Abstract
The prognosis of acute myeloid leukemia (AML) is influenced by both disease-intrinsic and patient-related factors. In particular, AML following myelodysplastic syndrome (MDS) (AML with myelodysplasia-related changes, AML-MRC) has a poor prognosis. We hypothesized that patients with cytopenias prior to AML, but no known prior MDS, may share biologic features with AML-MRC. We evaluated 140 AML patients without prior MDS who had complete blood count (CBC) data available 6–36 months prior to their diagnosis. Cytopenia, defined as clinically unexplained thrombocytopenia or macrocytic anemia, was present in 29/140 (21%) patients. Compared to non-cytopenic patients, AML patients with prior cytopenia were older and more often met morphologic or cytogenetic criteria for AML-MRC. Prior cytopenia was associated with shorter survival in patients with intermediate-risk cytogenetics (median OS 4.2 versus 24.1 months, p < 0.0001), but not in patients with adverse-risk cytogenetics (median OS 4.4 versus 6.0 months, p = 0.57). Prior thrombocytopenia, but not macrocytic anemia, was significantly associated with shorter overall survival (p = 0.01) independent of treatment approach, karyotype risk, and age on multivariable analysis. Our data suggest that AML patients with prior cytopenias have features similar to AML-MRC, and in particular support the use of prior unexplained thrombocytopenia as an independent marker of high-risk disease.
Keywords: Acute myeloid leukemia, Myelodysplastic syndrome, Cytopenia
1. Introduction
Acute myeloid leukemia (AML) is a clonal myeloid neoplasm that is generally defined by the presence of ≥20% myeloid blasts in the peripheral blood or bone marrow. The clinical prognosis of the disease is influenced by both the intrinsic tumor biology (currently determined using the karyotype and mutational status of specific oncogenes) as well as patient-specific factors such as age and performance status. History of an antecedent myeloid neoplasm, such as a myelodysplastic syndrome (MDS), or prior exposure to cytotoxic agents, are associated with adverse prognosis, and as a result are distinguished as specific AML entities in the 2008 WHO Classification of myeloid neoplasms [1]. A substantial proportion of patients with AML have had antecedent MDS, a preleukemic clonal neoplasm characterized by cytopenia(s) and morphologic dysplasia of hematopoietic cells. Such cases are diagnosed as AML with myelodysplasia-related changes (AML-MRC), an AML subtype associated with a particularly poor prognosis [2]. This diagnosis can also be made in patients lacking a history of MDS but demonstrating MDS-associated cytogenetic abnormalities or significant morphologic dysplasia (at least 50% of cells in two or more lineages) [1,3]; however, the prognostic relevance of multilineage dysplasia alone, without an established preceding diagnosis of MDS, remains controversial [4,5]. Nevertheless, AML-MRC does appear to have a molecular mutation pattern that is distinct from that of de novo AML [6]. Patients who do not fulfill WHO criteria for AML-MRC or other defined subtypes are diagnosed with AML, not otherwise specified (AML-NOS), which captures a heterogeneous group of disorders with variable prognosis [7].
Current WHO Classification criteria require an established pathologic diagnosis of MDS in order to classify a case as AML-MRC, in the absence of specific cytogenetic abnormalities or multilineage dysplasia. However, patients typically are only diagnosed with MDS when they present with cytopenias, which then prompt a diagnostic bone marrow biopsy. Cytopenic patients with elevated MCV and red cell distribution width (RDW) are more likely to be diagnosed with MDS than patients who have normocytic anemia, thrombocytopenia, or leukopenia [8]. In patients diagnosed with MDS, macrocytic anemia is present in 80–85%, thrombocytopenia in 30–45%, and neutropenia in 40%, and therefore unexplained anemia, particularly when macrocytic, should prompt consideration of an MDS diagnosis [9]. Anemia is relatively common in elderly patients, seen in an estimated 10% of adults over 65 years, and approximately one third remain unexplained after clinical workup. Of those, approximately 15% have concomitant macrocytosis, thrombocytopenia or leukopenia, raising the possibility of an underlying MDS [10–12], which may prompt bone marrow biopsy to confirm or exclude the diagnosis [13,14]. In one retrospective series reviewing bone marrow biopsies performed for unexplained cytopenias, approximately 30% of patients were diagnosed with probable or definite MDS; the incidence of MDS in cytopenic patients who do not undergo bone marrow sampling is not known [8].
Some investigators have used persistent (>6 months) cytopenias (in addition to a pathologically confirmed MDS) as indicative of an “antecedent hematologic disorder” for the purposes of clinical trials [15]. To our knowledge, however, there are no prior studies examining the features of AML developing in patients with antecedent cytopenias who have no prior history of hematologic malignancy as a way to identify patients who may have adverse outcome similar to AML-MRC. In the current study, we hypothesized that unexplained cytopenias, defined as macrocytic anemia or prior thrombocytopenia, prior to an AML diagnosis may represent an undiagnosed MDS in many of these patients, and therefore may be associated with clinical and prognostic features similar to AML-MRC.
2. Materials and methods
We searched the pathology records from two hospitals (Massachusetts General Hospital [MGH] from 2003 to 2013 and Brigham and Women’s Hospital [BWH] from 2007 to 2012) for adult (age > 18) patients with a new diagnosis of AML. 622 (345 from BWH, 277 from MGH) patients were identified and their electronic medical records reviewed to identify patients with complete blood count (CBC) data available between 6 and 36 months prior to diagnosis. We used data from 177 de novo AML patients from MGH lacking a prior CBC as a control group. Patients with macrocytic anemia or thrombocytopenia due to Vitamin B12 or folate deficiency, aplastic anemia, liver failure, renal failure, or treatment-responsive immune thrombocytopenic purpura were excluded. For patients with CBCs performed at the time of a surgical procedure, pre-operative values were used. Patient characteristics including age, co-morbidities at the time of CBC draw, and reason for CBC testing were recorded. A prior cytopenia was defined as macrocytic anemia or thrombocytopenia (outside each hospital’s normal reference range for hematocrit, MCV, and platelet count, according to patient gender); neutropenia was not included as a criterion since WBC differentials were not available for a large proportion of patients. Clinical data at the time of AML diagnosis included CBC, ECOG performance status, and AML therapy. AML therapies were categorized as supportive care only (including blood product and growth factor support and/or hydroxyurea), low-intensity chemotherapies (including hypomethylating agents), and induction chemotherapy. Patients were also categorized according to whether they underwent allogeneic hematopoietic stem cell transplantation. Overall survival (OS) was defined as the time from AML diagnosis until patient death, with censoring at last known alive date during survival analysis.
All AML cases were diagnosed according to the 2008 WHO Classification, as well as by the FAB system when data concerning myeloperoxidase expression and monocytic features were available. Slides were reviewed for all AML cases with morphologic dysplasia described in the pathology report; these cases were classified as AML-MRC if they fulfilled WHO Classification morphologic criteria [1]. Karyotypes were reported using the International System for Human Cytogenetic Nomenclature and cytogenetic risk group stratification was based on the United Kingdom Medical Research Council trials (UKMRC) classification [16]. The presence of FLT3 and/or NPM1 mutations was recorded when available.
Fisher’s exact test and Mann–Whitney tests were used to compare categorical and continuous variables between groups, respectively. Overall survival (OS) and disease-specific survival (DSS) from diagnosis was estimated using the method of Kaplan and Meier; the log-rank test was used to compare OS between groups. For DSS, patients who died of causes unrelated to AML were censored at the time of death. Univariate Cox proportional hazards regression was performed to assess the impact of variables on OS, and values significant at the 0.20 level were included in multivariable Cox proportional hazards regression. A 2-sided p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Clinical features of patient groups
A total of 140 AML patients with prior CBC data were identified (85 from MGH and 55 from BWH). The circumstances in which the CBC was obtained were as follows: evaluation of a medical condition (52), routine primary care visit (39), surgical procedure (19), emergency room visit/trauma (10), and obstetric visit/delivery (2). The reason for the visit was unknown or undocumented for 18 patients. Overall, according to the prior CBC, 59 patients (42%) had prior anemia (including 15 [11%] with macrocytic anemia), 20 (14%) had prior thrombocytopenia, and 33 (24%) had prior leukopenia. Twenty-nine patients (21%) had macrocytic anemia and/or thrombocytopenia, and were considered the “AML with prior cytopenia group” (AML-cytopenic) for the purposes of this study. The remaining 111 patients comprised the AML-non-cytopenic group, which lacked macrocytic anemia or thrombocytopenia; this group included 20 patients with leukopenia and 33 patients with normocytic or microcytic anemia. At the time of the CBC prior to AML diagnosis, AML-cytopenic patients were older than AML-non-cytopenic patients (p = 0.001), but had no significant difference in co-morbidities that may be associated with macrocytic anemia or thrombocytopenia (Table 1). There was no significant difference in the time from prior CBC to AML diagnosis between the AML-cytopenic and AML-non-cytopenic groups (median 10 versus 12 months, respectively; p = 0.36). In addition, 177 de novo AML patients from MGH diagnosed during the same time period but lacking a prior CBC were identified as a control group. Compared to the AML-non-cytopenic patients, the AML patients without any prior CBC were younger (median age 58 versus 65 years, p = 0.007), but showed no difference in gender distribution (p = 0.33), ECOG performance status (p = 0.32), hematocrit (p = 0.85), white blood count (p = 0.33), or platelet count (p = 0.89) at the time of AML presentation.
Table 1.
Characteristics of AML patients with (AML-cytopenic) and without (AML-non-cytopenic) prior cytopenia at the time their CBC was drawn.
| AML-cytopenic (n = 29) | AML-non-cytopenic (n = 111) | p-Value | |
|---|---|---|---|
| Age at AML diagnosis, median (range) | 71 (37–93) | 65 (21–88) | 0.001 |
| Gender (M:F) | 18:11 | 56:55 | 0.30 |
| Co-morbidities that may be associated with anemia or thrombocytopenia | 6/29 | 14/111 | 0.37 |
| Cardiac valvular disease | 0 | 4 | |
| Beta thalassemia trait | 1 | 1 | |
| Chronic lymphocytic leukemia | 1 | 1 | |
| Gastrointestinal bleeding history | 2 | 1 | |
| Autoimmune disease | 0 | 4 | |
| Status post solid-organ transplant | 1 | 1 | |
| Chronic renal failure | 0 | 1 | |
| Chronic hepatitis B infection | 1 | 0 | |
| Von Willebrand disease | 0 | 1 | |
| Months between prior CBC and AML diagnosis, median (range) | 10 (6–35) | 12 (6–35) | 0.36 |
| Circumstances of visit when CBC was obtained | 0.65 | ||
| Routine primary care visit | 9 (31%) | 30 (27%) | |
| Medical condition | 10 (34%) | 42 (38%) | |
| Surgery or surgical follow-up | 3 (10%) | 16 (14%) | |
| Emergency room/trauma | 4 (14%) | 6 (5%) | |
| Obstetric visit/delivery | 1 (3%) | 1 (1%) | |
| Unknown | 2 (7%) | 15 (14%) | |
| Hematocrit, %, median (range) | 35.0 (29.3–41.5) | 40.8 (26.2–49.0) | <0.0001 |
| Mean corpuscular volume, fL, median (range) | 95 (61–126) | 90 (67–110) | 0.022 |
| Platelet count, ×109/L, median (range) | 129 (30–397) | 237 (150–685) | <0.0001 |
| White blood count, ×109/L, median (range) | 4.8 (2.3–16.5) | 6.8 (2.1–51.9a) | 0.014 |
Patient with white blood count of 51.9 × 109/L had concomitant chronic lymphocytic leukemia.
3.2. Cytogenetic and molecular genetic abnormalities
There was a significant difference in the distribution of cytogenetic abnormalities at AML diagnosis between these two groups (p = 0.02, Table 2). Cytogenetically favorable risk AML was not observed in any of the AML-cytopenic patients, but comprised 17% of the AML-non-cytopenic patients. Conversely, adverse risk karyotypes were more frequent in AML-cytopenic (38%) compared to AML-non-cytopenic (20%) patients. Chromosomal aberrations in adverse-risk cases were similar between AML-cytopenic and AML-non-cytopenic groups: −7/del(7q) (3/11 AML-cytopenic versus 10/22 AML-non-cytopenic, p = 0.46), −5/del(5q) (7/11 AML-cytopenic versus 15/22 AML-non-cytopenic, p = 1.0), complex (≥3 chromosomal abnormalities) karyotype (8/11 AML-cytopenic versus 18/22 AML-non-cytopenic, p = 0.66), and number of cytogenetic abnormalities (median 6 [range 1–12] for AML-cytopenic and median 7 [range 1–15] for AML-non-cytopenic, p = 0.50). Full karyotypes of all 29 AML-cytopenic patients are shown in Supplemental Table 1. Among those with molecular testing, 18/59 (30%) AML-non-cytopenic patients and 3/11 (27%) AML-cytopenic patients had FLT3-ITD mutations, while 18/57 (32%) AML-non-cytopenic and 5/11 (45%) AML-cytopenic patient had NPM1 mutations. There was no difference the distribution of AML cytogenetic risk groupings (p = 0.49) between AML-non-cytopenic patients and the 177 AML patients without any prior CBC.
Table 2.
Characteristics of AML patients at diagnosis based on the presence (AML-cytopenic) or absence (AML-non-cytopenic) of prior macrocytic anemia or thrombocytopenia.
| AML-cytopenic (n = 29) | AML-non-cytopenic (n = 111) | p-Value | |
|---|---|---|---|
| White blood count, ×109/L, median (range) | 2.6 (0.7–125.2) | 4.5 (0.1–268.6) | 0.15 |
| Peripheral blast %, median (range) | 2 (0–91) | 8.5 (0–98) | 0.16 |
| Hematocrit, %, median (range) | 27.9 (14.3–40.8) | 27.1 (13.1–39.1) | 0.88 |
| Mean corpuscular volume, fL, median (range) | 93.5 (67–118) | 96 (77–116) | 0.64 |
| Platelet count, ×109/L, median (range) | 60 (16–172) | 68 (7–358) | 0.26 |
| Marrow cellularity, %, median (range) | 80 (20–100) | 80 (5–100) | 0.32 |
| Marrow blast %, median (range) | 48 (9–82) | 49 (5–95) | 0.79 |
| ECOG Performance Status (0/1/2/3/4)a | 7/9/9/3/1 | 30/51/17/10/2 | 0.07(0–1 versus 2–4) |
| UKMRC risk grouping | 0.02 | ||
| Favorable | 0 (0%) | 18 (16%) | |
| Intermediate | 18 (62%) | 69 (62%) | |
| Adverse | 11 (38%) | 22 (20%) | |
| Not available | 0 | 2 (2%) | |
| WHO Classification | 0.0002 | ||
| AML-RGA | 0 (0%) | 21 (20%) | |
| t(15;17) | 0 | 9 | |
| inv(16) | 0 | 9 | |
| t(8;21) | 0 | 0 | |
| 11q23 variant | 0 | 2 | |
| t(6;9) | 0 | 1 | |
| t(3;3)/inv(3), t(1;22) | 0 | 0 | |
| AML-NOS | 10 (34%) | 58 (52%) | |
| AML-MRC | 19 (66%) | 30 (27%) | |
| AML-MRC karyotype (with or without multilineage dysplasia) | 13 | 21 | |
| Multilineage dysplasia only | 6 | 9 | |
| Not available (missing karyotype) | 0 (0%) | 2 (2%) | |
| Mutation status | |||
| FLT3 ITD mutation | 3/11 (27%) | 18/59 (31%) | 1 |
| NPM1mutation | 5/11 (45%) | 18/57 (32%) | 0.49 |
| NPM1 mutated/FLT3 wild type | 3/11 (27%) | 8/56 (14%) | 0.37 |
| FAB Classification | 0.55 | ||
| M0 | 2 (7%) | 4 (4%) | |
| M1 | 3 (10%) | 14 (13%) | |
| M2 | 6 (21%) | 17 (15%) | |
| M3 | 0 | 8 (7%) | |
| M4 | 7 (24%) | 28 (25%) | |
| M5 | 3 (10%) | 10 (9%) | |
| M6 | 2 (7%) | 4 (4%) | |
| M7 | 1 (3%) | 0 | |
| Not determined | 5 (18%) | 26 (23%) | |
| Treatments administered | 0.02 | ||
| Supportive care only | 9 (31%) | 10 (9%) | |
| Low-intensity agents without stem cell transplant | 4b (%) | 19c (%) | |
| Induction chemotherapy without stem cell transplant | 8 (28%) | 42 (38%) | |
| Stem cell transplant | 8 (28%) | 40 (36%) |
ECOG performance status unknown for one patient.
Hypomethylating agents (3 patients) and gefitinib (1 patient).
Hypomethylating agents (16 patients), cloretazine (1 patient), low-dose cytarabine (1 patient), and midostaurin with mTOR inhibitor (1 patient).
3.3. Patient survival
AML-cytopenic patients were more often treated with supportive care than AML-non-cytopenic patients (Table 2) and showed a trend to be treated less often with intensive therapy (induction chemotherapy and/or stem cell transplant, p = 0.07). These differences likely reflect the older median age of the AML-cytopenic patients, as there was no significant difference in the distribution of treatments between AML-cytopenic and AML-cytopenic patients aged >60 (p = 0.16). The median OS of all patients was 14.7 months and median DSS was 19.4 months. The association of specific factors with OS in univariate analysis is shown in Table 3. Compared to AML-non-cytopenic patients, AML-cytopenic patients had a significantly worse median OS (4.4 versus 21 months, p < 0.0001) and DSS (4.7 versus 24.7 months, p < 0.0001) (Fig. 1A and B). Regarding specific cytopenic parameters, the presence of prior anemia p = 0.008), and particularly macrocytic anemia (p = 0.0001), was associated with worsened OS. Prior thrombocytopenia alone (p = 0.002) was also associated with shorter OS, while prior leukopenia was not (p = 0.32). Other factors associated with worse OS on univariate analysis were ECOG performance status of 2–4 versus 0–1 (p < 0.0001), older age (p < 0.0001), adverse-risk karyotype (p = 0.005 versus intermediate risk), and WHO AML-MRC diagnosis (p < 0.0001). In contrast, improved OS was associated with favorable-risk karyotype (p = 0.005), treatment with low-intensity therapies (p < 0.0001) or induction therapy (p < 0.0001) versus supportive care, and treatment with stem cell transplant (p < 0.0001). There was no association between OS and peripheral blood counts (hematocrit, WBC, platelet count, or blast percentage) at the time of AML diagnosis, BM cellularity, or BM blast percentage (Table 3). There was also no association between OS and the time between prior CBC draw and AML diagnosis. There was no difference in the distribution of treatment types (p = 0.19) or in OS (median 20.2 versus 21 months, respectively, p = 0.85) between the 177 AML patients without prior CBC data and AML-non-cytopenic patients.
Table 3.
Association of variables with hazard ratios for OS in univariate analysis.
| Factor | HR | 95%CI | p-Value |
|---|---|---|---|
| Age at AML diagnosis (per year increase) | 1.047 | 1.030–1.065 | <0.0001 |
| ECOG performance status (2–4 versus 0–1) | 2.608 | 1.695–4.014 | <0.0001 |
| Cytopenias 6–36 months before AML diagnosis | |||
| Anemia | 1.772 | 1.156–2.717 | 0.008 |
| Macrocytic anemia | 6.281 | 2.478–15.913 | 0.0001 |
| Thrombocytopenia | 3.186 | 1.543–6.464 | 0.002 |
| Macrocytic anemia or thrombocytopenia | 4.098 | 2.182–7.698 | <0.0001 |
| Leukopenia | 0.786 | 0.486–1.270 | 0.32 |
| Counts at AML diagnosis | |||
| Hematocrit | 1.025 | 0.988–1.064 | 0.18 |
| Platelet count | 0.998 | 0.995–1.001 | 0.22 |
| White blood count | 1.002 | 0.998–1.006 | 0.26 |
| Peripheral blood blast percentage | 0.999 | 0.992–1.006 | 0.77 |
| Bone marrow blast percentage | 0.997 | 0.988–1.006 | 0.52 |
| Bone marrow cellularity | 0.999 | 0.990–1.007 | 0.77 |
| AML karyotype risk group (versus intermediate) | |||
| Favorable | 0.235 | 0.085–0.650 | 0.005 |
| Adverse | 2.016 | 1.283–3.170 | 0.002 |
| AML with myelodysplasia-related changes | 2.43 | 1.600–3.691 | <0.0001 |
| NPM1/FLT3 mutation status | |||
| FLT3 ITD mutation | 1.042 | 0.710–2.965 | 0.31 |
| NPM1 mutation | 1.253 | 0.643–2.442 | 0.51 |
| NPM1 mutated/FLT3 wild-type | 1.552 | 0.713–3.380 | 0.27 |
| Type of AML therapy (versus supportive care) | |||
| Low-intensity agents | 0.156 | 0.079–0.308 | <0.0001 |
| Induction chemotherapy | 0.048 | 0.025–0.092 | <0.0001 |
| Treatment with stem cell transplant | 0.384 | 0.239–0.617 | <0.0001 |
Fig. 1.
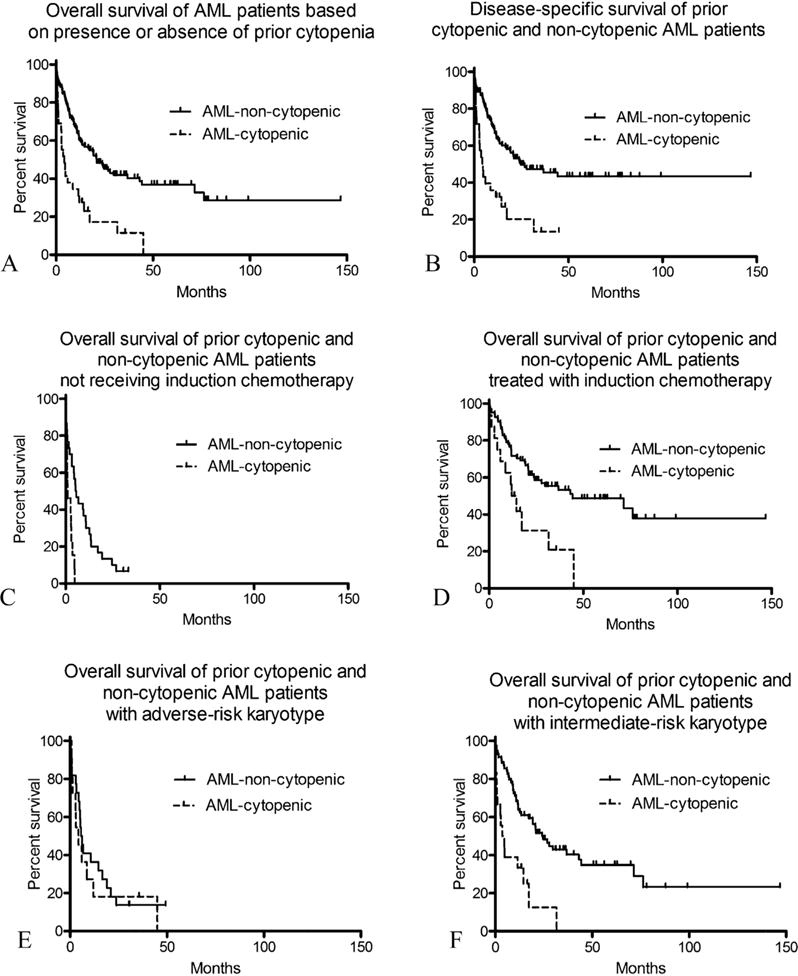
Comparison of outcome of AML patients with prior cytopenia compared to AML patients without prior cytopenia. (A) Overall survival (OS) of AML-cytopenic patients (n = 29) is shorter than that of AML-non-cytopenic patients (n = 111) (p < 0.0001). (B) Disease-specific survival (DSS) of AML-cytopenic patients is significantly shorter that of AML-non-cytopenic patients (p < 0.0001). (C) Among patients not receiving induction chemotherapy, OS of AML-cytopenic patients (n = 13) is significantly shorter than that of AML-non-cytopenic patients (n = 29) (p = 0.0003). (D) Among patients treated with induction chemotherapy (with or without subsequent stem cell transplant), OS of AML-cytopenic patients (n = 16) is significantly shorter than that of AML-non-cytopenic patients (n = 82) (p = 0.004). (E) Among patients with UKMRC adverse-risk karyotype, there is no significant difference in OS between AML-cytopenic patients (n = 11) and AML-non-cytopenic patients (n = 22) (p = 0.57). (F) Among patients with UKRMC intermediate-risk karyotype, OS of AML-cytopenic patients (n = 18) is significantly shorter than that of AML-non-cytopenic patients (n = 69) (p < 0.0001).
AML-cytopenic patients had an inferior OS compared to AML-non-cytopenic patients both for those receiving induction chemotherapy (median OS 13.3 months versus 44.3 months for AML-non-cytopenic, p = 0.004) (Fig. 1D), as well as for those who were not treated with induction chemotherapy (median OS 1.1 versus 5.6 months, p = 0.0004) (Fig. 1C). Among adverse cytogenetic risk patients, there was no difference in OS between AML-cytopenic patients (median 4.4 months) and AML-non-cytopenic patients (median 6.0 months, p = 0.57) (Fig. 1E); however, among intermediate cytogenetic risk patients, AML-cytopenic patients had a much shorter OS (median 4.2 months) compared to AML-non-cytopenic patients (median 24.1 months, p < 0.0001) (Fig. 1F).
Stepwise multivariable Cox regression analysis was performed including variables significant to the p < 0.20 level in univariate analysis (age, ECOG performance status, prior cytopenia, UKMRC cytogenetic risk group, WHO diagnosis of AML-MRC, treatment groups [induction chemotherapy, and low-intensity therapies versus supportive care, and stem cell transplant]). Because favorable-risk karyotype patients are known to have markedly superior survival (and all were in the non-cytopenic group), these 18 patients were excluded from the multivariable analysis. Adverse-risk karyotype (p = 0.003, HR 2.062 [1.274–3.337]) and prior cytopenia (p = 0.02, HR 1.927 [1.115–3.329]) were independently associated with shorter OS, while treatment with stem cell transplant (p < 0.0001, HR 0.349 [0.197–0.617]) and use of induction chemotherapy (p < 0.0001, HR 0.140 [0.059–0.331]) or lower intensity therapies (p < 0.0001, HR 0.212 [0.100–0.450]) compared to supportive care were associated with longer OS; neither age nor ECOG performance status were independently associated with OS in this model. However, when we analyzed prior thrombocytopenia and macrocytic anemia separately in the multivariable analysis, prior thrombocytopenia, but not macrocytic anemia, was independently associated with inferior OS (Table 4); in this final model, older age also had a borderline association with shorter OS.
Table 4.
Factors independently influencing OS in multivariable analysis.
| Factor | HR | 95% CI | p-Value |
|---|---|---|---|
| Prior thrombocytopenia | 2.184 | 1.208–3.951 | 0.01 |
| Age (per year increase) | 1.018 | 0.996–1.040 | 0.12 |
| UKMRC adverse karyotype (versus intermediate) | 1.942 | 1.193–3.161 | 0.008 |
| Treatment with low-intensity agents (versus supportive care only) | 0.186 | 0.090–0.385 | <0.0001 |
| Treatment with induction chemotherapy (versus supportive care only) | 0.138 | 0.058–0.326 | <0.0001 |
| Treatment with stem cell transplant | 0.331 | 0.186–0.591 | <0.0001 |
4. Discussion
In this study we evaluated whether patients with AML who have prior cytopenias without an established diagnosis of MDS have a worsened clinical prognosis, and therefore whether prior cytopenia may carry prognostic significance. Because macrocytic anemia and thrombocytopenia are common in patients who are diagnosed with MDS, we used these to define a group of AML patients who had prior unexplained cytopenia [9]. In our patient cohort, we found that prior macrocytic anemia as well as prior thrombocytopenia were associated with adverse outcome. Our data suggest that prior macrocytic anemia and/or thrombocytopenia define a group of AML patients with adverse outcomes similar to those patients with AML and prior MDS or to patients with adverse-risk cytogenetics. Estey et al. also examined the significance of cytopenias prior to diagnoses of AML and MDS with excess blasts, and similarly found that patients with cytopenias prior to diagnosis had an inferior prognosis [15]. In comparison to the Estey et al. study, we focused more narrowly on thrombocytopenia and macrocytic anemia and used a stricter cut-off of six months prior to diagnosis. Neutropenia is also present in many patients who are diagnosed with MDS, but because many of the patients in our series had had no WBC differential, neutropenia was not included as a criterion. Prior leukopenia was not associated with adverse outcome in our cohort.
Overall, 20.2% (29/129) of the patients had macrocytic anemia and/or thrombocytopenia preceding their AML diagnosis. The patients with and without prior cytopenias had similar bone marrow cellularity, blast counts, and peripheral blood counts at the time of AML presentation. Although the patients with prior cytopenias were older than those without cytopenia, both groups had similar co-morbidities that could potentially cause anemia or thrombocytopenia, suggesting that the cytopenias were not due to other diseases. A subset of patients in both groups had prior malignancies (melanoma, breast, urothelial, lung, vulvar, laryngeal, and prostate carcinomas and chronic lymphocytic leukemia), but none had been treated with cytotoxic therapies or with radiation therapy that exposed hematopoietic marrow, and hence would not be considered therapy-related AML. Aside from younger age, we found no differences between demographics, cytogenetic risk grouping, treatment approach, or outcome between a comparison group of patients who presented with AML but had no prior CBC and those who had a prior non-cytopenic CBC, suggesting that this is a representative group of AML patients despite having had a CBC drawn prior their AML diagnosis.
Strikingly, none of the patients who presented with AML with recurrent genetic abnormalities (AML-RGA) had prior cytopenia; since these genetic types of AML occur more commonly in younger patients, we cannot absolutely exclude the possibility that this reflects the older age of the cytopenic group. AML with t(15;17), inv(16), t(8;21), and MLL rearrangements have relatively few recurrent mutations compared with other AML cytogenetic subgroups, which may indicate fewer genetic events required for leukemogenesis in these AML subtypes and thus potentially a shorter time to develop leukemia from normal marrow [17]. The fact that the AML-RGA patients in our study all lacked significant cytopenias prior to their AML diagnosis as well as the observation that the AML-RGA subtypes inv(16), t(8;21), and t(9;11) almost never occur after a prior diagnosis of bona fide MDS [18] support the notion that AML-RGA develops rapidly from a functionally normal marrow. Conversely, prior cytopenia was common in AML with adverse karyotypes and in AML-MRC, suggesting an undiagnosed prior MDS may have been present in at least a subset of these cases. Nonetheless, a significant number of AML cases with high-risk or complex karyotypes arose in patients without a prior identifiable cytopenic prodrome, including patients with a documented normal CBC as recently as 6 months prior to their AML diagnosis. Moreover, among patients with adverse risk cytogenetics, patients with and without a prior cytopenia had a similar proportion of complex karyotypes and number of independent karyotypic abnormalities. Thus, the genetic events that underlie complex-karyotype AML may develop without a cytopenic prodrome. It is uncertain whether the lack of cytopenia in this patient subgroup reflects a prior normal marrow with rapid transition to AML, or an abnormal precursor state that has effective hematopoiesis without causing cytopenias.
Prior cytopenia did not have any effect on the prognosis of patients with adverse risk cytogenetics, which was overall poor. However, among patients with intermediate-risk karyotype, patients with prior cytopenia had a significantly poorer outcome than patients without prior cytopenia, and more closely approximated those patients with adverse-risk disease. Overall, 18 patients with an intermediate-risk karyotype had cytopenia preceding their diagnosis of AML, and only 8 of these patients were classified as AML-MRC (6 based on morphologic dysplasia criteria and 2 based on AML-MRC-defining karyotype). In multivariable analysis, prior thrombocytopenia, but not macrocytic anemia, along with cytogenetic risk, was an independent prognostic risk factor for OS. An AML-MRC designation was not independently associated with survival, suggesting that prior thrombocytopenia may be more important than morphologic dysplasia in identifying high-risk AML in patients with normal karyotype and no antecedent hematologic malignancy. Although prior macrocytic anemia was strongly associated with shorter OS in univariate analysis, it was not a significant factor in multivariable analysis, suggesting that its adverse influence on outcome may be captured by cytogenetic risk grouping.
Our study was limited by its retrospective nature: we cannot with certainty infer that the prior cytopenias indicated a clonal bone marrow abnormality, nor could we absolutely exclude cytopenias that may have been caused by extrinsic factors in some patients. In order to mitigate the latter possibility, we used a control group of patients who had record of prior CBCs drawn in a similar time frame to the cytopenic patients, but did not show macrocytic anemia or thrombocytopenia. This control group encompassed patients receiving medical care for a similar range of conditions as the cytopenic patient group; we excluded patient who had been exposed to any prior cytotoxic chemotherapy or radiotherapy from both groups. The fact that the adverse prognosis observed in the prior cytopenia group was independent of patient age, comorbidities, or treatment with induction chemotherapy suggests that prior cytopenia identifies a more aggressive disease, rather than merely a more morbid patient group. Since we included patients diagnosed prior to 2008 in this study, many did not have available FLT3, NPM1, or CEBPA mutation analysis and we could not include these variables in the multivariate analysis. Among the patients tested, we did find NPM1 mutation in a subset of the AML patients with prior cytopenia. Although usually associated with de novo AML, NPM1 mutation can also occur in secondary AMLs and in such cases is associated with a mutational profile that differs from de novo NPM1-mutated AML [19]. Molecular analysis may eventually be able to define MDS-associated features more effectively than either morphology or prior cytopenia [18]; however, expanded mutational analyses are not widely available in most centers. In the absence of such testing, cytopenia preceding a diagnosis of AML appears to be a helpful marker that suggests MDS-related disease. Of note, several recent studies have found that recurrent AML- and MDS-associated mutations occur in the blood cells of apparently healthy individuals and are associated with increased risk of subsequent hematologic malignancies, including AML [20,21]. It would be of interest to determine whether cytopenias identify a subgroup of patients with such mutations who manifest ineffective hematopoiesis and thus may be at the highest risk for developing AML or other hematologic malignancies.
In conclusion, we found that AML in patients with preceding (≥6 months before AML diagnosis) macrocytic anemia or thrombocytopenia is associated with shorter OS. Prior thrombocytopenia was an independent prognostic risk factor for worse OS in multivariable analysis. Macrocytic anemia was associated with adverse outcome in univariate, but not multivariable analysis. These data suggest that documented prior unexplained macrocytic anemia or thrombocytopenia may be used to identify high-risk AML with features similar to MDS-related AML. Prior cytopenias may suggest a longer period of disease evolution, associated with greater genetic complexity, which has been associated with therapy resistance and aggressive behavior in AML [22]. Future studies are needed to determine whether genetic events in AML following cytopenia are similar to those that follow a documented diagnosis of MDS.
Supplementary Material
Acknowledgments
We thank Stephen Conley for his assistance with the figure.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.leukres.2015.06.012
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–2088. [PubMed] [Google Scholar]
- 3.Weinberg OK, Seetharam M, Ren L, Seo K, Ma L, Merker JD, et al. Clinical characterization of acute myeloid leukemia with myelodysplasia-related changes as defined by the 2008 WHO classification system. Blood. 2009;113(9):1906–1908. doi: 10.1182/blood-2008-10-182782. [DOI] [PubMed] [Google Scholar]
- 4.Wandt H, Schäkel U, Kroschinsky F, Prange-Krex G, Mohr B, Thiede C, et al. MLD according to the WHO classification in AML has no correlation with age and no independent prognostic relevance as analyzed in 1766 patients. Blood. 2008;111(4):1855–1861. doi: 10.1182/blood-2007-08-101162. [DOI] [PubMed] [Google Scholar]
- 5.Miesner M, Haferlach C, Bacher U, Weiss T, Macijewski K, Kohlmann A, et al. Multilineage dysplasia (MLD) in acute myeloid leukemia (AML) correlates with MDS-related cytogenetic abnormalities and a prior history of MDS or MDS/MPN but has no independent prognostic relevance: a comparison of 408 cases classified as “AML not otherwise specified” (AML-NOS) or “AML with myelodysplasia-related changes” (AML-MRC) Blood. 2010;116(15):2742–2751. doi: 10.1182/blood-2010-04-279794. [DOI] [PubMed] [Google Scholar]
- 6.Devillier R, Gelsi-Boyer V, Brecqueville M, Carbuccia N, Murati A, Vey N, et al. Acute myeloid leukemia with myelodysplasia-related changes are characterized by a specific molecular pattern with high frequency of ASXL1 mutations. Am J Hematol. 2012;87(7):659–662. doi: 10.1002/ajh.23211. [DOI] [PubMed] [Google Scholar]
- 7.Lahortiga I, Cools J. New opportunities and new problems for acute myeloid leukemia treatment. Haematologica. 2012;97(6):796. doi: 10.3324/haematol.2012.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckstein R, Jang K, Friedlich J, Zhang L, Reis M, Chesney A, et al. Estimating the prevalence of myelodysplastic syndromes in patients with unexplained cytopenias: a retrospective study of 322 bone marrows. Leuk Res. 2009;33(10):1313–1318. doi: 10.1016/j.leukres.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Foran JM, Shammo JM. Clinical presentation, diagnosis, and prognosis of myelodysplastic syndromes. Am J Med. 2012;125(7 Suppl):S6–S13. doi: 10.1016/j.amjmed.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Woodman R, Ferrucci L, Guralnik J. Anemia in older adults [Miscellaneous article] Curr Opin Hematol. 2005 Mar;12(2):123–128. doi: 10.1097/01.moh.0000154030.13020.85. [DOI] [PubMed] [Google Scholar]
- 11.Eisenstaedt R, Penninx BWJH, Woodman RC. Anemia in the elderly: current understanding and emerging concepts. Blood Rev. 2006;20(4):213–226. doi: 10.1016/j.blre.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 13.Khan AM. Why are myelodysplastic syndromes unrecognized and underdiagnosed? A primary care perspective. Am J Med. 2012;125(7):S15–S17. doi: 10.1016/j.amjmed.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 14.Makipour S, Kanapuru B, Ershler WB. Unexplained anemia in the elderly, Semin. Hematol. 2008;45(4):250–254. doi: 10.1053/j.seminhematol.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estey E, Thall P, Beran M, Kantarjian H, Pierce S, Keating M. Effect of diagnosis (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or acute myeloid leukemia [AML]) on outcome of AML-type chemotherapy. Blood. 1997;90(8):2969–2977. [PubMed] [Google Scholar]
- 16.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2014 doi: 10.1182/blood-2014-11-610543. http://dx.doi.org/10.1182/blood-2014-11-610543 e-pub ahead of print 30 December 2014. [DOI] [PMC free article] [PubMed]
- 19.Schnittger S, Haferlach C, Nadarajah N, Alpermann T, Meggendorfer M, Perglerová K, et al. In AML secondary to MDS NPM1 mutations are late events, less frequent, and associated with a different pattern of molecular mutations than in de novo AML. Blood. 2014;124(21) abstract 700. [Google Scholar]
- 20.Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rücker FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119(9):2114–2121. doi: 10.1182/blood-2011-08-375758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


