Abstract
Studies have suggested that elevated tumor mitotic rate (MR) is linked to overall survival in thin melanoma. Recently, promising data regarding anti-phosphohistone 3 (pHH3) immunohistochemistry and its ability to aid in calculation of MR have emerged. The authors retrospectively analyzed original biopsies from 13 thin melanomas with positive sentinel node (SN) status and 16 thin melanomas with negative SN status. Both anti-pHH3 immunohistochemistry and the hematoxylin and eosin (H&E) stain were used to evaluate MR by 2 dermatopathologists blinded to SN status using the “hot spot” method. Intraclass coefficient values were attained to measure interobserver concordance and reliability of the pHH3 stain. By generating a receiver operating characteristic curve and analyzing the overall area under the curve, pHH3 was found to have good interobserver reliability. The relationship between MR and SN involvement was also evaluated, but this correlation was not statistically significant.
INTRODUCTION
Recent statistics reveal that more than half of newly diagnosed melanomas are thin melanoma (Breslow depth less than 1 mm).1 Studies examining the histologic variables that predict poor outcome within this group of patients2–13 confirm that in addition to Breslow depth and ulceration, that mitotic rate (MR), is the most likely predictor of disease recurrence and survival. This finding prompted the addition of MR to the microstaging criteria for thin melanoma in the recent 7th edition of the American Joint Committee on Cancer (AJCC) Melanoma Staging criteria.3 This makes it imperative that we develop accurate and reliable methodology for identification and quantification of mitotic figures in these lesions.
The current guidelines recommend counting mitotic figures using hematoxylin and eosin (H&E)-stained sections in a “hot spot” of mitotic activity within lesions.10 We recently reported that anti-pHH3 immunohistochemistry (IHC) could be a useful adjunct to H&E analysis for identifying mitotic figures in thin melanomas.16 The pHH3 marker has the ability to stain mitotic figures in all phases of mitosis and is useful for detecting the hot spot and as a confirmatory staining technique. The current study used pHH3 IHC to quantify MR in thin melanomas and determine the interobserver concordance and reliability of the stain.
There is considerable debate about the need for sentinel lymph node biopsy (SLNB) for patients with thin melanomas (≤1 mm). Some investigators argue that identification of a single mitotic figure, which classifies the lesion as a stage T1b, should trigger an SLNB while others have shown that MR is not linked to SN status in thin melanomas.14,15 MR and SN status have been correlated in larger studies,5,6,8,14,15,17,18 and we sought to further explore correlation between these 2 factors once MR was counted using both pHH3 IHC and the H&E stain.
MATERIALS AND METHODS
After Institutional Review Board approval, primary tumor slides were reviewed from 13 patients with thin melanomas (depth < 1 mm) and positive SNs and from a control group of 16 patients with SN-negative thin melanomas. Primary tumors were stained with H&E and anti-PHH3 (ser10; Cell Signaling Technology, Danvers, MA) as per the Casper protocol.16 Two dermatopathologists, blinded to both the SN status and to each other’s results, counted the MR per square millimeter using the hot spot method. Interobserver concordance was determined as was the reliability of the anti-pHH3 stain. Patient confidentiality was maintained to the extent permitted by law.
The intraclass coefficient (ICC) method was used to measure interrater reliability between values of the PHH3 and H&E stain. The ICC ranges from −1 to 1, with higher absolute values correlating with better agreement. Sensitivity and specificity were calculated using H&E stain as the gold standard, and a receiver operating characteristic (ROC) curve and the area under the curve (AUC) were created. The Mann–Whitney U statistic test was used to compare the overall AUC from the different markers.
RESULTS
Among the 29 patients included in the study, 19 were male and 10 were female. The mean age was 54.6 with a range from 35 to 75 years. Table 1 summarizes other primary tumor factors for each of these patients, including primary tumor location, Breslow depth, MR by H&E and pHH3 counted by 2 blinded observers, and SN status.
TABLE 1.
Demographic and Primary Tumor Factors
| SN Status | Age | Gender | Location | Breslow Depth (mm) | Observer 1 | Observer 2 | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| H&E | pHH3 | H&E | pHH3 | |||||
| Negative | 62 | M | Right upper arm | 0.87 | 0 | 0 | 0 | 0 |
| Negative | 52 | F | Right lateral back | 0.79 | 1 | 4 | 1 | 4 |
| Negative | 70 | M | Left preauricular | 0.7 | 1 | 0 | 0 | 0 |
| Negative | 40 | F | Left upper paraspinal | 0.9 | 0 | 0 | 3 | 2 |
| Negative | 46 | F | Left lower medial leg | 0.88 | 0 | 5 | 2 | 6 |
| Negative | 52 | F | Right hip | 0.8 | 0 | 3 | 1 | 2 |
| Negative | 40 | M | Right upper back | 0.85 | 1 | 0 | 1 | 3 |
| Negative | 53 | M | Left forearm | 0.62 | 2 | 3 | 2 | 1 |
| Negative | 62 | F | Left posterior leg | 0.84 | 1 | 0 | 2 | 4 |
| Negative | 64 | M | Left lower abdomen | 0.73 | 2 | 5 | 4 | 4 |
| Negative | 56 | M | Left upper back | 0.8 | 8 | 11 | 7 | 34 |
| Negative | 68 | M | Left upper back | 0.88 | 0 | 0 | 0 | 0 |
| Negative | 74 | M | Left neck | 0.72 | 1 | 0 | 1 | 1 |
| Negative | 52 | M | Right upper back | 0.62 | 0 | 0 | 1 | 1 |
| Negative | 49 | M | Scalp | 0.68 | 6 | 20 | 6 | 26 |
| Negative | 62 | F | Left shoulder | 0.74 | 0 | 0 | 0 | 0 |
| Positive | 43 | F | Left buttock | 0.9 | 0 | 2 | 2 | 4 |
| Positive | 58 | F | Upper right leg | 0.95 | 1 | 2 | 2 | 4 |
| Positive | 55 | M | Left lower lateral back | 0.8 | 3 | 4 | 3 | 6 |
| Positive | 72 | M | Left neck | 0.95 | 2 | 4 | 2 | 12 |
| Positive | 54 | F | Left hand | 0.85 | 2 | 13 | 5 | 21 |
| Positive | 56 | M | Left neck | 0.95 | 0 | 3 | 0 | 1 |
| Positive | 45 | M | Right upper arm | 0.6 | 2 | 3 | 2 | 5 |
| Positive | 63 | M | Right scapula | 0.98 | 2 | 4 | 2 | 3 |
| Positive | 48 | M | Right lower leg | 0.8 | 0 | 1 | 0 | 1 |
| Positive | 35 | F | Left posterior arm | 0.8 | 0 | 0 | 3 | 3 |
| Positive | 38 | M | Right upper arm | 0.8 | 8 | 5 | 3 | 13 |
| Positive | 38 | M | Left torso | 1 | 2 | 6 | 4 | 8 |
| Positive | 75 | M | Right preauricular | 0.9 | 2 | 4 | 4 | 5 |
H&E indicates hematoxylin and eosin; F, female; M, male; pHH3, phosphohistone 3; SN, sentinel node.
The ICC values were attained to determine the predictability and positive correlation of the 2 stains when counting mitotic figures. The ICC value for the pHH3 stain was 0.842 (P < 0.001), with a 95% confidence interval between 0.659 and 0.927. For H&E, the ICC value was 0.816 (P < 0.001) with a 95% confidence interval between 0.602 and 0.915.
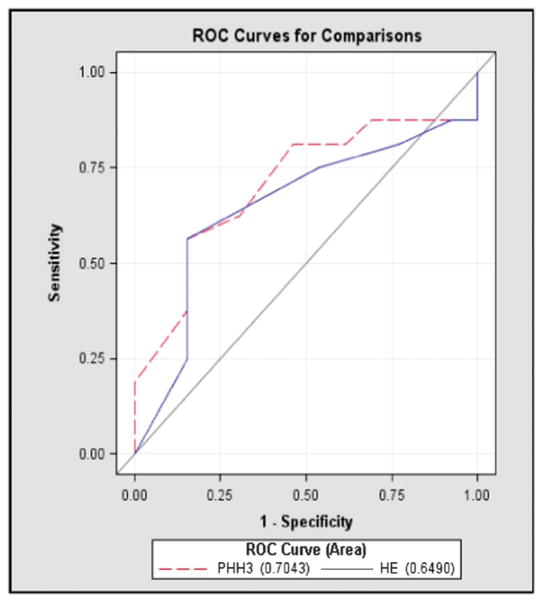
The ROC curves for the pHH3 marker and H&E are depicted in Figure 1. The PHH3 curve provided an overall AUC of 0.7043, whereas the H&E curve had an overall AUC of 0.649. The highest AUC achieved was 0.705. These results were not statistically significant with a P value of 0.3659.
FIGURE 1.
ROC curves for the pHH3 marker and the H&E stain.
The relationship between MR and SN involvement was also evaluated using both stains. In patients with negative SNs, the median value for MR by pHH3 was 0, with a range of 0–34, and in patients with a positive SN, the median pHH3 was 4, with a range of 2–21 (Table 2). However, no significant difference was found with a P value of 0.1649. Although the median value of PHH3 in SN-positive and -negative samples was different (4 vs. 0), the standard deviation in both the groups was quite large (5.55 in SN negative and 3.92 in SN positive). The median value for H&E in SN-negative patients was 1, with a range of 0–8, whereas it was 2 in SN-positive patients, with a range of 0–8. The difference in MR by H&E staining in thin SN-positive melanomas and SN-negative melanomas was not statistically significant (Table 3).
TABLE 2.
PHH3 MR Counts and SN Involvement
| No. Observation | Mean | SD | Median | No. Miss | |
|---|---|---|---|---|---|
| SN negative | 16 | 3.40 | 5.55 | 0 | 1 |
| SN positive | 13 | 3.92 | 3.17 | 4 | 0 |
TABLE 3.
H&E MR Counts and SN Involvement
| No. Observation | Mean | SD | Median | No. Miss | |
|---|---|---|---|---|---|
| SN negative | 16 | 1.53 | 2.36 | 1 | 1 |
| SN positive | 13 | 1.85 | 2.12 | 2 | 0 |
The mean tumor thickness in both SN-positive and SN-negative patients was also calculated. The mean thickness in SN-positive patients was 0.867, whereas the mean thickness in SN-negative patients was 0.776.
DISCUSSION
Approximately 65% of the nearly 70,000 cases of melanomas diagnosed annually in the United States are thin melanomas.1 Although typically associated with a favorable prognosis, the 10-year survival rate is highly variable (85%–99%).2 There is debate concerning the optimal surgical intervention in primary localized thin melanoma—although wide local excision is standard, the role of SN biopsy is more controversial, with metastasis rates reported between 3% and 8.4%.4–6,8–10,18,19 These findings stress the need to find reliable prognostic markers to stratify risk in patients with thin melanomas.
There are numerous potential predictors of disease outcome in melanoma, with thickness and ulceration identified as the most important prognostic attributors.4–6,8–10,12,13 More recently, the impact of primary tumor MR on survival probability has been recognized. Staging alterations were recently implemented in the 7th edition AJCC Melanoma Staging Manual, with MR replacing Clark level of invasion and T1b tumors now defined as having tumor thickness <1.0 mm and the presence of >0 mitoses/mm2 or ulceration.3
Because of this emerging data, it becomes imperative to accurately and reliably determine mitotic activity in thin melanomas. We sought to determine the utility of the pHH3 stain given the promising data published in our previous study.16 MR was measured both conventionally using H&E and also with phh3 IHC. Interrater reliability as assessed by the ICC value revealed that both H&E and pHH3 had acceptable predictability and correlation, with ICC values greater than 0.81 for both the stains. This indicates near-perfect agreement between the dermatopathologists on each stain with a strong interrater reliability, supporting the reproducibility of the stain.
To compare MR counts among the 2 stains, we determined the area under the ROC curve for each stain, which represents the average sensitivity of the biomarkers over the range of specificities. The highest AUC was achieved was 0.705, an acceptable discrimination based on the guidelines provided by Hosmer and Lemeshow.19 As displayed in Figure 1, the pHH3 stain seemed to perform similarly to the H&E stain overall, with an overall AUC of 0.7043 versus 0.649 for H&E. This supports the use of this supplemental stain, as it should enhance the identification of mitotic figures and of hot spots to aid the reader. The pHH3 marker has advantages that include the ability to stain mitotic figures in all phases of mitosis, more rapid and efficient detection of the hot spot, and potential use as a confirmatory staining technique to verify what is already seen on H&E. However, the potential for overstaining must be recognized and skilled dermatopathologists must be able to decipher between stained melanocytic mitosis and other neighboring cells (ie, lymphocytes and keratinocytes) in the M phase.16 Use of this marker should not significantly add to the turnaround time for melanoma diagnosis because other immunohistochemical stains such as Melan-A are commonly used to ascertain diagnosis and tumor depth in this scenario. The potential aid provided by this stain to help identify MR and potential candidates for SN biopsies may in itself justify the cost of the stain.
There are mixed reports in the literature concerning the association with MR and SN status.5,6,8,14,15,17,18 Kesmodel et al6 studied thin melanomas with a vertical growth phase, revealing that in the presence of MR > 0, SN positivity was found to be 8.7%; factoring in tumor thickness >0.76 mm, this risk increased to 12.3%. Similarly, Karakousis et al5 published a study using regional lymph node disease as a surrogate for SN positivity and concluded that thin melanomas with MR > 0 and vertical growth phase corresponded with a regional nodal disease rate of 11.9%. Han et al performed an analysis and correlated both ulceration and MR > 1/mm2 with SN metastasis. They found that although ulceration was a significant predictor of SN metastasis, MR > 1/mm2 trended toward significance but was ultimately not significantly associated with SN status (OR, 2.50; 95% CI, 0.95–6.60; P = 0.06).15
Our secondary end point was to also explore correlation between MR and SN status. The median MR value reported in those cases with SN positivity was 4 with the pHH3 stain and 2 when using H&E, whereas the value was 0 and 1, respectively, in cases who were SN negative. These results were not statistically significant given the small sample size and large standard deviation attained from our data.
In summary, we retrospectively analyzed MR in 29 thin melanoma cases and explored correlation between MR and SN status and between the pHH3 and H&E stains. Our results highlighted the strong reproducibility and interrater reliability of the pHH3 stain but found no significant correlation between MR and SN status using either stain. Our previous study showed that pHH3 immunostaining facilitates determination of MR by providing a more sensitive and simplified manner to quickly screen tumors for mitotic figures.16 A 7% increase in the detection of mitotic figures was demonstrated when using the pHH3 marker. The stain is most useful in identifying areas of increased mitotic activity, which is why our recommendations are that it should be used as an adjunct to H&E to help identify these hot spots.
Footnotes
Disclosures: Supported by the University of South Florida and Moffitt Cancer Center. The authors declare no conflicts of interest.
References
- 1.Allen AC, Spitz S. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer. 1953;6:1–45. doi: 10.1002/1097-0142(195301)6:1<1::aid-cncr2820060102>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Devesa SS, Hartge P, et al. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–683. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: an analysis of 3661 patients from a single center. Cancer. 2003;97:1488–1498. doi: 10.1002/cncr.11196. [DOI] [PubMed] [Google Scholar]
- 5.Karakousis GC, Gimotty PA, Botbyl JD, et al. Predictors of regional nodal disease in patients with thin melanomas. Ann Surg Oncol. 2006;13:533–541. doi: 10.1245/ASO.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Kesmodel SB, Karakousis GC, Botbyl JD, et al. Mitotic rate as a predictor of sentinel lymph node positivity in patients with thin melanomas. Ann Surg Oncol. 2005;12:449–458. doi: 10.1245/ASO.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Slingluff CL, Jr, Vollmer RT, Reintgen DS, et al. Lethal “thin” malignant melanoma. Identifying patients at risk. Ann Surg. 1988;208:150–161. doi: 10.1097/00000658-198808000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sondak VK, Taylor JM, Sabel MS, et al. Mitotic rate and younger age are predictors of sentinel lymph node positivity: lessons learned from the generation of a probabilistic model. Ann Surg Oncol. 2004;11:247–258. doi: 10.1245/aso.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 9.Thompson JF, Soong SJ, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: an analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. J Clin Oncol. 2011;29:2199–2205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gimotty PA, Guerry D, Ming ME, et al. Thin primary cutaneous malignant melanoma: a prognostic tree for 10-year metastasis is more accurate than American Joint Committee on Cancer staging. J Clin Oncol. 2004;22:3668–3676. doi: 10.1200/JCO.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 12.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 13.Balch CM, Gershenwald JE, Soong SJ, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28:2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner JD, Gordon MS, Chuang TY, et al. Predicting sentinel and residual lymph node basin disease after sentinel lymph node biopsy for melanoma. Cancer. 2000;89:453–462. doi: 10.1002/1097-0142(20000715)89:2<453::aid-cncr34>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Han D, Yu D, Zhao X, et al. Sentinel node biopsy is indicated for thin melanomas >0.76 mm. Ann Surg Oncol. 2012;19:3335–3342. doi: 10.1245/s10434-012-2469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casper DJ, Ross KI, Messina JL, et al. Use of anti-phosphohistone H3 immunohistochemistry to determine mitotic rate in thin melanoma. Am J Dermatopathol. 2010;32:650–654. doi: 10.1097/DAD.0b013e3181cf7cc1. [DOI] [PubMed] [Google Scholar]
- 17.Phan GQ, Messina JL, Sondak VK, et al. Sentinel lymph node biopsy for melanoma: indications and rationale. Cancer Control. 2009;16:234–239. doi: 10.1177/107327480901600305. [DOI] [PubMed] [Google Scholar]
- 18.Cascinelli N, Clemente C, Bifulco C, et al. Sentinel lymph node biopsy in cutaneous melanoma: the WHO Melanoma Program experience. Ann Surg Oncol. 2000;7:469–474. doi: 10.1007/s10434-000-0469-z. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer WD, Lemeshow S. Applied Logistic Regression. 2. New York, NY: John Wiley and Sons; 2000. p. 392. [Google Scholar]