Abstract
BACKGROUND
St. Louis encephalitis virus is a mosquito-borne flavivirus that infrequently causes epidemic central nervous system infections. In the United States, blood donors are not screened for St. Louis encephalitis virus infection, and transmission through blood transfusion has not been reported. During September 2015, St. Louis encephalitis virus infection was confirmed in an Arizona kidney transplant recipient. An investigation was initiated to determine the infection source.
STUDY DESIGN AND METHODS
The patient was interviewed, and medical records were reviewed. To determine the likelihood of mosquito-borne infection, mosquito surveillance data collected at patient and blood donor residences in timeframes consistent with their possible exposure periods were reviewed. To investigate other routes of exposure, organ and blood donor and recipient specimens were obtained and tested for evidence of St. Louis encephalitis virus infection.
RESULTS
The patient presented with symptoms of central nervous system infection. Recent St. Louis encephalitis virus infection was serologically confirmed. The organ donor and three other organ recipients showed no laboratory or clinical evidence of St. Louis encephalitis virus infection. Among four donors of blood products received by the patient via transfusion, one donor had a serologically confirmed, recent St. Louis encephalitis virus infection. Exposure to an infected mosquito was unlikely based on the patient’s minimal outdoor exposure. In addition, no St. Louis encephalitis virus-infected mosquito pools were identified around the patient’s residence.
CONCLUSION
This investigation provides evidence of the first reported possible case of St. Louis encephalitis virus transmission through blood product transfusion. Health care providers and public health professionals should maintain heightened awareness for St. Louis encephalitis virus transmission through blood transfusion in settings where outbreaks are identified.
St. Louis encephalitis virus (SLEV) is a mosquito-borne flavivirus closely related to West Nile virus (WNV). These viruses share the same mosquito vectors, and their associated disease presentations are clinically indistinguishable.1 Like WNV, SLEV is primarily maintained and amplified through cycles between Culex species mosquitoes and avian hosts, and their geographic and temporal distribution dictates the occurrence of human infections.2–5 Most SLEV infections are asymptomatic, but they also can result in a nonspecific febrile illness.3 Less than 1% of human SLEV infections lead to severe neuroinvasive disease, which can present as encephalitis, meningitis, or acute flaccid paralysis; individuals ages 55 years and older are at higher risk for developing SLEV neuroinvasive disease.3,6–8 SLEV infection can be diagnosed by molecular or serologic testing. The viremic period is transient; thus, ribonucleic acid (RNA) is seldom detectable in acute infections that are evaluated after the onset of symptoms, and serology is the mainstay of diagnosis. However, because antibodies against SLEV and WNV readily cross-react on immunoglobulin (Ig)M diagnostic tests, confirmatory neutralizing antibody testing is required to identify the specific infecting flavivirus.9
SLEV was first identified in St. Louis, Missouri, in 1933, and more than 50 outbreaks have been reported in the United States since then.10,11 Reports of SLEV neuroinvasive disease declined considerably after WNV was first detected in 1999; however, isolated cases and limited outbreaks of SLEV disease still occur sporadically in the United States.1,2,12 SLEV is a nationally notifiable infectious disease. In 2015, Arizona state and local health authorities identified an outbreak of SLEV during which infection was confirmed in 23 symptomatic persons. Arizona was the only US state to report human SLEV disease cases to the Centers for Disease Control and Prevention (CDC) that year. In addition, acute WNV disease cases were also confirmed, making this the first documented concurrent outbreak of WNV and SLEV in the United States.13 The majority of SLEV disease cases were reported in Maricopa County, which is the largest urban area in Arizona. Although the blood supply in the United States has attained an unprecedented level of safety, it remains vulnerable to emerging infectious agents.14 WNV transmission by blood transfusion in the United States has been well documented and is rarely reported since the implementation of routine screening of blood donors for WNV infection by nucleic-acid testing (NAT).15–17 Blood donor screening for Zika virus, another related flavivirus, was implemented in 2016.18 SLEV transmission by blood transfusion has not previously been reported.17 There are no US Food and Drug Administration (FDA)-licensed blood donor screening tests for SLEV.
During September 2015, the United Network for Organ Sharing alerted CDC of suspected neuroinvasive SLEV disease in a kidney transplant recipient in Maricopa County on the basis of the detection of SLEV IgM antibodies in his serum. This patient is referred to hereinafter as the SLEV recipient. The organ recipient care teams, Arizona Department of Health Services, and Maricopa County Department of Public Health were notified. The objectives of the ensuing public health investigation were to confirm the diagnosis of SLEV infection in the recipient and to determine the source of his infection.
MATERIALS AND METHODS
SLEV recipient
The SLEV recipient was interviewed regarding exposure history and clinical course, and his medical records were reviewed. Residual serum specimens collected before organ transplantation and after neuroinvasive disease symptom onset, as well as residual cerebrospinal fluid (CSF) collected after symptom onset were obtained for SLEV and WNV testing. There were no remaining specimens collected between day of transplant and symptom onset.
Case definition
An SLEV disease case was classified according to clinical and laboratory criteria stipulated in the national case definition for reporting of arboviral diseases.19 In addition, any persons who had laboratory evidence of recent SLEV infection within 4 weeks after receipt of an organ or blood component from a donor with evidence of recent SLEV infection was considered to have a possible transplant-transmitted or transfusion-transmitted infection, respectively. SLEV infection was considered confirmed if there was molecular detection of SLEV RNA or if serologic testing was positive for SLEV IgM with the detection of neutralizing antibodies to SLEV at a titer 4-fold higher than WNV.
Organ donor and other organ recipients
The organ donor’s medical records were reviewed, and residual serum, plasma, and lymph node DNA lysate collected before organ recovery were obtained for flavivirus testing. Other recipients of organs from the same donor were contacted to obtain symptom history and serum specimens for SLEV and WNV testing.
Blood donors and other blood product recipients
Medical records were reviewed to determine whether the SLEV recipient had received any blood products before symptom onset. The blood collection center initiated a lookback investigation to determine whether there were any remaining in-date co-components that needed to be quarantined and tested for SLEV RNA. The donors of all identified blood products were contacted to obtain symptom history, residence address at the time of donation, and serum specimens for SLEV testing.
Laboratory testing
Serum and CSF samples were tested for the presence of anti-SLEV and anti-WNV IgM and IgG using an enzyme-linked immunosorbent assay at the state public health laboratory, and for anti-WNV IgM using a microsphere-based immunosorbent assay at the CDC.20 For serum specimens with IgM test results reported as positive or nonspecific, confirmatory plaque reduction neutralization testing (PRNT) using a 90% reduction in the number of plaques (PRNT90) for SLEV and WNV was performed at the CDC.21 Tissue specimens were tested by real-time polymerase chain reaction (RT-PCR) and immunohistochemistry at the CDC.
Mosquito surveillance
The Maricopa County Environmental Services (MCES) Vector Control Division routinely conducted mosquito surveillance in 2015 for WNV and SLEV because of the circulation of both viruses in the region. Traps were placed throughout Maricopa County to collect Culex species mosquitoes. MCES recorded the number and location of mosquito traps and tested all mosquitoes captured using RT-PCR for both WNV and SLEV; mosquitoes from the same trap were tested in pools.22 To determine whether SLEV was circulating in the areas in which the SLEV patient might have been exposed to mosquitoes, suggesting a greater likelihood of vector-borne transmission, MCES reviewed data from mosquito surveillance conducted during the 30 days before the date of his illness onset for both the area of his residence and the hospital where he was admitted. Similarly, MCES reviewed the data for areas of residence of any implicated donors for 30 days before organ or blood donation. A 5-mile radius around each of the sites was chosen based on the average maximum flight range combined for both adult C. tarsalis and C. quinquefasciatus mosquitoes from a previous dispersal study.23
RESULTS
SLEV recipient
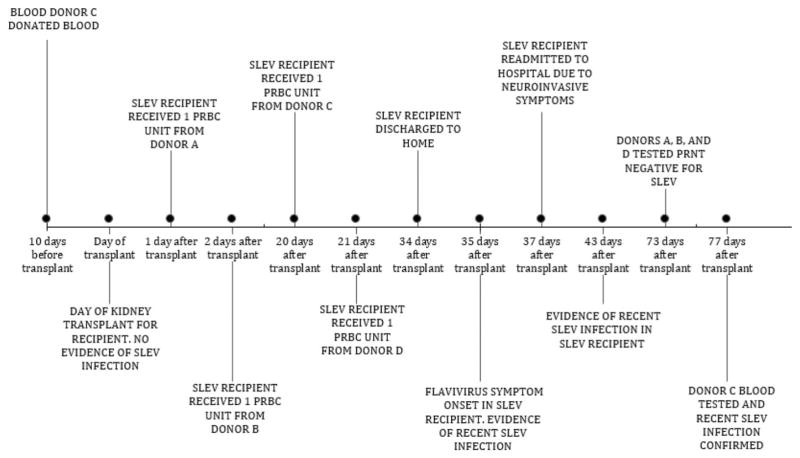
The SLEV recipient was a 69-year-old man who was admitted to the hospital in late July 2015 for a kidney transplant because of end-stage renal disease, which was attributed to diabetic nephropathy and hypertension (Fig. 1). He received methylprednisolone and induction therapy with basiliximab to prevent organ rejection; tacrolimus was added 2 days after transplant. The patient received transfusions of 4 units of leukocyte-reduced red blood cells (pRBCs) on Days 1, 2, 20, and 21 after transplant and was discharged home 34 days after transplant.
Fig. 1.
Investigation timeline of possible St. Louis encephalitis virus transmission through blood transfusion relative to the timing of transplantation of the infected kidney recipient: Arizona, 2015.
The day after discharge, he experienced headache, fever, fatigue, nausea, diarrhea, shortness of breath, and chills, which progressed to rigors. Upon initial consultation, his physicians attributed his symptoms to bacteremia secondary to ureteral stent removal; however, he was readmitted 37 days after transplant, when his symptoms progressed to lower extremity paralysis and respiratory distress, requiring a tracheostomy. By Day 43, the patient’s mental status began to deteriorate. Serum and CSF specimens were collected on Day 43 to test for possible flavivirus infection, and empiric antibiotics were started for possible bacterial infection. The patient received intravenous immunoglobulin and interferon therapy from Day 44 to Day 48 after transplant and 1 pRBC unit each on Days 49 and 56. On Day 51, the patient exhibited gradual motor function improvement, and he had cognitive improvement by Day 59. The patient was discharged to home on Day 105 and experienced some neurologic sequelae after discharge, including memory loss and weakness.
Residual serum specimens collected 16 days before and on the day of his transplant, respectively, were obtained for testing. The serum specimens had nonspecific results on WNV and SLEV IgM testing but had no detectable neutralizing antibodies to SLEV or WNV, ruling out prior infection. The IgM results likely reflect either prior flavivirus infection or reactivity to other serum factors. Testing of subsequent samples, which were collected on the day of symptom onset and beyond, confirmed seroconversion on PRNT as well as a greater than fourfold increase in SLEV neutralizing antibody titers between serum collected on Day 35 (PRNT = 20) and Day 43 (PRNT = 1280). Neutralizing antibody titers against SLEV were also more than four-fold higher than titers against WNV (Table 1). The SLEV recipient’s presentation and test results thus met the clinical and laboratory criteria for neuroinvasive SLEV infection. Upon subsequent interview, the patient reported no febrile illness, exposure to mosquitoes, or travel out of state before transplant. The SLEV recipient spent minimal time outdoors during the weeks before symptom onset, because he was hospitalized with limited mobility. The only noted history of flavivirus exposure was receipt of a yellow fever vaccination in 1965. The patient continued to recover; and, by April 2016, he was reportedly back to neurological baseline.
TABLE 1.
West Nile virus and St. Louis encephalitis virus serologic laboratory results for St. Louis encephalitis virus recipient before and after transplant
| Event | Date | Specimen | SLEV
|
WNV
|
||
|---|---|---|---|---|---|---|
| MIA | PRNT | MIA | PRNT | |||
| Before transplant | Jun 23 | Serum | NS | <10 | NS | <10 |
| Day of transplant | Jul 28 | Serum | NS | <10 | NS | <10 |
| Day of symptom onset | Sep 1 (Day 35)* | Serum | Positive | 20 | Negative | <10 |
| After symptom onset | Sep 9 (Day 43)* | Serum | NS | 1,280 | NS | <10 |
| Sep 10 (Day 44)* | Cerebrospinal fluid | Negative | NA | Negative | NA | |
| Oct 9 (Day 73)* | Serum | Positive | 20,480 | Negative | <10 | |
NS = nonspecific (i.e., a sample that reacts with the negative antigen such that the result using the viral antigen cannot be interpreted); NA = not done; MIA = microsphere immunoassay.
After transplant.
Organ donor and other organ recipients
The cadaveric donor of the left kidney transplanted into the SLEV recipient died in July 2015 in Illinois. His heart, liver, and right kidney were also recovered and transplanted. According to his medical record, he had no symptoms suggestive of flavivirus infection. The heart and liver recipients were Illinois residents, and the right kidney recipient was an Arizona resident; none of these recipients had symptoms compatible with flavivirus infection after transplantation. Organ donor serum collected 1 day before organ recovery contained no detectable SLEV RNA, and IgM antibody test results were negative. There was no SLEV RNA or SLEV antigen detected in residual lymph node tissue by RT-PCR or immunohistochemistry, respectively. Serum specimens collected 6 weeks after organ transplant from the heart recipient and right kidney recipient had no evidence of recent infection. No specimens were available for testing from the liver recipient.
Blood donors and other blood product recipients
The four pRBC units that the SLEV recipient received before symptom onset were collected from four blood donors (Donors A, B, C, and D) whose blood products were delivered by transfusion on Day 1 (Donor A), Day 2 (Donor B), Day 20 (Donor C), and Day 21 (Donor D) after transplant. The donated blood products had been screened by WNV NAT at the blood collection agency and were negative. There were no remaining blood products available for testing. Serum was collected from these donors from 73 to 85 days after the date of donation for SLEV and WNV testing. Specimens from Donors A, B, and D contained no detectable IgM or neutralizing antibodies against SLEV or WNV. Serum from Donor C, collected 77 days after donation, tested positive for SLEV and negative for WNV IgM by microsphere-based immunosorbent assay. The specimen from blood Donor C had detectable neutralizing antibodies against SLEV (PRNT = 320) but not against WNV (PRNT <10). This donor did not report any symptoms compatible with flavivirus infection before or after donating blood.
The recipient of the fresh-frozen plasma co-component from Donor C’s donation was a 77-year-old woman who received a transfusion after she was admitted to the hospital in late July 2015. She had altered mental status before the blood transfusion and received multiple units of fresh-frozen plasma and platelets while admitted. After approximately 2 weeks of hospitalization, she was transferred to hospice, where she died 3 days later. Her primary diagnoses upon discharge to hospice were toxic metabolic encephalopathy, subdural hematoma, and possible hemolytic anemia/thrombotic thrombocytopenic purpura. The cause of death was not suspected to be a flavivirus infection. No autopsy was conducted. Although no specimens were available for testing, a medical records review did not indicate febrile illness or exacerbation of neurologic features after the transfusion.
Mosquito surveillance
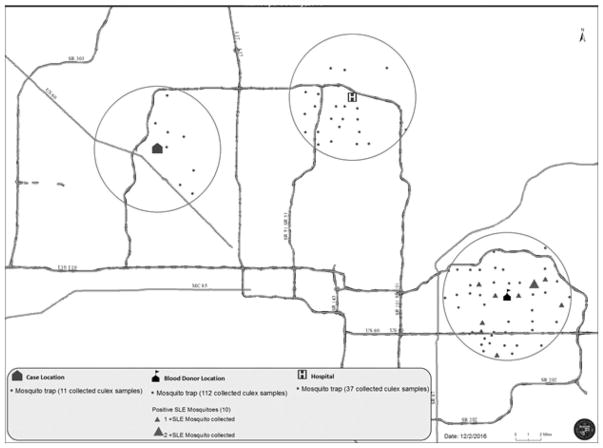
Approximately 50 traps had been placed within a 5-mile radius of the SLEV recipient’s residence, Donor C’s residence, and the hospital where the SLEV recipient was admitted. Traps were observed for mosquitoes by MCES on a weekly basis. No SLEV-infected mosquitoes were identified from 11 mosquito pools from traps collected within a 5-mile radius around the index patient’s residence (Fig. 2) (only traps that trapped live mosquitoes are shown in the figure). No SLEV-infected mosquitoes were identified from 37 mosquito pools from traps collected within a 5-mile radius around the hospital location. Ten SLEV-infected mosquitoes (nine C. quinquefasciatus and one C. tarsalis species) were identified from 112 mosquito pools from traps collected within a 5-mile radius around Donor C’s residence.
Fig. 2.
Mosquito surveillance* for St. Louis encephalitis virus in a 5-mile radius of case locations and donor location for a 30-day period before recipient symptom onset and donor blood donation: Maricopa County, Arizona, 2015. *Fifty traps were located within each 5-mile radius, and only traps that contained mosquitoes are shown; empty traps are not displayed.
DISCUSSION
To our knowledge, this is the first reported case of possible SLEV transmission through blood product transfusion. The patient experienced a clinical illness compatible with SLEV infection 15 days after receiving blood products from an asymptomatic donor with serologically confirmed, recent SLEV infection. The interval between transfusion and symptom onset is consistent with the estimated incubation period for mosquito-transmitted SLEV infection (range, 5–15 days), which has been documented as prolonged (median, 13.5 days) in immunocompromised recipients with transfusion-associated WNV infection.16 The timing of SLEV infection after the implicated transfusion is also supported by seroconversion from neutralizing antibodies being undetectable before the transfusion to being detectable and progressively increasing after the transfusion. This case was likely detected because of enhanced surveillance and testing systems in place for the concurrent WNV and SLEV outbreaks in the region.13 Mosquito surveillance data demonstrated the detection of SLEV infected mosquitoes around Donor C’s residence but not around the SLEV recipient’s residence or the hospital, indicating that the SLEV recipient might have been at lower risk of mosquito-borne exposure. However, these data were not directly comparable, because many more mosquito pools were tested in the region of the blood donor’s residence. In addition, the SLEV recipient spent all of his potential exposure period indoors while hospitalized, except for 1 day spent at home. Serologic test results indicating recent SLEV infection in both the index patient, after the implicated transfusion, and Blood Donor C support SLEV transmission through Blood Donor C’s blood products.
Human SLEV infection is most commonly acquired from Culex species mosquito bites; however, other less common routes of transmission are possible. One case of laboratory-acquired SLEV infection has been previously described.24 Although transmission of SLEV through blood transfusion has not been documented in the published literature, transmission of flavivirus infections through blood transfusion is known to occur.15,25–27 In Arizona, blood products are routinely screened for WNV by NAT.18,28 There is no FDA requirement or AABB standard for screening blood products for SLEV, and there are no SLEV NAT assays commercially available and approved for blood donor screening.
The benefit of blood product screening for blood-borne pathogens depends on several factors, including disease incidence and severity of outcome; screening has proven effective in reducing transfusion transmission of WNV, for example.29 Reported SLEV disease incidence nationwide is extremely low; an average of seven SLEV disease cases were reported annually in the United States during 2004 through 2013 compared with an average of 2540 WNV disease cases reported annually during that same period. This would limit the benefit of screening blood donors for SLEV infection.30 The low US incidence makes the positive predictive value of any SLEV blood product screening test much lower than that for WNV. The duration of donor SLEV infectious risk is assumed to be similar to that for WNV; and, in this case, the local blood collection agency issued a recommendation once confirmatory testing was completed for the asymptomatic blood donor for a 120-day donor deferral period based on the same period stipulated for WNV NAT-positive blood donors.31
This investigation had multiple limitations. There were no residual specimens from Blood Donor C’s implicated donation to confirm the presence of SLEV in any of the donated blood products, which would have strengthened evidence for transfusion-related transmission of SLEV. We also could not definitely state when Blood Donor C’s SLEV viremia occurred, because we only had serologic confirmation of recent infection. Specimens were also unavailable for the plasma recipient, because the investigation took place several weeks after she had died.
This investigation provides evidence to support the possibility that SLEV, like WNV, can be transmitted through blood products, reinforcing the importance of public health surveillance for SLEV disease, especially during an outbreak. Public health officials could determine whether there is an increased risk for SLEV transmission in an area and alert physicians to consider SLEV if there is neuroinvasive disease in a blood transfusion recipient. This should prompt a public health investigation to determine whether the infection might have been transfusion or transplant derived. Current public health surveillance data may under represent the true burden of SLEV disease because of the infrequent availability of SLEV laboratory testing and cross-reactivity with WNV testing. In the absence of systematic data on the risk of transfusion transmission of SLEV, there is currently no justification for routine blood screening; however, continued vigilance for cases like that described herein should inform risk-based decisions on whether further mitigation measures are required. Health care providers and public health professionals should maintain heightened awareness for SLEV transmission through blood transfusion in settings in which SLEV transmission is identified.
SLEV TRANSMISSION INVESTIGATION TEAM
The St. Louis Encephalitis Virus Transmission Investigation Team: Melissa Kretschmer, Lia Koski, Andrew Strumpf, and the Maricopa County Department of Public Health; Hayley Yaglom, Kenneth Komatsu, Lydia Plante, and the Arizona Department of Health Services; Dan Damien and Maricopa County Environmental Services Vector Control Division; Janna Huskey, Hasan Khamash, and Mayo Clinic Hospital; Michael Harmon and the Gift of Hope Organ and Tissue Donor Network; Debbie Freeman and the Illinois Department of Public Health; Tiana Riley and the University of Chicago Medical Center; Becca Craven and the Kovler Organ Transplantation Center; Toni Slaughter and United Blood Services; and Kristine Bisgard and the Centers for Disease Control and Prevention.
Acknowledgments
This investigation was supported by the Centers for Disease Control and Prevention, the Arizona Department of Health Services, the Maricopa County Department of Public Health, and Blood Systems, Inc., as part of their routine activity.
ABBREVIATIONS
- CSF
cerebrospinal fluid
- MCES
Maricopa County Environmental Services
- PRNT
confirmatory plaque reduction neutralization testing
- SLEV
St Louis encephalitis virus
- WNV
West Nile virus
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
References
- 1.Reimann CA, Hayes EB, DiGuiseppi C, et al. Epidemiology of neuroinvasive arboviral disease in the United States, 1999–2007. Am J Trop Med Hyg. 2008;79:974–9. [PubMed] [Google Scholar]
- 2.Reeves WC, Hammon WM, Longshore WA, Jr, et al. Epidemiology of the arthropod-borne viral encephalitides in Kern County, California, 1943–1952. Publ Public Health Univ Calif. 1962;4:1–257. [PubMed] [Google Scholar]
- 3.Monath TP, Tsai TF. St. Louis encephalitis: lessons from the last decade. Am J Trop Med Hyg. 1987;37:40S–59S. doi: 10.4269/ajtmh.1987.37.40s. [DOI] [PubMed] [Google Scholar]
- 4.McLean RG, Webb JP, Campos EG, et al. Antibody prevalence of St. Louis encephalitis virus in avian hosts in Los Angeles, California, 1986. J Am Mosq Control Assoc. 1988;4:524–8. [PubMed] [Google Scholar]
- 5.Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111–38. doi: 10.1146/annurev.ento.46.1.111. [DOI] [PubMed] [Google Scholar]
- 6.Brinker KR, Paulson G, Monath TP, et al. St Louis encephalitis in Ohio, September 1975: clinical and EEG studies in 16 cases. Arch Intern Med. 1979;139:561–6. [PubMed] [Google Scholar]
- 7.Lindsey HS, Calisher CH, Mathews JH. Serum dilution neutralization test for California group virus identification and serology. J Clin Microbiol. 4:503–10. doi: 10.1128/jcm.4.6.503-510.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) St. Louis encephalitis epidemiology & geographic distribution. Atlanta (GA): CDC; 2015. [cited 2017 May 10] Available from: http://www.cdc.gov/sle/technical/epi.html. [Google Scholar]
- 9.Martin DA, Noga A, Kosoy O, et al. Evaluation of a diagnostic algorithm by using immunoglobulin M enzyme-linked immunosorbent assay to differentiate human West Nile virus and St. Louis encephalitis virus infections during the 2002 West Nile virus epidemic in the United States. Clin Diagn Lab Immunol. 2004;11:1130–3. doi: 10.1128/CDLI.11.6.1130-1133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumsden LL. St. Louis encephalitis in 1933; observations on epidemiological features. Public Health Rep. 1958;73:340–53. [PMC free article] [PubMed] [Google Scholar]
- 11.Kopp A, Gillespie TR, Hobelsberger D, et al. Provenance and geographic spread of St. Louis encephalitis virus. MBio. 2013;4:e00322–13. doi: 10.1128/mBio.00322-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–14. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 13.Venkat H, Krow-Lucal E, Hennessey M, et al. Concurrent outbreaks of St. Louis encephalitis virus and West Nile virus disease—Arizona, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:1349–50. doi: 10.15585/mmwr.mm6448a5. [DOI] [PubMed] [Google Scholar]
- 14.Chamberland ME. Emerging infectious agents: do they pose a risk to the safety of transfused blood and blood products? Clin Infect Dis. 2002;34:797–805. doi: 10.1086/338787. [DOI] [PubMed] [Google Scholar]
- 15.Harrington T, Kuehnert MJ, Kamel H, et al. West Nile virus infection transmitted by blood transfusion. Transfusion. 2003;43:1018–22. doi: 10.1046/j.1537-2995.2003.00481.x. [DOI] [PubMed] [Google Scholar]
- 16.Pealer L, Marfin AA, Petersen LR, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–45. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 17.Stramer SL, Hollinger FB, Katz LM, et al. Emerging infectious disease agents and their potential threat to transfusion safety. Transfusion. 2009;49(Suppl 2):1S–29S. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration (FDA) Guidance for industry: donor screening recommendations to reduce the risk of transmission of Zika virus by human cells, tissues, and cellular and tissue-based products. Silver Spring (MD): FDA; 2016. [cited 2017 May 10] Available from: https://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplian-ceregulatoryinformation/guidances/tissue/ucm488582.pdf. [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Arboviral diseases, neuroinvasive and non-neuroinvasive 2015 case definition. Atlanta (GA): CDC; 2015. [cited 2017 May 10] Available from: http://wwwn.cdc.gov/nndss/conditions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive/case-definition/2015/ [Google Scholar]
- 20.Johnson AJ, Noga AJ, Kosoy O, et al. Duplex microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin M antibodies. Clin Diagn Lab Immunol. 2005;12:566–74. doi: 10.1128/CDLI.12.5.566-574.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AJ, Cheshier RC, Cosentino G, et al. Validation of a microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin m antibodies. Clin Vaccine Immunol. 2007;14:1084–93. doi: 10.1128/CVI.00115-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutherland GL, Nasci RS. Detection of West Nile virus in large pools of mosquitoes. J Am Mosq Control Assoc. 2007;23:389–95. doi: 10.2987/5630.1. [DOI] [PubMed] [Google Scholar]
- 23.Verdonschot PFM, Besse-Lototskaya AA. Flight distance of mosquitoes (Culicidae): a metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica. 2014;45:69–79. [Google Scholar]
- 24.Von Magnus H. Laboratory infection with St. Louis encephalitis virus. Acta Pathol Microbiol Immunol Scand. 1950;27:276–82. [Google Scholar]
- 25.Lederman E, Warkentien T, Bavaro M, et al. Transfusion-related transmission of yellow fever vaccine virus—California, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:34–7. [PubMed] [Google Scholar]
- 26.Tambyah PA, Koay E, Poon M, et al. Transfusion-Transmitted Dengue Infection Study Group. Dengue hemorrhagic fever transmitted by blood transfusion. N Engl J Med. 2008;359:1526–7. doi: 10.1056/NEJMc0708673. [DOI] [PubMed] [Google Scholar]
- 27.Motta IJF, Spencer BR, Cordeiro da Silva SG, et al. Evidence for transmission of Zika virus by platelet transfusion. N Engl J Med. 2016;375:1101–3. doi: 10.1056/NEJMc1607262. [DOI] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration (FDA) Guidance for industry: use of nucleic acid tests to reduce the risk of transmission of West Nile virus from donors of whole blood and blood components intended for transfusion. Silver Spring (MD): FDA; 2009. [cited 2017 Jun 15] Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/Guidance-ComplianceRegulatoryInformation/Guidances/Blood/UCM189464.pdf. [Google Scholar]
- 29.Custer B, Busch MP, Marfin AA, et al. The cost-effectiveness of screening the US blood supply for West Nile virus. Ann Intern Med. 2005;143:486–92. doi: 10.7326/0003-4819-143-7-200510040-00007. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) West Nile virus final cumulative maps and data for 1999–2015. Atlanta (GA): CDC; 2016. [cited 2017 Jun 15] Available from: http://www.cdc.gov/westnile/statsmaps/cummapsdata.html. [Google Scholar]
- 31.US Food and Drug Administration (FDA) Guidance for industry: assessing donor suitability and blood and blood product safety in cases of known or suspected West Nile virus infection. Silver Spring (MD): FDA; 2005. [cited 2017 Jun 15] Available from: http://www.fda.gov/downloads/Biolog-icsBloodVaccines/GuidanceComplianceRegulatoryInforma-tion/Guidances/Blood/ucm080286.pdf. [Google Scholar]