Abstract
The use of antibiotics in livestock production in North America and possible association with elevated abundance of detectable antimicrobial resistance genes (ARG) is a growing concern. Real-time, quantitative PCR (RT-qPCR) was used to determine the relative abundance and diversity of ARG in fecal composite and catch basin samples from 4 beef feedlots in Alberta. Samples from a surrounding waterway and municipal wastewater treatment plants were also included to compare the ARG profile of urban environments and fresh water with that of feedlots. The relative abundance of 18 resistance genes across 5 antibiotic families including sulfonamides, tetracyclines, macrolides, fluoroquinolones, and β-lactams was examined. Sulfonamide, fluoroquinolone, and β-lactam resistance genes predominated in wastewater treatment samples, while tetracycline resistance genes predominated in cattle fecal composite samples. These results reflect the types of antibiotic that are used in cattle versus humans, but other factors such as co-selection of ARG and variation in the composition of bacterial communities associated with these samples may also play a role.
Résumé
En Amérique du Nord, l’utilisation des antibiotiques dans la production du bétail et l’association possible avec une abondance élevée détectable de gènes de résistance aux antimicrobiens (GRA) est une préoccupation grandissante. Une épreuve d’amplification en chaîne par la polymérase quantitative en temps réel a été utilisée afin de déterminer l’abondance relative et la diversité des GRA dans des échantillons composites de fèces et de bassin de rétention de quatre parcs d’engraissement de bovins en Alberta. Des échantillons d’un cours d’eau avoisinant et de l’usine municipale de traitement des eaux usées ont également été inclus afin de comparer le profil des GRA provenant d’un milieu urbain et d’eau fraîche à celui des parcs d’engraissement. L’abondance relative de 18 gènes de résistance issus de cinq familles d’antibiotiques incluant les sulfonamides, les tétracyclines, les macrolides, les fluoroquinolones, et les β-lactames fut examinée. Les gènes de résistance aux sulfonamides, aux fluoroqunolones, et aux β-lactames prédominaient dans les échantillons d’eaux usées, alors que les gènes de résistance à la tétracycline étaient prédominants dans les échantillons composites de fèces des bovins. Ces résultats reflètent les types d’antibiotiques qui sont utilisés chez les bovins versus les humains, mais d’autres facteurs tels que la co-sélection de GRA et la variation dans la composition des communautés bactériennes associées à ces échantillons peuvent également jouer un rôle.
(Traduit par Docteur Serge Messier)
Introduction
The acquisition of antimicrobial resistance genes (ARG) by bacterial pathogens is a serious concern that impedes the successful treatment of infectious diseases (1). Antibiotics used in livestock production are often analogues of those used in human medicine, raising the possibility that ARGs with implications for the efficacy of antimicrobial use in humans arise within agricultural production systems.
In the Canadian beef feedlot industry, a number of antimicrobials are approved for administration to cattle in feed or drinking water, including aminoglycosides, macrolides, tetracyclines, and sulfonamides (2). Bacteria residing in the bovine gastrointestinal tract may become resistant to these antibiotics and once disseminated into the environment, transfer ARG to human pathogens (3,4). Furthermore, residual antibiotics may enter the environment through runoff or manure, exposing bacteria to these antibiotics and possibly selecting for antimicrobial resistance (5,6).
Real-time, quantitative PCR (RT-qPCR) is a useful tool that can provide an approximation of the abundance of ARG in the environment (7) and has been used to study the levels of ARG in livestock and poultry systems (8–10) and in wastewater from urban environments (11,12). In previous studies, tetracycline and sulfonamide resistance genes have been noted as prevalent in beef cattle environments (10,13,14) while in municipal environments fluoroquinolone resistance genes appear to dominate (15). However, these previous studies have been rather narrow with regard to the scope of ARG investigated. The objective of this study was to use RT-qPCR to compare the types and relative abundance of ARG in feedlot cattle feces to those in feedlot catch basins, a surrounding waterway and municipal wastewater, treatment plants in Alberta.
Materials and methods
Ethics statement
The research study was reviewed and approved by the University of Calgary Animal Care Committee, under the guidelines of the Canadian Council of Animal Care. Access to municipal wastewater treatment plants was arranged directly with the facilities and samples collected by their staff. Permission was not required for environmental sampling as collection of samples was non-disruptive.
Study area and sample collection
Sample collection occurred from April to October 2014. Four beef feedlots (designated A to D) and 2 municipal (human) wastewater treatment plants located in Alberta upstream and downstream of most of Alberta’s feedlot industry were selected for this study (Table I). Antibiotic usage in all feedlots was recorded (Table II). In feedlots A, B, and C, conventional production pens associated with the catch basins of interest at each feedlot were identified and 20 pens in each feedlot were randomly selected. At feedlot D, pens were stratified by production type with 15 conventional pens (Dc) and 5 natural pens (Dn) randomly selected. Conventional pens contained cattle routinely administered antibiotics in their feed while natural pens contained cattle that were not receiving any antibiotics. Twenty fresh fecal pats were sampled from each pen and composited to provide one fecal sample per pen per feedlot. Three composite samples were then arbitrarily chosen from each feedlot (or within each production strata for feedlot D) for RT-qPCR. After collection, fecal samples were transported to the lab on ice, flash-frozen in liquid nitrogen within 24 h, and stored at −80°C for DNA extraction.
Table I.
Summary of samples collected.
| Sample type and ID | Type of production | Average number of cattle per pen/catch basin |
|---|---|---|
| Feedlot fecal composite | ||
| A (n = 3) | Conventional | 237 |
| B (n = 3) | Conventional | 200 |
| C (n = 3) | Conventional | 371 |
| DC (n = 3) | Conventional | 251 |
| DN (n = 3) | Natural | 232 |
| Catch basin | ||
| CB (n = 5) | n/a | 13 673 |
| Sewage treatment | ||
| Influent (n = 2) | n/a | n/a |
| Effluent (n = 2) | n/a | n/a |
| Surface water | ||
| Ephemeral creek (n = 2) | n/a | n/a |
n/a — Not available.
Table II.
Summary of antibiotics used at sampled feedlots.
| Feedlot | Antibiotic family | Antibioitc | Route |
|---|---|---|---|
| A | Tetracycline | Chlortetracycline | In feed |
| Oxytetracycline | Parenteral | ||
| Ionophore | Monensin | In feed | |
| Lasalocid | In feed | ||
| Macrolide | Tylosin | In feed/parenteral | |
| Tulathromycin | Parenteral | ||
| Phenicol | Florfenicol | Parenteral | |
| Cephalosporin | Ceftiofur | Parenteral | |
| Fluoroquinolone | Enrofloxacin | Parenteral | |
| Potentiated sulfonamide | Sulfadoxine | Parenteral | |
| Sulfonamide combination | Sulfanilamide, sulfathiozole, sulfamethazine | Oral administration | |
| B, C, Dc | Tetracycline | Chlortetracycline | In Feed |
| Oxytetracycline | Parenteral | ||
| Ionophore | Monensin | In Feed | |
| Lasalocid | In Feed | ||
| Macrolide | Tylosin | In Feed/parenteral | |
| Tulathromycin | Parenteral | ||
| Tilmicosin | Parenteral | ||
| Phenicol | Florfenicol | Parenteral | |
| Cephalosporin | Ceftiofur | Parenteral | |
| Fluroquinolone | Enrofloxacin | Parenteral | |
| Potentiated sulfonamide | Sulfadoxine | Parenteral | |
| Sulfonamide combination | Sulfanilamide, sulfathiozole, sulfamethazine | Oral administration |
DC — conventional pens at feedlot D.
Catch basins, which collected runoff from the feedlot pens, were also sampled once at each feedlot. Sewage influent and effluent samples were collected from wastewater treatment plants located at 2 different municipal centers. Surface water was collected from a creek that was adjacent to feedlot C, which drains land that receives regular manure application. Samples were transported on ice to the lab and stored at 4°C until processed. Catch basin, sewage treatment, and surface water samples were processed by centrifugation (30 mL for catch basin and 80 mL for sewage influent; 15 500 × g) or filtration (sewage effluent and surface water) through a 0.45 μm nitrocellulose filter membrane (until the filter was saturated) within 24 h of collection. The filter membrane or pellet was stored at −80°C.
DNA extraction
Total DNA was extracted from the pellet or from the filter for water samples and from fecal composites (approximately 350 mg). Subsamples of the pellet or feces (100 mg) were suspended in 300 μL of buffer (600 mM NaCl, 120 mM Tris-HCl, 60 mM EDTA, 200 mM guanidine isothyocynate) or 800 μL for filters. For feces and the pellet, aliquots (1 mL) were transferred to 2 mL microfuge tubes containing 0.4 g of sterile zirconia beads (0.3 g of 0.1 mm and 0.1 g of 0.5 mm). For filtered samples, beads were added directly to the vial containing filter paper. β-Mercaptoethanol (5 μL) and 200 μL (70°C) of 10% sodium lauryl sulfate (SDS) were added to the tubes and gently mixed. Cells were lysed (Qiagen TissueLyser; QIAGEN, Germantown, Maryland, USA) for 3 min at maximum speed (setting = 30), or for filters using an Omni Bead Ruptor, (3.25 m/s for 5 min; Omni International, Kennesaw, Georgia, USA). Samples were gently shaken at 70°C for 15 min. Filter paper was removed and samples were centrifuged at 4°C for 5 min at 16 000 × g, and the supernatant was transferred to a 2-mL microfuge tube. The pellet was suspended in 800 μL of buffer and the bead-beating process repeated. Duplicate lysates were subject to isopropanol precipitation of nucleic acid and the pellet was suspended in 100 μL Tris-EDTA, pH 7.4 (TE). Nucleic acids in TE were pretreated with 2 μL of DNase-free RNase (10 mg/mL) per 200 μL of sample and incubated at 37°C for 15 min. Resulting DNA was further purified using a QIAamp DNA Stool Mini Kit (Qiagen) with inclusion of proteinase K (Kit handbook), and the final elution accomplished using nuclease-free water. Extracted DNA was assessed for PCR inhibitors using 16S primers (Table III) and if the absence or low yield of a PCR product was noted, an additional purification step using sepharose 2B was undertaken as described by Miller et al (16). Purification was required for all catch basin samples (n = 5). Purity of the DNA was determined using a spectrophotometer (NanoDrop 2000; Thermo Scientific, Waltham, Maine, USA) and the DNA was quantified using a (Quant-iT PicoGreen dsDNA Assay Kit with a Nanodrop 3300 fluorospectrometer Thermo Scientific).
Table III.
Primers used in real-time, quantitative polymerase chain reaction analysis.
| Gene | Primer pair | Sequence (5′-3′) | Annual temperature (°C) | Amplicon size (bp) | Number of cycles | Slope | Intercept point | Efficiency (%) | R2 | LLOQa copies | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16S-rRNA | F | CTCCTACGGGAGGCAGCAGT | 60 | 156 | 30 | −3.4 | 35.2 | 97.4 | 0.999 | 1 × 105 | This study |
| R | TTACCGCGGCTGCTGGCAC | ||||||||||
| sul1 | F | CGCACCGGAAACATCGCTGCAC | 55 | 162 | 40 | −3.6 | 42.7 | 88.3 | 1.000 | 1 × 102 | (11) |
| R | TGAAGTTCCGCCGCAAGGCTCG | ||||||||||
| sul2 | F | TCCGGTGGAGGCCGGTATCTGG | 60 | 190 | 40 | −3.7 | 43.0 | 85.6 | 0.998 | 1 × 102 | (11) |
| R | CGGGAATGCCATCTGCCTTGAG | ||||||||||
| tet(A) | F | GCTACATCCTGCTTGCCTTC | 64 | 210 | 35 | −3.4 | 38.9 | 96.3 | 0.999 | 5 × 101 | (44) |
| R | CATAGATCGCCGTGAAGAGG | ||||||||||
| tet(B) | F | ACACTCAGTATTCCAAGCCTTTG | 60 | 205 | 40 | −3.6 | 40.8 | 90.2 | 0.999 | 5 × 101 | (19) |
| R | GATAGACATCACTCCCTGTAATGC | ||||||||||
| tet(M) | F | TGGACAAAGGTACAACGAGGACGG | 64 | 224 | 35 | −3.5 | 36.4 | 94.4 | 0.998 | 5 × 101 | This study |
| R | ACGAGTTTGTGCTTGTACGCCA | ||||||||||
| tet(O) | F | ACGGARAGTTTATTGTATACC | 53 | 171 | 40 | −3.4 | 40.1 | 95.5 | 0.999 | 5 × 101 | (45) |
| R | TGGCGTATCTATAATGTTGAC | ||||||||||
| tet(Q) | F | AGAATCTGCTGTTTGCCAGTG | 64 | 167 | 35 | −3.7 | 42.4 | 86.8 | 0.998 | 1 × 102 | (45) |
| R | CGGAGTGTCAATGATATTGCA | ||||||||||
| tet(W) | F | GAGAGCCTGCTATATGCCAGC | 64 | 168 | 35 | −3.7 | 38.4 | 86.0 | 0.997 | 5 × 101 | (45) |
| R | GGGCGTATCCACAATGTTAAC | ||||||||||
| erm(A) | F | CCTTCTCAACGATAAGATAGC | 55 | 207 | 35 | −3.4 | 37.6 | 97.2 | 0.998 | 5 × 101 | This study |
| R | ATGGAGGCTTATGTCAAGTG | ||||||||||
| erm(B) | F | TTCAATTCCCTAACAAACAGAG | 55 | 161 | 40 | −3.6 | 45.5 | 88.5 | 0.994 | 5 × 101 | This study |
| R | TGTTCGGTGAATATCCAAGG | ||||||||||
| erm(C) | F | GAGGTGTAATTTCGTAACTGCC | 55 | 189 | 35 | −3.7 | 38.6 | 85.0 | 0.997 | 5 × 101 | This study |
| R | TTGCGTATTATATCCGTACTTATG | ||||||||||
| erm(F) | F | GCCCGAAATGTTCAAGTTGTCGGTTG | 55 | 164 | 35 | −3.6 | 38.7 | 90.3 | 0.998 | 1 × 103 | This study |
| R | TGAAGGACAATGGAACCTCCCAGA | ||||||||||
| mef(A) | F | GGAGCTACCTGTCTGGATGG | 60 | 179 | 40 | −3.3 | 36.3 | 100.3 | 1.000 | 5 × 101 | (46) |
| R | CAACCGCCGGACTAACAATA | ||||||||||
| qnrS | F | ATGCAAGTTTCCAACAATGC | 60 | 240 | 35 | −3.5 | 37.9 | 91.5 | 0.998 | 5 × 101 | (12) |
| R | CTATCCAGCGATTTTCAAACA | ||||||||||
| oqxB | F | TCCTGATCTCCATTAACGCCCA | 64 | 131 | 35 | −3.4 | 38.4 | 96.0 | 1.000 | 5 × 101 | (47) |
| R | ACCGGAACCCATCTCGATGC | ||||||||||
| blaSHV | F | CGCTTTCCCATGATGAGCACCTTT | 64 | 110 | 35 | −3.5 | 38.7 | 94.7 | 0.999 | 5 × 101 | (48) |
| R | TCCTGCTGGCGATAGTGGATCTTT | ||||||||||
| blaTEM1 | F | TTGGGTGCACGACTGGGT | 64 | 504 | 35 | −3.8 | 36.7 | 84.8 | 0.997 | 5 × 101 | (49) |
| R | TAATTGTTGCCGGGAAGC | ||||||||||
| blaCTX-M | F | CTATGGCACCACCAACGATA | 60 | 103 | 35 | −3.6 | 36.9 | 90.4 | 0.999 | 5 × 101 | (12) |
| R | ACGGCTTTCTGCCTTAGGTT |
LLOQ — Lower limit of quantification.
Quantification of antimicrobial resistance genes
Real-time, quantitative PCR (RT-qPCR) was used to estimate the copy numbers of 18 resistance genes across 5 antibiotic families including sulfonamides (sul1 and sul2), tetracyclines [tet(A), tet(B), tet(M), tet(O), tet(Q), and tet(W)], macrolides [erm(A), erm(B), erm(C), erm(F), and mef(A)], fluoroquinolones (qnrS and oqxB), and β-lactams (blaSHV, blaTEM1, and blaCTX-M). Primers for the 16S-ribosomal RNA (rRNA) gene were also included to estimate the total amount of bacteria associated with each sample and to normalize the abundance of ARG in collected samples. All RT-qPCR assays were done using a PCR system (Applied Biosystems 7500 Fast RT-PCR System; Applied Biosystems, Foster City, California, USA) using primers and conditions as described in Table III.
To generate primer sets for ARG, the encoding sequence was downloaded from the GenBank Database (http://www.ncbi.nlm.nih.gov/) and aligned using computer software (Geneious, version 8.1; Auckland, New Zealand) to identify a consensus sequence for primer design. Forward and reverse primers that annealed to regions of the consensus sequence were designed and verified using the BLAST alignment tool (http://www.ncbi.nlm.nih.gov/blast/).
Each reaction was carried out in a total volume of 25 μL, containing 2 μL of template, 0.2 μM of each primer and 1 × iQ SYBR (Green Supermix; Bio-Rad, Saint-Laurent, Quebec). All RT-qPCR reactions included an initial step of 95°C for 3 min, followed by 30 to 40 cycles, with denaturation at 95°C for 15 s, annealing at the respective temperature for 30 s, and an extension at 72°C for 30 s, with the exception of blaTEM1 which was extended for 40 s. Melt curve (55°C to 95°C) analysis was done to verify amplicon uniformity.
Standard curves using known quantities of cloned or synthesized target genes were used to quantify gene copy numbers. Standards for tet(A), qnrS, oqxB, and blaSHV were synthesised (Eurofins Scientific, Lancaster, Pennsylvania, USA) while tet(W), blaTEM1 and blaCTX-M were synthesised separately (Integrated DNA Technologies, San Diego, California, USA). The remaining standards were cloned and the presence of the target gene was verified by sequencing. Dilutions of cloned target genes ranging from 108 to 50 copies per reaction were amplified in duplicate to generate standard curves for each RT-qPCR assay. No template controls were included in duplicate for each RT-qPCR assay to detect contamination. If contamination was detected, the assay was repeated. All RT-qPCR reactions were done in triplicate.
Statistical analysis
To estimate the relative abundance of resistance genes, values for each DNA sample were divided by 16S-rRNA copy numbers (copies of ARGs/copies of 16S-rRNA). Data were natural log (ln) transformed to achieve normality prior to statistical analyses using computer software (MIXED procedure, SAS version 9.4; SAS Institute, Cary, North Carolina, USA). The model consisted of sample type [feedlot = A (n = 3), B (n = 3), C (n = 3), DC (n = 3), DN (n = 3), catch basin = CB (n = 5), sewage influent = Influent (n = 2), sewage effluent = Effluent (n = 2), and creek = Ephemeral creek (n = 2)] as a fixed effect and the relative abundance of each ARG as the dependant variable. The LSMEANS statement was used to separate means with significance declared at a P-value ≤ 0.05. Statistical analysis of oqxB and blaSHV could not be done due to a lack of detection of resistance genes in replicates.
Results
Antibiotics from the tetracycline, macrolide, phenicol, cephalosporin, fluoroquinolone, and sulfonamide families as well as ionophores were used at feedlots A, B, C, and DC (Table II). Among the 18 target resistance genes, all genes with the exception of those associated with fluoroquinolone (qnrS and oqxB) and β-lactam resistance (blaSHV and blaCTX-M) were detected in fecal composite and catch basin water samples. Both the sewage influent and effluent samples possessed all genes except erm(A), oqxB, and blaSHV, coding for macrolide, fluoroquinolone, and β-lactam resistance, respectively. Only 7 [sul1, sul2, tet(O), tet(Q), tet(W), erm(C), mef(A)] of the 18 ARG were detected in creek water. In some cases, ARG were detected in samples (Table IV) at copy numbers that were outside the standard curve and as a result they were not included in the analysis.
Table IV.
Raw copies of each gene quantified by quantitative polymerase chain reaction (qPCR).
| Sample ID | Replicate | Sample type | Technical replicate | 16S rRNA | sul1 | sul2 | tetA | tetB | tetM | tetO | tetQ | tetW | ermA | ermB | ermC | ermF | mefA | qnrS | oqxB | blaSHV | blaCTX-M | blaTEM1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 1 | Fecal | 1 | 135316848.00 | 20470.49 | 8431.89 | 2851.00 | 1350.12 | 471012.72 | 8856329.00 | 48037360.01 | 1674731.50 | 844.93 | 2187571.00 | 40.85a | 205467.56 | 10500624.00 | 50.30 | ||||
| 2 | 131714168.01 | 17365.96 | 7998.46 | 2735.85 | 1447.68 | 308166.19 | 9390889.00 | 49510743.99 | 1604023.00 | 684.48 | 1602458.00 | 217129.28 | 7118568.00 | 55.96 | ||||||||
| 3 | 129662864.01 | 18957.58 | 8862.46 | 2650.42 | 1514.21 | 267133.56 | 9903522.00 | 51098516.02 | 1701210.50 | 974.48 | 1671816.50 | 33.60a | 211299.39 | 5797324.00 | ||||||||
| A | 2 | Fecal | 1 | 99899663.99 | 21102.22 | 40312.38 | 8108.91 | 439.11 | 441146.84 | 6130922.00 | 40700640.01 | 1327763.50 | 4753.59 | 1276430.12 | 118.17 | 116988.45 | 3365209.25 | 86.74 | ||||
| 2 | 103062087.99 | 18706.49 | 51132.17 | 7737.51 | 378.95 | 423588.78 | 5981603.50 | 39793516.00 | 1322640.25 | 5150.98 | 1038140.06 | 130.73 | 117221.91 | 3204971.25 | ||||||||
| 3 | 100093440.01 | 21806.00 | 43241.84 | 8301.56 | 315.31 | 360798.62 | 6061510.50 | 38425511.99 | 1265414.38 | 5476.85 | 1235953.88 | 122629.25 | 3724736.50 | 81.03 | ||||||||
| A | 3 | Fecal | 1 | 111629152.01 | 25474.63 | 22078.60 | 5601.00 | 7009.02 | 584707.06 | 6507431.00 | 53670336.00 | 1515935.88 | 4014.69 | 2352380.00 | 1708.55 | 336977.94 | 3663204.25 | 236.53 | ||||
| 2 | 122443199.99 | 28652.35 | 22681.12 | 5607.93 | 6733.56 | 543316.44 | 6483575.50 | 53856492.00 | 1373533.13 | 3135.03 | 2622462.25 | 1310.36 | 327080.19 | 3506120.00 | 287.97 | |||||||
| 3 | 119955575.99 | 31465.53 | 22883.97 | 6646.54 | 7191.74 | 426515.75 | 6950969.00 | 58135256.00 | 1445576.13 | 3889.44 | 2624452.75 | 991.10 | 348714.59 | 4450952.00 | 398.74 | |||||||
| B | 1 | Fecal | 1 | 159316384.01 | 111101.95 | 165836.91 | 6728.63 | 12306.90 | 282245.50 | 10369626.00 | 61502276.00 | 2224887.75 | 117338.26 | 900.45 | 282343.72 | 9378331.00 | 2818.12 | |||||
| 2 | 146688688.00 | 113397.43 | 118706.06 | 6479.08 | 12935.85 | 239815.77 | 9428455.00 | 59709327.99 | 2088906.13 | 110939.27 | 2213242.00 | 268473.81 | 8159826.50 | 2728.29 | ||||||||
| 3 | 161305823.98 | 116997.45 | 156138.98 | 6362.95 | 13036.56 | 10377423.00 | 68864232.01 | 2255860.75 | 117087.73 | 2805674.50 | 473.71 | 279472.25 | 6569808.50 | 3095.71 | ||||||||
| B | 2 | Fecal | 1 | 81552063.99 | 98154.14 | 110756.03 | 5701.19 | 59988.39 | 302924.81 | 6453254.00 | 33777476.01 | 1422558.87 | 73125.31 | 2202396.75 | 1074.36 | 168106.72 | 4872355.50 | |||||
| 2 | 85743295.99 | 97176.06 | 79365.62 | 5726.55 | 55252.45 | 242925.16 | 6351378.00 | 35357060.00 | 1371462.75 | 50762.96 | 2299559.75 | 177602.81 | 4400084.00 | 81.93 | ||||||||
| 3 | 79841408.01 | 80799.32 | 4670.81 | 50258.24 | 5654909.00 | 26986128.00 | 1183911.13 | 1886979.50 | 1082.50 | 152050.31 | 3529317.50 | 92.84 | ||||||||||
| B | 3 | Fecal | 1 | 183421056.01 | 47936.98 | 31062.49 | 19571.46 | 11931.66 | 65516.30 | 13083021.00 | 48467588.00 | 3201282.50 | 25943.95 | 8699972.00 | 1731.31 | 499035.00 | 9397475.00 | 91.60 | 880.61 | |||
| 2 | 174064223.98 | 44109.56 | 33776.75 | 19191.80 | 12006.78 | 91600.15 | 13306456.00 | 54075300.00 | 3315827.25 | 25552.25 | 9034571.00 | 1872.85 | 477091.50 | 12886706.00 | 105.42 | 796.97 | ||||||
| 3 | 194329407.99 | 46224.36 | 33637.20 | 21135.25 | 13638.56 | 105886.27 | 12358680.00 | 61737384.00 | 3250019.00 | 24779.23 | 8225782.50 | 1463.09 | 391836.84 | 10886674.00 | 783.58 | |||||||
| C | 1 | Fecal | 1 | 132025615.99 | 59021.54 | 198582.59 | 3159.40 | 557.26 | 1125759.62 | 7817069.50 | 49755600.00 | 1568247.75 | 30813.44 | 418717.34 | 363393.00 | 3167658.75 | 37.20a | |||||
| 2 | 131376103.98 | 59779.86 | 184612.44 | 3836.21 | 523.14 | 1045928.12 | 7809439.00 | 53673215.99 | 1574292.50 | 33599.78 | 482782.94 | 110.83 | 423221.34 | 2862075.50 | 22.55a | |||||||
| 3 | 120998464.00 | 56464.17 | 168604.11 | 3454.13 | 377.25 | 829512.44 | 7937674.50 | 46971811.99 | 1528236.12 | 32392.02 | 424963.19 | 72.78 | 373501.34 | 3173973.50 | 24.64a | |||||||
| C | 2 | Fecal | 1 | 122397007.99 | 72122.42 | 235897.00 | 13834.71 | 1891.63 | 1006640.69 | 6830077.00 | 60875284.02 | 1388427.00 | 30666.98 | 547956.31 | 19.39a | 687731.19 | 2188730.00 | 15.58a | 2384.82 | |||
| 2 | 122793696.00 | 66459.59 | 220050.12 | 13869.91 | 1745.99 | 950735.63 | 7544167.50 | 57674784.00 | 1519405.00 | 30872.13 | 566380.69 | 23.10a | 693207.62 | 2100251.50 | 17.83a | 2385.01 | ||||||
| 3 | 115632415.99 | 63934.21 | 163830.08 | 12835.12 | 2014.22 | 1051637.88 | 6819442.00 | 57393216.01 | 1415696.38 | 30387.80 | 501701.44 | 16.31a | 685353.69 | 2969424.25 | 2114.98 | |||||||
| C | 3 | Fecal | 1 | 137840176.00 | 46671.25 | 179057.03 | 18671.75 | 5545.93 | 113185.48 | 6309339.00 | 28700536.00 | 1571610.50 | 10945.18 | 1911604.63 | 650.14 | 130912.76 | 4955301.00 | 234.94 | ||||
| 2 | 145724783.99 | 44179.83 | 97156.34 | 17989.21 | 5403.88 | 100214.77 | 6724203.50 | 29734530.00 | 1668433.50 | 2173519.50 | 716.32 | 143671.91 | 5677877.00 | 281.04 | ||||||||
| 3 | 135602272.01 | 43281.54 | 130144.59 | 17046.96 | 5018.45 | 119425.29 | 6746098.00 | 28995622.00 | 1542394.00 | 8665.21 | 2137903.00 | 140682.22 | 5617090.50 | 220.93 | ||||||||
| DC | 1 | Fecal | 1 | 129925072.00 | 40353.48 | 373749.06 | 4017.37 | 823.61 | 168191.22 | 8200801.00 | 49411755.99 | 1973424.25 | 74038.43 | 11354147.00 | 6525.17 | 352319.09 | 9235876.00 | 295.75 | ||||
| 2 | 115599064.00 | 31477.65 | 429841.19 | 3568.97 | 608.41 | 165141.44 | 7787328.00 | 46216495.99 | 1773117.37 | 80707.81 | 9423764.00 | 6217.44 | 320004.37 | 7344657.50 | 288.50 | |||||||
| 3 | 118867728.01 | 38710.29 | 373662.66 | 1050.50 | 171959.63 | 7115402.00 | 45508143.99 | 1694098.62 | 84501.50 | 5095.33 | 305576.59 | 4809984.50 | 274.63 | |||||||||
| DC | 2 | Fecal | 1 | 129377624.00 | 22421.61 | 79879.81 | 2969.62 | 200728.61 | 8610279.00 | 43357992.01 | 1950964.50 | 13844.17 | 377.58 | 203484.56 | 8058277.00 | 466.72 | ||||||
| 2 | 141131536.00 | 25813.30 | 78503.76 | 3243.03 | 570.82 | 192892.69 | 9469729.00 | 50623836.01 | 1975484.00 | 14494.78 | 5714419.50 | 190509.19 | 8697406.00 | 499.05 | ||||||||
| 3 | 145017184.01 | 22801.69 | 76488.68 | 3596.93 | 669.44 | 147117.75 | 8740696.00 | 51748652.01 | 1996960.63 | 12459.60 | 5409304.00 | 408.99 | 218296.69 | 6174802.00 | 503.26 | |||||||
| DC | 3 | Fecal | 1 | 43101751.99 | 10237.82 | 13785.80 | 1305.03 | 517.25 | 111468.91 | 3666688.00 | 18258204.00 | 642751.06 | 17556.67 | 529002.56 | 3348.62 | 48692.04 | 1979577.50 | 13.06a | 100.59 | |||
| 2 | 41444508.01 | 12869.65 | 13924.53 | 1194.60 | 389.30 | 110159.65 | 3717029.25 | 17124558.00 | 604603.62 | 18156.68 | 566633.19 | 3043.43 | 43779.34 | 2204189.50 | 12.00a | 66.44 | ||||||
| 3 | 44755803.99 | 8382.83 | 13732.72 | 1946.21 | 407.02 | 123459.26 | 3919679.75 | 20919260.00 | 666029.56 | 17895.37 | 580689.13 | 4296.82 | 49713.02 | 2199199.50 | 20.35a | 77.79 | ||||||
| DN | 1 | Fecal | 1 | 37480983.99 | 12179.08 | 5156.01 | 241.47 | 12121.73 | 2141243.00 | 9101782.00 | 412804.25 | 2945.59 | 575923.06 | 119.52 | 26330.30 | 1817781.12 | 53.34 | |||||
| 2 | 36215332.00 | 6770.08 | 5549.21 | 227.09 | 10885.59 | 2091591.50 | 8376621.00 | 421088.66 | 3005.33 | 459052.22 | 24433.53 | 1735015.75 | 39.39a | |||||||||
| 3 | 37729760.00 | 7409.72 | 5326.78 | 316.66 | 270.52 | 12177.91 | 2101451.50 | 8117923.50 | 415199.91 | 2729.59 | 400774.44 | 120.27 | 23920.72 | 1349194.50 | ||||||||
| DN | 2 | Fecal | 1 | 44521535.99 | 16224.93 | 19464.00 | 371.90 | 217.13 | 55777.77 | 1691142.12 | 9644993.00 | 334087.06 | 7747.75 | 71843.28 | 70.28 | 18048.39 | 1409157.62 | 181.48 | ||||
| 2 | 43508420.00 | 12930.62 | 19531.71 | 376.52 | 309.44 | 40239.45 | 1635285.25 | 9207957.00 | 308672.87 | 7507.85 | 64030.17 | 16776.38 | 1433570.25 | |||||||||
| 3 | 46520511.99 | 19602.84 | 411.49 | 370.36 | 43530.51 | 1518456.75 | 9917966.00 | 301118.59 | 7760.27 | 70832.16 | 60.59 | 18279.78 | 219.55 | |||||||||
| DN | 3 | Fecal | 1 | 141902400.00 | 57814.70 | 310189.19 | 102429.73 | 25710.93 | 440572.31 | 6990363.50 | 47887328.01 | 2384895.00 | 50948.66 | 1549203.12 | 203043.66 | 6134732.50 | 446.82 | |||||
| 2 | 143139519.99 | 56497.85 | 301551.94 | 102248.34 | 23645.28 | 291791.06 | 6489048.00 | 51405652.01 | 2096934.12 | 53354.60 | 1735329.00 | 188.29 | 211177.77 | 9015095.00 | 594.74 | |||||||
| 3 | 143673359.99 | 62237.89 | 277239.03 | 115880.05 | 26122.71 | 335872.87 | 7051654.50 | 1673693.75 | 58052.32 | 1695864.37 | 216.24 | 221886.25 | 6697095.50 | 580.79 | ||||||||
| CB | 1 | Catch Basin | 1 | 18703190.00 | 146279.45 | 536011.19 | 398.38 | 59815.21 | 14823.55 | 7636.33 | 11995.24 | 106602.04 | 971.07 | 3513.79 | 10157.67 | 14.28a | ||||||
| 2 | 16186165.00 | 161438.92 | 468081.38 | 494.18 | 111.21 | 45805.99 | 15114.80 | 347608.06 | 6991.99 | 12288.96 | 109660.18 | 3364.80 | 7974.03 | 18.20a | ||||||||
| 3 | 18902718.00 | 155824.48 | 548441.31 | 450.63 | 140.99 | 57605.87 | 16031.42 | 409233.78 | 7820.98 | 12553.37 | 97607.95 | 856.06 | 3706.70 | 9697.13 | ||||||||
| CB | 2 | Catch Basin | 1 | 15063999.00 | 379057.69 | 414152.28 | 153.42 | 113603.36 | 25949.88 | 604500.81 | 17091.27 | 37464.08 | 86781.61 | 2903.46 | 11392.22 | 27065.91 | 16.38a | |||||
| 2 | 14237252.00 | 348791.00 | 420699.09 | 241.13 | 161.34 | 114591.84 | 24069.18 | 589924.69 | 14613.12 | 32663.29 | 90019.25 | 2947.21 | 9539.64 | 25740.40 | 11.30a | 18.42a | ||||||
| 3 | 16192147.00 | 390327.22 | 443561.25 | 328.65 | 113.33 | 122569.90 | 24550.94 | 664899.37 | 16414.96 | 32869.02 | 86747.30 | 2572.93 | 11765.55 | 29211.59 | 18.65a | |||||||
| CB | 3 | Catch Basin | 1 | 15566558.00 | 152165.77 | 156909.59 | 2241.81 | 7231.61 | 24904.78 | 175294.53 | 889.47 | 28864.72 | 15912.83 | 21920.78 | ||||||||
| 2 | 14022131.00 | 144100.55 | 112549.97 | 2045.47 | 8010.50 | 24839.63 | 170369.31 | 882.64 | 23696.39 | 14753.91 | 20901.29 | |||||||||||
| 3 | 15329532.00 | 151966.83 | 115317.68 | 1877.90 | 23883.96 | 187182.70 | 764.37 | 28468.64 | 16060.86 | 22305.12 | ||||||||||||
| CB | 4 | Catch Basin | 1 | 18983356.00 | 43344.68 | 381580.47 | 30048.64 | 886.17 | 95362.10 | 41749.20 | 845609.25 | 23801.66 | 74629.36 | 872866.75 | 4966.11 | 27366.06 | 47067.50 | 17.32a | 92.56 | |||
| 2 | 19714306.00 | 48460.45 | 395349.41 | 29622.58 | 979.91 | 100002.84 | 40699.20 | 868928.13 | 25048.52 | 79670.13 | 877625.31 | 5355.82 | 29033.96 | 50241.57 | 21.18a | 137.01 | ||||||
| 3 | 19069442.00 | 52214.59 | 382200.75 | 29031.36 | 812.59 | 99042.60 | 40327.45 | 874559.63 | 25561.80 | 79933.93 | 830863.19 | 4543.10 | 32221.33 | 54585.04 | 113.54 | |||||||
| CB | 5 | Catch Basin | 1 | 32513026.00 | 113575.21 | 200583.11 | 1063.29 | 552.15 | 137919.02 | 40425.26 | 1029176.44 | 43591.25 | 378695.41 | 2924847.50 | 26179.17 | 181086.98 | 70543.54 | 10.36a | 89.32 | |||
| 2 | 27277818.00 | 99679.20 | 209723.22 | 951.40 | 623.87 | 140190.69 | 41385.32 | 863914.69 | 39857.52 | 301237.62 | 2567297.75 | 21340.53 | 164171.45 | 59324.51 | 14.22a | 123.25 | ||||||
| 3 | 37447248.01 | 94858.66 | 211840.95 | 865.69 | 499.67 | 122132.66 | 35514.40 | 37923.13 | 279235.09 | 2484248.25 | 27479.65 | 162058.97 | 54576.43 | 13.50a | 96.00 | |||||||
| Influent | 1 | Sewage | 1 | 82388256.01 | 995124.75 | 187163.92 | 32838.39 | 6655.73 | 20719.75 | 387385.13 | 2711903.50 | 68745.53 | 1029.51 | 19532302.00 | 526.72 | 284718.87 | 83297.43 | 1309367.00 | 119.69 | 3109.30 | 1336.238403 | 65035.35 |
| Influent | 2 | 81109407.99 | 182969.22 | 33881.34 | 7084.83 | 22775.30 | 375026.16 | 2652150.75 | 62506.80 | 1220.07 | 18525322.00 | 283778.12 | 96386.62 | 1388566.87 | 119.31 | 3181.14 | 1412.349731 | 67611.09 | ||||
| 3 | 70840319.99 | 1072630.87 | 198985.41 | 32978.54 | 7330.73 | 21845.22 | 391636.34 | 2956557.75 | 79212.13 | 1418.44 | 20022754.00 | 683.35 | 279162.63 | 109074.01 | 1444878.25 | 124.47 | 3303.45 | 1588.18042 | 79525.66 | |||
| Influent | 2 | Sewage | 1 | 37155672.00 | 750581.50 | 115719.91 | 11906.55 | 2580.00 | 5449.93 | 150048.62 | 742904.75 | 23979.22 | 4121.26 | 8816599.00 | 597.24 | 109423.16 | 60308.86 | 1441790.87 | 87.47 | 2162.38 | 385.20 | 12393.67 |
| Influent | 2 | 37180852.00 | 716767.50 | 85876.12 | 12473.78 | 2750.65 | 6349.06 | 148901.39 | 699771.12 | 23660.55 | 3634.77 | 8749802.00 | 519.54 | 103746.18 | 56488.30 | 1472774.50 | 83.56 | 2472.96 | 389.61 | 11638.84 | ||
| 3 | 39584727.99 | 84036.18 | 13184.17 | 2932.60 | 5946.53 | 157894.20 | 793511.44 | 28515.30 | 4517.93 | 8972806.00 | 531.34 | 103627.99 | 61474.34 | 1560031.25 | 98.00 | 2225.99 | 346.83 | 13289.37 | ||||
| Effluent | 1 | Sewage | 1 | 4804898.00 | 89705.76 | 15962.68 | 945.28 | 9.00a | 349.75 | 4498.64 | 18368.64 | 1065.17 | 25.76a | 259306.09 | 375.98 | 3468.21 | 1561.33 | 7430.90 | 0.67a | 14.84a | 84.91 | |
| Effluent | 2 | 4818336.50 | 90243.05 | 17754.80 | 884.22 | 12.52a | 407.76 | 4331.12 | 17219.56 | 946.12 | 19.65a | 263796.94 | 417.42 | 3313.91 | 1714.50 | 7552.45 | 0.99a | 86.03 | 5.37a | |||
| 3 | 5012697.00 | 94629.18 | 17782.24 | 937.30 | 423.61 | 4921.21 | 17450.86 | 1169.20 | 279994.22 | 2545.83 | 1627.88 | 7556.24 | 0.63a | 21.92a | 77.70 | 5.98a | ||||||
| Effluent | 2 | Sewage | 1 | 2654580.00 | 52085.63 | 34527.16 | 719.34 | 140.03 | 182.84 | 3215.77 | 11869.75 | 643.48 | 98357.73 | 7390.41 | 1079.39 | 20827.89 | 0.63a | 30.92a | 12.09a | 118.66 | ||
| Effluent | 2 | 2738544.25 | 51803.52 | 36214.01 | 835.13 | 205.53 | 146.19 | 3144.79 | 13364.86 | 600.82 | 31.64a | 79695.05 | 74.09 | 6933.53 | 870.97 | 22355.65 | 1.02a | 116.86 | ||||
| 3 | 2343376.75 | 48166.34 | 32328.57 | 867.35 | 238.85 | 160.51 | 2449.20 | 11197.99 | 410.11 | 32.88a | 75651.98 | 28.49a | 6285.76 | 867.81 | 18702.66 | 31.38a | 7.56a | 143.13 | ||||
| Creek | 1 | Ephermal | 1 | 2768154.25 | 1015.00 | 10.07a | 209.42 | |||||||||||||||
| Creek | 2 | 2925811.00 | 1217.49 | 24.64a | 57.91 | 484.38 | 583.61a | 186.39 | 20.95a | 8.84a | ||||||||||||
| 3 | 2850461.25 | 1026.70 | 20.21a | 51.28 | 471.48 | 8.23a | 41.15a | 22.32a | 5.27a | |||||||||||||
| Creek | 2 | Ephermal | 1 | 8306417.50 | 6438.36 | 455.97 | 30.55a | 297.50 | 1736.26 | 499.33 | 680.35a | 189.26 | 36.38a | |||||||||
| Creek | 2 | 8646568.00 | 5641.77 | 484.49 | 237.73 | 1971.78 | 649.63 | 45.14a | 196.52 | |||||||||||||
| 3 | 8265799.50 | 6121.54 | 374.68 | 50.21a | 234.69 | 2211.98 | 558.36 | 218.96 | 31.16a |
Values outside standard curve range. Absence of a value indicates that the resistance determinant was not detected.
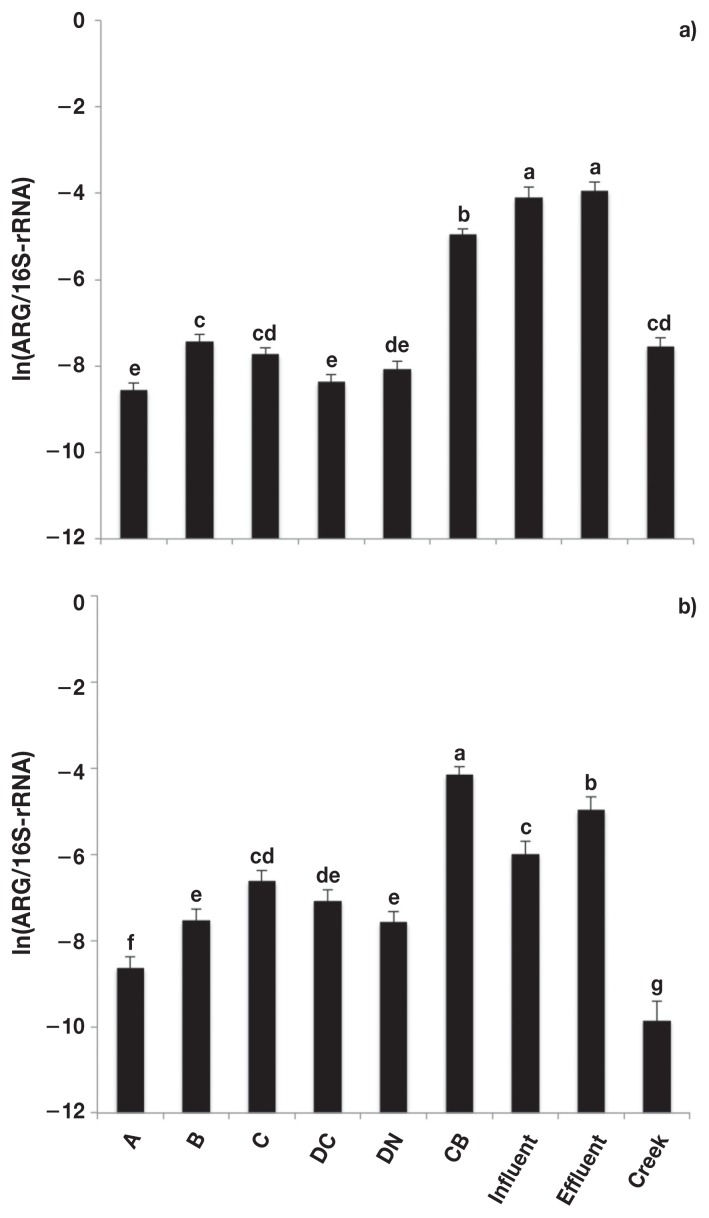
The relative abundance of sul1 and sul2 differed (P < 0.0001) among sample types, but were similar in fecal composites collected from the 4 feedlots (Figure 1). The relative abundance of both sul1 and sul2 was greater (P < 0.05) in catch basin and sewage samples compared to fecal composite and creek samples. There was no difference (P > 0.05) in sul1 or sul2 between conventional and natural production systems.
Figure 1.
Relative abundance [copies of antimicrobial resistance genes (ARG)/copies of 16S-rRNA] of sulfonamide resistance genes. a) sul1 and b) sul2. Error bars represent standard deviation of the mean. A — feedlot A, B — feedlot B, C — feedlot C, DC — feedlot D (conventional production), DN — feedlot D (natural production), CB — catch basin, Influent — sewage influent, Effluent — sewage effluent, and Creek — Ephemeral creek.
a,b,c,d,e,f,g Different letters represent significant difference (P ≤ 0.05).
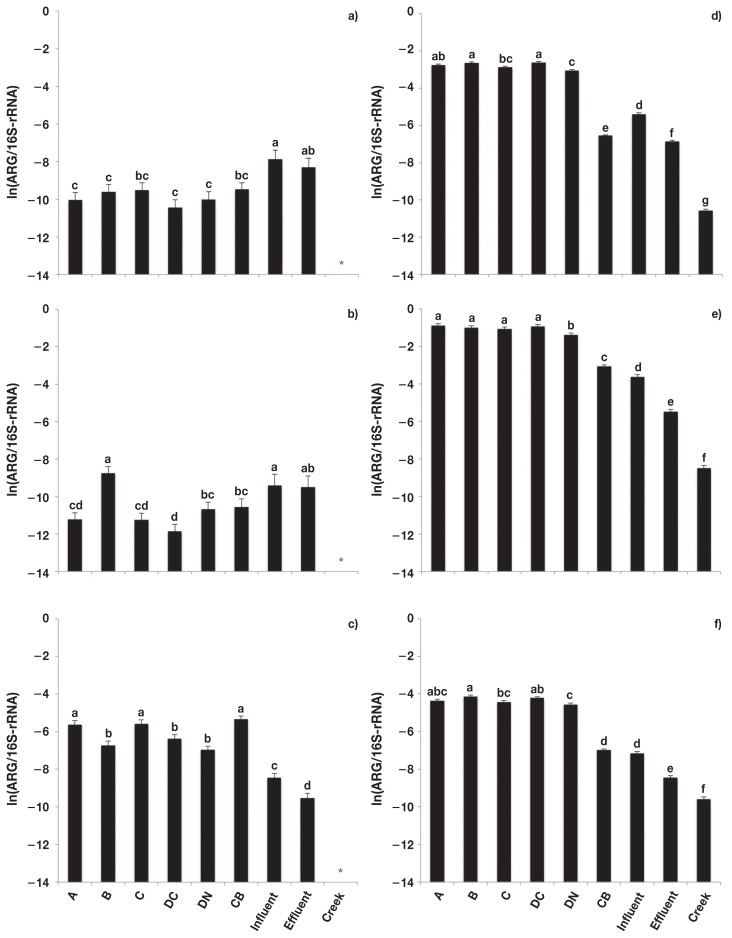
The relative abundance of tet genes also differed among sample types (P < 0.01, Figure 2). The relative abundance of tet(A) and tet(B) (Figures 2a, 2b) was similar across samples, but sewage influent and effluent samples were both lower (P < 0.05) in the relative abundance of tet(M) (Figure 2c) as compared to fecal composite and catch basin samples. There was also a greater relative abundance of tet(M) in sewage influent than effluent (P < 0.05). The catch basin, sewage influent, sewage effluent, and creek samples were all lower (P < 0.05) in the relative abundance of tet(O), tet(Q), and tet(W) than in fecal composite samples (Figures 2d, 2e, 2f). The sewage influent did not differ (P > 0.05) in the relative abundance of tet(W) in the catch basin, but was greater (P < 0.05) in tet(O) and lower (P < 0.05) in tet(Q). All 3 tet genes in sewage influent were greater (P < 0.05) than in sewage effluent and creek samples. The creek samples were lower (P < 0.05) in relative abundance of tet(O), tet(Q), and tet(W) than samples from all other environments. There was no difference (P > 0.05) in tet(A), tet(B), tet(M), tet(O), or tet(W) between fecal composites collected from cattle raised in conventional versus natural production systems. However, fecal composites collected from the conventional system had a greater (P < 0.05) relative abundance of tet(Q) than the natural system.
Figure 2.
Relative abundance [copies of antimicrobial resistance genes (ARG)/copies of 16S-rRNA] of tetracycline resistance genes. a) tet(A), b) tet(B), c) tet(M), d) tet(O), e) tet(Q), and f) tet(W). Error bars represent standard deviation of the mean. A — feedlot A, B — feedlot B, C — feedlot C, DC — feedlot D (conventional production), DN — feedlot D (natural production), CB — catch basin, Influent — sewage influent, Effluent — sewage effluent, and Creek — Ephemeral creek.
a,b,c,d,e,f,g Different letters represent significant difference (P ≤ 0.05).
* Unable to be detected/outside of standard curve.
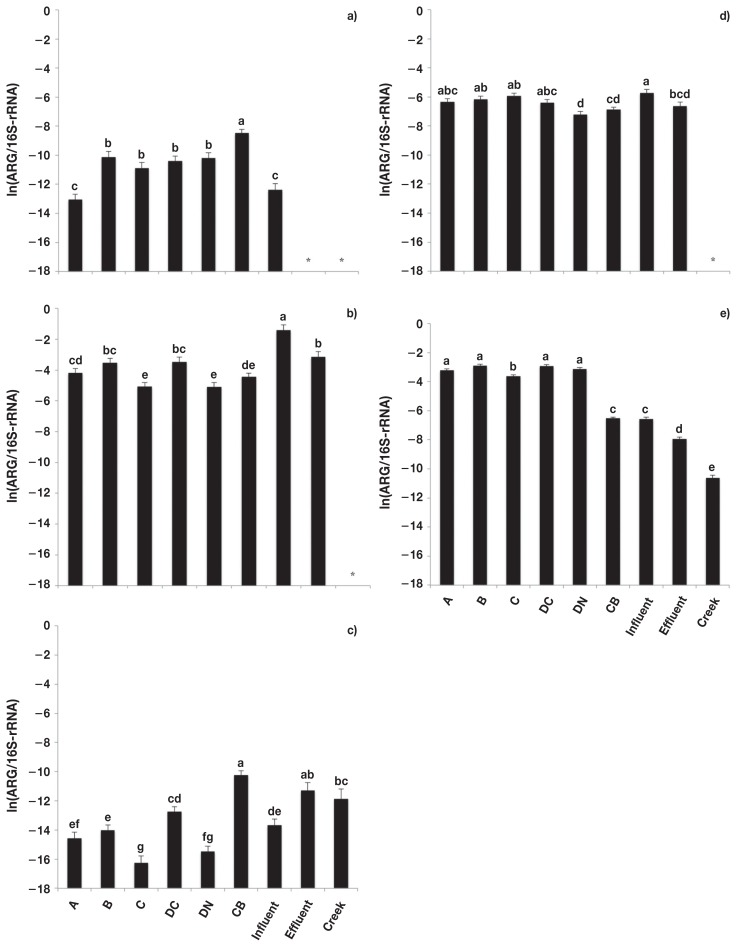
There was no difference (P > 0.05) in the relative abundance of macrolide ARG in fecal composites collected from conventional versus natural production systems, with the exception of erm(F), which was greater (P < 0.05) in conventional than natural production systems (Figure 3). The relative abundance of erm(A) and erm(C) (Figures 2a, 2c) was greater (P < 0.05) in catch basin samples than in fecal composites, whereas mef(A) (Figure 3e) was lower (P < 0.05). The relative abundance of erm(B) was greater (P < 0.05) in sewage influent than in fecal composites, catch basin or sewage effluent samples. The relative abundance of mef(A) in the catch basin, sewage influent and effluent and creek samples were all lower (P < 0.05) than fecal composites.
Figure 3.
Relative abundance [copies of antimicrobial resistance genes (ARG)/copies of 16S-rRNA] of macrolide resistance genes. a) erm(A), b) erm(B), c) erm(C), d) erm(F), and e) mef(A). Error bars represent standard deviation of the mean. A — feedlot A, B — feedlot B, C — feedlot C, DC — feedlot D (conventional production), DN — feedlot D (natural production), CB — catch basin, Influent — sewage influent, Effluent — sewage effluent, and Creek — Ephemeral creek.
a,b,c,d,e,f,g Different letters represent significant difference (P ≤ 0.05).
* Unable to be detected/outside of standard curve.
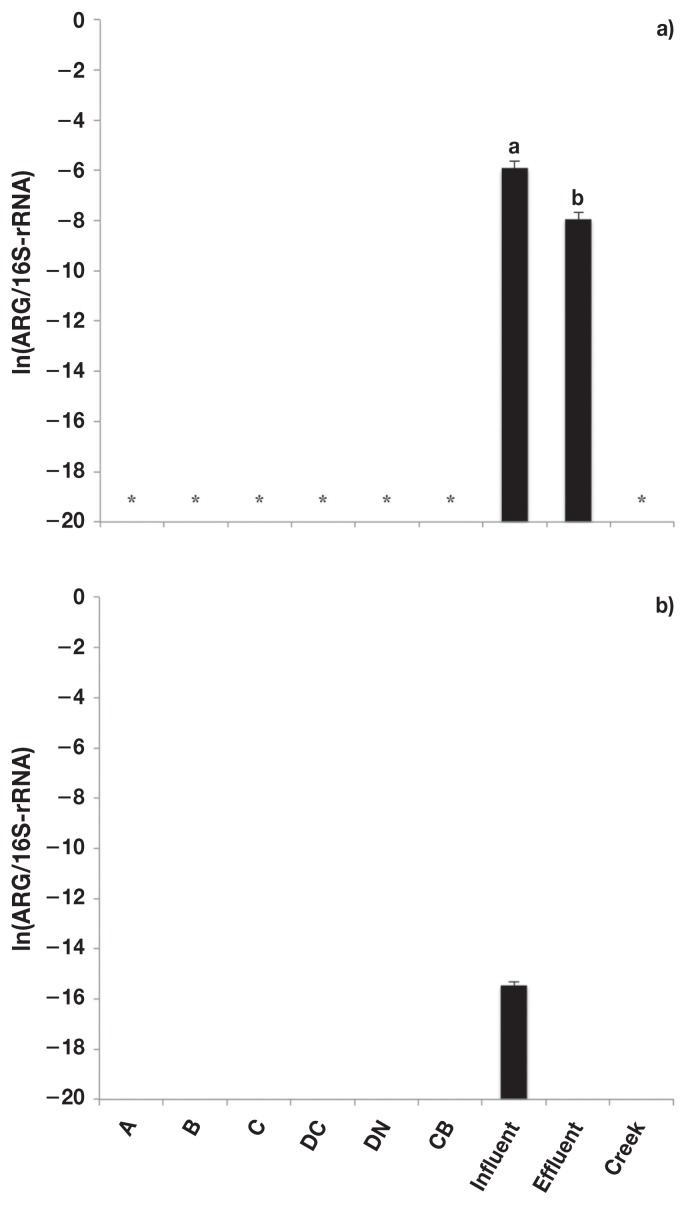
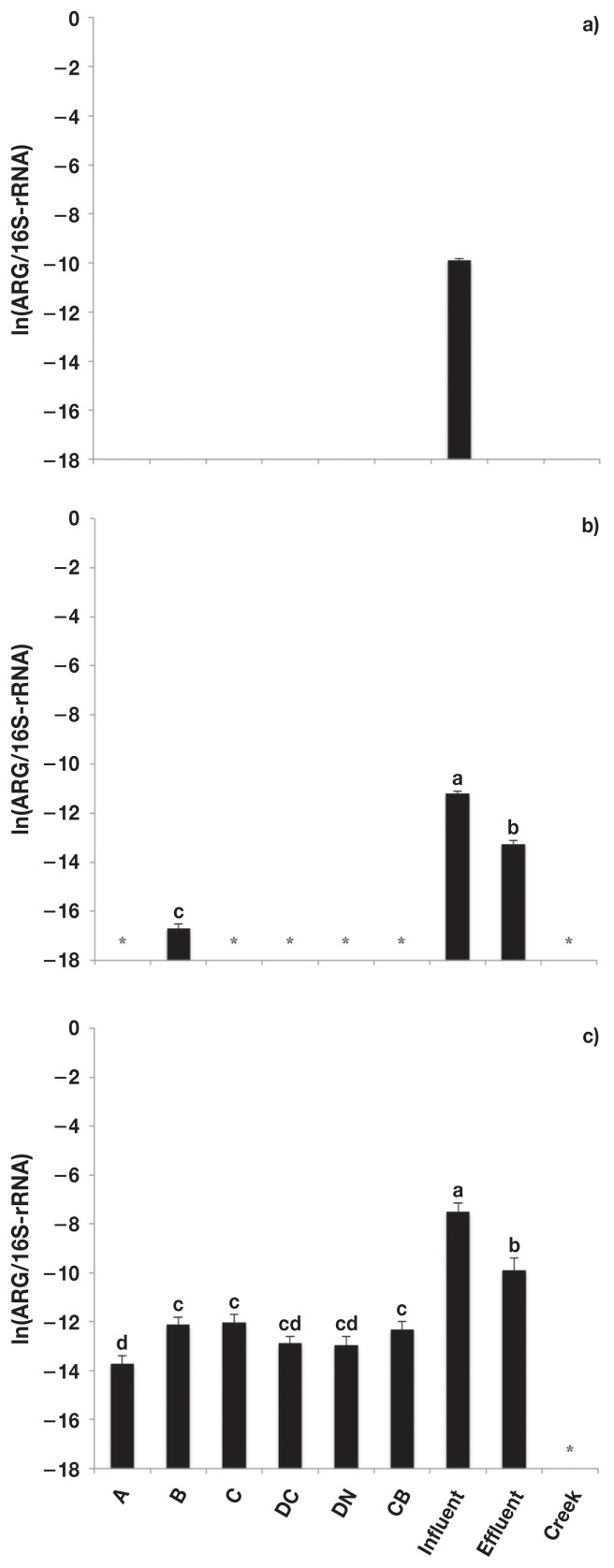
The fluoroquinolone resistance genes (qnrS and oqxB) were only detected in the sewage samples (Figure 4). The relative abundance of qnrS (Figure 4a) was greater (P < 0.05) in sewage influent than effluent. The oqxB gene (Figure 4b) was detected in both the sewage influent and effluent samples, but the copy number was below the detectable limit in effluent samples (Table IV).
Figure 4.
Relative abundance [copies of antimicrobial resistance genes (ARG)/copies of 16S-rRNA] of fluoroquinolone resistance genes. a) qnrS and b) oqxB. Error bars represent standard deviation of the mean. A — feedlot A, B — feedlot B, C — feedlot C, DC — feedlot D (conventional production), DN — feedlot D (natural production), CB — catch basin, Influent — sewage influent, Effluent — sewage effluent, and Creek — Ephemeral creek.
a,b Different letters represent significant difference (P ≤ 0.05).
* Unable to be detected/outside of standard curve.
Likewise, the β-lactam resistance gene blaSHV (Figure 4a) was detected in both sewage influent and effluent samples, but was below the range of the standard curve for effluent samples (Table IV). Of the bla genes, blaCTX-M (Figure 4b) was detected in sewage samples and in the fecal composites at feedlot B. The relative abundance of blaCTX-M was greatest (P < 0.05) for sewage influent and lowest (P < 0.05) for fecal composites at feedlot B. The relative abundance of blaTEM1 (Figure 5) was greater (P < 0.05) in sewage samples than in fecal composite or catch basin samples, but was greater (P < 0.05) for in sewage influent than effluent.
Figure 5.
Relative abundance (copies of antimicrobial resistance genes (ARG)/copies of 16S-rRNA) of β-lactam resistance genes. a) blaSHV, b) blaCTX-M, and c) blaTEM1. Error bars represent standard deviation of the mean. A — feedlot A, B — feedlot B, C — feedlot C, DC — feedlot D (conventional production), DN — feedlot D (natural production), CB — catch basin, Influent — sewage influent, Effluent — sewage effluent, and Creek — Ephemeral creek.
a,b,c,d Different letters represent significant difference (P ≤ 0.05).
* Unable to be detected/outside of standard curve.
Discussion
Real-time, quantitative PCR has been used to examine the abundance and distribution of ARG in beef cattle feces (13,17,18), feedlot wastewater lagoons (14,19,20), urban wastewater (21,22), and freshwater samples from a flowing river (23). In the past, the abundance of tetracycline and sulfonamide ARG in beef cattle feces has been the focus as these antibiotics are frequently used in beef cattle production (13,17,18). Previous studies have compared the abundance of ARG in urban wastewater to farm environments (15). Alberta accounts for most of Canada’s feedlot cattle production and the abundance of ARG in urban wastewater compared with samples collected from feedlot cattle production systems has not been undertaken. In this study, we aimed to examine the abundance and distribution of ARG across 5 antibiotic families and over a range of environments including beef cattle feces, feedlot catch water, municipal sewage samples and surface water from a creek, all collected within the same temporal period.
Studies have demonstrated that administration of antibiotics can increase the abundance of ARG, including in beef cattle feces (18,19). Consequently, antibiotic use in humans and in livestock production likely plays a role in the abundance and distribution of ARG within the environment. An aspect of this study was the collection of data related to antibiotic use from the feedlots sampled (Table II).
Sulfonamides were not administered to cattle at feedlots A, B, C, and D at the time of sampling, but they had been used to treat clinically ill cattle at all feedlots in the past (Table II). The absence of recent sulfonamide use likely resulted in the low relative abundance of sulfonamide resistance genes in fecal composites. Compared to other antibiotics used in feedlots, sulfonamides are more hydrophilic and this property combined with their low sorption to soil makes them among the most mobile of antibiotics (24). The relative abundance of sulfonamide ARG was greater in catch basin than fecal composite samples, possibly as a result of the accumulation of this antimicrobial in the catch basin as a result of run-off. The stability of sulfonamides in water may also explain the greater abundance of these ARG in sewage samples as sulfonamides are excreted in the urine and feces of humans (25). Testing samples for sulfonamide residues would help elucidate if this was the case. Another possible explanation of higher abundance in both the catch basin and sewage treatment samples may be due to differences in bacterial diversity in these different environments. Sulfonamide resistance genes are predominately associated with Gram-negative bacteria, particularly bacteria from the Enterobacteriaceae family but have also been reported in Gram-positive bacteria such as Enterococcus (26). It is possible that the bacterial composition of both the catch basin and sewage treatment samples were predominated by bacteria that typically harbor these resistance determinants.
In this study, tetracycline resistance genes encoding for efflux proteins [tet(A) and tet(B)] were present in all environments at similar levels, with the exception of the creek sample, in which they were not detected. In contrast, the genes encoding for ribosomal protection proteins [tet(M), tet(O), tet(Q) and tet(W)] were predominant in the fecal composites compared with other samples. In general, the abundance ARG coding for ribosomal protection proteins was also much higher than those encoding for efflux proteins. Ribosomal protection proteins are predominantly found in Gram-positive bacteria, which account for most bacteria in bovine feces (27,28), possibly accounting for the greater relative abundance of these genes. Efflux proteins; however, are largely identified in Gram-negative bacteria (27).
Tetracyclines are usually fed to feedlot cattle at low concentrations for the control of liver abscesses and other bacterial diseases. All conventional feedlots sampled in this study used chlortetracycline in their production practices (Table II) and at the time of sampling, most cattle were being administered chlortetracycline in their diet. This could account for the greater relative abundance of tet genes in fecal composites, as administration of tetracycline increases the relative abundance of tet genes in cattle feces (18). There was no difference between conventional and natural production systems for tet(A), tet(B), tet(M), tet(O), and tet(W). However, tet(Q) was more predominant in feces collected from the conventional compared with the natural production system, suggesting that in feed chlortetracycline may preferentially select for specific tet genes. In general, tetracycline ARG in DNA isolated from the catch basin, sewage, and creek samples were in low relative abundance compared to feces. Tetracyclines have a high sorption to soil compared to other antibiotics making them less mobile (24) and less likely to be transported in runoff into the catch basin or nearby waterways. Their lower mobility in water could also account for the reduced presence of tet genes in wastewater. Consequently, selection pressure for tetracycline resistance in the catch basin, sewage treatment and creek samples would be lower than in feces, accounting for the lower tet abundance in these environments.
Of the macrolide resistance genes assessed, differences were observed among samples for all genes. The genes conferring resistance to macrolides are mostly associated with Gram-positive bacteria, with the host range varying among genes (29). The nature of the bacterial microbiome within samples is likely to influence both the density and types of resistance determinants present, factors that may explain why the relative abundance of erm(A) and erm(C) was much lower than erm(B), erm(F), and mef(A) even though all determinants code for macrolide resistance. As with tetracycline, administration of macrolides to cattle also increases the abundance of macrolide ARG in cattle feces (18). While macrolides (tylosin, tulathromycin, and tilmicosin) were used at all conventional feedlots, only 1 out of the 3 conventional pens sampled from feedlot D were being administered macrolides at the time of sampling. This may explain why no difference was observed in the relative abundance of macrolide resistance genes in cattle feces collected from conventional versus natural pens for erm(A), erm(B), erm(C), and mef(A).
The macrolide resistance gene mef(A) was the dominant gene within fecal samples. Its greater relative abundance in cattle feces could be due to its common presence in enteric bacteria (29) or a reflection of co-selection along with other ARG. Many tetracycline ARG are linked to macrolide ARG through common mobile genetic elements. For example, erm(F) frequently shares a conjugative transposon with tet(Q) (30) and erm(B) has been shown to be associated with tet(M) on a Tn1545 conjugative transposon in enterococci (31). In our study, erm(B) was more abundant in sewage influent than in other samples. This gene has been identified in a number of bacterial species, including enterococci and Escherichia coli (32). Macrolides, such as erythromycin, are extensively used in humans (33). A practice that may account for the high abundance of erm(B) in sewage samples.
The fluoroquinolone ARG, qnrS, and oqxB, were only detected in sewage samples, likely as result of the frequent use of fluoroquinolones in humans and the infrequent use of members of this antibiotic family in cattle. There was a noticeable decrease in the relative abundance of qnrS when comparing sewage influent to effluent. The sewage treatment process has been shown to reduce the number of bacteria resistant to tetracycline and sulfonamides, although numbers of antibiotic resistant bacteria in the effluent still remained high (22). In this study, it appears the sewage treatment process resulted in a decline in fluoroquinolone resistant bacteria, as reflected by a reduction in the number of fluoroquinolone ARG detected. However, the fact that fluoroquinolone ARG were detected in sewage effluent shows that they still enter the environment. The fluoroquinolone ARG assessed in this study are predominantly plasmid-mediated and have been shown to be readily transferred to other bacteria (34,35). They are mostly associated with Gram-negative bacteria but have also been reported in Gram-positive bacteria (36).
Similar to the fluoroquinolone ARG, the β-lactamase ARG genes were predominantly found in sewage samples. The blaTEM1 confers resistance to ampicillin, penicillin, and first-generation cephalosporins (35) and was primarily detected in sewage samples, but low levels were also detected in the fecal composite and catch basin samples. Our results support those of Agga et al (15) describing a greater abundance of fluoroquinolone and β-lactamase resistance genes in sewage samples as compared to cattle feces. β-lactamase resistance genes are primarily reported in Gram-negative bacteria and are often linked with fluoroquinolone resistance genes (37). The association between fluoroquinolone and β-lactamase resistance genes, in particular qnrS and blaTEM1 could possibly indicate co-selection of these ARG in sewage (34).
Although the relative abundance of ARG can be influenced by the use of antibiotics, there is a growing body of literature highlighting the relationship between antibiotic use and ARG is complex and not necessarily linear. Jindal et al (38) demonstrated a high level of tylosin resistance persisted on swine farms years after antimicrobial use ceased. The ARG can also be detected in pristine environments not exposed to antibiotics and in which the corresponding antibiotic residues are absent (39,40). Furthermore, the abundance of ARG can be influenced by the species composition of the bacterial community, with some ARG being more common in certain bacterial species than others. Other studies have also demonstrated links between the ARG profile and the bacterial taxonomic profile (40,41). Changes in fecal bacterial communities through differential decay as they transition from primary fecal environments to secondary habitats including catch basins, urban wastewater, and streams would also impact the ARG profile (42,43). Bacterial composition and diversity among sample types was not examined in this study but it is likely to have influenced the distribution and abundance of ARG.
The results from this study demonstrate clear differences in the relative abundance of ARG among feedlot and human related samples. Although samples were only collected at one point in time, it is clear that sulfonamide, fluoroquinolone, and β-lactam resistance genes predominate in urban wastewater, while tetracycline resistance genes are more prevalent in cattle fecal composites. These differences appear to reflect differences in antibiotic use in cattle versus humans. However, other factors such as co-selection of ARG and differences in bacterial community diversity and distribution may also be playing a role. Antibiotic resistance is a complex issue with multiple factors influencing the selection and persistence of ARG. Nevertheless, this study has provided quantitative characterization of various types of ARG from cattle feedlot and urban environments in Alberta. It is apparent that both feedlot cattle and human waste represent different reservoirs of ARG that can enter the environment and possibly contribute to the spread of antibiotic resistance.
References
- 1.Centers for Disease Control and Prevention. Website on the Internet. Antibiotic resistance threats in the United States. 2013. [accessed July 24, 2015]. Available from: http://www.cdc.gov/drugresistance/threatreport-2013/Last.
- 2.Silbergel EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- 3.Andremont A. Commensal flora may play key role in spreading antibiotic resistance. ASM News. 2003;69:601–607. [Google Scholar]
- 4.Marshall BM, Ochieng DJ, Levy SB. Commensals: Underappreciated reservoirs of antibiotic resistance. Microbe. 2009;4:231–238. [Google Scholar]
- 5.Campagnolo ER, Johnson KR, Karpati A, et al. Antimicrobial residues in animal waste and water resources proximal to large-scale swine and poultry feeding operations. Sci Total Environ. 2002;299:89–95. doi: 10.1016/s0048-9697(02)00233-4. [DOI] [PubMed] [Google Scholar]
- 6.Heuer H, Solehati Q, Zimmerling U, et al. Accumulation of sulfonamide resistance genes in arable soils due to repeated application of manure containing sulfadiazine. Appl Environ Microbiol. 2011;77:2527–2530. doi: 10.1128/AEM.02577-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berendonk TU, Manaia CM, Merlin C, et al. Tackling antibiotic resistance: The environmental framework. Nat Rev Microbiol. 2015;13:310–317. doi: 10.1038/nrmicro3439. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y, Johnson TA, Su J, et al. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci USA. 2013;110:3435–3440. doi: 10.1073/pnas.1222743110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He L, Liu Y, Su H, et al. Dissemination of antibiotic resistance genes in representative broiler feedlots environments: Identification of indicator ARGs and correlations with environmental variables. Environ Sci Technol. 2014;48:13120–13129. doi: 10.1021/es5041267. [DOI] [PubMed] [Google Scholar]
- 10.Mu Q, Li J, Sun Y, Mao D, Wang Q, Luo Y. Occurrence of sulfonamide-, tetracycline-, plasmid-mediated quinolone- and macrolide-resistance genes in livestock feedlots in Northern China. Environ Sci Pollut Res. 2014;22:6932–6940. doi: 10.1007/s11356-014-3905-5. [DOI] [PubMed] [Google Scholar]
- 11.Negreanu Y, Pasternak Z, Jurkevitch E, Cytryn E. Impact of treated wastewater irrigation on antibiotic resistance in agricultural soils. Environ Sci Technol. 2012;46:4800–4808. doi: 10.1021/es204665b. [DOI] [PubMed] [Google Scholar]
- 12.Marti E, Jofre J, Balcazar JL. Prevalence of antibiotic resistance genes and bacterial community composition in a river influenced by a wastewater treatment plant. PLoS ONE. 2013;8:e78906. doi: 10.1371/journal.pone.0078906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faldynova M, Vidensk P, Havlickova H, et al. Prevalence of antibiotic resistance genes in faecal samples from cattle, pigs and poultry. Veterinarni Medicina. 2013;58:298–304. [Google Scholar]
- 14.McKinney CW, Loftin KA, Meyer MT, Davis JG, Pruden A. tet and sul antibiotic resistance genes in livestock lagoons of various operation type, configuration, and antibiotic occurrence. Environ Sci Technol. 2010;44:6102–6109. doi: 10.1021/es9038165. [DOI] [PubMed] [Google Scholar]
- 15.Agga GE, Arthur TM, Durso LM, Harhay DM, Schmidt JW. Antimicrobial-resistant bacterial populations and antimicrobial resistance genes obtained from environments impacted by livestock and municipal waste. PLoS ONE. 2015;10:e0132586. doi: 10.1371/journal.pone.0132586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller DN. Evaluation of gel filtration resins for the removal of PCR-inhibitory substances from soils and sediments. J Microbiol Meth. 2001;44:49–58. doi: 10.1016/s0167-7012(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 17.Yu Z, Michel FC, Hansen G, Witum T, Morrison M. Development and application of real-time PCR assays for quantification of genes encoding tetracycline resistance. Appl Environ Microbiol. 2005;71:6926–6933. doi: 10.1128/AEM.71.11.6926-6933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander TW, Yanke JL, Reuter T, et al. Longitudinal characterization of antimicrobial resistance genes in feces shed from cattle fed different subtherapeutic antibiotics. BMC Microbiol. 2011;11:19. doi: 10.1186/1471-2180-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peak N, Knapp CW, Yang RK, et al. Abundance of six tetracycline resistance genes in wastewater lagoons at cattle feedlots with different antibiotic use strategies. Environ Microbiol. 2007;9:143–151. doi: 10.1111/j.1462-2920.2006.01123.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Zhang T, Fang HH. Antibiotic resistance genes in water environment. Appl Microbiol Biotechnol. 2009;82:397–414. doi: 10.1007/s00253-008-1829-z. [DOI] [PubMed] [Google Scholar]
- 21.Lachmayr KL, Kerkhof LJ, DiRienzo AG, Cavanaugh CM, Ford TE. Quantifying nonspecific TEM β-lactamase (blaTEM) genes in a wastewater stream. Appl Environ Micorbiol. 2009;75:203–211. doi: 10.1128/AEM.01254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao P, Munir M, Xagoraraki I. Correlation of tetracycline and sulfonamide antibiotics with corresponding genes and resistant bacteria in a conventional municipal wastewater treatment plant. Sci Total Environ. 2012;421:173–183. doi: 10.1016/j.scitotenv.2012.01.061. [DOI] [PubMed] [Google Scholar]
- 23.Pei R, Kim S, Carlson KH, Pruden A. Effect of river landscape on the sediment concentrations of antibiotics and corresponding antibiotic resistance genes (ARG) Water Res. 2006;40:2427–2435. doi: 10.1016/j.watres.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Chee-Sanford JC, Mackie RI, Koike S, et al. Fate and transport of antibiotic residues and antibiotic resistance genes following land application of manure waste. J Environ Qual. 2009;38:1086–1108. doi: 10.2134/jeq2008.0128. [DOI] [PubMed] [Google Scholar]
- 25.Yang S, Cha J, Carlson K. Simultaneous extraction and analysis of 11 tetracycline and sulfonamide antibiotics in influent and effluent domestic wastewater by solid-phase extraction and liquid chromatography-electrospray ionization tandem mass spectrometry. J Chromatogr. 2005;1097:40–53. doi: 10.1016/j.chroma.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 26.Byrne-Bailey KG, Gaze WH, Kay P, Boxall AB, Hawkey PM, Wellington EM. Prevalence of sulfonamide resistance genes in bacterial isolates from manured agricultural soils and pig slurry in the United Kingdom. Antimicrob Agents Chemother. 2008;53:696–702. doi: 10.1128/AAC.00652-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts MC. Update on acquired tetracycline resistance genes. FEMS Microbol Lett. 2005;245:195–203. doi: 10.1016/j.femsle.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Shanks OC, Kelty CA, Archibeque S, et al. Community structures of fecal bacteria in cattle from different animal feeding operations. Appl Environ Microbiol. 2011;77:2992–3001. doi: 10.1128/AEM.02988-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts MC. Resistance to macrolide, lincosamides, streptogramin, ketolide, and oxazolidinone antibiotics. Mol Biotechnol. 2004;28:47–62. doi: 10.1385/MB:28:1:47. [DOI] [PubMed] [Google Scholar]
- 30.Chung WO, Young K, Leng Z, Roberts MC. Mobile elements carrying ermF and tetQ genes in Gram-positive and Gram-negative bacteria. J Antimicob Chemother. 1999;44:329–335. doi: 10.1093/jac/44.3.329. [DOI] [PubMed] [Google Scholar]
- 31.De Leener E, Martel A, Decostere A, Haesebrouck F. Distribution of the erm(B) gene, tetracycline resistance genes, and Tn1545-like transposons in macrolide- and lincosamides-resistant enterococci from pigs and humans. Microb Drug Resist. 2004;10:341–345. doi: 10.1089/mdr.2004.10.341. [DOI] [PubMed] [Google Scholar]
- 32.Roberts MC, Sutcliffe J, Courvalin P, Jensen LB, Rood J, Seppala H. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob Agents Chemother. 1999;43:2823–2830. doi: 10.1128/aac.43.12.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Website on the Internet. Critically important antimicrobials for human medicine. [Last accessed November 20, 2015]. Available from: http://apps.who.int/iris/bitstream/10665/77376/1/9789241504485_eng.pdf?ua=1.
- 34.Hata M, Suzuki M, Matsumoto M, et al. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob Agents Chemother. 2005;49:801–803. doi: 10.1128/AAC.49.2.801-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rupp ME, Fey PD. Extended spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. Drug. 2003;63:353–365. doi: 10.2165/00003495-200363040-00002. [DOI] [PubMed] [Google Scholar]
- 36.Strahilevitz J, Jacoby GA, Hooper DC, Robicsek A. Plasmid-mediated quinolone resistance: A multifaceted threat. Clin Microbiol Rev. 2009;22:664–689. doi: 10.1128/CMR.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaikh S, Fatima J, Shakil S, Rizci SM, Kamal MA. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J Biol Sci. 2015;22:90–101. doi: 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jindal A, Kocherginskaya S, Mehboob A, et al. Antimicrobial use and resistance in swine waste treatment systems. Appl Environ Microbiol. 2006;72:7813–7820. doi: 10.1128/AEM.01087-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Costa VM, King CE, Kalan L, et al. Antibiotic resistance is ancient. Nature. 2011;477:457–461. doi: 10.1038/nature10388. [DOI] [PubMed] [Google Scholar]
- 40.Durso LM, Miller DN, Wienhold BJ. Distribution and quantification of antibiotic resistant genes and bacteria across agricultural and non-agricultural metagenomes. PLoS ONE. 2012;7:e48325. doi: 10.1371/journal.pone.0048325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forsberg KJ, Patel S, Gibson MK, et al. Bacterial phylogeny structures soil resistomes across habitats. Nature. 2014;509:612–616. doi: 10.1038/nature13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson KL, Whitlock JE, Harwood VJ. Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl Environ Microbiol. 2005;71:3041–3048. doi: 10.1128/AEM.71.6.3041-3048.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang LL, Mankin KR, Marchin GL. Survival of fecal bacteria in dairy cow manure. Trans ASAE. 2004;47:1239–1246. [Google Scholar]
- 44.Ng LK, Martin I, Alfa M, Mulvey M. Multiplex PCR for the detection of tetracycline resistant genes. Mol Cell Probe. 2001;15:209–215. doi: 10.1006/mcpr.2001.0363. [DOI] [PubMed] [Google Scholar]
- 45.Aminov RI, Garrigues-Jeanjean N, Mackie RI. Molecular ecology of tetracycline resistance: Development and validation of primers for detection of tetracycline resistance genes encoding ribosomal protection proteins. Appl Environ Microbiol. 2001;67:22–32. doi: 10.1128/AEM.67.1.22-32.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szczepanowski R, Linke B, Krahn I, et al. Detection of 140 clinically relevant antibiotic-resistance genes in the plasmid metagenome of wastewater treatment plant bacteria showing reduced susceptibility to selected antibiotics. Microbiol. 2009;155:2306–2319. doi: 10.1099/mic.0.028233-0. [DOI] [PubMed] [Google Scholar]
- 47.Kim HB, Wang M, Park CH, Kim E, Jacoby GA, Hooper DC. oqxAB encoding a multidrug efflux pump in human clinical isolates of Enterobacteriaceae. Appl Agents Chemother. 2009;53:3582–3584. doi: 10.1128/AAC.01574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xi C, Zhang Y, Marrs CF, et al. Prevalence of antibiotic resistance in drinking water treatment and distribution systems. Appl Environ Micorbiol. 2009;75:5714–5718. doi: 10.1128/AEM.00382-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu RB, Alexander TW, Li JQ, Munns K, Sharma R, McAllister TA. Prevalence and diversity of class 1 integrons and resistance genes in antimicrobial-resistant Escherichia coli originating from beef cattle administered subtherapeutic antimicrobials. J Appl Microbiol. 2011;111:511–523. doi: 10.1111/j.1365-2672.2011.05066.x. [DOI] [PubMed] [Google Scholar]