Abstract
Patient: Female, 28
Final Diagnosis: Type I and II Brugada phenocopy
Symptoms: Fever • syncope
Medication: —
Clinical Procedure: —
Specialty: Cardiology
Objective:
Unusual clinical course
Background:
Brugada pattern on electrocardiogram (ECG) is seen when there are at least 2 mm J-point elevation and 1 mm ST-segment elevation in two or more of the right precordial leads, with right bundle-branch block (RBBB)-like morphology. Elevation of a coved-type shape in leads V1 and V2 is consistent with type I Brugada pattern, whereas elevation of a saddle-back configuration distinguishes type II Brugada. If accompanied by life-threatening arrhythmias or sudden cardiac death, Brugada syndrome (BrS) is diagnosed. The presence of Brugada ECG pattern in absence of the syndrome has come to be known as Brugada phenocopy (BrP).
Case Report:
We introduce a case of both Brugada type I and II patterns unmasked in a 28-year-old female with fever secondary to mastitis. Though fever-induced BrP is a universally known phenomenon, the presentation of both type I and II patterns presenting in a patient during a single hospitalization makes this case unique from others. The patient was brought to the emergency department after experiencing a syncopal episode that appeared classically vasovagal in nature. Once her fever resolved, her baseline ECG showed no abnormalities.
Conclusions:
Though Brugada ECG pattern may be very alarming, especially after syncope, appropriate management in the case of a fever-induced event would consist of observation with cardiac monitoring, immediate treatment of fever with antipyretics, and antibiotics for suspected infection. Close follow-up by a cardiologist as an outpatient is imperative to further ascertain if the patient is at high risk of life-threatening arrhythmias, significant for BrS.
MeSH Keywords: Brugada Syndrome, Electrocardiography, Fever, Syncope
Background
Brugada syndrome (BrS) was first described in 1992 as a hereditary disorder diagnosed on the basis of a clinically significant event: documented ventricular fibrillation, polymorphic ventricular tachycardia, nonvagal syncope, or sudden cardiac death [1,2]. Electrocardiogram (ECG) pattern is characterized by a minimum of 2 mm J-point elevation and 1 mm ST-segment elevation in at least two of the right precordial leads, V1–V3, with RSR’ of the QRS [1–3]. It was not until 2013 that the term Brugada phenocopy (BrP) was coined, describing the presence of Brugada ECG pattern without manifestations of the syndrome, though there multiple accounts of such phenomenon documented prior to its terminology [4,5]. The prevalence of Brugada ECG patterns is approximated to be 0.15%, with higher prevalence in Asia, and nine times more common in males than females [6–8]. In the United States, Brugada ECG pattern is an even rarer occurrence, with a prevalence of approximately 0.012% [7,9]. In a prospective study by Adler et al., type I Brugada pattern was 20 times more prevalent among febrile patients [10]. All aberrant ECGs were incidentally found in the febrile patients—none reported syncope or arrhythmia-related symptoms [10].
Case Report
A 28-year old, G4P4004, Caucasian female with no significant past medical history presented to the emergency department after a witnessed syncopal episode that occurred at a shopping mall. She described feelings of warmth, lightheadedness, and palpitations, but lost consciousness before she could sit down. As per bystanders, the patient was unconscious for less than one minute without tonic-clonic movements, tongue bite, and bladder or bowel incontinence. The patient denied confusion, slurred speech, or weakness upon awakening. The patient reported three similar episodes in the past, all experienced in the setting of pain – the first occurrence was 12 years prior to presentation. In this instance, the patient reported having left breast pain and subjective fevers for the past week, attributing pain to breastfeeding. The patient denied personal history of documented ventricular fibrillation or tachycardia, and any family history of arrhythmias or sudden cardiac death.
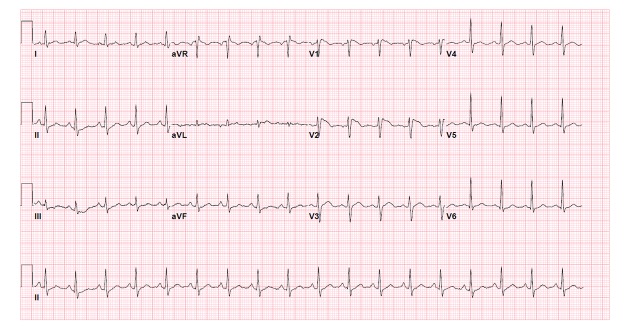
Admission vital signs showed temperature of 102.1°F (38.94°C), blood pressure of 104/93 mm Hg, and pulse of 128 beats per minute, with negative orthostatics. Physical examination revealed left breast tenderness without erythema or discharge. It was concluded that fever was due to left breast mastitis. Cardiac auscultation revealed no abnormalities. A 12-lead ECG completed on admission showed sinus tachycardia at 108 beats per minute, with normal axis, PR interval, QRS duration, and QTc interval. There was RSR’ morphology and ST segment elevations in leads V1 and V2 with coved-type pattern consistent with a type I Brugada (Figure 1). Troponin-I measured was 0.00. Other laboratory studies completed showed absence of hyponatremia with a sodium level of 135 mEq/L, and absence of hypokalemia or hyperkalemia with a potassium level of 3.9 mEq/L as a contributing factor to ECG abnormalities. Urine drug screen was also negative for illicit drugs or controlled substances.
Figure 1.
Patient’s admission ECG showing Brugada type I pattern (temperature 102.1°F (38.94°C).
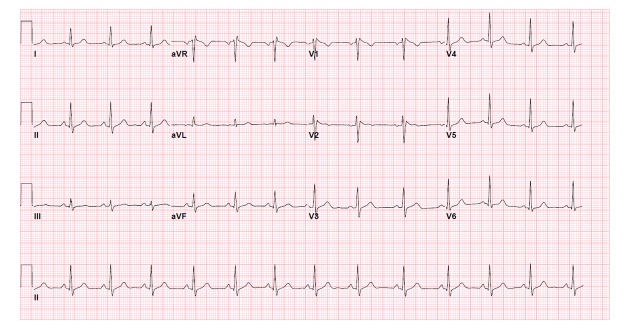
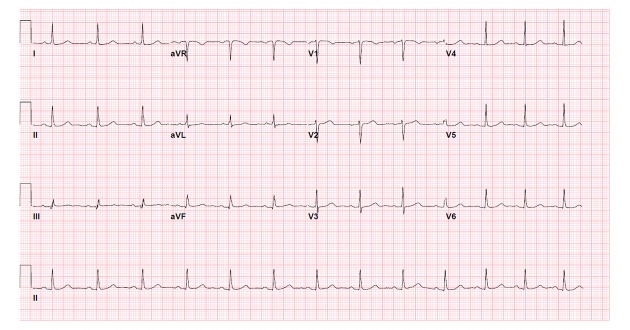
The patient was treated with acetaminophen and oral cephalexin. However, on her second day of stay, her leukocyte count increased from 11,800/μL to 17,900/μL and a decision was made to change from oral antibiotics to intravenous ampicillin/sulbactam. As her fever subsided and decreased to a temperature of 100°F (37.78°C), her ECG showed regression to type II Brugada, with ST segment elevations in leads V1 and V2 of saddle back configuration (Figure 2). At a temperature of 98.3°F (36.83°C), her ECG showed normal sinus rhythm without evidence of J-point or ST segment elevations (Figure 3). Echocardiogram showed no regional wall motion abnormalities, no right ventricular dilation or hypertrophy, and normal left ventricular ejection fraction. The review of telemonitoring during 48-hour stay showed absence of ventricular fibrillation or ventricular tachycardia. On the day of discharge, it was advised that the patient consider genetic testing and cardiac monitoring with an implantable loop recorder – however, she deferred further inpatient workup and was scheduled for a follow-up appointment with a cardiologist to evaluate her increased risk of sudden cardiac death. The cardiologist agreed with this plan, as he did not believe that patient’s syncopal episode was related to BrS but rather by vasovagal phenomenon, supported by history of previous similar syncopal events, and each time having quick recovery without medical intervention.
Figure 2.
Patient’s repeat ECG the day after admission showing Brugada type II pattern after decrease of temperature from 102.1°F (38.94°C) to 100°F (37.78°C).
Figure 3.
Patient’s ECG upon resolution of fever and Brugada pattern (temperature 98.3°F (36.83°C).
Discussion
The patient’s history of syncope in the setting of pain, with prodromal symptoms of warmth, palpitations, and lightheadedness seem to be vasovagal in nature, however, she had a fever-induced Brugada ECG pattern that may be a marker of increased risk. Once her fever dissipated, a normal ECG manifested suspicious for BrP. In a paper by Gottschalk, et al., BrP is categorized into three classes: class A where all mandatory diagnostic criteria are met, class B where BrP is highly suspected but not all diagnostic studies could be completed, and class C where BrP is highly suspected but provocative testing is not justified [11]. The patient presented fits in class B, and can be specified as having type IB and IIB BrP.
The prevalence of either type I or II Brugada ECG is approximately 0.15% [6,7], but the presentation of both type I and II Brugada ECG patterns in a single patient is undocumented.
Though there are numerous studies looking at the prevalence of Brugada ECG patterns in various countries, there lacks data in how many of those patients presented with more than one type of Brugada ECG pattern. Implantable cardioverter defibrillator (ICD) implantation is recommended for those with multiple risk factors of nonvagal syncope, familial sudden cardiac death, or inducible ventricular tachycardia or fibrillation on electrophysiology study [12,13]. This patient had no true known risk factors, as her syncope was likely unrelated to ventricular fibrillation or ventricular tachycardia. It remains controversial as to the most appropriate workup to further risk stratify this patient, but a reasonable approach was to conduct genetic testing and long-term cardiac monitoring to rule out ventricular arrhythmias as a cause of syncope. Other possible diagnostic tests include electrophysiology studies and provocative testing with a sodium channel blocker [11].
There are multiple gene mutations associated with the disease, but about 20% of BrS cases are due to abnormalities in cardiac sodium channels. Mutations in SCN5A gene lead to decreased functioning of these channels in the right ventricular epicardium, thereby believed to cause slow conduction and reentry [2,14]. Another theory proposes that voltage gradient across the cardiac layers generates dispersion of epicardial repolarization, making it vulnerable to premature impulses that lead to ventricular arrhythmias [15,16]. The mechanism for fever-induced Brugada ECG pattern is not completely understood. Many hypothesize that under higher temperatures, sodium channels are prone to enter their slow inactivated state [17]. Additionally, sinus tachycardia often seen in febrile patients reduces the time between consecutive depolarization and does not allow enough time for mutated channels to recover from inactivation [17]. Fever can help unmask BrS, but it can also produce electrocardiographic findings of BrP in otherwise healthy individuals.
The most reasonable approach to patients with incidental fever-induced BrP is supportive care with antipyretics and clearance of infection with antibiotics. Pharmacologic therapies such as antiarrhythmics or beta-blockers do not protect affected individuals. Close follow-up as an outpatient is crucial in identifying high-risk patients with Brugada ECG pattern, as treatment requires placement of ICD for prevention of sudden cardiac death [12,13]. In patients with documented ventricular fibrillation, polymorphic ventricular tachycardia, or aborted cardiac death, ICD placement is the only management that decreases mortality [12,13].
Conclusions
Brugada ECG pattern, or rather BrP, is a rare phenomenon – even more unique is the presentation of both type I and II BrP in a single patient. This begs the question whether these individuals, though incidentally found, could be at higher risk for sudden cardiac death when compared to individuals presenting with either only type I or II Brugada ECG. Fever-induced BrP may be benign and can be treated with monitoring and antipyretics. However, it is critical to identify patients that may have an increased incidence of sudden cardiac death. Risk stratification must be done for patients who are at an intermediate risk. When type I or II Brugada pattern is recognized and the patient also has documented ventricular fibrillation or polymorphic ventricular tachycardia, syncope highly suspected to be due to a ventricular arrhythmia, or familial sudden cardiac death, an ICD is the only effective treatment shown to decrease mortality.
Footnotes
Conflicts of Interest
None.
References:
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20(6):1391–96. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Brugada P, Brugada J, Roy D. Brugada syndrome 1992–2012: 20 years of scientific excitement, and more. Eur Heart J. 2013;34(47):3610–15. doi: 10.1093/eurheartj/eht113. [DOI] [PubMed] [Google Scholar]
- 3.Brugada J, Brugada R, Brugada P. Right bundle-branch block and ST-segment elevation in leads V1 through V3: A marker for sudden death in patients without demonstrable structural heart disease. Circulation. 1998;97:457–60. doi: 10.1161/01.cir.97.5.457. [DOI] [PubMed] [Google Scholar]
- 4.Anselm D, Barachuk A. Brugada phenocopy: Redefinition and updated classification. Am J Cardiol. 2013;111(3):453. doi: 10.1016/j.amjcard.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Anselm D, Evans J, Baranchuk A. Brugada phenocopy: A new electrocardiogram phenomenon. World J Cardiol. 2014;6(3):81–86. doi: 10.4330/wjc.v6.i3.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuo K, Akahoshi M, Nakashima E, et al. The prevalence, incidence, and prognostic value of the Brugada-type electrocardiogram: A population-based study of four decades. J Am Coll Cardiol. 2001;38(3):765–70. doi: 10.1016/s0735-1097(01)01421-8. [DOI] [PubMed] [Google Scholar]
- 7.Kamakura S. Epidemiology of Brugada syndrome in Japan and rest of the world. J Arrhythmia. 2013;29:52–55. [Google Scholar]
- 8.Benito B, Sarkozy A, Mont L, et al. Gender differences in clinical manifestations of Brugada syndrome. J Am Coll Cardiol. 2008;52(19):1567–73. doi: 10.1016/j.jacc.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 9.Patel SS, Anees S, Ferrick KJ. Prevalence of a Brugada pattern electrocardiogram in an urban population in the United States. Pacing Clin Electrophysiol. 2009;32:704–8. doi: 10.1111/j.1540-8159.2009.02354.x. [DOI] [PubMed] [Google Scholar]
- 10.Adler A, Topaz G, Heller K, et al. Fever-induced Brugada pattern: How common is it and what does it mean? Heart Rhythm. 2013;10(9):1375–82. doi: 10.1016/j.hrthm.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gottschalk B, Anselm D, Baranchuk A. Brugada phenocopy: Morphological classification and importance of provocative testing. Ann Noninvasive Electrocardiol. 2014;19(6):604–5. doi: 10.1111/anec.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Priori S, Gasparini M, Napolitano C, et al. Risk stratification in Brugada syndrome: Results of the PRELUDE (PRogrammed ELectrical stimUlation pre-Dictive valuE) registry. J Am Coll Cardiol. 2012;59(1):37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 13.Delise P, Allocca G, Sitta N, Di Stefano P. Event rates and risk factors in patients with Brugada syndrome and no prior cardiac arrest: A cumulative analysis of the largest available studies distinguishing ICD-recorded fast ventricular arrhythmias and sudden death. Heart Rhythm. 2014;11(2):252–58. doi: 10.1016/j.hrthm.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 14.Keller D, Huang H, Zhao J, et al. A novel SCN5A mutation, F1344S, identified in a patient with Brugada syndrome and fever-induced ventricular fibrillation. Cardiovasc Res. 2006;70:521–29. doi: 10.1016/j.cardiores.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 15.Meregalli P, Wilde A, Tan H. Pathophysiological mechanisms of Brugada syndrome: Depolarization disorder, repolarization disorder, or more? Cardiovasc Res. 2005;67(3):367–78. doi: 10.1016/j.cardiores.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Wilde A, Postema P, Diego J, et al. The pathophysiological mechanism underlying Brugada syndrome. Depolarization versus repolarization. J Mol Cell Cardiol. 2010;49(4):543–53. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amin A, Klemens C, Verkerk A, et al. Fever-triggered ventricular arrhythmias in Brugada syndrome and type 2 long-QT syndrome. Neth Heart J. 2010;18(3):165–69. doi: 10.1007/BF03091755. [DOI] [PMC free article] [PubMed] [Google Scholar]