting and addiction: Neurobiological insights. Curr Opin Psychol 15:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabihi S, Dong SM, Durosko NE, Leuner B (2014): Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front Behav Neurosci 8:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA (2010): Cortico‐Basal Ganglia reward network: Microcircuitry. Neuropsychopharmacology 35:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC (1998): The Mini‐International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview. J Clin Psychiatry 59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Stack EC, Balakrishnan R, Numan MJ, Numan M (2002): A functional neuroanatomical investigation of the role of the medial preoptic area in neural circuits regulating maternal behavior. Behav Brain Res 131:17–36. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M (2011): Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev 35:826–847. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M (2007): Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy‐terminated rats. Behav Neurosci 121:907–919. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Kim S (2013): Mothers' amygdala response to positive or negative infant affect is modulated by personal relevance. Front Neurosci 7:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Mayes LC (2010): Cocaine addiction in mothers: Potential effects on maternal care and infant development. Ann N Y Acad Sci 1187:172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR (2008): What's in a smile? Maternal brain responses to infant facial cues. Pediatrics 122:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR (2009): Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology 34:2655–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2009): The NSDUH report: Substance use among women during pregnancy and following childbirth. In: U.S. Department of Health and Human Services, editor. Rockville, MD.
- Substance Abuse and Mental Health Services Administration . (2014) Results from the 2013 National Survey on Drug Use and Health: Summary of national findings. In: U.S. Department of Health and Human Services, editor. Rockville, MD.
- Suchman NE, DeCoste C, Castiglioni N, McMahon TJ, Rounsaville B, Mayes L (2010): The Mothers and Toddlers Program, an attachment‐based parenting intervention for substance using women: Post‐treatment results from a randomized clinical pilot. Attach Hum Dev 12:483–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L (2007): Brain basis of early parent‐infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry 48:262–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, Abel KM (2014): Approaching the biology of human parental attachment: Brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Res 1580:78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2014): The health consequences of smoking–50 years of progress: A report of the Surgeon General. In: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, editors. Atlanta, GA.
- Vernotica EM, Rosenblatt JS, Morrell JI (1999): Microinfusion of cocaine into the medial preoptic area or nucleus accumbens transiently impairs maternal behavior in the rat. Behav Neurosci 113:377–390. [DOI] [PubMed] [Google Scholar]
- Vertes RP (2004): Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51:32–58. [DOI] [PubMed] [Google Scholar]
- Wolf ME (2002): Addiction: Making the connection between behavioral changes and neuronal plasticity in specific pathways. Mol Interv 2:146–157. [DOI] [PubMed] [Google Scholar]
- Wulczyn F, Ernst M, Fisher P (2011): Who Are the Infants In Out‐Of‐Home Care? An Epidemiological and Developmental Snapshot. Chicago, IL: Chapin Hall at the University of Chicago. [Google Scholar]


