Abstract
Methods for the detection of plasmid loss in natural environments have typically relied on replica plating, selective markers and PCR. However, these traditional methods have the limitations of low sensitivity, underestimation of specific cell populations, and lack of insightful data for non-homogeneous environments. We have developed a non-invasive microscopic analytical method to quantify local plasmid segregational loss from a bacterial population within a developing biofilm. The probability of plasmid segregational loss in planktonic and biofilm cultures of Pseudomonas putida carrying the TOL plasmid (pWWO::gfpmut3b) was determined directly in situ, in the absence of any applied selection pressure. Compared to suspended liquid culture, we report that the biofilm mode of growth enhances plasmid segregational loss. Results based on a biofilm-averaged analysis reveal that the probability of plasmid loss in biofilm cultures (0.016 ± 0.004) was significantly greater than that determined in planktonic cultures (0.0052 ± 0.0011). Non-invasive assessments showed that probabilities of plasmid segregational loss at different locations in a biofilm increased dramatically from 0.1% at the substratum surface to 8% at outside layers of biofilm. Results suggest that higher nutrient concentrations and subsequentially higher growth rates resulted in higher probability of plasmid segregational loss at the outer layers of the biofilm.
Keywords: biofilm plasmid loss, non-invasive biofilm diagnostics, mathematical modeling
Graphical Abstract
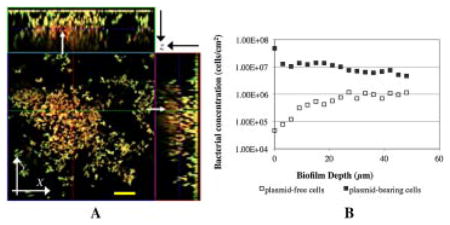
In situ estimation of plasmid segregational loss in a developing biofilm. A. Spatial distribution of yellow luorescent host cells (P. putida TUM-PP12 (mini-Tn5Putdsred) harboring the TOL plasmid pWWO::gfpmut3b)) and red fluorescent segregants (P. putida TUM-PP12 (miniTn5Putdsred)). The stack mage was collected on Day 4 of continuous biofilm formation. Arrows indicate the red fluorescent egregants embedded in microcolonies. Bar size: 20 μm. B. Distribution of both plasmid-bearing () and lasmid-free () cells at the different biofilm depths.
Waste contamination in nature profoundly affects human health and the environment. Due to the advantages of low cost and non-toxic by-products, biological treatment to remove hazardous wastes in nature has gained considerable interest during the past two decades. For example, bioremediation has been employed for the clean-up of organic and radioactive contaminants such as lead, mercury, plutonium and the herbicide 2, 4-dichlorophenoxyacetic acid (2, 4-D) (Bathe et al., 2004; Mathewson and Grubbs, 1988; Wuertz et al., 2004). The capacity of many microorganisms to biodegrade toxicants is directly correlated to the presence of various conjugative plasmids (Lajoie et al., 1993; Ramos et al., 1987; Yoon, 2005). Such plasmids contain genes that encode for particular pathways of biodegradation and are readily transferred to neighboring bacteria. It has been shown that 2, 4-D degradation was successfully enhanced with the indirect transfer of degradative plasmid pJP4 from donor strains to the indigenous microbial population (Bathe et al., 2004; Dejonghe et al., 2000; DiGiovanni et al., 1996). Several groups have also studied the treatment of petroleum contamination in soil by transferring TOL (pWWO) plasmid, which carries genes encoding the capacity to degrade certain organic compounds such as benzyl alcohol, from a donor strain of Pseudomonas putida to the indigenous bacterial consortia (Christensen et al., 1998; Molbak et al., 2003). These studies have provided useful insights into the application of direct conjugational plasmid transfer to enhance the degradative ability of microbes for the treatment of toxicants in natural environment.
To better understand plasmid transfer mediated approaches to bioremediation, there is an increasing concern about the stability of plasmid DNA under non-selective conditions. Plasmids are not stably inherited without some selective pressures; plasmid DNA either undergoes molecular rearrangement (structural instability) or is not absolutely inherited by progeny (segregational instability). It has been established, both experimentally and theoretically, that plasmid maintenance and recombinant gene expression can reduce the overall growth rate of the plasmid-bearing cell relative to the plasmid-free cell (Kaiser and Losick, 1993; Ollis, 1982; Ollis and Chang, 1982; Uhlin and Nordstrom, 1978; Uhlin et al., 1979). Reduction of copy number (Lauffenburger, 1987) and loss of plasmids from populations grown in continuous suspended culture have been reported (Grandi et al., 1981; Wood and Peretti, 1991; Wood et al., 1990), even in the presence of selective pressures (Kadam et al., 1987; Wood et al., 1990; Wood and Peretti, 1991). Plasmid loss has been shown to occur in Escherichia coli (Kaiser and Losick, 1993; Peretti et al., 1989; Seo and Bailey, 1985; Uhlin and Nordstrom, 1978; Uhlin et al., 1979), Bacillus (Fredrickson, 1977; Grandi et al., 1981), and Pseudomonas (Nakazawa, 1978) strains. Recent studies have shown that, even though the rate of segregational plasmid loss is low; rarely formed segregants can become the majority population when the plasmid loss is significant enough to result in a faster growing population of plasmid-free cells (De Gelder et al., 2005).
Owing to methodological limitations, plasmid stability and survival in biofilms have only been investigated in a limited number of cases even though bacterial biofilms, matrices formed by microbial cells and their extracellular polymers associated with a substratum, are far more common than free-living planktonic organisms in the environment (Huang et al., 1994; Imran et al., 2005; O’Connell et al., 2007; Sharp et al., 1998). Due to low segregation rates, traditional methods that rely on replica plating, selective markers and PCR detection (Smalla et al., 2000) suffer from low sensitivity and underestimation of the various cell populations (Amann et al., 1995; Torsvik et al., 1990).
In this study, we have developed a non-invasive method coupled with a mathematical model to quantify the segregational stability of TOL plasmid expressing the fluorescent marker gfpmut3b in planktonic bacteria and developing biofilms without an applied selection pressure. The TOL plasmid, which contains genes coded for the degradation of toluene and benzyl alcohol, was used in this study. Confocal Laser Scanning Microscopy (CLSM) analyses were applied to identify two communities of plasmid-bearing and plasmid-free bacteria locally within a developing biofilm. The number of viable plasmid-bearing and plasmid-free bacteria were quantified non-invasively using an approach developed previously by our group, which is based on the correlation between fluorescence intensity values and cell number per area (Ma and Bryers, 2010).
Materials and Methods
Bacterial Strains and Media
Pseudomonas putida TUM-PP12 was chosen as a model strain to investigate plasmid segregational loss in our studies (Ma and Bryers, 2013). This double-labeled donor strain was derived from a strain of P. putida KT2442 and was chromosomally tagged with the DsRed gene by transposon insertion, which provides a stable approach to maintain the DsRed fluorescent reporter gene (Nancharaiah et al., 2003). In addition, this strain also contains a conjugative GFPmut3b-modified plasmid TOL (pWWO) that expresses the green fluorescent protein. The TOL (pWWO) plasmid is a well-characterized plasmid that codes for the degradation of certain organic compounds, including toluene and benzyl alcohol (Haagensen et al., 2002; Molbak et al., 2003). The green fluorescence derived from GFPmut3b used in this study has a half-life of over 7 days and remains completely stable within the timeframe of the biofilm formation experiments. LB Broth (Bacto tryptone 10 g/L, Bacto yeast extract 5 g/L, NaCl 4 g/L) and chemically defined medium (Na3C6H5O7, 129 mg/L; (NH4)2SO4, 2 g/L; Na2HPO4·2H2O, 6 g/L; KH2PO4, 3 g/L; NaCl, 3 g/L; MgCl2, 93 mg/L; CaCl2; 11 mg/L; Trace Metal solution, 1 mL/L) were used for batch suspended cell cultures. The trace metal solution is composed of CaSO4·2H2O, 200 mg/L; FeSO4·7H2O, 200 mg/L; MnSO4·H2O, 20 mg/L; CuSO4·5H2O; 20 mg/L; ZnSO4·7H2O; 10 mg/L; CoSO4·7H2O, 10 mg/L; Na2MoO4·H2O; 10 mg/L; H3BO3, 5 mg/L (Ma and Bryers, 2010). Glucose was supplied as the sole carbon source with the final concentrations of 20 mg/L in suspended culture. The expression of GFP and DsRed is very stable in both complicated and chemically defined medium (Tombolini, 1997). To cultivate cells in suspension culture, a sample of one colony from the streaked plate was collected by a sterile loop and added to 25 mL of 10 g/L LB Broth, then incubated at 30°C overnight.
Biofilm Reactor Systems
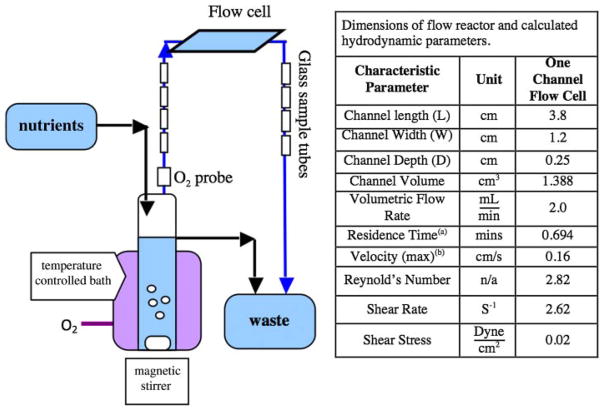
A stainless steel flow cell (Protofab, Bozeman, MT) was used for biofilm cultivation and non-invasive microscopic analyses (Fig. 1). The flow cell consists of a single flow-through channel. Glass cover slips (Erie Scientific) (48 mm wide × 65 mm long × 0.13–0.17 mm thick) were used to form the top and bottom of the flow channel. The entire system, except the flow cells, was sterilized by autoclaving. To sterilize the stainless steel flow cell, all parts were soaked in 5% NaOCl2 (Sodium hypochlorite) solution for 4 h, then rinsed with sterilized Millipore™ ultra-pure water and assembled under UV light. 70% ethanol in filtered sterile water was delivered to the flow cell for 5 min. All parts of the flow cell were assembled aseptically and placed into the system line. Four glass tubes (3.5 mm diameter, 20 mm long) were also connected, in this system, both up-stream and down-stream of the flow cell. The surface area of two glass tubes was approximately the same as the surface area of the cover glass in the flow cell. At each sample time, two glass tubes were removed and placed in 1 mL of PBS solution. The samples were then sonicated by a probe sonicator with a frequency of 20 kHZ to remove the cells from the glass, and the bacterial cell number per surface area was determined by direct counts using epifluorescent microscopy. Efficiency experiments showed that sonication of the glass tubes removed all of the adherent cells in one application. Destructive samples of the glass tubes were used to quantify the adherent cell density (cell number/area) and to verify the cell density results determined non-invasively (from the sealed flow cell) by microscopy, as described in more detail below.
Figure 1.
Schematic of chemostat reactor system used for biofilm formation experiments.
Fluorescent Microscopy and Image Analysis
Biofilms that developed on the surface of the flow cells were examined periodically throughout an experiment. The flow cell was clamped at both ends and placed on the microscope stage for imaging. A Zeiss confocal laser scanning microscope LSM510 (Jena, Germany) mounted on an Axiovert 100 M inverted microscope was used for all optical sectioning experiments. A 63× oil immersion lens with a numerical aperture of 1.4 was used to obtain images of cells expressing green and red fluorescent proteins. Green fluorescent (Ex/Em: 505/515 nm) microspheres 2.5 μm in diameter with 100% relative intensity (InSpeck™ Microscope Image Intensity Calibration Kit, Invitrogen, USA) were used as calibration standards during CLSM quantitative analysis. Two “tracks” were used to collect signals from the red and green fluorescent proteins, separately; a “Track” is a data-recording channel where excitation and emission wavelength information observed at specific locations is stored. The composite superimposed images were re-constructed from each track image collected at different z-depths. “Track One” was set up at the excitation wavelength of 488 nm (argon laser) and a 505 nm to 530 nm band-pass (BP) emission filter to detect GFP-expressing bacteria. “Track Two” was excited at 543 nm with a HeNe laser and a 590 long–pass (LP) emission filter to recognize DsRed-expressing bacteria. All image analysis related to biofilm fluorescent density calibration was accomplished using “Image J” version 1.30v software (Research Services Branch, National Institutes of Health, Bethesda, Maryland; http://rsb.info.nih.gov/ij/).
To satisfy our goal of quantifying cell numbers from fluorescent densities, all of the control parameters in the image analysis software were maintained constant between each image. A non-invasive method based on a calibration of cell fluorescence to known cell number concentrations was applied here to determine the local bacterial concentrations within a biofilm (Ma and Bryers, 2010).
Non-Invasive Determination of Plasmid Segregational Loss
The probability of segregational loss of the TOL-gfpmut3b plasmid from donor strains was evaluated both in suspended and biofilm cultures. The TOL-gfpmut3b plasmid bearing donor strain, P. putida TUM-PP12, was grown in liquid culture without the presence of any antibiotic pressure. Plasmid-bearing cells of P. putida TUM-PP12 are termed “donors”, and any P. putida plasmid-free daughter cells that arise by random segregational plasmid loss from the parents donors are called “segregants”.
Strains were cultivated individually in batch culture overnight. Suspended pure culture cells of P. putida TUM-PP12 in exponential phase were centrifuged at 8,000xg, washed, re-suspended, and centrifuged again. Flow cells were then inoculated with a diluted suspension of 107 cells/mL. The volumetric flow rate was set at 120 mL/h and the cell suspension was recycled (no effluent) through the flow cells for 2–3 h. After inoculation, sterile modified FAB chemically defined medium with concentrations of 20 mg/L glucose as the carbon source was supplied in a once-through mode at a constant flow rate of 120 mL/h using a peristaltic pump (Master flex model 7528-10). In order to ensure carbon was the rate limiting nutrient, pure medical degree O2 was supplied via aeration to the system. Biofilms that developed on the surface of the flow cells were examined periodically throughout an experiment over a 7-day period, until the biofilm matured and developed. The flow cell was clamped at both ends and placed on the microscope stage for imaging.
The number of plasmid-containing and plasmid-free cells within a biofilm was counted in a series of microscopic x–y images from several z-stacks. The spatial distribution of plasmid-free and plasmid-bearing cells within a 3-D native hydrated biofilm were determined non-invasively and verified by direct cell counts of destructive samples of cultivated biofilms.
Cell per volume concentrations in suspended batch cultures and cell per area concentrations in biofilm cultures for both plasmid-free and plasmid-bearing cell populations were used in two appropriate mathematical models (Huang et al., 1993, 1994) to estimate the probability of plasmid segregational loss, P. Model details and assumptions are provided in the Supplementary File (S1).
Verification of Plasmid Segregational Loss by Flow Cytometry and Colony-Direct PCR
Verification of plasmid segregational loss was performed as previously described (Ma and Bryers, 2013). Segregants were isolated from biofilm populations and subsequently characterized by multiplex polymerase chain reaction (PCR) to confirm the loss of GFP-based TOL-gfpmut3b plasmid from donor cells of P. putida TUM-PP12. Briefly, the segregants were detected and isolated from mixed destructive samples of biofilms by the use of a BD Aria fluorescent-activated cell sorter (FACS; Becton Dickinson Biosciences, San Jose, CA, USA), and then diluted and placed on agar plates to form single colonies. Colony-direct PCR was applied to detect GFP-containing sequences on the TOL-gfpmut3b plasmid and DsRed-containing sequences on chromosome DNA in order to exclude the possibility of false-reading of donors as segregants.
Statistics
A Student’s t-test (two-tailed) was used for statistical analysis. A “P-value” less than 0.05 was used to reveal a significant difference.
Results
Non-Invasive Biofilm Diagnostics and Estimations of Plasmid Segregational Loss on Average
A non-invasive 1-photon CLSM method was used to determine local and overall bacterial species concentrations within a biofilm. Adherent monolayers of bacteria are easy to quantify by epifluorescence microscopy but as the depth and cell density of a biofilm increases, individual cells are difficult to count. Briefly, our method is based upon a calibration of “single cell fluorescence” (when individual cells can be discerned) to independently determine cell numbers. At advanced stages of biofilm development (when individual cells cannot be counted) the total fluorescence per x–y horizontal slice is determined by CLSM and then converted to cell numbers using the “per cell fluorescence” calibration. Non-invasive CLSM estimates of local cell concentrations of single population biofilms of gfpmut3b-expressing or DsRed-expressing cells, or mixed populations, cultivated in a parallel-plate flow cell, were verified by direct cell counts of destructive samples of the biofilms (Ma and Bryers, 2010).
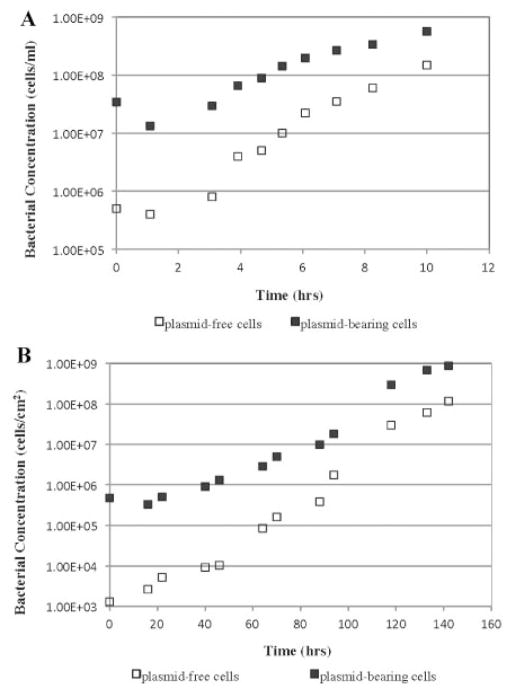
We quantified the probability of plasmid segregational loss (P) from the donor strain, both in batch suspended culture as well as in flow-cell cultivated biofilm culture, without any antibiotic selection pressure. We hypothesized that, compared to the stability of a plasmid in planktonic cell culture, biofilm formation would detrimentally affect the plasmid segregational stability. The probability of segregational loss of the TOL-gfpmut3b plasmid was evaluated both in suspended and biofilm cultures based upon two system-appropriate mathematical models (Huang et al., 1993, 1994). Planktonic cultures were carried out in a classic well-mixed batch system. A once-through parallel-plate flow cell system was used for all biofilm formation studies (Fig. 1). The population dynamics of plasmid-bearing and plasmid-free cells in planktonic culture (Fig. 2A) allowed us to estimate, based on our mathematical model, P in planktonic culture (P =0.0052 ± 0.001). The biofilm-averaged value of P was estimated to be 0.016 ± 0.004 based on the population dynamics in biofilm culture and our mathematical model (Fig. 2B). A “P-value” of 0.0083 (<0.05) reveals that the probability of plasmid loss (P) changes significantly when experimental environments were changed from planktonic to biofilm culture.
Figure 2.
Distribution of population dynamics of plasmid-bearing cells (■) and plasmid-free cells (□) of Pseudomonas putida TUM PP12 in a batch suspended culture (A) and a biofilm culture (B) at 30°C.
Non-Invasive Estimation of Biofilm Plasmid Segregational Loss In Situ
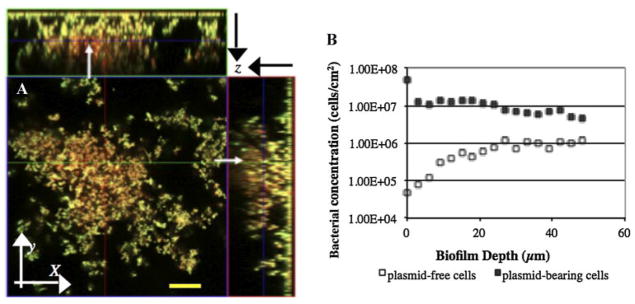
One must realize that estimating P using biofilm-averaged populations, as described above, provides a distorted homogenized estimate of plasmid instability averaged over the entire biofilm. However, the accumulation of biofilms is heterogeneous and may result in distinct distribution of cell populations and P values at different biofilm locations. From CLSM slices at multiple x–y planes taken with depth (z-plane) within an established biofilm (Fig. 3A), we also quantified local plasmid-bearing and plasmid-free cell concentrations (Fig. 3B). The majority of cells expressing DsRed (plasmid-free cells that have lost their pDNA, also called segregants, red fluorescence) preferentially appeared on the top layers of existing microcolonies of the donor cells (yellow fluorescence), although a few segregants were also found deep inside the biofilms. The distribution of cells changes dramatically with depth moving from the substratum to the outside layers of biofilm (Fig. 3B). Less than 1.0% of the cells at the substratum were plasmid-free cells, but this number was increased to 12.5% at the upper layers.
Figure 3.
A: In situ monitoring of plasmid segregational loss in a biofilm cultivated at 30°C. Spatial distribution of yellow fluorescent donor cells (P. putida TUM-PP12 [miniTn5Putdsred] harboring the TOL plasmid [pWWO::gfpmut3b]) and red fluorescent segregants (P. putida TUM-PP12 [miniTn5Putdsred]). The micrograph is displayed in an orthogonal view to show the biofilm structure in three sets of two-dimensional sections; XY, XZ, and YZ. The stack image was collected on Day 4 of continuous biofilm formation. This single image slice was acquired at an axial position in the flow cell approximately 1.9 cm from the inlet in the center of the flow cell; overall biofilm thickness was 70 μm. The arrows indicate the red fluorescent segregants embedded in microcolonies. Bar size: 20 μm. B: Distribution of both plasmid-bearing (■) (P. putida TUM-PP12 [miniTn5Putdsred] harboring the TOL plasmid [pWWO::gfpmut3b]) and plasmid-free (□) (P. putida TUM-PP12 [miniTn5Putdsred]) cells at the different biofilm depths sampled on Day 4 in a continuous flow chamber.
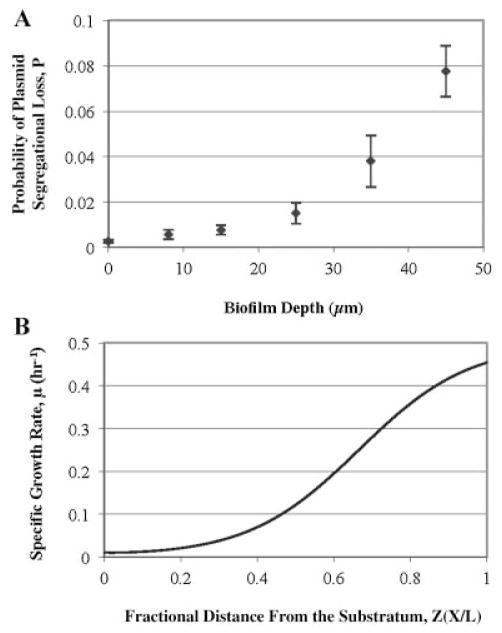
Hypothetically, cells in the outer layers of a biofilms may have a higher probability to lose their TOLgfpmut3b plasmids than cells embedded in deeper layers due to their higher growth rates. However, this hypothesis cannot be verified by only visualizing spatial distribution of plasmid-bearing and plasmid-free cells since the growth of segregants will also contribute to the formation of more red fluorescent cells on top layers of microcolonies. Thus, we applied a dynamic model (Huang et al., 1993, 1994) combined with our non-invasive quantification method to estimate the P value at different spatial localizations in the biofilms. From the cell concentrations shown in Figure 3B, we can determine P values as a function of biofilm depth in the z-direction away from the glass substratum (Fig. 4A). Local values of P varied spatially in depth from 0.001 to 0.08 over a 60 μm-thick biofilm and were verified by destructive sampling and direct population counts (data not shown).
Figure 4.
Probability of plasmid segregational loss (A) and predicted specific growth rate (B) as a function of biofilm depth. Biofilm depth in figure (B) was normalized to a fractional distance from the substratum. Biofilms were cultivated for 7 days within a continuous parallel flow cell at 30°C. Each data point was calculated by using a mathematical model (SI).
In order to investigate the nutrient effects on plasmid segregational loss, a one-dimensional external mass transfer resistance model was used to evaluate the glucose concentration profile within the biofilms over a depth of 60 μm. In this model, the Thiele modulus (Φ), defined as the ratio of a maximum total first order reaction rate to a maximum diffusion rate, was utilized to estimate internal mass transfer resistance in the biofilms (Grady and Lim, 1980). The specific growth rate estimated by the model with an Φ value of 6.88 (at P. putida optimal growth temperature of 30°C) presented an increasing profile within the biofilms from the substratum to the outer layer (Fig. 4B). This calculation also estimated that the glucose concentration in the middle of biofilm is only 10% of the bulk liquid concentration.
Characterization and Quantification of Plasmid Segregational Loss via Destructive Biofilm Sampling Followed by FACS and PCR
The cultivated biofilms collected from the sample procedure were destructively removed from the substratum and suspended in PBS for FACS. In order to validate the enumeration of bacterial populations by direct microscopic counts based on CLSM and image analysis, FACS and epi-fluorescence microscopy counts (EMC) were also performed to quantify plasmid-free and plasmid-bearing cells. Plasmid loss was expressed as the ratio of plasmid-free cells to the total cells (Table I). Although experimental biases may be caused by the procedure of collecting destructive biofilm samples, all three methods of EMC, FACS and CLSM analyses provided comparable results for the percentage of plasmid loss. The fine correlation among the cell numbers observed by all three techniques confirmed the effectiveness of the developed non-invasive CLSM analysis.
Table I.
Enumeration of plasmid-free and plasmid-bearing biofilm cells of P. putida TUM-PP12 by EMC, FACS, and CLSM.
| Method | Plasmid-free cells (cells/cm2) | Total cells (cells/cm2) | Plasmid loss (%) |
|---|---|---|---|
| EMC | (9.48 ± 1.30) × 107 | (8.12 ± 1.10) × 108 | 11.38 ± 0.95 |
| FACS | (8.73 ± 1.96) × 107 | (8.62 ± 0.84) × 108 | 10.06 ± 1.94 |
| CLSM | (11.4 ± 1.37) × 107 | (9.76 ± 0.89) × 108 | 11.72 ± 1.43 |
EMC, epi-fluorescence microscopy counts. Enumeration of cells by direct counts from destructive samples using epi-fluorescent microscopy.
FACS, fluorescence activated cell sorting. Enumeration of cells by flow cytometry using the defined gates.
CLSM, confocal laser scanning microscopy. Estimation of plasmid-free and plasmid-bearing cells by CLSM combined with quantitative image analysis.
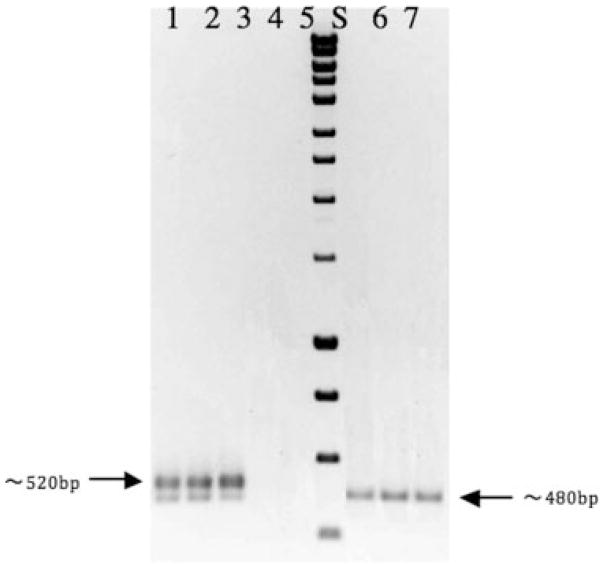
The populations of sorted cells collected from FACS were subsequently cultured on agar plates and utilized as PCR templates for characterization of segregational loss genes. Specific sized bands based on different primer designs were detected by the agarose gel electrophoresis (Fig. 5). For donor cells experiencing segregational plasmid loss, only one band corresponding to the DsRed-containing sequence on the chromosomal DNA was observed. In contrast, donor cells retaining the plasmid, a GFP-containing sequence on the TOL-gfpmut3b plasmid and a DsRed-containing sequence on the chromosomal DNA show as two bands on the gel. Although the two sizes of the targeting sequences are close, the differences were still clearly shown in the Figure 5. The detection of genetic traits at the molecular level confirmed the gfp-based plasmid segregational loss from the host cells and exclude the possibility of false-reading of plasmid-bearing cells as plasmid-free cells.
Figure 5.
Colony PCR analyses of donors and segregants cells obtained by plating cells sorted by flow cytometry equipped with FACS machine on LB plates. Lane 1, 2, 3—positive controls, using donor cells (dual labeling) as DNA templates; Lane 4, 5—negative controls, using recipient cells (no color) and diH2O as DNA templates; Lane 6, 7, 8—using segregants as DNA templates; Lane S—standard DNA HyperLadder I (Bioline), sizes are 400, 600, 800, and 1,000 bp for the last four bands from the bottom. Both DsRed and GFP-specific primers were used for all PCR reactions.
Discussion
Mathematical models have been widely used to complement laboratory studies and predict plasmid dynamics in natural habitats. Early mathematical models to simulate plasmid stability were based on suspended cultures and simple mass action balances, which resulted in the underestimation of cell growth and plasmid segregational loss (Dionisio et al., 2002; Gordon, 1992; Licht et al., 1999). Subsequent models of plasmid loss and transfer were applied to biofilm cultures since the majority of microbial activities are associated with biofilms in natural environments. However, these models assume that the biofilm is a completely mixed, homogeneous architecture (Christensen et al., 1998; DeAngelis and Gross, 1992; Lagido et al., 2003; Pinedo and Smets, 2005), thus these models cannot provide any information on spatial gradients of cell population density at different locations within a biofilm. Recently, three-dimensional (3-D) image analysis coupled with CLSM has revealed the real 3-D structure of biofilms can be spatially heterogeneous, containing tortuous channels around dense clusters of microbes and extracellular matrix (Haagensen et al., 2002; Molin and Tolker-Nielsen, 2003; Wuertz et al., 2001). Through the use of a non-invasive quantification method coupled with a mathematical model (Huang et al., 1993, 1994), we have directly evaluated the segregational stability of the TOL-gfpmut3b plasmid, both in averaged samples of planktonic bacteria and locally within developing biofilms, without any applied selection pressure. In the present study, CLSM analyses were applied to identify two communities of plasmid-bearing and plasmid-free bacteria.
We found that a biofilm-averaged probability of plasmid loss, P, in biofilm cultures was significantly greater than P for suspension cultures (“P-value” <0.05). It has been reported that plasmid segregational loss is affected by many factors, such as: specific growth rates of plasmid-free and plasmid-bearing cells, plasmid distribution between daughter cells during division (Imanaka and Aiba, 1981; Ollis, 1982; Ollis and Chang, 1982; Popov et al., 2011), host cell genotype (James et al., 1982), plasmid copy number (Summers, 1991), and plasmid multimerization (Summers and Sherratt, 1984). The additional metabolic burden to form the extracellular polymer matrix of a biofilm would explain the higher probability of plasmid loss compared with liquid suspension cultures, which was proven in our prior findings (Huang et al., 1993, 1994). It has been suggested that the copy number variance between batch and biofilm cultures can be correlated with the probability of plasmid loss (Huang et al., 1993; Stewart and Carlson, 1986). However, copy number may not be a key factor affecting our estimated P values since the TOL plasmid used in this study is a low copy number plasmid. Moreover, unlike batch cultures, biofilms in flow cells can be cultivated over an extended time period, without depletion of substrate. The accumulation of faster growing plasmid-free cells versus plasmid-bearing cells in the continuous biofilm cultures could result in the much higher probability of plasmid loss, when compared to planktonic cultures, which are terminated by substrate depletion at the end of the batch.
The focus of this study was to investigate the plasmid stability within a biofilm in situ. In the experiments above, the local values of P in biofilm cultures increased dramatically from the substratum to the biofilm-fluid interface. The spatial distributions of plasmid-bearing and plasmid-free cells within the biofilms were detected within mushroom- or tulip-shaped microcolonies and clusters, while single cells and chains of bacteria were also observed in layers close to the glass substratum. The plasmid-bearing cells were the dominating population within the biofilms close to the substratum, but the distribution of cells changed dramatically with depth moving away from the surface to the upper layers of the biofilm. As shown previously, many environmental and biological factors can affect plasmid segregational stability. However, for the biofilm cultures in this study, biological factors such as copy number may not act as the key factors affecting plasmid stability. The TOL plasmid used here is a low copy number plasmid. The plasmid copy number variance within a biofilm developed in a continuously flow condition with antibiotic-free media was found to be relatively low (O’Connell et al., 2007). In contrast, growth rate directly affects the overall generation of plasmid-free cells. It is well-known that biofilms can exhibit significant mass transfer resistance, resulting in major differences in cell growth rates in thick biofilms (Yang and Lewandowski, 1995). This heterogeneous structure often leads to variations of segregational plasmid instability spatially within a biofilm. We hypothesized that nutrient limitation may be partially responsible for the distribution patterns of plasmid-bearing and plasmid-free cells within biofilm cultures.
To validate the above hypothesis, a spatially dynamic model of plasmid loss within biofilms at different localizations was used to estimate the probability of plasmid segregational loss locally. When biofilms were exposed and developed at the optimal growth temperature of 30°C, probability of plasmid segregational loss increased dramatically from 0.1% to 8%, respectively from the substratum to the outer layers of biofilm. The difference in the lowest and highest segregational loss determined locally versus values determined from biofilm-averaged values were relatively large (86% lower and 81% highest, respectively). Our local spatial data coupled with our mathematical model point out the significant discrepancy that can arise using math models that assume biofilms are well-mixed and have the same growth rates through the biofim (Christensen et al., 1998; Pinedo and Smets, 2005). Bacterial cells deep inside biofilms can experience different nutrient environments compared to cells at outer layers of biofilms due to nutrient diffusion limitations. Nutrient limitations can reduce local bacterial growth rates and subsequently affect plasmid stabilities at different depth of biofilms (Marsh et al., 1983; McKenney and Allison, 1997). In the present study, a simple one-dimensional external mass transfer resistance model used to evaluate the glucose concentration profile and specific growth rate within the biofilm revealed that populations at the outer layers of the biofilm most likely exhibited a higher growth rate due to higher nutrient concentrations. These results suggest that active plasmid-bearing cells at the outer layers of a biofilm have higher overall plasmid loss rates. Bacterial cells at the top layer of the biofilms “see” more substrate, which results in higher growth rates.
In summary, we have developed a non-invasive microscopic analytical method to quantify local plasmid segregational loss between bacterial populations within a developing biofilm. Overall plasmid segregational loss is higher in biofilm-bound populations compared to their planktonic counterparts, which agrees well with our prior findings (Huang et al., 1993, 1994). Non-invasive detection of plasmid-bearing and plasmid-free cell populations, coupled with culture specific mathematical models, allowed estimation of the probability of plasmid segregational loss at different depths of biofilm. The plasmid segregational loss was lowest at the inner depths of a biofilm cluster and increased to the highest values at the outer, upper layers of the biofilm cluster.
Supplementary Material
Acknowledgments
This work was partially funded by grants to J.D.B. from the National Science Foundation (CBET-0450253) and the National Institutes of Health (R01 EB007575). K.N.K. gratefully acknowledges the NSF for a Graduate Research Fellowship.
Contract grant sponsor: National Science Foundation
Contract grant number: CBET-0450253
Contract grant sponsor: National Institutes of Health
Contract grant number: R01 EB007575
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59(1):143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathe S, Mohan TV, Wuertz S, Hausner M. Bioaugmentation of a sequencing batch biofilm reactor by horizontal gene transfer. Water Sci Technol. 2004;49(11–12):337–344. [PubMed] [Google Scholar]
- Christensen BB, Sternberg C, Andersen JB, Eberl L, Moller S, Givskov M, Molin S. Establishment of new genetic traits in a microbial biofilm community. Appl Environ Microbiol. 1998;64(6):2247–2255. doi: 10.1128/aem.64.6.2247-2255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gelder L, Vandecasteele FP, Brown CJ, Forney LJ, Top EM. Plasmid donor affects host range of promiscuous IncP-1beta plasmid pB10 in an activated-sludge microbial community. Appl Environ Microbiol. 2005;71(9):5309–5317. doi: 10.1128/AEM.71.9.5309-5317.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis D, Gross LJ. Individual-based models and approaches in ecology: Populations, communities and ecosystems. New York: Chapman and Hall; 1992. [Google Scholar]
- Dejonghe W, Goris J, El Fantroussi S, Hofte M, De Vos P, Verstraete W, Top EM. Effect of dissemination of 2,4-dichlorophenoxyacetic acid (2,4-D) degradation plasmids on 2,4-D degradation and on bacterial community structure in two different soil horizons. Appl Environ Microbiol. 2000;66(8):3297–3304. doi: 10.1128/aem.66.8.3297-3304.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiovanni GD, Neilson JW, Pepper IL, Sinclair NA. Gene transfer of Alcaligenes eutrophus JMP134 plasmid pJP4 to indigenous soil recipients. Appl Environ Microbiol. 1996;62(7):2521–2526. doi: 10.1128/aem.62.7.2521-2526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio F, Matic I, Radman M, Rodrigues OR, Taddei F. Plasmids spread very fast in heterogeneous bacterial communities. Genetics. 2002;162(4):1525–1532. doi: 10.1093/genetics/162.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson AG. Behavior of mixed cultures of microorganisms. Annu Rev Microbiol. 1977;31:63–87. doi: 10.1146/annurev.mi.31.100177.000431. [DOI] [PubMed] [Google Scholar]
- Gordon DM. Rate of plasmid transfer among Escherichia coli strains isolated from natural populations. J Gen Microbiol. 1992;138(1):17–21. doi: 10.1099/00221287-138-1-17. [DOI] [PubMed] [Google Scholar]
- Grady CP, Lim CH. Biological waste water treatment: theory and application. New York, NY: Marcel Dekker, Inc; 1980. pp. 519–528. [Google Scholar]
- Grandi G, Mottes M, Sgaramella V. Specific pattern of instability of Escherichia coli HisG gene cloned in Bacillus subtilis via the Staphylococcus aureus plasmid pCS194. Plasmid. 1981;6(1):99–111. doi: 10.1016/0147-619x(81)90057-3. [DOI] [PubMed] [Google Scholar]
- Haagensen JA, Hansen SK, Johansen T, Molin S. In situ detection of horizontal transfer of mobile genetic elements. FEMS Microbiol Ecol. 2002;42(2):261–268. doi: 10.1111/j.1574-6941.2002.tb01016.x. [DOI] [PubMed] [Google Scholar]
- Huang CT, Peretti SW, Bryers JD. Plasmid retention and gene expression in suspended and biofilm cultures of recombinant Escherichia coli DH5alpha(pMJR 1750) Biotechnol Bioeng. 1993;41(2):211–220. doi: 10.1002/bit.260410207. [DOI] [PubMed] [Google Scholar]
- Huang CT, Peretti SW, Bryers JD. Effects of medium carbon-to-nitrogen ratio on biofilm formation and plasmid stability. Biotechnol Bioeng. 1994;44(3):329–336. doi: 10.1002/bit.260440310. [DOI] [PubMed] [Google Scholar]
- Imanaka T, Aiba S. A perspective on the application of genetic engineering: stability of recombinant plasmid. Ann N Y Acad Sci. 1981;369:1–14. doi: 10.1111/j.1749-6632.1981.tb14172.x. [DOI] [PubMed] [Google Scholar]
- Imran M, Jones D, Smith H. Biofilms and the plasmid maintenance question. Math Biosci. 2005;193(2):183–204. doi: 10.1016/j.mbs.2004.10.008. [DOI] [PubMed] [Google Scholar]
- James AA, Morrison PT, Kolodner R. Genetic recombination of bacterial plasmid DNA. Analysis of the effect of recombination-deficient mutations on plasmid recombination. J Mol Biol. 1982;160(3):411–430. doi: 10.1016/0022-2836(82)90305-9. [DOI] [PubMed] [Google Scholar]
- Kadam KL, Wollweber KL, Grosch JC, Jao YC. Investigation of plasmid instability in amylase-producing B. subtilis using continuous culture. Biotechnol Bioeng. 1987;29(7):859–872. doi: 10.1002/bit.260290708. [DOI] [PubMed] [Google Scholar]
- Kaiser D, Losick R. How and why bacteria talk to each other. Cell. 1993;73(5):873–885. doi: 10.1016/0092-8674(93)90268-u. [DOI] [PubMed] [Google Scholar]
- Lagido C, Wilson IJ, Glover LA, Prosser JI. A model for bacterial conjugal gene transfer on solid surfaces. FEMS Microbiol Ecol. 2003;44(1):67–78. doi: 10.1016/S0168-6496(02)00453-1. [DOI] [PubMed] [Google Scholar]
- Lajoie CA, Zylstra GJ, DeFlaun MF, Strom PF. Development of field application vectors for bioremediation of soils contaminated with polychlorinated biphenyls. Appl Environ Microbiol. 1993;59(6):1735–1741. doi: 10.1128/aem.59.6.1735-1741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA. Bacteriocin production as a method of maintaining plasmid-bearing cells in continuous culture. Trends Biotechnol. 1987;5(4):87–89. [Google Scholar]
- Licht TR, Christensen BB, Krogfelt KA, Molin S. Plasmid transfer in the animal intestine and other dynamic bacterial populations: the role of community structure and environment. Microbiology. 1999;145(Pt 9):2615–2622. doi: 10.1099/00221287-145-9-2615. [DOI] [PubMed] [Google Scholar]
- Ma H, Bryers JD. Non-invasive method to quantify local bacterial concentrations in a mixed culture biofilm. J Ind Microbiol Biotechnol. 2010;37(10):1081–1089. doi: 10.1007/s10295-010-0756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Bryers JD. Non-invasive determination of conjugative transfer of plasmids bearing antibiotic-resistance genes in biofilm-bound bacteria: effects of substrate loading and antibiotic selection. Appl Microbiol Biotechnol. 2013;97(1):317–328. doi: 10.1007/s00253-012-4179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh PD, Hunter JR, Bowden GH, Hamilton IR, McKee AS, Hardie JM, Ellwood DC. The influence of growth rate and nutrient limitation on the microbial composition and biochemical properties of a mixed culture of oral bacteria grown in a chemostat. J Gen Microbiol. 1983;129(3):755–770. doi: 10.1099/00221287-129-3-755. [DOI] [PubMed] [Google Scholar]
- Mathewson RJ, Grubbs RB. Innovative techniques for the bioremedi-ation of contaminated soils. 2nd Annual CWPCA Industrial and Hazardous Waste Information Exchange; Oakland, CA. 1988. [Google Scholar]
- McKenney D, Allison DG. Influence of growth rate and nutrient limitation on susceptibility of Burkholderia cepacia to ciprofloxacin and tobramycin. J Antimicrob Chemother. 1997;40(3):415–417. doi: 10.1093/jac/40.3.415. [DOI] [PubMed] [Google Scholar]
- Molbak L, Licht TR, Kvist T, Kroer N, Andersen SR. Plasmid transfer from Pseudomonas putida to the indigenous bacteria on alfalfa sprouts: characterization, direct quantification, and in situ location of trans-conjugant cells. Appl Environ Microbiol. 2003;69(9):5536–5542. doi: 10.1128/AEM.69.9.5536-5542.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molin S, Tolker-Nielsen T. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr Opin Biotechnol. 2003;14(3):255–261. doi: 10.1016/s0958-1669(03)00036-3. [DOI] [PubMed] [Google Scholar]
- Nakazawa T. TOL plasmid in Pseudomonas aeruginosa PAO: thermo-sensitivity of self-maintenance and inhibition of host cell growth. J Bacteriol. 1978;133(2):527–535. doi: 10.1128/jb.133.2.527-535.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancharaiah YV, Wattiau P, Wuertz S, Bathe S, Mohan SV, Wilderer PA, Hausner M. Dual labeling of Pseudomonas putida with fluorescent proteins for in situ monitoring of conjugal transfer of the TOL plasmid. Appl Environ Microbiol. 2003;69(8):4846–4852. doi: 10.1128/AEM.69.8.4846-4852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell HA, Niu C, Gilbert ES. Enhanced high copy number plasmid maintenance and heterologous protein production in an Escherichia coli biofilm. Biotechnol Bioeng. 2007;97(3):439–446. doi: 10.1002/bit.21240. [DOI] [PubMed] [Google Scholar]
- Ollis DF. Industrial fermentations with (unstable) recombinant cultures. Phil Trans R Soc Lond B. 1982;297:617–629. [Google Scholar]
- Ollis DF, Chang HT. Batch fermentation kinetics with (unstable) recombinant cultures. Biotechnol Bioeng. 1982;24(11):2583–2586. doi: 10.1002/bit.260241120. [DOI] [PubMed] [Google Scholar]
- Peretti SW, Bailey JE, Lee JJ. Transcription from plasmid genes, macromolecular stability, and cell-specific productivity in Escherichia coli carrying copy number mutant plasmids. Biotechnol Bioeng. 1989;34(7):902–908. doi: 10.1002/bit.260340704. [DOI] [PubMed] [Google Scholar]
- Pinedo CA, Smets BF. Conjugal TOL transfer from Pseudomonas putida to Pseudomonas aeruginosa: effects of restriction proficiency, toxicant exposure, cell density ratios, and conjugation detection method on observed transfer efficiencies. Appl Environ Microbiol. 2005;71(1):51–57. doi: 10.1128/AEM.71.1.51-57.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov M, Petrov S, Nacheva G, Ivanov I, Reichl U. Effects of a recombinant gene expression on ColE1-like plasmid segregation in Escherichia coli. BMC Biotechnol. 2011;11:18. doi: 10.1186/1472-6750-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos JL, Wasserfallen A, Rose K, Timmis KN. Redesigning metabolic routes: manipulation of TOL plasmid pathway for catabolism of alkylbenzoates. Science. 1987;235(4788):593–596. doi: 10.1126/science.3468623. [DOI] [PubMed] [Google Scholar]
- Seo JH, Bailey JE. A segregated model for plasmid content and product synthesis in unstable binary fission recombinant organisms. Biotechnol Bioeng. 1985;27(2):156–166. doi: 10.1002/bit.260270209. [DOI] [PubMed] [Google Scholar]
- Sharp RR, Bryers JD, Jones WG. Activity and stability of a recombinant plasmid-borne TCE degradative pathway in biofilm cultures. Biotechnol Bioeng. 1998;59(3):318–327. doi: 10.1002/(sici)1097-0290(19980805)59:3<318::aid-bit8>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Smalla K, Krögerrecklenfort E, Heuer H, Dejonghe W, Top E, Osborn M, Niewint J, Tebbe C, Barr M, Bailey M, Greated A, Thomas C, Turner S, Young P, Nikolakopoulou D, Karagouni A, Wolters A, van Elsas JD, Drønen K, vandaa R, Borin S, Brabhu J, Grohmann E, Sobecky P. PCR-based detection of mobile genetic elements in total community DNA. Microbiology. 2000;146(Pt 6):1256–1257. doi: 10.1099/00221287-146-6-1256. [DOI] [PubMed] [Google Scholar]
- Stewart GJ, Carlson CA. The biology of natural transformation. Annu Rev Microbiol. 1986;40:211–235. doi: 10.1146/annurev.mi.40.100186.001235. [DOI] [PubMed] [Google Scholar]
- Summers DK. The kinetics of plasmid loss. Trends Biotechnol. 1991;9(8):273–278. doi: 10.1016/0167-7799(91)90089-z. [DOI] [PubMed] [Google Scholar]
- Summers DK, Sherratt DJ. Multimerization of high copy number plasmids causes instability: CoIE1 encodes a determinant essential for plasmid monomerization and stability. Cell. 1984;36(4):1097–1103. doi: 10.1016/0092-8674(84)90060-6. [DOI] [PubMed] [Google Scholar]
- Tombolini RA, Unge ME, Davey FJ, de Brujin FJ, Jansson JK. Flow cytometric and microscopic analysis of GFP-tagged Pseudomonas fluorescens bacteria. FEMS Microbiol Ecol. 1997;22:17–28. [Google Scholar]
- Torsvik V, Goksoyr J, Daae FL. High diversity in DNA of soil bacteria. Appl Environ Microbiol. 1990;56:782–787. doi: 10.1128/aem.56.3.782-787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin BE, Nordstrom K. A runaway-replication mutant of plasmid R1drd-19: temperature-dependent loss of copy number control. Mol Gen Genet. 1978;165(2):167–179. doi: 10.1007/BF00269904. [DOI] [PubMed] [Google Scholar]
- Uhlin BE, Molin S, Gustafsson P, Nordstrom K. Plasmids with temperature-dependent copy number for amplification of cloned genes and their products. Gene. 1979;6(2):91–106. doi: 10.1016/0378-1119(79)90065-9. [DOI] [PubMed] [Google Scholar]
- Wood TK, Peretti SW. Effect of chemically-induced, cloned-gene expression on protein synthesis in E. coli. Biotechnol Bioeng. 1991;38(4):397–412. doi: 10.1002/bit.260380410. [DOI] [PubMed] [Google Scholar]
- Wood TK, Kuhn RH, Peretti SW. Enhanced plasmid stability through post-segregational killing of plasmid-free cells. Biotechnol Tech. 1990;4:39–44. [Google Scholar]
- Wuertz S, Hendrickx L, Kuehn M, Rodenacker K, Hausner M. In situ quantification of gene transfer in biofilms. Methods Enzymol. 2001;336:129–143. doi: 10.1016/s0076-6879(01)36585-0. [DOI] [PubMed] [Google Scholar]
- Wuertz S, Okabe S, Hausner M. Microbial communities and their interactions in biofilm systems: an overview. Water Sci Technol. 2004;49(11–12):327–336. [PubMed] [Google Scholar]
- Yang S, Lewandowski Z. Measurement of local mass transfer coefficient in biofilms. Biotechnol Bioeng. 1995;48(6):737–744. doi: 10.1002/bit.260480623. [DOI] [PubMed] [Google Scholar]
- Yoon KP. Stabilities of artificially transconjugated plasmids for the bioremediation of cocontaminated sites. J Microbiol. 2005;43(2):196–203. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.