Abstract
The process of tissue morphogenesis, especially for tissues reliant on the establishment of a specific cytoarchitecture for their functionality, depends a balanced interplay between cytoskeletal elements and their interactions with cell adhesion molecules. The microtubule cytoskeleton, which has many roles in the cell, is a determinant of directional cell migration, a process that underlies many aspects of development. We investigated the role of microtubules in development of the lens, a tissue where cell elongation underlies morphogenesis. Our studies with the microtubule depolymerizing agent nocodazole revealed an essential function for the acetylated population of stable microtubules in the elongation of lens fiber cells, which was linked to their regulation of the activation state of myosin. Suppressing myosin activation with the inhibitor blebbistatin could attenuate the loss of acetylated microtubules by nocodazole and rescue the effect of this microtubule depolymerization agent on both fiber cell elongation and lens integrity. Our results also suggest that acetylated microtubules impact lens morphogenesis through their interaction with N-cadherin junctions, with which they specifically associate in the region where lens fiber cell elongate. Disruption of the stable microtubule network increased N-cadherin junctional organization along lateral borders of differentiating lens fiber cells, which was prevented by suppression of myosin activity. These results reveal a role for the stable microtubule population in lens fiber cell elongation, acting in tandem with N-cadherin cell-cell junctions and the actomyosin network, giving insight into the cooperative role these systems play in tissue morphogenesis.
Keywords: lens, microtubules, acetylation, N-cadherin, myosin, morphogenesis
Graphical Abstract

INTRODUCTION
The formation of tissues during embryonic development involves highly coordinated spatiotemporal interactions of the component cells that are dependent on dynamic changes in cell-cell junctions and the cytoskeleton. The microtubule cytoskeleton has been implicated, together with actin-myosin cytoskeletal filaments, in determining cell-shape changes, establishing cell polarity, and directing cellular movements, processes considered central to generating a tissue’s cytoarchitecture (1–8). Much of what we know about the function of microtubules in development comes from studies of axon extension and guidance (9,10). However, there is still much to learn about the role of microtubules in determining tissue morphogenesis, particularly their function in coordinating how differentiating cells become organized into highly-ordered structures. The developing lens is ideal for investigating microtubule function in tissue morphogenesis. Lens formation is governed by the extensive, directional elongation of its differentiating fiber cells, the unique cell-type that predominates lens tissue, and the formation of complex lateral interactions as they elongate. Fiber cell extension and elongation is dependent on the movement of their apical tips along the anterior surfaces of the overlying undifferentiated lens epithelium in an N-cadherin-dependent manner (11), which is coordinated with movement of their basal surfaces along the posterior basement membrane capsule that surrounds the lens, a region rich in integrin matrix receptors. These morphogenetic movements require dynamic creation and remodeling of cell-cell and cell-matrix adhesions by the lens fiber cells as they differentiate (12–21). In this study, we investigated how microtubules and their stabilization function in regulation of lens fiber cell elongation and the directionality of movement to impact tissue morphogenesis.
Microtubules are multifunctional cytoskeletal structures that have many well-characterized roles in the cell including as determinants of cell division, as the highways for vesicle transport, in the positioning and movement of cellular organelles, and as determinants of the directionality of cell migration (22–24). They are polarized filaments comprised of α- and β-tubulin heterodimers. While dynamic microtubules rapidly interconvert between polymerized and depolymerized states (25–27), microtubules are stabilized by their association with Microtubule Associated Proteins (MAPs) (9,28–34) and by post-translational modifications (PTMs) of tubulin (35–39), including tubulin acetylation. The stabilization of microtubules by tubulin acetylation is implicated in orienting cells and providing directionality to migration (9,27,33,38,40–44). Microtubules can also be stabilized by tubulin detyrosination (37,44,45). In addition, the association of microtubule tip proteins, like EB1, with the microtubule plus (+) end, the growing end of the filament, are linked to downstream signaling events that impact microtubule post-translational modifications, microtubule stability and apical positioning (46).
Microtubules can influence cell movement by coordinating the function other cytoskeletal elements of the cell. They maintain the polarized distribution of actin-dependent protrusions at the leading edge of motile cells (47) and can stimulate activation of Rac1, which induces formation of the branched actin network that underlies the lamellipodial protrusions necessary for cellular movement (42,48). Microtubules not only interact with the actin cytoskeleton but also with the actomyosin machinery, and microtubule acetylation and stabilization is known to regulate cellular contractility in migrating systems (41). In addition, microtubule interactions with cell-cell junctional proteins are becoming recognized for their role in regulating cellular polarization and movement (49).
In the lens, cytoarchitecture depends greatly on cytoskeletal signaling networks and their association with cell-cell junctions (2,15,50–53). Early work with lens epithelial cell cultures suggested a role for microtubules in their elongation (54,55). However, despite this early insight, how microtubules function in lens fiber cell elongation and lens morphogenesis remains unknown. To enhance our understanding of the role that microtubules play in tissue morphogenesis, we investigated the impact of microtubule stability on lens development and the link between these microtubules, myosin activation, and N-cadherin junctions in the lens morphogenetic process.
MATERIALS AND METHODS
Lens microdissection
Lenses were isolated from chicken embryos (B&E Eggs, York Springs, PA; Poultry Futures) at embryonic day 10 and microdissected into epithelial (E), cortical fiber (FP), and nuclear fiber (FC) zones as previously described (19).
Preparation of ex vivo whole lens organ cultures
E10 lenses were placed in Complete Medium (Medium 199 [Life Technologies, 11150–059] with 10% fetal bovine serum, 1% penicillin and 1% streptomycin) at 37 °C as described previously (56). After two hours in culture isolated lenses free of opacities were exposed to inhibitors or the vehicle control DMSO (0.1% Me2SO; Sigma, 276855) for 24 h. Inhibitor treatments included nocodazole (10ng/ml, 1μg/ml, 10μg/ml; Sigma Aldrich) or colchicine (5μM, 10μM; Millipore Sigma), both of which act by inducing microtubule depolymerization, and/or the myosin inhibitor Blebbistatin (50μM, Sigma).
Antibodies
Primary antibodies used in these studies include N-cadherin (NCD-2) (Invitrogen, 13–2100), N-cadherin (BD Transduction Laboratories, 610921), myosin II (LifeSpan BioSciences, Inc., LS-C84042), phospho-myosin light chain 2 Thr18/Ser19 (Cell Signaling, 3674), α-tubulin (abcam, ab18251), acetylated tubulin (Sigma-Aldrich, T6793), EB1 (MAPRE1) (abcam, ab50188), and β-catenin (BD Biosciences, 610154).
Immunoblotting
Lens samples were extracted in Triton (1% Triton X-100, 100mM NaCl, 1mM MgCl2, 5mM EDTA, 10mM imidazole, pH 7.4), Triton/Octylglucoside (OG) (44.4mM n-Octyl β-D glucopyranoside, 1% Triton X-100, 100mM NaCl, 1mM MgCl2, 5mM EDTA, 10mM imidazole, pH 7.4), or RIPA (5mM EDTA, 150mM NaCl, 1% NP40, 0.1% Sodium deoxycholate, 0.1% SDS, 50mM Tris-HCl, pH 7.4) buffers, as specified in each study. Each extraction buffer contained 1mM sodium vanadate, 0.2mM H2O2, Protease/Phosphatase Inhibitor Cocktail (Cell Signaling, 5872S and Halt™ Phosphatase Inhibitor Cocktail (Thermo Scientific, 1862495). The concentration of protein was determined using the BCA assay (Thermo Scientific, 23223, 23224). 15 μg of protein extracts were subjected to SDS-PAGE on precast 8–16% tris/glycine gels (Invitrogen, EC6045BOX). Proteins were electrophoretically transferred onto Immobilon-P membranes (Millipore Corp., IPVH00010). Membranes were blocked in 5% skim milk or 5% BSA for 1 h and probed for primary antibodies at 4 °C overnight followed by secondary antibodies conjugated to horseradish peroxidase (BIO-RAD, 170–6515, 170–6516). Protein bands were detected using ECL reagent or ECL plus reagent (Thermo Fisher Scientific Inc. Rockford, 32106, 80197) and images were acquired using the FluorChem E & M imager from Protein Simple (FM0418). The FluorChem E & M imager is a digital darkroom technology with an 8.3 megapixel CCD (charge-coupled device) resolution and flat field calibration that corrects for non-uniformities in light gathering.
Immunoprecipitation
For these studies the E, FP, and the FC zones were isolated from 25 E10 lenses by microdissection and extracted with OG/T buffer. To determine association of total and acetylated tubulin with N-cadherin the entire E, FP, and FC fractions were immunoprecipitated with primary antibody to N-cadherin (4°C overnight), followed by incubation with TrueBlot immunoprecipitation beads from eBiosciences (00-8800-25) for 1 h. The immunoprecipitates and whole cell lysates were subjected to SDS-PAGE, transferred to Immobilon-P membranes and the association of N-cadherin with α-tubulin and acetylated tubulin determined using western blot techniques as described above. For co-immunoprecipitation studies the membrane was cut into molecular weight regions for analysis of both immunoprecipitated and co-immunoprecipitated proteins on the same membrane.
Immunostaining
Freshly isolated lenses and lenses from organ culture were fixed in 3.7% formaldehyde, cryopreserved in 30% sucrose, and prepared for cryosectioning. 20-μm thick sections were cut. Samples were then permeabilized with 0.25% Triton X-100 in DPBS buffer (2.7 mM KCl, 1.5 mM KH2PO4, 137.9 mM NaCl, 8.1 mM Na2HPO4–7 H2O [Corning, 21-0310CV]) for 12 min, incubated in blocking buffer (5% goat serum, 0.5 g BSA in 50 ml DPBS) for 1 h, and then incubated sequentially in primary antibody (3 h or overnight at 37 °C), followed by a fluorescent-conjugated secondary antibody for 2 h (Jackson ImmunoResearch Laboratories, 111-295-144, 115-545-003, 115-295-008). F-actin was localized with Alexa448 or 633-conjugated phalloidin (Invitrogen-Molecular Probes, A12379). Nuclei were counterstained with TO-PRO-3 (Invitrogen-Molecular Probes, T3605). All sections were cut serially in the anterior to posterior direction.
Image analysis
Confocal microscopy was performed using either the Zeiss LSM510 META or Zeiss LSM800 confocal microscope. Single optical planes were selected from z-stacks, each 1-μm thick, unless otherwise indicated, using the LSM5 Image Browser or ZEN blue software. For quantification of immunostaining results ImageJ Analysis Software was used to import Zeiss LSM510META confocal microscope images. Representative areas measuring 200μm × 200μm from both the epithelium and fiber cell zones were outlined to generate pixel intensity value plots from which image histogram readouts were generated.
N-cadherin lens-specific conditional knockout mice
The N-cadherin MLR10 lens-specific conditional knockout mouse (N-cadΔlens) was generated and characterized in a previous study (11).
RESULTS
Differentiating Lens Fiber Cells are Rich in Microtubules Stabilized by Acetylation
Despite early research into the potential of microtubules as a determinant of lens cell elongation (54,55), very little is understood about the role of microtubules in lens fiber cell elongation and how this cytoskeletal element impacts lens morphogenesis. We began our studies by determining the localization, organization, and stability of microtubules in the embryonic chick lens as a factor of lens cell differentiation state. Immunolabeling of chick embryo lens sections at embryonic day (E)10 for α-tubulin, a protein component of all microtubules, showed that microtubules were abundantly expressed throughout the developing lens, in both lens epithelial cells and differentiating lens fiber cells, and were distributed from the basal to the apical aspects of these cells (Fig. 1A). Microtubules were most highly concentrated in the cells of the lens equatorial epithelium (Fig. 1A, arrowhead) and the opposing newly differentiating secondary fiber cells (Fig. 1A, arrow). These microtubule-rich lens cell populations span the lens fulcrum, where epithelial cells transition to lens fiber cells, and extend along the region of the lens where the elongating lens fiber cells first interact with the adjacent lens epithelial cells (Fig, 1A,C), along which they migrate as they elongate (11).
FIGURE 1.
Acetylated Microtubules are Enriched in Differentiating Lens Fiber Cells. Cryosections of E10 chick embryo lenses were immunostained for α-tubulin (A,C), acetylated-tubulin (B,C,D,F) and the microtubule plus-end tracking protein EB1 (E,F). Microtubules were present throughout the developing lens, most highly localized to equatorial epithelial (A, arrowhead) and differentiating fiber cells (A, arrow). Acetylated microtubules were most concentrated in the apical aspects of lens equatorial epithelial cells (B, arrowhead) and newly differentiated fiber cells (B, arrow). EB1 co-localized with acetylated stable microtubules throughout the lens transition zone (D–F), suggesting that microtubules in both lens epithelial cells (E, arrowhead) and differentiating lens fiber cells (E, arrow) grew in the direction of the cells apical domains. (Mag bar=20μm; n=3; images shown are projections of Z-stacks acquired by confocal microscopy imaging)
Microtubules are dynamic structures that rapidly polymerize and depolymerize from their (+) ends. The elongation of microtubules is facilitated by microtubule plus-end tracking proteins (+TIPs) such as the end-binding (EB) protein, EB1 (57,58), which can regulate microtubule dynamics and promote microtubule acetylation (46,59). Immunolabeling for EB1 in the E10 lens showed that it is most highly localized to the more anterior regions of both lens equatorial epithelial cells (Fig. 1E, arrowhead) and differentiating fiber cells (Fig. 1E, arrow), indicating that the microtubules in the developing lens grow primarily in the direction of the cells’ apical domains (Fig. 1E,F).
Although microtubules are characterized by their dynamic instability (60), subpopulations of microtubules can selectively acquire PTMs to become stable populations with resistance to turnover (61–63). One of the most well-documented PTMs for conveying stability to microtubules is acetylation of tubulin at lysine residue 40 (40). The polarized regulation of microtubule dynamics and stability in motile cells can convey directionality of movement (27,64). Immunofluorescence analysis of the acetylation of the microtubule population in the developing lens provided insight into the potential role of microtubule stability in the lens morphogenetic process. Stable, acetylated tubulin populations were found throughout the developing lens (Fig 1B,C,D,F), but were most prominent at the apical aspects of lens equatorial epithelial cells (Fig. 1B, arrowhead, and 1D) and newly differentiating fiber cells (Fig. 1B, arrow, and 1D). These acetylated tubulin populations were localized in close proximity to where the apical surfaces of the fiber cells contact the apical surfaces of the overlying epithelium (the Epithelial Fiber Cell Interface (EFI)) (Fig. 1B,C,D,F), along which the fiber cells migrate as they elongate (11). It is therefore highly likely that the stabilized microtubule population, often associated with guidance and directionality of migration (27), is important in establishing the migration and elongation of new fiber cells. Thus, we investigated the potential role of acetylated microtubules in regulating lens fiber cell elongation and lens morphogenesis.
Tubulin Acetylation Suppressed Following Exposure of Embryonic Lenses to the Microtubule Depolymerization Drug Nocodazole
The role of microtubules in cellular processes is typically investigated by exposure of cells to the microtubule depolymerization drug nocodazole. Prior to using this drug to investigate the role of stable, acetylated microtubules in lens morphogenesis, we examined the efficacy of moderate (1μg/ml) and high (10μg/ml) doses of nocodazole for suppression of tubulin acetylation. E10 chick embryo lenses were exposed to nocodazole for 24 hr in whole lens ex vivo culture, with control lenses exposed to the vehicle DMSO, and microdissected into differentiation-state specific fractions to provide isolated lens epithelium (E), newly differentiating cortical fiber cells from the lens periphery (FP), and differentiated fiber cells from the lens nucleus (FC) for analysis (modeled in Fig. 2A). These microdissected lens fractions were western blotted for both acetylated tubulin and α-tubulin (Fig. 2B), the levels of acetylated and α-tubulin quantified (Figs. 2C and D, respectively), and the ratio of acetylated-tubulin/α-tubulin determined (Fig. 2E). The results showed that both moderate and high doses of nocodazole caused a significant decrease in tubulin acetylation in lens cells at all stages of differentiation (Fig 2E), and therefore the loss of stable microtubule populations in these cells. While we do not know the specific concentration of nocodazole that reaches each differentiation-state specific zone of the lenses exposed to this inhibitor in whole lens culture, these results show similar effects of nocodazole on tubulin acetylation throughout each region of the lens (Fig. 2B,C).
FIGURE 2.
Differentiation-State Specific Analysis of Effects of Nocodazole Exposure on Tubulin Acetylation. E10 chick embryo lenses grown ex vivo for 24 hrs in whole lens culture in the presence of the vehicle DMSO, 1μg/ml nocodazole, or 10μg/ml nocodazole were microdissected to separate lens epithelial cells (E), cortical fiber cells (FP) and central fiber cells (FC) (modeled in A), and these differentiation-state specific fractions were immunoblotted for both α-tubulin and acetylated-tubulin (B). Protein bands were quantified from the acetylated-tubulin (C) and α-tubulin (D) immunoblots, and the ratio determined of acetylated tubulin to α-tubulin (E). Both 1μg/ml and 10μg/ml nocodazole caused a significant decrease in tubulin acetylation in all regions of the developing lens. (p<0.05*; p<0.01**; p<0.001***; n=5)
Acetylated Microtubules are Specifically Linked to N-cadherin Junctions in Elongating Lens Fiber Cells
The cell-cell junctional receptor N-cadherin localizes along apical and lateral cell interfaces of lens epithelial and fiber cells (11,15). Our previous studies reveal a requisite role for apical N-cadherin junctions in the lens fiber cell elongation process through their role in mediating the migration of the apical tips of newly differentiating lens fiber cells along the apical surfaces of the overlying epithelium (11). While N-cadherin junctions are best known for their linkage to and stabilization by the actin cytoskeleton (65–68), cadherin junctions also have been shown to interact with microtubules, particularly in collectively migrating cells (69,70). We examined whether there was differentiation-state specificity to microtubule association with N-cadherin junctions in the developing lens, and how linkage between microtubules and N-cadherin was impacted by exposure to nocodazole. E10 lenses were exposed for 24 hrs in ex vivo culture to the vehicle DMSO to determine normal N-cadherin/microtubule associations, or to nocodazole at either 1μg/ml or 10μg/ml, and microdissected into the differentiation-state specific lens fractions of undifferentiated lens epithelial cells (E), elongating cortical lens fiber cells (FP), and differentiated central lens fiber cells (FC) prior to analysis (Fig. 3A). Each fraction was immunoprecipitated for N-cadherin and immunoblotted for N-cadherin, α-tubulin (total microtubule population), and acetylated tubulin (stable microtubule population) (Fig. 3B). The results revealed a high specificity for microtubule linkage to N-cadherin junctions in the population of elongating, differentiating fiber cells (FP), and that this association included microtubules stabilized by acetylation (Fig. 3B, quantified and represented as the ratio of acetylated-tubulin/N-cadherin (C), and of α-tubulin/N-cadherin (D)).
FIGURE 3.
Acetylated Microtubules are Linked to N-cadherin Junctions Specifically in Differentiating Cortical Fiber Cells of the Developing Lens. E10 chick embryo lenses grown ex vivo for 24 hrs in whole lens culture in the presence of the vehicle DMSO, 1μg/ml nocodazole, or 10μg/ml nocodazole were microdissected to separate lens epithelial cells (E), cortical fiber cells (FP) and central fiber cells (FC) (modeled in A), and these differentiation-state specific fractions were immunoprecipitated for N-cadherin and immunoblotted for N-cadherin, acetylated-tubulin and α-tubulin (B). Protein bands were quantified, and the ratio of acetylated tubulin (C) and α-tubulin (D) to N-cadherin determined. These co-immunoprecipitation studies demonstrated that acetylated tubulin was highly associated with N-cadherin junctional complexes in the differentiating cortical fiber cells of the developing lens. The association between acetylated-tubulin and N-cadherin junctions was lost following treatment with 1μg/ml or 10μg/ml nocodazole. Since stable N-cadherin junctions are typically Triton X-100 insoluble, E10 chick embryo lenses grown ex vivo for 24 hrs in whole lens culture in the presence of the vehicle DMSO, 1μg/ml nocodazole, or 10μg/ml nocodazole were serially extracted in Triton X-100, Triton X-100/OG and RIPA buffers, and immunoblotted for N-cadherin (E). Results were quantified and are presented as the percent distribution between soluble (Triton X-100 plus Triton X-100/OG) and insoluble (RIPA) N-cadherin populations (F). Exposure to nocodazole resulted in a small but significant decrease in the soluble N-cadherin soluble population and a relative increase in N-cadherin insolubility, reflecting an overall increase in N-cadherin junction formation (F). (p<0.05*; p<0.01**; p<0.001***; n=4)
Exposure to nocodazole impacted the linkage of microtubules to N-cadherin (Fig. 3B). Quantification showed a nearly complete loss of N-cadherin association with acetylated-tubulin in differentiating fiber cells, and a significant decrease in N-cadherin linkage to α-tubulin (Fig 3C, D), demonstrating that stable microtubules were the principle form of this cytoskeletal structure that was linked to N-cadherin in the cell population undergoing elongation. These results suggest that microtubules may have an important function in regulating the role of N-cadherin in lens fiber cell elongation and lens morphogenesis.
Dynamically remodeling N-cadherin junctions are typically soluble in detergents such as Triton X-100 and Octylglucoside (OG), while stabilized N-cadherin junctions are Triton X-100/OG insoluble. These properties of N-cadherin junctions were used to investigate whether loss of stable microtubules affects the organization of N-cadherin junctions during lens development. Whole lenses exposed to 1μg/ml or 10μg/ml nocodazole as above were sequentially extracted in Triton X-100, Triton X-100/OG and RIPA buffers and the fractions immunoblotted for N-cadherin (Fig. 3E). Immunoblots were quantified and presented as the distribution between soluble (Triton X-100 plus Triton X-100/OG) and insoluble (RIPA) N-cadherin populations (Fig. 3F). At both moderate and high doses of nocodazole there was a small but significant decrease in the soluble N-cadherin population and a concomitant increase in the insoluble pool of N-cadherin junctions. Since N-cadherin junctions are typically stabilized by association with the actin cytoskeleton (65–68), the increase in stable N-cadherin junctions when microtubules were depolymerized suggested possible effects on the organization of the actomyosin cytoskeleton and the morphogenesis of the developing lens.
Depolymerization of Stable Microtubules Leads to Aberrant Lens Morphogenesis
To investigate the function of microtubules in lens fiber cell elongation, we used the microtubule depolymerizing drug nocodazole in the whole lens culture system, which allowed us to follow the real-time effects of loss of microtubules on lens structure and morphogenesis. E10 chick embryo lenses were exposed for 24 hrs in ex vivo culture to a low dose of nocodazole (10ng/ml) that targets only dynamic microtubules for depolymerization (71), or to higher doses of nocodazole (1μg/ml and 10μg/ml) that depolymerize both stable and dynamic microtubules (72), with vehicle DMSO as control. At the low dose of 10ng/ml nocodazole there were no noticeable morphogenetic effects on the developing lens (Fig. 4E–H). The cells of these lenses retained a high level of microtubules (Fig. 4E, F), lens fiber cells elongated normally and retained their connectivity to the overlying epithelium (Fig 4E–G), and the lenses maintained clarity (Fig. 4H). These findings suggest that depolymerization of the population of dynamic, unstable microtubules did not significantly impair lens morphogenesis.
FIGURE 4.
Disassembly of Stable Microtubules Induces Lens Dysmorphogenesis and Opacity. Cryosections of E10 chick embryo lenses grown ex vivo for 24 hrs in whole lens culture in the presence of the vehicle DMSO (A–D), 10ng/ml nocodazole (E–H), 1μg/ml nocodazole (I–L) or 10μg/ml nocodazole (M–P) and labelled for α-tubulin (A,E,I,M), acetylated tubulin (B,F,J,N) and F-actin (C,G,K,O). 10ng/ml nocodazole, which disassembles only dynamic microtubule populations, had no major effect on lens cell organization or morphology (E–G), and the lenses remained clear (H). Exposure to 1μg/ml nocodazole decreased that total microtubule population (I) and reduced levels of acetylated-tubulin with complete loss in the lens epithelium (J). F-actin labeling, which highlights lens cytoarchitecture, reveals separation of the apical surfaces of lens equatorial epithelial cells and newly differentiating fiber cells at the epithelial-fiber interface (EFI) along the transition zone (K, arrowhead), also denoted by arrowhead in I,J, resulting in a cortical opacity (L). Morphogenetic defects were even greater when lenses were grown ex vivo in the presence of 10μg/ml nocodazole, with a very large separation along an extended region of the EFI (M–O, arrowhead), associated with major reduction in both stable (N) and total (M) microtubule populations and nuclear as well as cortical opacities (P). (Mag bar=20μm; n=5; immunostaining images shown are projections of z-stacks acquired by confocal microscopy)
Next, we examined whether the stable, acetylated microtubule population played a requisite role in lens morphogenesis. Immunolabeling of sections from moderate-dose (1μg/ml, Fig. 4I,J) and high-dose (10μg/ml, Fig. 4M,N) nocodazole-treated lenses showed that stable microtubules were lost from both lens epithelial and fiber cells (Fig. 4J,N), with the higher nocodazole dose causing a more complete loss of the acetylated microtubule population (Fig. 4N). While acetylated microtubules were almost completely lost from the lens equatorial epithelial cells at both moderate and high doses of nocodazole, a small population of stable microtubules was resistant to depolymerization and was retained in lens fiber cells. Co-labeling with fluorescent-conjugated phalloidin, which binds to F-actin, showed that depolymerization of stable microtubules resulted in an increased organization of actin filaments along lateral cell-cell borders of both lens epithelial and fiber cells (Fig. 4K, O). F-actin-labeling, which emphasizes the cytoarchitecture of the lens (Fig. 4C,G,K,O), also revealed that at 1μg/ml nocodazole the loss of stable microtubules caused significant defects in lens morphogenesis that resulted in an overt separation between the apical surfaces of the equatorial epithelial cells and the newest secondary fiber cells (Fig. 4K, arrowhead). This defect appears to be caused by a failure in elongation of these new fiber cells and in their interaction with neighboring epithelial cells at the EFI. At this concentration of nocodazole the elongation defect was limited to the differentiating lens fiber cells located just beyond the lens fulcrum (Fig. 4I–K, arrowheads), and resulted in a cortical opacity (Fig. 4L). The separation along the EFI was more extensive at 10μg/ml nocodazole, extending deeper into the lens, and involving the loss of previously established connections between lens epithelial and fiber cell apical domains (Fig. 4M–O, arrowhead). This result suggested that, in addition to a failure in the elongation of newly differentiating fiber cells, there is a direct impact on the ability of lens epithelial and fiber cells to establish and/or maintain cell-cell contact along the EFI, possibly reflecting a contraction of these lens fiber cells associated with the increased localization of F-actin along their lateral interfaces. While newly differentiating fiber cells maintained lateral contact and overall organization, the cells of the adjacent equatorial epithelium were somewhat disorganized. The morphogenetic defects induced by loss of stable microtubules at 10μg/ml nocodazole caused both dense cortical and nuclear opacities (Fig. 4P).
Labeling of nocodazole-treated lenses for F-actin also showed that the loss of stable microtubules disrupted the directionality of movement of the anterior tips of lens fiber cells as they elongated (Fig 4K). In contrast to control lenses, in which each subsequent layer of differentiating secondary fiber cells curves inward to encompass previous layers and form an intact elliptical structure (Fig. 4A–C), the apical tips of fiber cells exposed to nocodazole instead bend out toward the equatorial lens capsule. This finding is consistent with the established function for stable microtubules in directional cell migration (73).
Exposure of E10 chick embryo lenses in whole lens culture to colchicine, a microtubule depolymerization drug distinct from nocodazole, yielded similar results to nocodazole. 5μM colchicine induced a limited separation between the apical surfaces of the equatorial epithelial cells and the adjacent secondary fiber cells and caused the apical tips of fiber cells to bend toward the equatorial region (Supplemental Fig. 1B), while exposure to 10μM colchicine induced an extensive separation along the EFI (Supplemental Fig. 1C). These findings demonstrate that the lens morphogenetic defects caused by nocodazole resulted from a direct effect on microtubule depolymerization. Microtubules impact many cellular processes that are effected by their depolymerization. Since a downstream effect of nocodazole-induced microtubule depolymerization is the induction of LATS2, a molecule that can inhibit Wnt/β-catenin-mediated transcription (Li et al Cell Rep. 2013), we investigated whether Wnt/β-catenin signaling was impacted in nocodazole-treated lenses. Immunostaining of sections from lenses exposed to either DMSO or 10μg/ml nocodazole with antibody to β-catenin showed no evidence that microtubule depolymerization impacted nuclear localization of β-catenin (Supplemental Figure 2), suggesting that Wnt/β-catenin signaling was not altered by exposure to nocodazole.
Loss of Stable Microtubules Induces Myosin Activation
Since the morphogenetic impact of the loss of stable microtubules during lens development centered on a failure to establish and/or maintain interactions between lens epithelial and fiber cells along the EFI, with actin filaments increased along lateral cell-cell interfaces, we examined whether the observed defects in lens morphogenesis might reflect changes in myosin activation. Myosin II associates with actin to cause force generation necessary for cellular migration as well as for cell contractility (74–81). Interestingly, microtubules also can interact directly with myosin (82). It has been suggested that stable microtubules play a role in regulating myosin II activation to impact cell contractility (83), and that microtubule depolymerization can result in an increase in cell contractility (84,85).
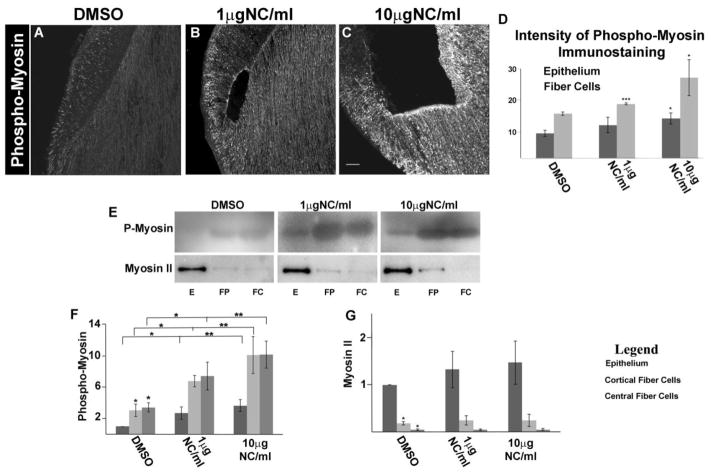
Exposure of lenses to nocodazole for 24 hrs in whole lens culture resulted in a dose-dependent increase in myosin activation as shown by immunolocalization studies with antibody to dually-phosphorylated myosin (Fig 5A–C). Myosin activation was elevated in both lens epithelial and fiber cells, along their cell-cell borders and at their apical tips. The increase in p-myosin immunofluorescence was quantified and represented as histograms generated with Image J (Fig. 5D). Immunoblot analysis following microdissection into lens epithelial (E), cortical fiber (FP) and central fiber (FC) cells (Fig. 5E), confirmed the immunolabeling results. Quantification of the immunoblot studies showed significant, nocodazole dose-dependent increases in myosin activation (p-myosin) in each of the zones of differentiation (Fig. 5F), with no significant change in expression of myosin II (Fig. 5G). These results showed that myosin activation is misregulated in the absence of acetylated microtubules, and suggested that the resultant overactivation of myosin could be responsible for the morphogenetic failures that occur in these developing lenses.
FIGURE 5.
Disassembly of Microtubules in the Developing Lens Results in Increased Myosin Activation. E10 chick embryo lenses grown ex vivo for 24 hrs in whole lens culture in the presence of the vehicle DMSO (A), 1μg/ml nocodazole (B), or 10μg/ml nocodazole (C) were immunolabeled for activated myosin (phospho-myosin, A–C). Exposure to nocodazole resulted in a significant increase in myosin activity, as shown by histogram analysis of immunostaining intensity for phospho-myosin in both epithelium and fiber cell regions of the developing lens (D). Immunoblot studies (E) with antibodies to phospho-myosin and myosin II of lenses grown ex vivo in the presence of DMSO, 1μg/ml nocodazole or 10μg/ml nocodazole following microdissection to separate epithelial (E), cortical fiber (FP), and central fiber (FC) fractions. Quantification of immunoblot results for phospho-myosin (F) and myosin II (G) confirmed the increase in myosin activity when microtubules were depolymerized, and showed there was no significant change in expression of myosin II. (mag bar=20μm p<0.05*; p<0.01**; p<0.001***; n=5; immunostaining images shown are projections of z-stacks acquired by confocal microscopy)
Inhibition of Myosin Activation Prevents Nocodazole from Inducing Dysmorphogenesis along the Lens Epithelial Fiber Interface
The increase in myosin activation that occurred when stable microtubules were depolymerized by nocodazole emerged as a likely downstream effector with a direct connection to the observed lens dysmorphogenesis. Therefore, we investigated if suppression of myosin activation could prevent the defects in fiber cell elongation and lens morphogenesis induced by nocodazole. For these studies we used blebbistatin, a specific myosin II inhibitor that acts on the myosin ATPase domain to inhibit myosin activation (86), without impacting myosin phosphorylation by myosin light chain kinase. Lenses were pretreated with blebbistatin (50μM) for 2 hrs in whole lens culture prior to exposure to both blebbistatin and nocodazole (1μg/ml and 10μg/ml) for 24 hrs.
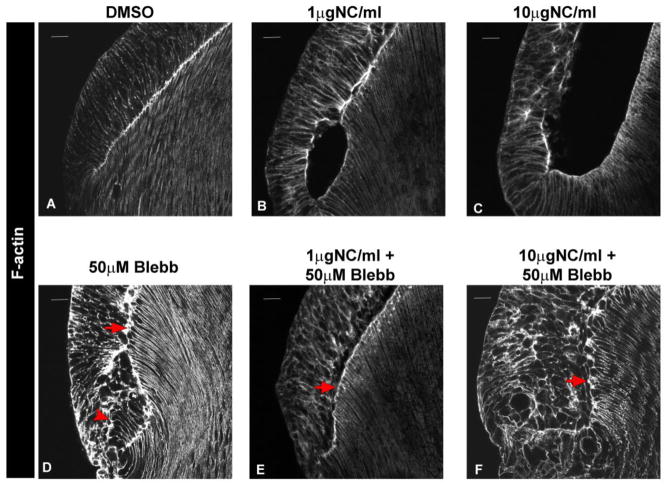
Treatment of lenses with blebbistatin alone did not alter the interaction between lens epithelial and fiber cells along the EFI (Fig 6D, arrow). The differentiating fiber cells in these lenses maintained their linear alignment and the organization of actin filaments along their lateral interfaces. While the equatorial epithelial cells also maintained lateral actin filament structures, the exposure to blebbistatin caused changes in the organization and morphology of these cells, and cells in the posterior-most equatorial epithelium appeared to have an increased length (Fig 6D, arrowhead). When lenses were exposed to blebbistatin and nocodazole in combination (at both moderate and high doses), blocking myosin activation prevented the defects in fiber cell elongation and the separation of lens epithelial and fiber cell apical domains along the EFI (Fig. 6E,F, arrows) that occurred when the lenses were exposed to nocodazole alone (Fig. 6B,C). These results suggest that activation of myosin was a key element in the dysmorphogenesis of the lens that occurred when its microtubules were destabilized by exposure to nocodazole. The elongated state of the posterior equatorial epithelial cells when myosin II activity was suppressed may contribute to the observed morphogenetic rescue of the effects of nocodazole on lens morphogenesis by blebbistatin.
FIGURE 6.
Blocking Myosin Activation Prevents Nocodazole-Induced Lens Dysmorphogenesis. E10 chick embryo lenses grown ex vivo for 24 hrs in whole lens culture in the presence of the vehicle DMSO (A), 1μg/ml nocodazole (B) 10μg/ml nocodazole (C), 50μM Blebbistatin (D), 1μg/ml nocodazole plus 50μM Blebbistatin following 2 hr exposure to 50μM Blebbistatin alone (E) or 10μg/ml nocodazole plus 50μM Blebbistatin following 2 hr exposure to 50μM Blebbistatin alone (F) and labeled for F-actin. 50μM Blebbistatin alone altered cellular organization in the region of the transition zone (D, arrowhead), but had no effect on association of lens epithelial and fiber cell apical domains along the EFI (D, arrow). Blocking myosin activation prevented the separation between lens epithelial and fiber cells along the EFI that is induced by exposure to nocodazole (E, F, arrows), rescuing lens dysmorphogenesis caused by this microtubule-depolymerizing agent (B, C). (mag bar=20μm; n=8; immunostaining images shown are a single 1μm optical plane acquired by confocal microscopy)
Inhibition of Myosin Activation Prevents Nocodazole-Induced Loss of Acetylated Microtubules
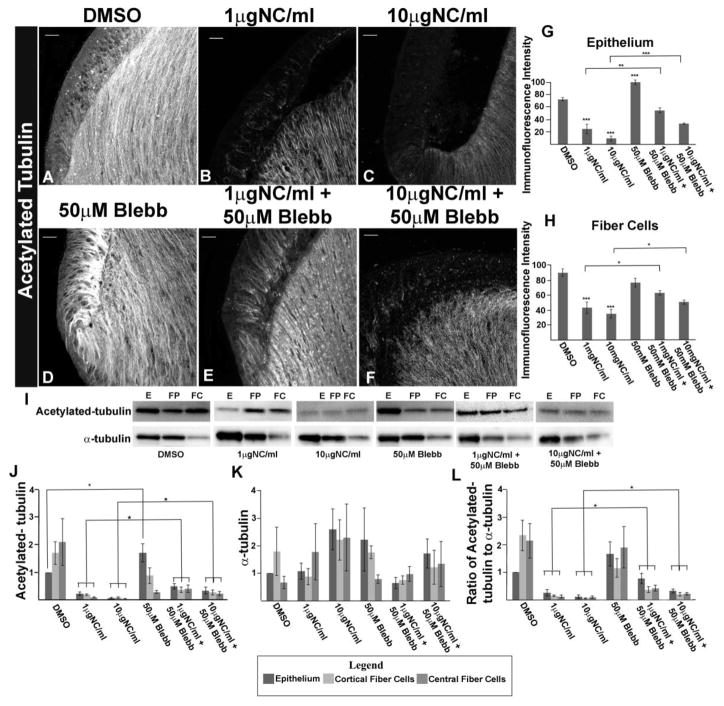
In previous studies, the inhibition of myosin activation has been shown to lead to an increase in acetylation of microtubules (41,42,76). To better understand the mechanism of the morphogenetic rescue that exposure to blebbistatin caused in nocodazole treated lenses, we examined whether blebbistatin prevented the loss of acetylated microtubules when developing lenses were exposed to the microtubule depolymerization agent nocodazole. Immunolocalization analyses showed that exposure to blebbistatin alone increased the presence of acetylated microtubules in the lens epithelium with no noticeable change in microtubule acetylation in lens fiber cells (Fig. 7D, fluorescence intensity quantified in G,H). This finding was confirmed by immunoblotting for acetylated tubulin in blebbistatin-treated lenses following microdissection to yield epithelial and fiber cell zones of differentiation (Fig. 7I, quantified in Fig. 7J). Therefore, it was possible that suppressing myosin activation in developing lenses could impact the stabilization of microtubules through induction of tubulin acetylation.
FIGURE 7.
Blocking Myosin Activation Prevents Loss of Acetylated Tubulin in Nocodazole-Treated Lenses. E10 chick embryo lenses grown ex vivo for 24 hrs in whole lens culture in the presence of the vehicle DMSO (A), 1μg/ml nocodazole (B), 10μg/ml nocodazole (C), 50μM Blebbistatin (D), 1μg/ml nocodazole plus 50μM Blebbistatin following 2 hr exposure to 50μM Blebbistatin alone (E), or 10μg/ml nocodazole plus 50μM Blebbistatin following 2 hr exposure to 50μM Blebbistatin alone (F) and immunostained for acetylated-tubulin (A–F). Exposure to Blebbistatin alone caused an increase in acetylated microtubules especially in the lens epithelium (D). Exposure to nocodazole in the presence of Blebbistatin (E, F) rescued a population of acetylated microtubules, especially notable in the cells of the equatorial epithelium. Histogram analysis of the immunostaining intensity in both lens epithelial (G) and fiber (H) cells confirmed that exposure to blebbistatin rescued acetylated tubulin populations in both lens epithelial and fiber cells. Immunoblot studies (I) of lenses grown ex vivo in the presence of DMSO, 1μg/ml nocodazole, or 10μg/ml nocodazole, in the presence and absence of 50μM blebbistatin following microdissection to separate epithelial (E), cortical fiber (FP), and central fiber (FC) fractions with antibodies to acetylated tubulin and α-tubulin. Protein bands were quantified from the acetylated-tubulin (J) and α-tubulin (K) immunoblots, and the ratio determined of acetylated tubulin to α-tubulin (L). The immunoblot results confirmed that preventing myosin activation partially rescued the acetylated microtubule population. (mag bar=20μm p<0.05*; p<0.01**; p<0.001***; n=5; immunostaining images shown are projections of z-stacks acquired by confocal microscopy)
Immunolabeling for acetylated tubulin in sections from lenses pretreated for 2 hrs with blebbistatin and then exposed for 24 hrs in whole lens culture to both blebbistatin and nocodazole showed that suppression of myosin activity resulted in a significant protection of acetylated microtubules in the presence of nocodazole (Fig. 7E, F). The observed increase in immunolabeling of acetylated microtubules in both lens epithelial and fiber cell populations in the presence of blebbistatin, with and without nocodazole, was quantified and plotted as a histogram (Fig. 7G, H). This analysis confirmed the significance of the rescue of tubulin acetylation by blebbistatin in lenses exposed to either 1μg/ml or 10μg/ml nocodazole. Lenses exposed to both concentrations of nocodazole in the presence or absence of blebbistatin were microdissected to separate lens epithelial cells (E), cortical lens fiber cells (FP) and central lens fiber cells (FC) and immunoblotted for both acetylated tubulin and α-tubulin (Fig. 7I). Tubulin acetylation and α-tubulin levels were quantified (Fig. 7J, K, respectively) and represented as the ratio of acetylated-tubulin/α-tubulin (Fig. 7L). Quantification showed a significant increase in tubulin acetylation when lenses were exposed to nocodazole in the presence of blebbistatin (Fig 7L). These results suggest that suppressing myosin activation maintains the interaction between the apical domains of lens epithelial and fiber cells in the presence of nocodazole by maintaining a microtubule population stabilized by acetylation. Furthermore, these studies support the conclusion that microtubule stability is essential for lens morphogenetic development.
Loss of Stable Microtubules Induces N-cadherin Junction Assembly Along Lateral Interfaces of Differentiating Lens Fiber Cells
The impact of acetylated microtubules on the organization of N-cadherin junctions in the developing lens was investigated by immunolocalization analysis for N-cadherin in E10 chick embryonic lenses exposed to moderate and high doses for nocodazole for 24hrs in ex vivo culture. The loss of stable microtubules impacted the organization of N-cadherin junctions, with increased immunolabeling of N-cadherin along cell-cell interfaces of differentiating lens fiber cells (Fig. 8C, E). Quantification of these N-cadherin immunolocalization studies confirmed the dose-dependent increase in N-cadherin junction assembly in differentiating fiber cells, and showed a loss of N-cadherin junctions in the lens equatorial epithelium (Fig. 8M). However, when embryonic lenses were exposed to blebbistatin for 2hrs prior to nocodazole, the organization of N-cadherin junctions at lateral epithelial and fiber cell interfaces was retained at near normal levels (Fig. 8I,K,M). Interestingly, immunolabeling of E14.5 eyes from lens N-cadherin conditional knockout mice (N-cadΔlens) (11) for acetylated tubulin showed that the loss of N-cadherin did not prevent formation of acetylated microtubules (Fig 8N). These findings suggest that the stable microtubule population of the developing lens impacts lens fiber cell elongation and lens morphogenesis through the regulation of N-cadherin junction assembly and stability.
FIGURE 8.
Increased Assembly of N-cadherin Junctions at Cell-Cell Interfaces of Fiber cells in Nocodazole-treated Lenses Prevented by Blocking Myosin Activation. E10 chick embryo lenses grown ex vivo for 24 hrs in whole lens culture in the presence of the vehicle DMSO (A,B), 1μg/ml nocodazole (C,D), 10μg/ml nocodazole (E,F), 50μM Blebbistatin (G,H), 1μg/ml nocodazole plus 50μM Blebbistatin following 2 hr exposure to 50μM Blebbistatin alone (I,J) or 10μg/ml nocodazole plus 50μM Blebbistatin following 2 hr exposure to 50μM Blebbistatin alone (K,L) and labeled for N-cadherin (A,C,E,G,I,K) and F-actin (B,D,F,H,J,L). Histogram analysis was performed to analyze immunostaining intensity for N-cadherin in the epithelium and fiber cells of these lenses (M). Blocking myosin activity maintained the level of N-cadherin junctions at lateral interfaces of lens fiber cells at near-normal levels. Immunolabeling of cryosections from eyes of E14.5 wildtype mice or lens N-cadherin conditional knockout mice (N-cadΔlens) for acetylated tubulin (N) demonstrated that the absence of N-cadherin did not prevent formation of acetylated microtubules. (mag bar=20μm p<0.05*; p<0.01**; p<0.001***; n=5; immunostaining images shown are projections of z-stacks acquired by confocal microscopy)
DISCUSSION
Cytoskeletal elements interact cooperatively to regulate cellular processes including directed cellular migration (48,87). Among these elements, microtubules have been suggested to regulate the directionality or polarity of migration (47,88,89) by promoting lamellipodial protrusions (90,91), and through regulation of cell adhesion and contraction (69,70,92). In the lens, a tissue in which proper tissue architecture is integral to its function, it was likely that the process of lens fiber cell migration and elongation during development would rely on coordination of cytoskeletal elements, such as microtubules (54,55), with the actin-myosin cytoskeletal machinery.
Our findings demonstrate that microtubule polymerization and stability have an important role in lens development and morphogenesis, with the stable population of microtubules linked to proper fiber cell elongation (modeled in Figure 9). As in other systems (44,93), it is the stable population of microtubules that is involved in the directed cell migration of lens fiber cells, as the loss of dynamic microtubules alone did not affect lens fiber cell elongation or the directionality of their migration. The impairment of fiber cell elongation associated with the loss of stabilized microtubules was associated with an increase in F-actin along the interfaces of differentiating lens fiber cells, suggesting that stable microtubules may impact the assembly state of actin. Our previous studies show that N-cadherin junctions are epicenters of actin organization in lens fiber cells (15). Since we now find that stabilized microtubules are associated with N-cadherin junctions specifically in the population of elongating fiber cells and play a role in regulating N-cadherin junction organization along the lateral borders of these cells, it is possible that N-cadherin and acetylated microtubules are involved in a coordinate regulation of actin organization during the complex process of lens fiber cell morphogenesis (modeled, Fig. 9).
Figure 9.
Role of Acetylated Microtubules in Lens Fiber Cell Elongation. Model of the impact of loss of acetylated microtubules on lens fiber cell elongation and lens morphogenesis. The elongation failure of newly differentiating lens fiber cells and failure to form and/or maintain the epithelial fiber interface (EFI) is shown to involve increased myosin activity and assembly of N-cadherin junctions along cell-cell interfaces.
Our results demonstrate that the function of stable microtubules in lens fiber cell elongation involves their regulation of myosin activation. The observed increase in myosin activation along lateral interfaces of both epithelial and differentiating lens fiber cells following the loss of microtubules could negatively impact fiber cell elongation by inducing excessive actomyosin contraction, that could lead to the observed morphological defects associated with failure to establish and/or maintain the interactions along the EFI during development. Microtubules can have both direct and indirect roles in regulating cellular contractility (81,94,95), and microtubule depolymerization can increase traction forces, at least in part through an activation of myosin (85). Therefore, the depolymerization of microtubules in the lens may increase contractility of fiber cells and lead to morphogenetic defects of lens fiber cells that prevent or destabilize the interaction between apical domains of lens epithelial and fiber cells. Additionally, as myosin II is known to have roles in creating and maintaining cell shape by regulating cell curvature (96), the increase in myosin activity following microtubule depolymerization could impair curvature of newly differentiating fiber cells as they elongate, and contribute to the defects in directionality of migration of new secondary fiber cells observed in nocodazole-treated lenses.
The maintenance of normal lens fiber cell elongation when lenses are exposed to nocodazole in the presence of the myosin inhibitor blebbistatin was associated with the maintenance of a stable, acetylated tubulin population, a property that could impact N-cadherin junction organization along the lateral borders of differentiating lens fiber cells (modeled, Fig. 9). These findings suggest that there is a connection between myosin activity, N-cadherin junction assembly and tubulin acetylation in processes central to normal lens morphogenesis. In other cell types, myosin II activity has been shown to inhibit microtubule acetylation while inhibition or ablation of microtubules promotes myosin activity (41,76). Similarly, myosin II activity has also been linked to regulation of N-cadherin distribution and function in migrating tissues (97–99). Active myosin II has been shown to regulate the concentration of cadherin junctions at cell-cell interfaces (97,100) and this myosin activity may depend on microtubule stability (101). These properties may explain the stable microtubule-dependent, myosin activity-mediated organization of N-cadherin junctions in differentiating lens fiber cells.
CONCLUSIONS
Our results suggest that there is a coordination of function between acetylated microtubules, N-cadherin cell-cell junctions, myosin activity and the actin cytoskeleton required for normal lens fiber cell elongation and for the establishment and maintenance of the interactions of fiber cells with the overlying lens epithelium necessary for lens morphogenesis.
Supplementary Material
HIGHLIGHTS.
In the developing lens, N-cadherin junctions become linked to acetylated microtubules, a stabilized form of this cytoskeletal element, specifically in the region of lens fiber cell elongation.
Stabilized microtubules are essential to the elongation of differentiating lens fiber cells and the maintenance of their association with the lens epithelium.
Loss of stable microtubules in the developing lens results in an increase in myosin activation state, which negatively impacts lens fiber cell morphogenesis.
The increased myosin activity that results from disassembly of stable microtubules alters N-cadherin junction assembly along lateral interfaces of differentiating lens fiber cells.
Acknowledgments
We thank Dr. Janice Walker for critical reading of the manuscript and Brigid Bleaken for assistance in figure preparation.
Funding: This work was supported by the National Institutes of Health [EY014258 to ASM]
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions: CML helped design experiments, performed most of the experiments, analyzed the results, and wrote the paper. CB helped perform experiments and contributed to figure preparation. ASM designed the study, was involved in interpretation of data, and co-wrote the manuscript. All authors analyzed the results and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heng YW, Koh CG. The international journal of biochemistry & cell biology. 2010;42:1622–1633. doi: 10.1016/j.biocel.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Rao PV, Maddala R. Semin Cell Dev Biol. 2006;17:698–711. doi: 10.1016/j.semcdb.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher DA, Mullins RD. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams G, Jr, Zhou J, Wang W, Wu H, Quan J, Liu Y, Xia P, Wang Z, Zhou S, Jiang J, Mo F, Zhuang X, Thomas K, Hill DL, Aikhionbare FO, He P, Liu X, Ding X, Yao X. The Journal of biological chemistry. 2016;291:20692–20706. doi: 10.1074/jbc.M116.732719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barth AI, Caro-Gonzalez HY, Nelson WJ. Seminars in cell & developmental biology. 2008;19:245–251. doi: 10.1016/j.semcdb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao D, Su Z, Wang W, Wu H, Liu X, Akram S, Qin B, Zhou J, Zhuang X, Adams G, Jin C, Wang X, Liu L, Hill DL, Wang D, Ding X, Yao X. The Journal of biological chemistry. 2015;290:23766–23780. doi: 10.1074/jbc.M115.673517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George SP, Chen H, Conrad JC, Khurana S. Journal of cell science. 2013;126:312–326. doi: 10.1242/jcs.116244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wakida NM, Botvinick EL, Lin J, Berns MW. PLoS One. 2010;5:e15462. doi: 10.1371/journal.pone.0015462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouquet C, Soares S, von Boxberg Y, Ravaille-Veron M, Propst F, Nothias F. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:7204–7213. doi: 10.1523/JNEUROSCI.2254-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayas CL, Avila J. Journal of Alzheimer’s disease: JAD. 2014;40(Suppl 1):S17–22. doi: 10.3233/JAD-132315. [DOI] [PubMed] [Google Scholar]
- 11.Logan CM, Rajakaruna S, Bowen C, Radice GL, Robinson ML, Menko AS. Developmental biology. 2017 doi: 10.1016/j.ydbio.2017.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira-Cornwell MC, Veneziale RW, Grunwald GB, Menko AS. Experimental cell research. 2000;256:237–247. doi: 10.1006/excr.2000.4819. [DOI] [PubMed] [Google Scholar]
- 13.Sue Menko A. Experimental eye research. 2002;75:485–490. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- 14.Cammas L, Wolfe J, Choi SY, Dedhar S, Beggs HE. Investigative ophthalmology & visual science. 2012;53:3067–3081. doi: 10.1167/iovs.11-9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leonard M, Zhang L, Zhai N, Cader A, Chan Y, Nowak RB, Fowler VM, Menko AS. Developmental biology. 2011;349:363–377. doi: 10.1016/j.ydbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hay ED. Cell differentiation and development: the official journal of the International Society of Developmental Biologists. 1990;32:367–375. doi: 10.1016/0922-3371(90)90052-x. [DOI] [PubMed] [Google Scholar]
- 17.Ireland ME, Braunsteiner A, Mrock L. Developmental biology. 1993;160:494–503. doi: 10.1006/dbio.1993.1323. [DOI] [PubMed] [Google Scholar]
- 18.Menko S, Philp N, Veneziale B, Walker J. Annals of the New York Academy of Sciences. 1998;842:36–41. doi: 10.1111/j.1749-6632.1998.tb09629.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker JL, Menko AS. Developmental biology. 1999;210:497–511. doi: 10.1006/dbio.1999.9277. [DOI] [PubMed] [Google Scholar]
- 20.Zelenka PS. The International journal of developmental biology. 2004;48:857–865. doi: 10.1387/ijdb.041871pz. [DOI] [PubMed] [Google Scholar]
- 21.Wederell ED, de Iongh RU. Seminars in cell & developmental biology. 2006;17:759–776. doi: 10.1016/j.semcdb.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Hancock WO. Current biology: CB. 2014;24:R968–970. doi: 10.1016/j.cub.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Venghateri JB, Jindal B, Panda D. Expert opinion on therapeutic targets. 2015;19:957–972. doi: 10.1517/14728222.2015.1018823. [DOI] [PubMed] [Google Scholar]
- 24.Yount AL, Zong H, Walczak CE. Experimental cell research. 2015;334:70–77. doi: 10.1016/j.yexcr.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchison T, Kirschner M. Nature. 1984;312:237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 26.Hotani H, Horio T. Cell motility and the cytoskeleton. 1988;10:229–236. doi: 10.1002/cm.970100127. [DOI] [PubMed] [Google Scholar]
- 27.Mimori-Kiyosue Y. Cytoskeleton (Hoboken, NJ) 2011;68:603–618. doi: 10.1002/cm.20540. [DOI] [PubMed] [Google Scholar]
- 28.Bartolini F, Ramalingam N, Gundersen GG. Mol Biol Cell. 2012;23:4032–4040. doi: 10.1091/mbc.E12-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, Riazuddin SA, Hejtmancik JF. Am J Hum Genet. 2011;88:827–838. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikegami K, Setou M. Cell structure and function. 2010;35:15–22. doi: 10.1247/csf.09027. [DOI] [PubMed] [Google Scholar]
- 31.Kadavath H, Hofele RV, Biernat J, Kumar S, Tepper K, Urlaub H, Mandelkow E, Zweckstetter M. Proc Natl Acad Sci U S A. 2015;112:7501–7506. doi: 10.1073/pnas.1504081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee A, Fischer RS, Fowler VM. Developmental dynamics: an official publication of the American Association of Anatomists. 2000;217:257–270. doi: 10.1002/(SICI)1097-0177(200003)217:3<257::AID-DVDY4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Takemura R, Okabe S, Umeyama T, Kanai Y, Cowan NJ, Hirokawa N. Journal of cell science. 1992;103(Pt 4):953–964. doi: 10.1242/jcs.103.4.953. [DOI] [PubMed] [Google Scholar]
- 34.Tegha-Dunghu J, Bausch E, Neumann B, Wuensche A, Walter T, Ellenberg J, Gruss OJ. Journal of cell science. 2014;127:5007–5013. doi: 10.1242/jcs.136457. [DOI] [PubMed] [Google Scholar]
- 35.Akisaka T, Yoshida H, Takigawa T. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2011;59:630–638. doi: 10.1369/0022155411405334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belmadani S, Pous C, Fischmeister R, Mery PF. Mol Cell Biochem. 2004;258:35–48. doi: 10.1023/b:mcbi.0000012834.43990.b6. [DOI] [PubMed] [Google Scholar]
- 37.Bulinski JC, Richards JE, Piperno G. The Journal of cell biology. 1988;106:1213–1220. doi: 10.1083/jcb.106.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perdiz D, Mackeh R, Pous C, Baillet A. Cellular signalling. 2011;23:763–771. doi: 10.1016/j.cellsig.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Song Y, Kirkpatrick LL, Schilling AB, Helseth DL, Chabot N, Keillor JW, Johnson GV, Brady ST. Neuron. 2013;78:109–123. doi: 10.1016/j.neuron.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webster DR, Borisy GG. Journal of cell science. 1989;92(Pt 1):57–65. doi: 10.1242/jcs.92.1.57. [DOI] [PubMed] [Google Scholar]
- 41.Joo EE, Yamada KM. Nat Commun. 2014;5:3510. doi: 10.1038/ncomms4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takesono A, Heasman SJ, Wojciak-Stothard B, Garg R, Ridley AJ. PLoS One. 2010;5:e8774. doi: 10.1371/journal.pone.0008774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Montagnac G, Meas-Yedid V, Irondelle M, Castro-Castro A, Franco M, Shida T, Nachury MV, Benmerah A, Olivo-Marin JC, Chavrier P. Nature. 2013;502:567–570. doi: 10.1038/nature12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gundersen GG, Bulinski JC. Proc Natl Acad Sci U S A. 1988;85:5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulinski JC, Gundersen GG. BioEssays: news and reviews in molecular, cellular and developmental biology. 1991;13:285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- 46.Kim DJ, Martinez-Lemus LA, Davis GE. Blood. 2013;121:3521–3530. doi: 10.1182/blood-2012-11-470179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasiliev JM, Gelfand IM, Domnina LV, Ivanova OY, Komm SG, Olshevskaja LV. Journal of embryology and experimental morphology. 1970;24:625–640. [PubMed] [Google Scholar]
- 48.Rodriguez OC, Schaefer AW, Mandato CA, Forscher P, Bement WM, Waterman-Storer CM. Nature cell biology. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 49.Plestant C, Strale PO, Seddiki R, Nguyen E, Ladoux B, Mege RM. Journal of cell science. 2014;127:1660–1671. doi: 10.1242/jcs.131284. [DOI] [PubMed] [Google Scholar]
- 50.Menko AS. Experimental eye research. 2002;75:485–490. doi: 10.1006/exer.2002.2057. [DOI] [PubMed] [Google Scholar]
- 51.Maddala R, Chauhan BK, Walker C, Zheng Y, Robinson ML, Lang RA, Rao PV. Developmental biology. 2011;360:30–43. doi: 10.1016/j.ydbio.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maddala R, Nagendran T, Lang RA, Morozov A, Rao PV. Developmental biology. 2015;406:74–91. doi: 10.1016/j.ydbio.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maddala R, Skiba N, Vasantha Rao P. Differentiation. 2007;75:713–725. doi: 10.1111/j.1432-0436.2007.00173.x. [DOI] [PubMed] [Google Scholar]
- 54.Piatigorsky J. Annals of the New York Academy of Sciences. 1975;253:333–347. doi: 10.1111/j.1749-6632.1975.tb19211.x. [DOI] [PubMed] [Google Scholar]
- 55.Piatigorsky J, de Webster HF, Wollberg M. The Journal of cell biology. 1972;55:82–92. doi: 10.1083/jcb.55.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Basu S, Rajakaruna S, Reyes B, Van Bockstaele E, Menko AS. Autophagy. 2014;10:1193–1211. doi: 10.4161/auto.28768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schuyler SC, Pellman D. Cell. 2001;105:421–424. doi: 10.1016/s0092-8674(01)00364-6. [DOI] [PubMed] [Google Scholar]
- 58.Coquelle FM, Vitre B, Arnal I. Biochemical Society transactions. 2009;37:997–1001. doi: 10.1042/BST0370997. [DOI] [PubMed] [Google Scholar]
- 59.Nehlig A, Molina A, Rodrigues-Ferreira S, Honore S, Nahmias C. Cellular and molecular life sciences: CMLS. 2017 doi: 10.1007/s00018-017-2476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saxton WM, Stemple DL, Leslie RJ, Salmon ED, Zavortink M, McIntosh JR. The Journal of cell biology. 1984;99:2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulze E, Asai DJ, Bulinski JC, Kirschner M. The Journal of cell biology. 1987;105:2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schulze E, Kirschner M. The Journal of cell biology. 1987;104:277–288. doi: 10.1083/jcb.104.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kreis TE. The EMBO journal. 1987;6:2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaverina I, Straube A. Seminars in cell & developmental biology. 2011;22:968–974. doi: 10.1016/j.semcdb.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jamora C, DasGupta R, Kocieniewski P, Fuchs E. Nature. 2003;422:317–322. doi: 10.1038/nature01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jamora C, Fuchs E. Nature cell biology. 2002;4:E101–108. doi: 10.1038/ncb0402-e101. [DOI] [PubMed] [Google Scholar]
- 67.Vasioukhin V, Bauer C, Yin M, Fuchs E. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 68.Verma S, Shewan AM, Scott JA, Helwani FM, den Elzen NR, Miki H, Takenawa T, Yap AS. The Journal of biological chemistry. 2004;279:34062–34070. doi: 10.1074/jbc.M404814200. [DOI] [PubMed] [Google Scholar]
- 69.Revenu C, Streichan S, Dona E, Lecaudey V, Hufnagel L, Gilmour D. Development (Cambridge, England) 2014;141:1282–1291. doi: 10.1242/dev.101675. [DOI] [PubMed] [Google Scholar]
- 70.Peglion F, Llense F, Etienne-Manneville S. Nature cell biology. 2014;16:639–651. doi: 10.1038/ncb2985. [DOI] [PubMed] [Google Scholar]
- 71.Vasquez RJ, Howell B, Yvon AM, Wadsworth P, Cassimeris L. Mol Biol Cell. 1997;8:973–985. doi: 10.1091/mbc.8.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Brabander M, Van de Veire R, Aerts F, Geuens S, Hoebeke J. Journal of the National Cancer Institute. 1976;56:357–363. doi: 10.1093/jnci/56.2.357. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe T, Noritake J, Kaibuchi K. Trends in cell biology. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 74.Arnold TR, Stephenson RE, Miller AL. Experimental cell research. 2017 doi: 10.1016/j.yexcr.2017.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bergert M, Chandradoss SD, Desai RA, Paluch E. Proc Natl Acad Sci U S A. 2012;109:14434–14439. doi: 10.1073/pnas.1207968109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Nature cell biology. 2007;9:299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- 77.Munjal A, Lecuit T. Development (Cambridge, England) 2014;141:1789–1793. doi: 10.1242/dev.091645. [DOI] [PubMed] [Google Scholar]
- 78.Murrell MP, Gardel ML. Proc Natl Acad Sci U S A. 2012;109:20820–20825. doi: 10.1073/pnas.1214753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roper K. Current topics in developmental biology. 2015;112:103–127. doi: 10.1016/bs.ctdb.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 80.van den Dries K, Meddens MB, de Keijzer S, Shekhar S, Subramaniam V, Figdor CG, Cambi A. Nat Commun. 2013;4:1412. doi: 10.1038/ncomms2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou J, Kim HY, Wang JH, Davidson LA. Development (Cambridge, England) 2010;137:2785–2794. doi: 10.1242/dev.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shimo-Oka T, Hayashi M, Watanabe Y. Biochemistry. 1980;19:4921–4926. doi: 10.1021/bi00562a034. [DOI] [PubMed] [Google Scholar]
- 83.Foe VE, von Dassow G. The Journal of cell biology. 2008;183:457–470. doi: 10.1083/jcb.200807128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu BP, Chrzanowska-Wodnicka M, Burridge K. Cell adhesion and communication. 1998;5:249–255. doi: 10.3109/15419069809040295. [DOI] [PubMed] [Google Scholar]
- 85.Rape A, Guo WH, Wang YL. Journal of cell science. 2011;124:4233–4240. doi: 10.1242/jcs.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. The Journal of biological chemistry. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 87.Goode BL, Drubin DG, Barnes G. Current opinion in cell biology. 2000;12:63–71. doi: 10.1016/s0955-0674(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 88.Small JV, Kaverina I. Current opinion in cell biology. 2003;15:40–47. doi: 10.1016/s0955-0674(02)00008-x. [DOI] [PubMed] [Google Scholar]
- 89.Small JV, Stradal T, Vignal E, Rottner K. Trends in cell biology. 2002;12:112–120. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- 90.Rinnerthaler G, Geiger B, Small JV. The Journal of cell biology. 1988;106:747–760. doi: 10.1083/jcb.106.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED. Nature cell biology. 1999;1:45–50. doi: 10.1038/9018. [DOI] [PubMed] [Google Scholar]
- 92.Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Current biology: CB. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- 93.Wittmann T, Waterman-Storer CM. Journal of cell science. 2001;114:3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- 94.Krendel M, Zenke FT, Bokoch GM. Nature cell biology. 2002;4:294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- 95.Hui KL, Upadhyaya A. Proc Natl Acad Sci U S A. 2017;114:E4175–e4183. doi: 10.1073/pnas.1614291114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elliott H, Fischer RS, Myers KA, Desai RA, Gao L, Chen CS, Adelstein RS, Waterman CM, Danuser G. Nat Cell Biol. 2015;17:137–147. doi: 10.1038/ncb3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yashiro H, Loza AJ, Skeath JB, Longmore GD. Mol Biol Cell. 2014;25:2956–2969. doi: 10.1091/mbc.E14-04-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ng MR, Besser A, Danuser G, Brugge JS. The Journal of cell biology. 2012;199:545–563. doi: 10.1083/jcb.201207148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Menko AS, Bleaken BM, Walker JL. Experimental cell research. 2014;322:133–148. doi: 10.1016/j.yexcr.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. Mol Biol Cell. 2005;16:4531–4542. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stehbens SJ, Paterson AD, Crampton MS, Shewan AM, Ferguson C, Akhmanova A, Parton RG, Yap AS. Journal of cell science. 2006;119:1801–1811. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.