Abstract
The significance of kidneys in regulation of sodium and water balance and hemodynamics has been demonstrated both in patients and animal models. In the present study, we tested our hypothesis that kidneys play an essential role in control of sex differences in Ang II-dependent hypertension.
Kidney transplantations (KTXs) were performed between male and female C57BL/6 mice (F, female; M, male; donor→recipient: F→F; M→M; F→M; and M→F). Radio-telemetry transmitters were implanted for measurement of mean arterial pressure (MAP) during the infusion of Ang II (600ng/kg/min). Gene expressions and inflammatory responses in the transplanted grafts were assessed.
We found that same-sex-KTX mice still exhibited sex differences in Ang II-dependent hypertension (31.3±0.8 mmHg in M→M vs. 12.2±0.6 mmHg in F→F), which were reduced between M and F when they received kidneys of the opposite sex (32.9±1 mmHg in M→F vs. 22.3±0.7 mmHg in F→M). The sex differences in gene expressions, including AT1, AT1/AT2, ET1, ETA, NHE3, αENaC and γENaC, were unaltered in same-sex KTX and much lessened in cross-sex KTXs. In addition, the cross-sex KTXs exhibited more robust inflammatory responses reflected by higher expression of IL-6, TNFα, and KC than same-sex KTX.
Our results indicate that kidneys play an essential role in sex differences of Ang II-dependent hypertension. KTX of male kidneys to females augmented the BP response while KTX of female kidneys to males attenuated the BP response. The host’s extra-renal systems modulate expressions of many genes and inflammatory response, which may also contribute to the sex differences in blood pressure regulation.
Keywords: mouse kidney transplantation, sex difference, Ang II, mean arterial blood pressure
Introduction
Hypertension affects about 1 in 3 U.S. adults and is the leading risk factor for cardiovascular diseases.1–3 Multiple regulatory systems, such as the vasculature, the heart, the central nervous system, and the kidneys, have been demonstrated to participate in the regulation of the blood pressure (BP).4 Among them, the kidneys have been demonstrated to play a vital role in the development of hypertension in both humans and experimental animal models.5,6 Kidneys transplanted from hypertensive donors increase BP in normotensive recipients, whereas kidneys transplanted from normotensive donors lower BP in hypertensive recipients both in clinical7–10 and experimental renal transplantation studies.11–14
Epidemiological studies indicate that the prevalence of hypertension is greater in men than premenopausal women regardless of race, ethnicity or country of origin.15–17 Women are protected from more cardiovascular events compared to men until menopause. Sex differences in BP are also observed in experimental hypertensive models.18,19 The effects of sex hormones, such as estrogen and testosterone, on the sex differences in BP regulations have been extensively studied in recent years.20–22 However, whether kidneys play an important role in control of the sex differences in BP has not been clarified.
In the present study, we tested our hypothesis that kidneys play an essential role in control of the sex differences in Ang II-dependent hypertension. The sex differences pattern in high BP will be altered between males and females when they receive kidneys of opposite sex. To test our hypothesis, we performed KTX between male and female mice, induced hypertension and measured gene expressions and inflammatory responses in the transplanted grafts. Our results indicate that while the kidneys play an essential role in the control of the sex differences in hypertension, the extra-renal mechanisms also contribute to the sex differences in BP regulation.
Methods
All procedures and experiments were approved by the Institutional Animal Care and Use Committee at the University of South Florida College of Medicine. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except as indicated.
Detailed methods and associated references are available in the online-only Data Supplement. (See online-only Data Supplement)
Statistical Analysis
All data were presented as mean values ± SE. MAP and HR curves between groups were compared using 2-way ANOVA with Tukey’s post-hoc tests as appropriate. The MAP response curves within animals were compared using Student paired t test. The mRNA expressions of all the factors were compared among multiple groups using 1-way ANOVA with Tukey’s post-hoc tests. A p- value of <0.05 was considered to be statistically significant. Statistical analysis was performed with GraphPad Prism, version 6.0h (GraphPad Software).
Results
Graft function assessment post KTX
After KTX, the mice were allowed to recover for 4 weeks. Plasma creatinine concentration, glomerular filtration rate (GFR) (n=7; Fig. S1A and 1B) and plasma testosterone and estradiol levels were all in normal range (n=7; Fig. S2A and S2B).
Changes in MAP and heart rate (HR) in responses to Ang II infusion
To determine sex differences in Ang II-induced hypertension, we measured the MAP with radio-telemetry system. The basal MAP were similar and within normal range in all groups of animals (Fig. 1A, Figure S3A and B).
Fig 1. The response of MAP to a subpressor dose of Ang II infusion.
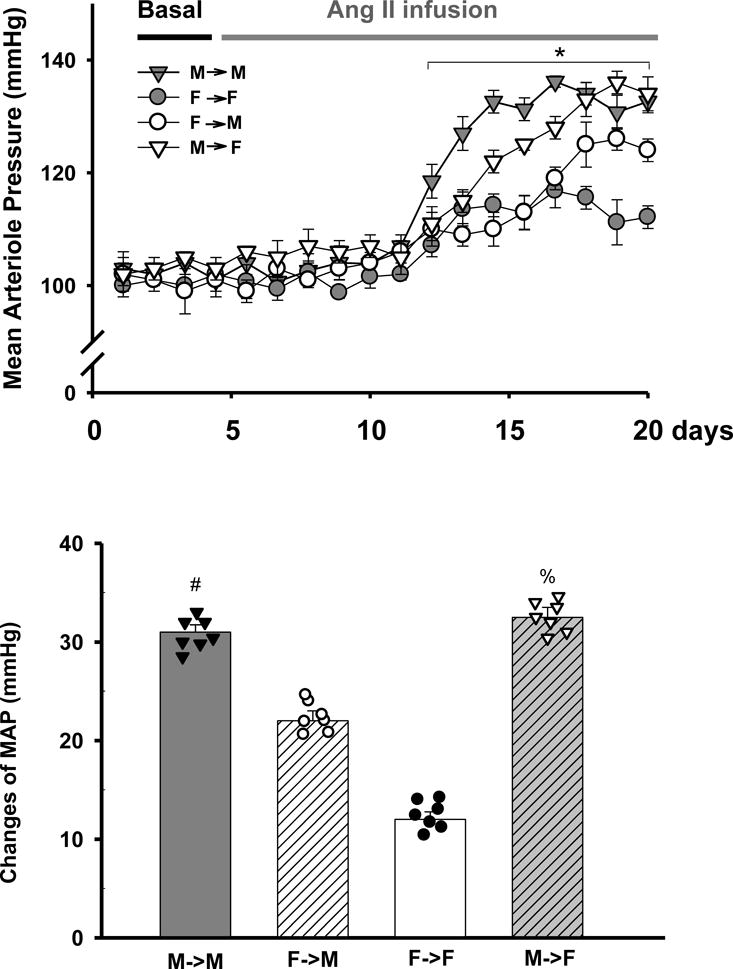
Mean arterial pressure (MAP) was measured with telemetry in response to a subpressor dose of Ang II infusion (600 ng/min/kg). A: MAP. (*p<0.01 vs. basal; by student’s t-test); B: Delta values of MAP (difference in the Ang II-induced peak response minus basal) for same- and cross-sex-KTX groups (#p<0.01 vs. F→F and F→M; %p<0.05 vs. F→F; n=7; by 2-way ANOVA) (donor→recipient; M: male, F: female).
The MAP started to increase from about the 4th day following Ang II infusion. The maximum increase in MAP was 31.3±0.8 mmHg in M→M and 12.2±0.6 mmHg in F→F in the same-sex-KTX groups (p< 0.01 Ang II infusion vs. basal; n=7; Fig. 1A). In cross-sex-KTX groups, the Ang II-induced maximum increase in MAP was 22.3±0.7 mmHg in F→M, which was significantly lower than M→M group but higher than F→F group. The maximum increase in MAP in M→F group was 32.9±1 mmHg, which was significantly higher than F→F group but similar to the M→M group. The results indicate that a female kidney in a male mouse attenuated the BP response while a male kidney in a female mouse had the same response as in a male mouse. The sex differences in MAP in response to Ang II infusion were present in the same-sex-KTX groups, but attenuated in cross-sex-KTX mice (p< 0.01; n=7; Fig. 1B).
Females showed higher basal HRs than males in the same-sex-KTX groups (613±11 bpm for F→F mice and 532±13 bpm for M→M mice). After cross-sex KTX, the HRs of female mice with implanted male kidneys (M→F) remained unchanged compared with F→F mice. However, the HRs increased about 40 bpm for male mice with implanted female kidneys (F→M) compared with M→M mice (p<0.01; n=7; Fig. 2A and B). Ang II infusion did not significantly change the HRs for either male or female mice.
Fig 2. The response of HRs to subpressor dose of Ang II infusion.
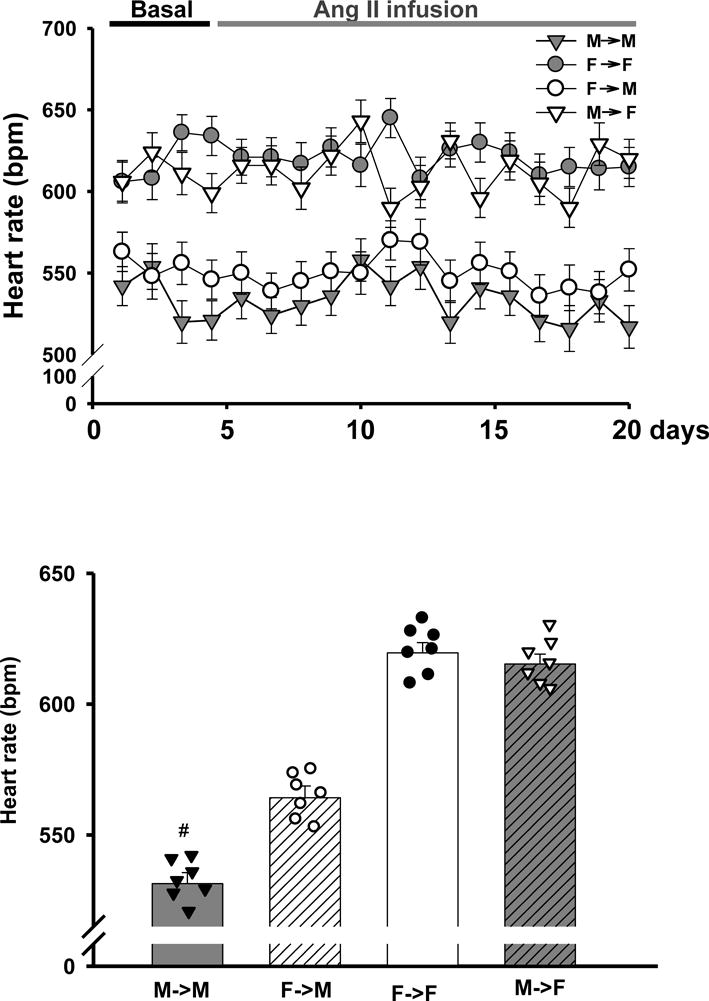
Heart rates (HRs) were measured with telemetry in response to a subpressor dose of Ang II infusion (600 ng/min/kg) (A) Heart rates over time; (B) Mean heart rates (# p<0.01 vs. F→M, n=7; by 2-way ANOVA) (donor→recipient; M: male, F: female)
Receptors and sodium cotransporters
To test if the recipient’s internal environment modulates gene expression of the transplanted grafts, we measured the expression levels of genes related to vascular contractility and the sodium reabsorption after 2-weeks of Ang II infusion. For all statistical comparisons, we focused on the comparison of the mRNA expressions of the donor kidneys between cross-sex recipients and the same-sex recipients (M→M vs. F→M and F→F vs. M→F) which were emphasized in the inset graphs in figures.
Angiotensin II receptors (ATR)
The mRNA levels of AT1R and AT2R for both males and females in the same-sex-KTX groups did not change significantly compared with their respective controls. AT1R mRNA was 2-fold higher in males than females for both same-sex-KTX and control groups (p<0.01 M→M vs. F→F and FC vs. MC; n=7; Fig. 3A). In the cross-sex-KTX groups, M→F mice showed a decrease in the expression level of AT1R mRNA compared with M→M mice. However, transplanted grafts from female donors to male recipients (F→M) resulted in a 50% increase of AT1R mRNA compared with F→F mice. Thus, the differences in the expression of AT1R between male and female grafts were attenuated after cross-sex KTX (p<0.05; n=7; Fig. 3A).
Fig 3. The gene expressions of the components in the RAAS and ET systems.
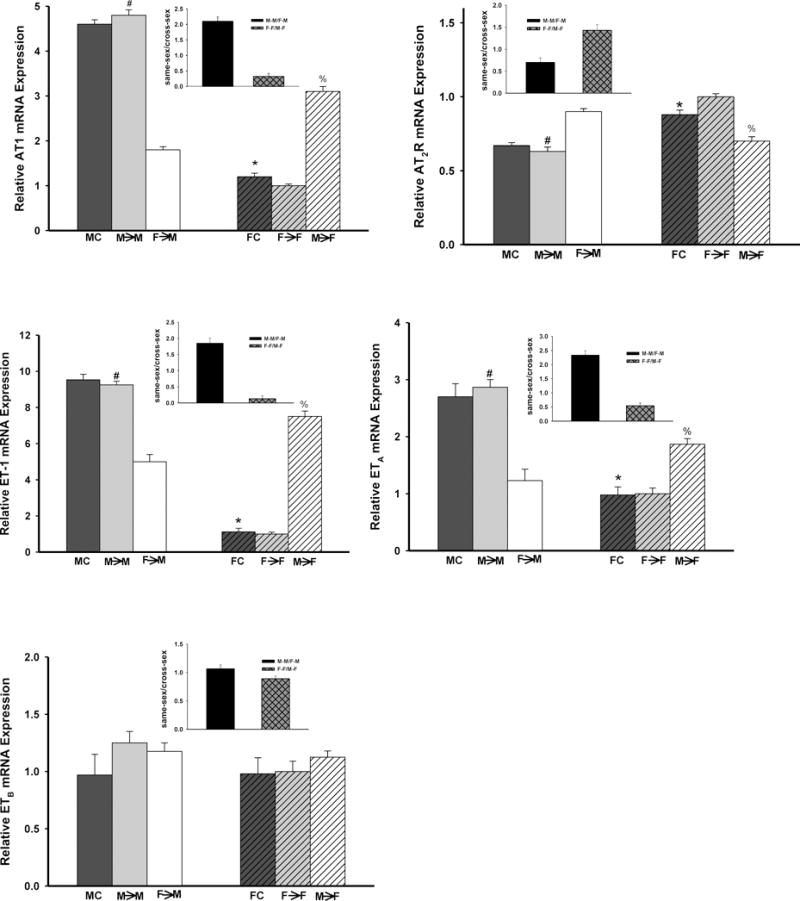
Expression levels of mRNA of the components in RAAS and ET systems in the transplanted kidneys following subpressor dose of Ang II infusion (600 ng/min/kg). (Insert graph showed the magnitude of the donor effect on measured parameters by comparing the sex differences in the gene expression in kidney grafts housed in same sex host) A: AT1R (*p<0.01 vs. MC; # p<0.01 vs. F→F and F→M; % p<0.05 vs. F→F); B: AT2R (*p<0.01 vs. MC; # p<0.01 vs. F→F and F→M; % p<0.05 vs. F→F); C: ET-1 (*p<0.01 vs. MC; # p<0.01 vs. F→F and F→M and % p<0.01 vs. F→F); D: ETA (*p<0.01 vs. MC; # p<0.01 vs. F→F and F→M; % p<0.01 vs. F→F) and E: ETB. (n=7; by 1-way ANOVA; donor→recipient; M: male, F: female)
AT2R mRNA expression levels were higher in female than male mice in same-sex-KTX and control groups (p<0.01 M→M vs. F→F and FC vs. MC; n=7; Fig. 3B). In cross-sex-KTX groups, the differences of AT2R levels between male and female grafts did not change much.
ET system
The level of ET-1 mRNA for both males and females in the same-sex-KTX groups did not change significantly compared with their respective controls. ET-1 mRNA level in male kidneys were about 8-fold higher than that in female kidneys. (p<0.01 M→M vs. F→F and FC vs. MC; n=7; Fig. 3C). In the cross-sex-KTX groups, the ET-1 mRNA level in the kidneys of M→F mildly decreased compared with M→M mice and showed a 5-fold increase in the kidneys of F→M compared with F→F mice (p<0.01 F→M vs. F→F; n=7; Fig. 3C). After cross-sex KTX, the sex differences of ET-1 mRNA expression was dramatically mitigated (p<0.01 M→M vs. F→M and M→F vs. F→F; n=7; Fig. 3C).
Male mice exhibited almost 2-fold higher of ETA mRNA expression than females in both control and same-sex-KTX groups (p<0.01 FC vs. MC and M→M vs. F→F; n=7; Fig. 3D). After cross-sex KTX, the abundance of the ETA mRNA was decreased in grafts of M→F group compared with M→M mice. The sex differences were attenuated in ETA mRNA expression in cross-sex-KTX groups in female recipients. No significant change in the expression of ETA mRNA was found in F→M group compared with F→F mice. (p<0.05; n=7; Fig. 3D) and there were no change of the sex differences in the ETA mRNA expression in cross-sex-KTX groups in male recipients. The expression levels of ETB did not differ in all groups (n=7; Fig. 3E).
Sodium transporters
Expressions of sodium transporter mRNAs were measured in whole kidney following 2-weeks of Ang II infusion. Fig. 4 summarized the observed decreases in NHE3 and ENaC mRNA expression and unaltered mRNA expression in NKCC2 and NCC between transplanted grafts and their respective controls.
Fig 4. The relative renal gene expression of the sodium transporters.
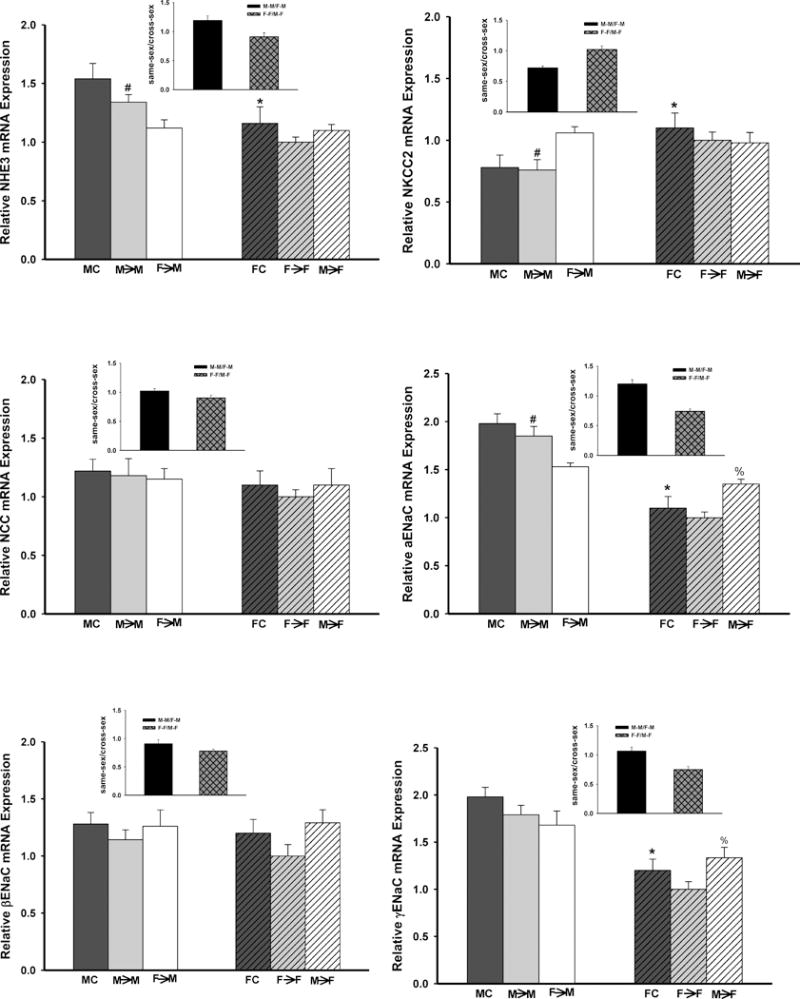
Expression levels of mRNA of sodium transporters in the transplanted kidneys following subpressor a dose of Ang II infusion (600 ng/min/kg). (Insert graph showed the magnitude of the donor effect on measured parameters by comparing the sex differences in the gene expression in kidney grafts housed in same sex host) A: NHE3 (*p<0.05 vs. F→F and MC; # p<0.01 vs. MC, F→F and F→M; @ p<0.05 vs. F→F); B: NKCC2 (*p<0.01 vs. MC; # p<0.01 vs. F→F and F→M); C: NCC; and D,E and F: α-,β- and γ- ENaC (*p<0.01 vs. MC; # p<0.01 vs. F→F and F→M; and % p<0.01 vs. F→F) (n=7; by 1-way ANOVA; donor→recipient; M: male, F: female;).
Male mice exhibited about 33%, 45% and 40% higher mRNA expression of NHE3 and two subunits of ENaC, α- and γ- ENaC, respectively, than female mice in the control and same-sex-KTX groups (p<0.01 M→M vs. F→F and FC vs. MC; n=7; Fig. 4A, 4D and 4F). Reductions of about 22%, 20% and 24% in NHE3, α- and γ- ENaC mRNAs were detected, respectively, in M→F mice compared with M→M mice. However, the expression of the NHE3, α- and γ- ENaC mRNA increased about 10%, 50% and 70%, respectively, in F→M mice compared with F→F mice. Thus, the sex differences in the expression of NHE3 and α- and γ- ENaC mRNA were lessened post cross-sex KTX compared with same-sex KTX (p< 0.05 F→M vs. M→M; and M→F vs. F→F; n=7; Fig 4A, 4D and 4F).
NKCC-2 mRNA in females (F→F) was 25% higher than in males (M→M) in same-sex-KTX groups (p<0.01 M→M vs. F→F and FC vs. MC; n=7; Fig. 4B). In cross-sex-KTX groups, the mRNA level of NKCC-2 in the female grafts did not change after transplanted into male recipients compared to that in female recipients. The mRNA level of NKCC-2 in the male grafts in the female recipients increased to the level of female grafts in female recipients. Sex differences in NKCC2 mRNA between male and female grafts did not change in the male recipients. However sex differences in NKCC2 mRNA between male and female grafts diminished in the female recipients. No significant sex differences in the mRNAs of NCC and β- ENaC have been detected in either same-sex-KTX or cross-sex-KTX groups (n=7; Fig. 4C and 4E).
Inflammatory response
Several typical inflammatory markers including IL-6, KC, TGFβ and TNFα were measured following Ang II infusion after KTX. Both same-sex and cross-sex-KTX groups exhibited an enhanced inflammatory response compared to control groups. Males exhibited higher levels of IL-6, KC and TNFα than females in the same-sex-KTX groups. Cross-sex KTX further stimulated the inflammation response for both males and females. In particular, the IL-6 levels were raised more than 4 folds in the cross-sex-KTX groups for both male and female kidneys when they were transplanted to opposite sex recipients. There were significant sex differences in the IL-6 level in the cross-sex KTX groups (p<0.01 F→M vs. M→M and M→F vs. F→F n=7; Fig 5A). No significant sex differences were found in the expression of KC and TGFβ mRNA in cross-sex-KTX groups.
Fig 5. The relative renal gene expression of the inflammatory factors.
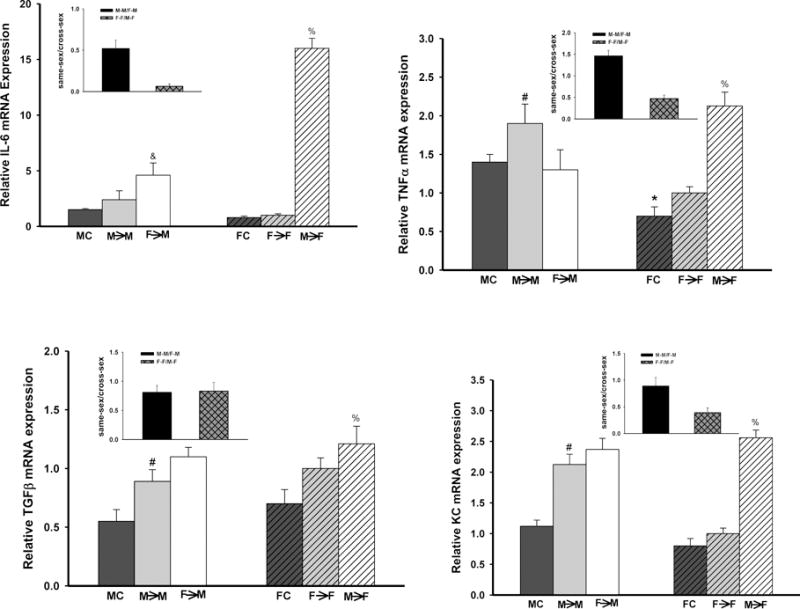
Inflammatory markers in the transplanted kidneys following a subpressor dose of Ang II infusion (600 ng/min/kg). (Insert graph showed the magnitude of the donor effect on measured parameters by comparing the sex differences in the gene expression in kidney grafts housed in same sex host) A: IL-6 (& p<0.01 vs. M→M; % p<0.01 vs. F→F); B: TNFα (*p<0.01 vs MC; # p<0.01 vs. F→F and F→M; % p<0.01 vs. F→F); C: TGFβ ((# p<0.01 vs. MC and F→M; and % p<0.01 vs. FC); and D: KC (# p<0.01 vs. MC and F→F; % p<0.01 vs. FC and F→F) (n=7; by 1-way ANOVA; donor→recipient; M: male, F: female).
Histology
Kidney histology was assessed with light microscopy. The kidney grafts in all groups demonstrated minor tubular injuries with focal intra-tubular hyaline casts. The tubular injuries were more severe in the M→F cohort compared with all other cohorts. No significant interstitial fibrosis or vascular abnormalities were evident in the kidney grafts (n=7; Fig 6).
Fig 6. Histology.
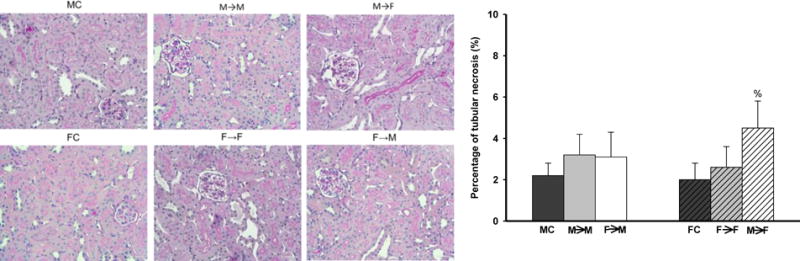
A. PAS staining showed minor tubular injuries with focal intra-tubular hyaline casts.
B. M→F cohort showed higher tubular injury scores than other groups. (% p<0.01; n=7; by 1-way ANOVA; donor→recipient; M: male, F: female)
Body and kidney weight
After KTX, the body weight of all the mice dropped about 10% in the first three days and gradually recovered in two weeks. At the end of the experiment, the body weight of all the groups was comparable to the mice without any operation for both sexes, respectively. The kidney weight of the M→M mice was about 32% heavier than that of F→F mice, however, the kidney weight between the M→F and the F→M transplanted mice was similar (*p<0.05 vs. other groups; n=7; Table S2).
Discussion
The present study examined the role of the kidneys in sex differences in Ang II-dependent hypertension by same- and cross-sex KTX in C57BL/6 mice. The sex differences in Ang II-induced hypertension still existed after same-sex KTX. The sex differences in hypertension were attenuated following cross-sex KTX, which were accompanied by lessened or diminished sex differences in mRNA levels of AT1, AT1/AT2 ratio, ET1, ETA, NHE3 and α and γ units of ENaC in the transplanted grafts. In addition, cross-sex KTX enhanced inflammatory response in the transplanted kidneys, reflected by significant increases in mRNA levels of IL-6, TNFα, and KC. These results indicated that kidneys play an essential role in sex differences in Ang II-dependent hypertension. Meanwhile the host’s extra-renal environment may contribute to the sex differences in BP by modulating gene expressions and inflammatory responses in the transplanted grafts.
Sex differences in BP are observed both in humans and experimental animal models with hypertension.23–25 However, the underlying mechanisms for the sex differences in BP have not been completely elucidated. The renal renin-angiotensin aldosterone system (RAAS) plays an essential role in the long-term control of arterial pressure. Chronic Ang II infusion is a widely-used method for experimental hypertension.26–28 In this study, we performed KTX between same sex and cross sexes mice and induced hypertension by chronic infusion of subpressor-dose of Ang II to test the significance of the kidneys in sex differences in BP regulation. Mice with same-sex KTX showed similar basal MAP compared with non-transplanted mice for both males and females,29 demonstrating that the transplant procedure and nephrectomy did not significantly affect BP. We observed that same-sex KTX did not change the sex differences in Ang II-induced hypertension compared with control groups. This result confirmed the previous finding that, in response to Ang II infusion, males exhibit greater responses compared to females.24,30,31 Interestingly, we found that the cross-sex KTX attenuated the sex differences in MAP in response to the chronic infusion of Ang II. The MAP in the group of M→F was simillar to that in the group of M→M. The presence of a kidney from a male donor in female recipient restored the magnitude of the hypertension to the levels similar to those observed in male mice. However, the MAP in the group of F→M was significantly higher than that in the group of F→F, but still significantly lower than that in the mice with male donors (M→M and M→F groups). Abundant evidence indicated that manipulation of sex steroid hormones through gonadectomy alters the course of hypertension.32,33 Here, we uncovered the impact of the sex steroid hormones on BP under physilogical condition. These data may indicate that while the kidneys play an important role in control of sex differences in BP, other factors also contribute to the regulation of BP after cross-sex KTX.
Multiple mechanisms have been reported to contribute to the sex differences in hypertension, including vascular contractility and tubular sodium reabsorption controlled by RAS,28 endothelin system,34,35 sodium transporters such as NHE3, NKCC2, NCC and ENaC36,37 and the immune system.38 Previously, we demonstrated the changes in gene expression and renal function after cross-sex KTX in normotensive animals.39 In the present study, we extended our investigation to hypertensive mice and the renal mechanisms of sex differences in hypertension.
We measured mRNA levels of the typical components of RAS, ET system and sodium transporters in the transplanted grafts. In the same-sex-KTX groups, males (M→M) showed a higher ratio of AT1/AT2 as well as higher expression of ET-1 and ETA than females (F→F), which are in similar patterns to native kidneys in previous studies.31,34,40,41 Cross-sex KTX significantly attenuated the sex differences in the expression of these genes. Regarding the sodium transporters, NKCC2 levels were higher in females and NHE3, and α- and γ- ENaC were more abundant in males in the same-sex-KTX groups, consistent with the previous studies in native kidneys.36,42,43 In the cross-sex-KTX groups, the sex differences in the expression of NKCC2, NHE3 and the α- and γ- ENaC were greatly attenuated or even reversed compared with same-sex-KTX groups. NCC mRNA expression levels did not differ between males and females in all sex combinations. These results indicated that the surgical procedure did not affect the gene expression patters in the same-sex KTX. It also revealed the complexity in sex differences in BP regulation, which may result from multiple factors of both intra- and extra-renal mechanisms. In addition, the sex difference patterns in gene expression levels in our recent study in normotensive mice39 were similar to the that in the present study in Ang II-induced hypertensive mice. These data indicate that the genotype of the transplanted grafts and the host internal environment may play an important role in regulation of the gene expressions in the grafts. Ang II infusion only played a minor role in modulating the gene expressions.
Inflammation is essential in the development of hypertension, including in Ang II–induced hypertension.44,45 Extensive evidence showed that there are sex differences in the magnitude of renal T-cell infiltration.46,47 In this study, we compared the sex differences of inflammatory response to Ang II-induced hypertension by measuring the renal expression of IL-6, TNF-α, TGFβ and KC after same-sex and cross-sex KTXs. Cross-sex KTX increased the inflammatory response to Ang II in both males and females, especially in the expression levels of IL-6 compared with same-sex-KTX groups. These data suggest that alterations in the extra-renal environment enhanced inflammatory responses, which may be significant in long-term graft function and could have potential translational significance in human KTXs. It should be noted that minor histocompatibility antigen rejection, in which female recipients reject grafts from the male donors due to incompatibility with the male H-Y antigen,48,49 may be one of the factors that induce higher inflammatory response in M→F group compared with other paired groups. The enhanced inflammatory response in M→F group may partially contribute to the high response of the MAP to the infusion of Ang II. Our results are in agreement with the findings from several clinical trials, in which female recipients from male donors exhibited a higher rate of graft failure.50,51 However, other clinical trials did not find the correlation between M→F group and graft function.52 These inconsistent observations may reflect the complexities in human KTX. It should be pointed out that implanted transmitters may enhance inflammatory response53 in the present study.
Sex steroids have been demonstrated to play an important role in modulating physiological factors in control of sex differences in BP.54 Ovariectomy, orchiectomy, central blockade or total knockout of estrogen or androgen receptors and administration of steroid have been applied to study the sex differences in hypertension.55,56 While these studies have provided valuable information and advanced our understanding of sex differences in many areas, all these strategies do not accurately mimic the physiological conditions. Here, we took advantage of cross-sex KTXs to provide a real opposite sex environment for the transplanted grafts and investigated the role of the kidneys in sex differences in hypertension. We believe that this approach would provide an additional tool in studying the significance of the kidneys in sex differences in both physiological and pathological situations.
Perspectives
The present study investigated the sex differences in Ang II-induced hypertension and assessed the expression of main components of RAS, endothelin system, and tubular sodium transporters in same-sex and cross-sex-KTX mice. We demonstrated that cross-sex KTX decreased sex differences in Ang II-induced hypertension compared with same-sex-KTX and control groups. These findings indicate that while the kidneys played an important role in determining the sex differences in BP, the hosts’ extra-renal environment also contributed to the sex differences in the regulation of renal hemodynamics by modulating the gene expressions in the transplanted grafts. Apparently enhanced inflammatory response in the grafts of cross-sex-KTX groups may influence the sex differences in hypertension and could have potential translational significance in the graft function in human KTXs. The results of the present study also revealed the complexity of the mechanisms involved in regulation of the gene expression, renal function and hemodynamics following KTX. Our findings may also provide some useful information in precision medicine based on gender for prevention and treatment of hypertension, as well as in improvement in graft function in KTXs.
Supplementary Material
Novelty and Significance.
What Is New?
This study investigated the role of kidneys in sex differences in blood pressure by same-sex and cross-sex-kidney transplantations in mice.
What Is Relevant?
We demonstrated novel information in understanding the underlying physiological mechanisms of sex differences in blood pressure regulation, which may provide new approaches in precision medicine based on gender in prevention and treatment of hypertension.
Summary
Kidneys play an essential role in determining sex differences in blood pressure. The hosts’ extra-renal environment also contributes to the sex differences in the regulation of renal hemodynamics by modulating the gene expressions and inflammations in the transplanted grafts.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health Grants DK099276 and DK098582 (RL).
Footnotes
Disclosures
No conflicts of interest, financial or otherwise, are declared by the author(s).
Reference List
- 1.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345:1291–1297. doi: 10.1056/NEJMoa003417. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Beiser A, Seshadri S, Larson MG, Kannel WB, D’Agostino RB, Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men. The Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 3.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief. 2013;(133):1–8. [PubMed] [Google Scholar]
- 4.Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med. 2011;17(11):1402–1409. doi: 10.1038/nm.2541. [DOI] [PubMed] [Google Scholar]
- 5.Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51(4):811–816. doi: 10.1161/HYPERTENSIONAHA.105.063636. [DOI] [PubMed] [Google Scholar]
- 6.Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim H-S, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. Journal of Clinical Investigation. 2005;115(4):1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis JJ, Luke RG, Dustan HP, Kashgarian M, Whelchel JD, Jones P, Diethelm AG. Remission of essential hypertension after renal transplantation. N Engl J Med. 1983;309:1009–1015. doi: 10.1056/NEJM198310273091702. [DOI] [PubMed] [Google Scholar]
- 8.Guidi E, Bianchi G, Rivolta E, Ponticelli C, Quarto di Palo F, Minetti L, Polli E. Hypertension in man with a kidney transplant: role of familial versus other factors. Nephron. 1985;41:14–21. doi: 10.1159/000183539. [DOI] [PubMed] [Google Scholar]
- 9.Guidi E, Menghetti D, Milani S, Montagnino G, Palazzi P, Bianchi G. Hypertension may be transplanted with the kidney in humans: a long-term historical prospective follow-up of recipients grafted with kidneys coming from donors with or without hypertension in their families. J Am Soc Nephrol. 1996;7(8):1131–1138. doi: 10.1681/ASN.V781131. [DOI] [PubMed] [Google Scholar]
- 10.Strandgaard S, Hansen U. Hypertension in renal allograft recipients may be conveyed by cadaveric kidneys from donors with subarachnoid haemorrhage. Br Med J [Clin Res ] 1986;292:1041–1044. doi: 10.1136/bmj.292.6527.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi G, Fox U, Di Francesco GF, Giovanetti AM, Pagetti D. Blood pressure changes produced by kidney cross-transplantation between spontaneously hypertensive rats and normotensive rats. Clin Sci Mol Med. 1974;47:435–448. doi: 10.1042/cs0470435. [DOI] [PubMed] [Google Scholar]
- 12.Churchill PC, Churchill MC, Bidani AK. Kidney cross transplants in Dahl salt-sensitive and salt-resistant rats. Am J Physiol. 1992;262(6 Pt 2):H1809–H1817. doi: 10.1152/ajpheart.1992.262.6.H1809. [DOI] [PubMed] [Google Scholar]
- 13.Dahl LK, Heine M. Primary role of renal homografts in setting chronic blood pressure levels in rats. Circ Res. 1975;36:692–696. doi: 10.1161/01.res.36.6.692. [DOI] [PubMed] [Google Scholar]
- 14.Rettig R, Stauss H, Folberth C, Ganten D, Waldherr R, Unger Y. Hypertension transmitted by kidneys from stroke-prone spontaneously hypertensive rats. Am J Physiol. 1989;257:F197–F203. doi: 10.1152/ajprenal.1989.257.2.F197. [DOI] [PubMed] [Google Scholar]
- 15.Doumas M, Papademetriou V, Faselis C, Kokkinos P. Gender differences in hypertension: myths and reality. Curr Hypertens Rep. 2013;15(4):321–330. doi: 10.1007/s11906-013-0359-y. [DOI] [PubMed] [Google Scholar]
- 16.Pimenta E. Hypertension in women. Hypertens Res. 2012;35(2):148–152. doi: 10.1038/hr.2011.190. [DOI] [PubMed] [Google Scholar]
- 17.Sandberg K, Ji H. Sex differences in primary hypertension. Biol Sex Differ. 2012;3(1):7. doi: 10.1186/2042-6410-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crofton JT, Ota M, Share L. Role of vasopressin, the renin-angiotensin system and sex in Dahl salt-sensitive hypertension. J Hypertens. 1993;11(10):1031–1038. doi: 10.1097/00004872-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Ganten U, Schröder G, Witt M, Zimmermann F, Ganten D, Stock G. Sexual dimorphism of blood pressure in spontaneously hypertensive rats: effects of anti-androgen treatment. J Hypertens. 1989;7:721–726. [PubMed] [Google Scholar]
- 20.Chappell MC, Westwood BM, Yamaleyeva LM. Differential effects of sex steroids in young and aged female mRen2.Lewis rats: a model of estrogen and salt-sensitive hypertension. Gend Med. 2008;5(Suppl A):S65–S75. doi: 10.1016/j.genm.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reckelhoff JF, Yanes LL, Iliescu R, Fortepiani LA, Granger JP. Testosterone supplementation in aging men and women: possible impact on cardiovascular-renal disease. Am J Physiol Renal Physiol. 2005;289(5):F941–F948. doi: 10.1152/ajprenal.00034.2005. [DOI] [PubMed] [Google Scholar]
- 22.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2009;296(4):F771–F779. doi: 10.1152/ajprenal.90389.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 24.Xue B, Pamidimukkala J, Hay M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart Circ Physiol. 2005;288:H2177–H2184. doi: 10.1152/ajpheart.00969.2004. [DOI] [PubMed] [Google Scholar]
- 25.Ma J, Stafford RS. Screening, treatment, and control of hypertension in US private physician offices, 2003–2004. Hypertension. 2008;51(5):1275–1281. doi: 10.1161/HYPERTENSIONAHA.107.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dickinson CJ, Lawrence JR. A slowly developing pressor response to small concentrations of angiotensin. Its bearing on the pathogenesis of chronic renal hypertension. Lancet. 1963 Jun 22;:1354–1356. doi: 10.1016/s0140-6736(63)91929-9. [DOI] [PubMed] [Google Scholar]
- 27.McCubbin JW, DeMoura RS, Page IH, Olmsted F. Arterial hypertension elicited by subpressor amounts of angiotensin. Science. 1965;149:1394–1395. doi: 10.1126/science.149.3690.1394. [DOI] [PubMed] [Google Scholar]
- 28.Kawada N, Imai E, Karber A, Welch WJ, Wilcox CS. A mouse model of angiotensin II slow pressor response: role of oxidative stress. J Am Soc Nephrol. 2002;13(12):2860–2868. doi: 10.1097/01.asn.0000035087.11758.ed. [DOI] [PubMed] [Google Scholar]
- 29.Tsukamoto Y, Mano T, Sakata Y, Ohtani T, Takeda Y, Tamaki S, Omori Y, Ikeya Y, Saito Y, Ishii R, Higashimori M, Kaneko M, Miwa T, Yamamoto K, Komuro I. A novel heart failure mice model of hypertensive heart disease by angiotensin II infusion, nephrectomy, and salt loading. Am J Physiol Heart Circ Physiol. 2013;305(11):H1658–H1667. doi: 10.1152/ajpheart.00349.2013. [DOI] [PubMed] [Google Scholar]
- 30.Gandhi SK, Gainer J, King D, Brown NJ. Gender affects renal vasoconstrictor response to Ang I and Ang II. Hypertension. 1998;31(1):90–96. doi: 10.1161/01.hyp.31.1.90. [DOI] [PubMed] [Google Scholar]
- 31.Miller JA, Cherney DZ, Duncan JA, Lai V, Burns KD, Kennedy CR, Zimpelmann J, Gao W, Cattran DC, Scholey JW. Gender differences in the renal response to renin-angiotensin system blockade. J Am Soc Nephrol. 2006;17(9):2554–2560. doi: 10.1681/ASN.2005101095. [DOI] [PubMed] [Google Scholar]
- 32.Crofton JT, Share L, Brooks DP. Gonadectomy abolishes the sexual dimorphism in DOC-salt hypertension in the rat. Clin Exp Hypertens [A] 1989;11:1249–1261. doi: 10.3109/10641968909038168. [DOI] [PubMed] [Google Scholar]
- 33.Hinojosa-Laborde C, Lange DL, Haywood JR. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension. 2000;35(1 Pt 2):484–489. doi: 10.1161/01.hyp.35.1.484. [DOI] [PubMed] [Google Scholar]
- 34.Kittikulsuth W, Sullivan JC, Pollock DM. ET-1 actions in the kidney: evidence for sex differences. Br J Pharmacol. 2013;168(2):318–326. doi: 10.1111/j.1476-5381.2012.01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, Carvalho MH. Endothelin, sex and hypertension. Clin Sci (Lond) 2008;114(2):85–97. doi: 10.1042/CS20070169. [DOI] [PubMed] [Google Scholar]
- 36.Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM. Sex differences in adaptive downregulation of pre-macula densa sodium transporters with ANG II infusion in mice. Am J Physiol Renal Physiol. 2010;298(1):F187–F195. doi: 10.1152/ajprenal.00088.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13(4):469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandberg K, Ji H, Hay M. Sex-specific immune modulation of primary hypertension. Cell Immunol. 2015;294(2):95–101. doi: 10.1016/j.cellimm.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Song J, Wang S, Buggs J, Chen R, Zhang J, Wang L, Rong S, Li W, Wei J, Liu R. Cross-sex transplantation alters gene expression and enhances inflammatory response in the transplanted kidneys. Am J Physiol Renal Physiol. doi: 10.1152/ajprenal.00039.2017. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva-Antonialli MM, Tostes RCA, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MHC, Fortes ZB, Nigro D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62:587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 41.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension. 2003;41(3 Pt 2):657–662. doi: 10.1161/01.HYP.0000048193.85814.78. [DOI] [PubMed] [Google Scholar]
- 42.Tiwari S, Li L, Riazi S, Halagappa VK, Ecelbarger CM. Sex and age result in differential regulation of the renal thiazide-sensitive NaCl cotransporter and the epithelial sodium channel in angiotensin II-infused mice. Am J Nephrol. 2009;30(6):554–562. doi: 10.1159/000252776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riazi S, Madala-Halagappa VK, Hu X, Ecelbarger CA. Sex and body-type interactions in the regulation of renal sodium transporter levels, urinary excretion, and activity in lean and obese Zucker rats. Gend Med. 2006;3(4):309–327. doi: 10.1016/s1550-8579(06)80219-6. [DOI] [PubMed] [Google Scholar]
- 44.De MC, Guo C, Lund H, Feng D, Mattson DL. Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am J Physiol Renal Physiol. 2011;300(3):F734–F742. doi: 10.1152/ajprenal.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindsey SH, Yamaleyeva LM, Brosnihan KB, Gallagher PE, Chappell MC. Estrogen receptor GPR30 reduces oxidative stress and proteinuria in the salt-sensitive female mRen2.Lewis rat. Hypertension. 2011;58(4):665–671. doi: 10.1161/HYPERTENSIONAHA.111.175174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pollow DP, Uhrlaub J, Romero-Aleshire MJ, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64(2):384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dierselhuis M, Goulmy E. The relevance of minor histocompatibility antigens in solid organ transplantation. Curr Opin Organ Transplant. 2009;14(4):419–425. doi: 10.1097/MOT.0b013e32832d399c. [DOI] [PubMed] [Google Scholar]
- 49.Pabon MA, Navarro CE, Martin R, Rodriguez M, Martin I, Gaitan L, Gomez A, Lozano E. Minor histocompatibility antigens as risk factor for poor prognosis in kidney transplantation. Transplant Proc. 2011;43(9):3319–3323. doi: 10.1016/j.transproceed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 50.Miller AJ, Kiberd BA, Alwayn IP, Odutayo A, Tennankore KK. Donor-Recipient Weight and Sex Mismatch and the Risk of Graft Loss in Renal Transplantation. Clin J Am Soc Nephrol. 2017;12(4):669–676. doi: 10.2215/CJN.07660716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGee J, Magnus JH, Islam TM, Jaffe BM, Zhang R, Florman SS, Hamm LL, Mruthinti N, Sullivan K, Slakey DP. Donor-recipient gender and size mismatch affects graft success after kidney transplantation. J Am Coll Surg. 2010;210(5):718–725. doi: 10.1016/j.jamcollsurg.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs SC, Nogueira JM, Phelan MW, Bartlett ST, Cooper M. Transplant recipient renal function is donor renal mass- and recipient gender-dependent. Transpl Int. 2008;21(4):340–345. doi: 10.1111/j.1432-2277.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 53.Helwig BG, Ward JA, Blaha MD, Leon LR. Effect of intraperitoneal radiotelemetry instrumentation on voluntary wheel running and surgical recovery in mice. J Am Assoc Lab Anim Sci. 2012;51(5):600–608. [PMC free article] [PubMed] [Google Scholar]
- 54.Maranon R, Reckelhoff JF. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 2013;125(7):311–318. doi: 10.1042/CS20130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;300(2):H555–H564. doi: 10.1152/ajpheart.00847.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji H, Menini S, Zheng W, Pesce C, Wu X, Sandberg K. Role of angiotensin-converting enzyme 2 and angiotensin(1–7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp Physiol. 2008;93(5):648–657. doi: 10.1113/expphysiol.2007.041392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


