Abstract
Peripheral nerves exhibit robust regenerative capabilities in response to selective injury among amniotes, but the regeneration of entire muscle groups following volumetric muscle loss is limited in birds and mammals. In contrast, lizards possess the remarkable ability to regenerate extensive de novo muscle after tail loss. However, the mechanisms underlying reformation of the entire neuromuscular system in the regenerating lizard tail are not completely understood. We have tested whether the regeneration of the peripheral nerve and neuromuscular junctions (NMJs) recapitulate processes observed during normal neuromuscular development in the green anole, Anolis carolinensis. Our data confirm robust axonal outgrowth during early stages of tail regeneration and subsequent NMJ formation within weeks of autotomy. Interestingly, NMJs are overproduced as evidenced by a persistent increase in NMJ density 120 and 250 days post autotomy (DPA). Substantial Myelin Basic Protein (MBP) expression could also be detected along regenerating nerves indicating that the ability of Schwann cells to myelinate newly formed axons remained intact. Overall, our data suggest that the mechanism of de novo nerve and NMJ reformation parallel, in part, those observed during neuromuscular development. However, the prolonged increase in NMJ number and aberrant muscle differentiation hint at processes specific to the adult response. An examination of the coordinated exchange between peripheral nerves, Schwann cells, and newly synthesized muscle of the regenerating neuromuscular system may assist in the identification of candidate molecules that promote neuromuscular recovery in organisms incapable of a robust regenerative response.
Keywords: neuromuscular junction, regeneration, de novo, lizard, reptile
Introduction
While salamanders, lizards, and teleost fish exhibit the capability to regrow entire appendages, the ability to regenerate complex, multi-tissue structures is limited in most mammals and birds (Bellairs and Bryant, 1985; Tsonis, 2000; Brockes and Kumar, 2005; Alibardi, 2014; Tanaka, 2016). Regeneration in urodele amphibians, such as the axolotl, has been the focus of research efforts, but studies in lizards could shed light on conserved genetic pathways as they are evolutionarily more closely related to mammals. Lizards such as the green anole, Anolis carolinensis, can readily self-amputate, or autotomize, their tails when threatened and regenerate a functional replacement (Cox, 1969; Gillis et al., 2009; Alibardi, 2014). Understanding the process of lizard tail regeneration may assist in discovering relevant cellular and molecular mediators of appendage regeneration in other amniotes such as humans.
The regeneration of a functional appendage requires dynamic and tightly regulated interactions between signaling centers, stem cell progenitors, and differentiated cell types (Sharma and Belmonte, 2001; Murphy et al., 2011; Reddien and Tanaka, 2016). An important goal has been to define the specific cellular subtypes and molecular signals that direct appendage regrowth (Tornini and Poss, 2014; Reddien and Tanaka, 2016). The regeneration of the neuromuscular system in the lizard tail is crucial for restoring appendage functionality and has been recognized for some time (Hughes and New, 1959; Bellairs and Bryant, 1985; Alibardi, 2014). The peripheral nerves comprised of axons and Schwann cells readily regrow following injury in both mammals and lizards and provide critical molecular cues that promote regeneration of other cell types (Hughes and New, 1959; Singer, 1961; Bosse et al., 2006; Pirotte et al., 2016). Birth dating experiments suggest that regenerating axons in the lizard tail are not derived from newly born neurons (Duffy, 1992; Alibardi, 1992). Instead, retrograde labeling experiments have shown that most of the newly formed axons in the tail are derived from sprouting of axotomized dorsal root ganglia (DRG) and spinal motor neurons rostral to the breakpoint (Hughes and New, 1959; Duffy, 1992; Cristino et al., 2000a; Cristino et al., 2000b; Cristino et al., 2000c). The expansion in the peripheral innervation field coincides with significant hypertrophy of neuronal cell bodies and altered gene expression in axotomized neurons located in spinal segments just rostral to the autotomy plane (Cristino et al., 2000a; Cristino et al., 2000b). In contrast to peripheral nerves, mammals do not regenerate muscle after extensive volumetric muscle loss (Turner and Badylak, 2011), whereas lizards exhibit robust regeneration of entire muscle groups following tail autotomy (Hughes and New, 1959; Alibardi, 1995; Fisher et al., 2012).
The reciprocal interaction between lower motor neuron axons and immature muscle during early development plays an important role in neuromuscular differentiation in amniotes (Lance-Jones and Landmesser, 1980; Harris, 1981; McLennan, 1983). However, the mechanisms that contribute to regeneration often involve unique, adult-specific processes in addition to reactivation of programs comparable to those used during development. Current evidence suggests this combinatorial model also applies to the lizard tail. Transcriptomic profiling efforts have identified changes in genes and microRNAs linked to developmental processes, in addition to adult-specific responses during lizard tail regeneration (Hutchins et al., 2014; Hutchins et al., 2016). The mature regenerated tail does not reproduce the anatomy of the non-injured tail in a number of ways (Hughes and New, 1959; Fisher et al., 2012; Ritzman et al., 2012; Hutchins et al., 2014). A central cartilage tube forms that encases the regenerated spinal cord composed of ependymal lining and descending axons without associated DRGs, instead of an ossified vertebral column containing articulating vertebrae, spinal cord white and grey matter, and segmented DRGs (Simpson, 1968; Duffy et al., 1990; Lozito and Tuan, 2015; Lozito and Tuan, 2016). Tail muscles are normally organized into epaxial and hypaxial quadrants, but regenerating muscle lacks clear interdigitating muscle segments and intramuscular septa (Alibardi, 1995; Fisher et al., 2012). Thus, the organization of musculoskeletal tissues in the regenerated tail differs markedly from that of the original pattern, formed during embryonic development. However, it is unknown whether the innervation of the de novo regenerated musculature is also markedly different or recapitulates processes seen during development, such as the stereotyped pattern of axonal outgrowth and synaptic innervation, the distinctive morphological maturation of NMJs, or Schwann cell remyelination (Darabid et al., 2014; Tintignac et al., 2015).
Here, we have examined the timing of interactions between regrowing axons, muscle, and Schwann cells during tail regeneration in the lizard A. carolinensis. Our findings demonstrate that substantial axonal regrowth and remyelination occurs rapidly following autotomy. Newly formed plaque-shaped motor endplates are also apparent within weeks of autotomy, but do not develop a morphologically mature, pretzel-like appearance for many months (Marques et al., 2000). Interestingly, NMJs are initially overproduced and persist at a higher density as compared to non-injured tails. Overall, our data suggest that adult neuromuscular regeneration in lizards recapitulates canonical neurodevelopmental processes. However, the final number of NMJs per muscle group remains increased relative to non-autotomized tails providing evidence of adult-specific programs. Further study of the precise timing and underlying interactions between peripheral nerves and de novo muscle in the regenerating lizard tail may inform approaches designed to promote neuromuscular regeneration in mammals.
Results
Regeneration of peripheral nerves following lizard tail autotomy
We analyzed the time course of peripheral nerve regeneration by immunostaining lizard tail sections with the neuron specific cytoskeletal protein, β-III Tubulin (TUJ1). In transverse sections of the original tail, the spinal cord is centrally located and enclosed within the neural arch of the vertebra (Fig. 1A). Muscle bundles exhibit radial symmetry. Longitudinal peripheral nerves are primarily localized along the muscle perimeter in the mesenchyme between the vertebral column and the inner surface of individual muscles or between the muscle and the surrounding epidermis (Fig. 1B–C). Adult regenerated tails were first analyzed at 15 days post-autotomy (DPA) caudal to the breakpoint (Fig. 1D). At this stage, early ependyma outgrowth has initiated from the severed stump and a rich plexus of TUJ1+ fibers could be detected at the very tip of the tail, in line with previous studies (Hughes and New, 1959; Fisher et al., 2012). There was noticeable outgrowth of regenerating peripheral axons situated near newly formed muscle groups (Fig. 1D–F). Peripheral nerve bundles were primarily localized to the inner surface of each muscle group similar to the original tail; however, many smaller TUJ1 immunoreactive axon branches were detected throughout the tail (Fig. 1D–F).
Figure 1.
Peripheral axons regrow early in regeneration and are subsequently remodeled. Transverse sections of original and regenerated Anolis carolinensis tails distal to the site of autotomy following immunostaining with β-III tubulin (TUJ1, red) and nuclei (DAPI, blue). (A–C) Representative confocal images showing the anatomical organization of nerves in the original tail in a region 2–5 mm distal to the site of autotomy (n=5). The spinal cord (sc) is enclosed by the vertebrae (v) and prominent scales (s) are visible. (D–F) Peripheral nerves (pn) and the ependyma (ep) readily regrow into the regenerated tail at 15 DPA (n=3). (G–I) At 30 DPA peripheral nerves continue to regenerate and cartilage (ct) has begun to differentiate and form around the ependymal tube 2–5 mm past the breakpoint (n=5). (J–L) Peripheral nerves localize deep to newly synthesized muscle at 120 DPA in the region 2–5 mm distal to the autotomy zone (n=4). (M) Quantification of axonal density in non-injured, 30 DPA, and 120 DPA regenerated tails. A statistically significant increase in TUJ1+ pixel density relative to non-injured tails was detected at 30 DPA during early regeneration and outgrowth (n=3). At 120 DPA, axonal density was decreased relative to 30 DPA, but increased relative to the original tail. Data are presented as mean sd; statistical analysis performed by one-way ANOVA followed by Tukey’s post-hoc test. **p<0.001, *p<0.05. The non-parametric, Kruskal-Wallis rank test followed by Dunn’s post-hoc test further confirmed the statistical significance of these changes (Dunn’s post-hoc p=0.049). Insets show a single muscle and its peripheral innervation. Scale bars A, D, G, J=200 µm; C, F, I, L=100 µm.
By 30–120 DPA substantial tail regeneration had occurred. In 30 DPA tails, many peripheral axons were labeled deep to the muscle (Fig. 1G–I). However, there are distinct differences in overall muscle anatomy consistent with previous studies (Hughes and New, 1959; Fisher et al., 2012; Ritzman et al., 2012). Comparisons between original and regenerated muscle from 30–120 DPA shows they share similar concentric organization around the cartilage tube (Fig. 1G–L).
We quantified axonal innervation of the muscle in cross-sections of non-injured and regenerated tails at 30 DPA and 120 DPA by analyzing the density of TUJ1+ pixels (Fig. 1M). Significant outgrowth of the tail at 30 DPA is accompanied by 3.08-fold increase in axonal density in the muscle relative to original tails (Fig. 1M). By 120 DPA, axonal density remains increased by 1.53-fold relative to original tails, but is decreased by 2.01–fold when compared to 30 DPA tails (Fig. 1M). Overall, these data indicate substantial axonal regrowth at early stages of regeneration resulting in a persistently elevated extent of axonal innervation at 120 DPA relative to the non-injured lizard tail.
Regenerating axons are rapidly myelinated
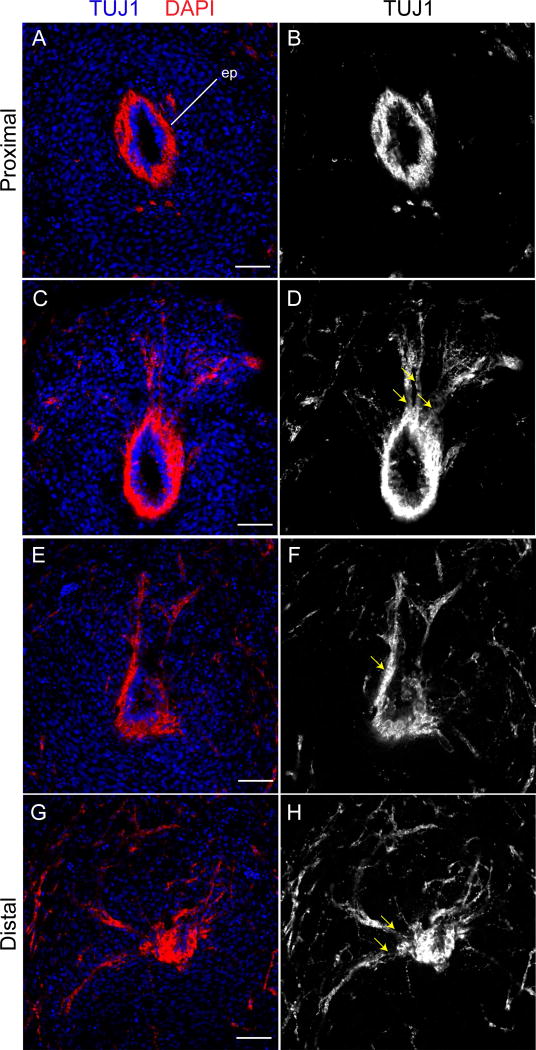
Axon function and regeneration in the peripheral nervous system is substantially influenced by Schwann cells (Feltri et al., 2002; Chen and Strickland, 2003; Yu et al., 2005). In amphibians, Schwann cell production of Anterior Gradient Protein has been shown to promote limb regeneration (Kumar et al., 2007; Pirotte et al., 2016). We examined Schwann cell myelination by double immunolabeling sections in the regenerated lizard tail with TUJ1 and Myelin Basic Protein (MBP) antibodies. To examine changes in myelination along the rostro-caudal axis, 30 DPA transverse sections were collected from the breakpoint to the tip of the regenerated tail. In proximal sections, the regenerating lizard tail exhibited significant MBP expression around TUJ1+ axons in the periphery and the ependymal lining (Fig. 2A–H). Surprisingly, TUJ1+ peripheral nerves near the tip of the regenerating lizard tail exhibited significantly reduced MBP expression (Fig. 2I–P). Presumptive oligodendrocytes surrounding axons in the ependyma did not demonstrate a similar reduction in MBP expression in distal sections (Fig. 2I–L). Similar results were obtained in 15 DPA samples (Supplemental Material Fig. 1A–F). These data show that regenerating axons are rapidly myelinated along a proximal-distal wave in the periphery following autotomy.
Figure 2.
Concurrent myelination of regenerating axons occurs along the rostro-caudal axis. Immunofluorescent micrographs of myelinated peripheral nerves stained with myelin basic protein (MBP, green) and β-III tubulin (TUJ1, red). Myelinated peripheral nerves are more abundant in (A–H) proximal sections when compared to (I–P) distal sections (n=3). Scale bar: 200 µm.
Morphology and reformation of de novo neuromuscular junctions
During development, formation of the neuromuscular junction (NMJ) coincides with the expression and aggregation of postsynaptic acetylcholine receptors at the motor endplate in the muscle (Sharma and Belmonte, 2001; Darabid et al., 2014). To identify putative NMJs at motor endplates acetylcholine receptors of the post-synaptic membrane were labeled with fluorescently-conjugated α-bungarotoxin. Motor endplates of non-injured tails exhibit a mature perforated, pretzel-like appearance (Fig. 3A) (Marques et al., 2000). Following autotomy, scarce, granular expression of acetylcholine receptors was detectable in newly formed muscle by 15 DPA (Fig. 3B) and had assembled into diffuse patches by 30 DPA (Fig. 3C). These early aggregated clusters resembled compact but simple plaques (Fig. 3B–C). Maturation of skeletal muscle, as shown by expression of fast twitch myosin (MY32), is detectable by 30 DPA (Supplemental Material Fig. 2A–C). Between 70–120 DPA, the motor endplate appeared to further elongate, morphologically differentiate, and develop a relatively uniform density of BTX labeling (Fig. 3E–F) (Slater, 1982; Marques et al., 2000; Sleigh et al., 2014). Numerous morphologically mature NMJs were observed by 120–250 DPA, characterized by a highly-perforated appearance and distinctive pretzel-like structures (Fig. 3G). Mature 250 DPA regenerated tails did not exhibit significant differences in overall morphology of many NMJs when compared to the original tail; however, some immature appearing NMJs persisted. In summary, the progressive morphological maturation of NMJs during de novo regeneration of the neuromuscular system is analogous to development, but occurs over a relatively protracted time course.
Figure 3.
Morphology and formation of the neuromuscular junction in the regenerating tail. Representative morphology of BTX-labeled neuromuscular junctions in high resolution confocal images of (A) original, (B) 15 DPA, (C) 30 DPA, (D) 70 DPA, (E) 90 DPA, (F) 120 DPA, (G) and 250 DPA tails (n=at least 3 tails for each time point). Axons were labeled via β-III tubulin staining (TUJ1, red) and acetylcholine receptors by Alexa-fluor 488-conjugated α-bungarotoxin (BTX, green). Scale bar: 200 µm.
Dynamic changes in neuromuscular junction density during initial tail regeneration and subsequent maturation
Axonal re-innervation of denervated, but otherwise intact, muscle has been well-studied following selective nerve injury (Rich and Lichtman, 1989; Lømo, 2003). It is not known if de novo neuromuscular regeneration in the lizard tail mimics events that occur during normal development or utilizes unique processes. We first estimated the number of bungarotoxin-labeled NMJs in the muscle in transverse sections of original and regenerated tails 2–5 mm caudal to the autotomy zone. The number of NMJs per cross sectional area of muscle was measured in non-injured tails and regenerated tails at 120 and 250 DPA (Fig. 4A–D). Our results show a statistically significant increase in motor endplate density at 120 and 250 DPA relative to non-injured tails (Fig. 4D). NMJ density at 250 DPA is increased by 2.06-fold when compared to original tails, but was 1.58-fold less than in 120 DPA tails. At both stages of regeneration, a proportion of NMJs were localized to presumably ectopic sites within the muscle body in addition to NMJs located at expected sites along the muscle perimeter (Fig. 4B–C).
Figure 4.
Spatiotemporal changes in regenerated NMJ number and distribution. Representative confocal images of motor endplate distribution within a single muscle group labeled with TUJ1 (red), α-bungarotoxin (BTX, green), and DAPI (blue) in (A) original, (B) 120 DPA, (C) and 250 DPA tails. (D) Quantification of NMJ density between each time point following regeneration of the lizard tail. At 120 DPA (n=3), NMJ density is statistically increased relative to original tails (n=5) and 250 DPA (n=3) regenerated tails. Though lower than 120 DPA, NMJ density at 250 DPA is still greater than original tails. Data are represented as mean sd; statistical analysis performed by one-way ANOVA analysis test followed by Tukey’s post-hoc test. **p <0.001, *p < 0.05, n.s. = not significant. The non-parametric, Kruskal-Wallis rank test followed by Dunn’s post-hoc test further confirmed the statistical significance of the changes in first regenerated tails (Dunn’s post-hoc p<0.05). R1: first regenerated tail, R2: second regenerated tail. Scale bars: 50 µm.
Preconditioning of mature nerves via crush or selective injury has been shown to accelerate the rate of adult nerve regeneration in response to a subsequent injury (McQuarrie and Grafstein, 1973; Franz et al., 2009; Hoffman-Kim et al., 2010). Lizards are capable of regenerating the tail multiple times raising the question of whether differences in neuromuscular regeneration occur during a second tail regeneration (Bryant and Bellairs, 1967; Maderson and Salthe, 1971; Alibardi, 2014). We examined lizard tails 250 days following a second autotomy and found that NMJ density remained elevated relative to original tails (Fig 4D). However, no significant difference in NMJ density was observed when first and second 250 DPA regenerated tails were compared (Fig. 4D).
Spinal cord derived axons transit across foramina in the cartilaginous endoskeletal tube
Nerves in the regenerated tail are derived from axonal sprouting of neurons rostral to the breakpoint, but it is unknown whether they can exit the cartilage tube or strictly arise from axons outside of the rostral vertebral column. At 30 DPA, axons within the cartilage tube and adjacent to the ependyma are evident along the entire rostro-caudal axis between the breakpoint and the distal tip (Fig. 5A–B). Terminal TUJ1+ axons within 1 mm of the tip were also observed branching away from the ependyma and projecting radially into the periphery in agreement with previous reports (Fig. 5C–H) (Kamrin et al., 1955). These data demonstrate that one additional conduit by which peripherally projecting axons may reach muscle is via the ependymal tip prior to cartilage maturation.
Figure 5.
Central axons radiate from the terminal neuroependyma into the periphery. Transverse sections within 1 mm of the regenerated tail tip at 30 DPA (n=3). (A–B) The ependyma (ep) projects towards the distal tip. (C–H) Regenerating axons radiate from the terminal neuroependyma and radiate outwards towards peripheral tissue. Yellow arrows indicate axons exiting the ependyma. Scale bars: 50 µm.
At 30 DPA we could also detect occasional foramina in the cartilage tube (Fig. 6A). In previous work, foramina in the hyaline cartilage tube were found to serve as passageways for blood vessels (Fisher et al., 2012). Interestingly, a few TUJ1+ axons could be detected traversing through these foramina (Fig. 6B–C). These labeled axons appeared contiguous with the ependyma when viewed in sagittal sections (Fig. 6D–F). These foramina traversing axons are rare in number, but suggest the presence of multiple neuroanatomical conduits for regenerating axons (Fig. 6G) that may play a previously unappreciated role in the functional innervation of the regenerated lizard tail.
Figure 6.
Foramina in the cartilaginous tube serve as passageways for axons from the neuroependyma to the periphery. (A–C) Transverse sections through 30 DPA tails (n=3) and (D–F) sagittal sections through 90 DPA tails (n=3) stained for axons (TUJ1, red) and nuclei (DAPI, blue). Insets show axons traversing foramina found in the hyaline cartilage tube into peripheral tissues. Yellow arrows indicate TUJ1 labeled axons, yellow dashed lines label boundary of the cartilage tube. (G) Schematic representation of proposed neuroanatomical conduits of regenerating axons. Scale bars: 200 µm
Discussion
Lizard tail regeneration provides a remarkable example of appendage replacement in amniote vertebrates. Rapid and efficient restoration of neuromuscular functionality in the newly formed lizard tail is thought to be evolutionarily advantageous for behaviors important for predator evasion, such as balancing and leaping (Ballinger, 1973; Gillis et al., 2009). The mechanisms of neuromuscular development have been well-studied, whereas the time course and cellular events underlying de novo neuromuscular regeneration in the adult lizard tail has not. Our data show that while axonal regrowth, morphological maturation of NMJs, and myelination qualitatively mimic strategies utilized during embryogenesis, the number of NMJs was sustained at a higher level providing evidence of processes specific to adult tails.
Neuromuscular development versus regeneration
The initial development of the neuromuscular system involves complex reciprocal interactions between axons, Schwann cells, and skeletal muscle. Early stages of muscle formation occur relatively independently of nerve interactions, including some degree of acetylcholine receptor (AChR) clustering (Lin et al., 2001; Lømo, 2003; Darabid et al., 2014). Subsequent neuronal innervation modulates later stages of muscle differentiation into distinct fiber types and long-term muscle survival (Condon et al., 1990; McLennan, 1994; DiMario and Stockdale, 1997; Hughes and Salinas, 1999). For example, experimental misrouting of motor axons has been shown to alter muscle fiber type identity (Vogel and Landmesser, 1987; Maggs et al., 2008).
Peripheral nerves and muscle maintain remarkable regenerative capabilities in adult amniotes; however, most studies have focused on the effects of focal nerve injury or selective muscle damage. These models typically preserve key extracellular structures, such as the synaptic basal lamina and epineurium. Regenerating axons are able to recapitulate the original pattern of growth and synaptic innervation in response to residual Schwann cell-lined endoneurial tubes or synaptic basal lamina that serve as scaffolds after nerve and/or muscle have degenerated (Sanes et al., 1978; Rich and Lichtman, 1989; Son and Thompson, 1995; Nguyen et al., 2002; Li et al., 2011; Kang and Lichtman, 2013). The dynamics of complete neuromuscular regeneration during de novo tail regeneration in lizards are less understood.
Our findings further demonstrate that axons quickly regenerate in the regenerating lizard tail (Hughes and New, 1959; Fisher et al., 2012; Hutchins et al., 2014). We show that regenerating axons appear to form morphologically identifiable NMJs on newly formed muscle between 15–30 DPA. We also detected a significant increase in the density of axonal labeling in muscle at 30 and 120 DPA when compared to the original tail. Differences in the level of expression, or distribution of βIII-tubulin in regenerating axons relative to original tail axons may contribute to this observation. However, these data are consistent with some degree of axonal overgrowth during early stages of tail regeneration. We have also identified a rare, but previously unappreciated source of axons traversing foramina in the cartilage tube whose precise origin have yet to be identified. Past transcriptomic analyses have identified altered expression of putative regulators of axon growth and guidance in the regenerating tail at 25 DPA, including slit2, slit3, robo2, unc5c, and ndnf (Hutchins et al., 2014). Future studies could identify the precise cellular source and functional contribution of these candidate molecules to axonal regrowth.
During mammalian development, multiple motor neurons innervate a single motor endplate on an embryonic muscle fiber (Bennett and Pettigrew, 1974; Tapia et al., 2012). As development proceeds, axons are pruned in an activity-dependent manner from polyneuronally innervated synapses to generate the adult, mononeuronal pattern (Tintignac et al., 2015). In regenerating lizard tails, we observed a reduction in axonal density in the muscle between 30 and 120 DPA. In combination with the decrease in NMJ density that occurs between 120 and 250 DPA, our data suggest axonal and synaptic pruning plays a key role in sculpting the final pattern of neuromuscular connectivity during de novo neuromuscular regeneration (Tapia et al., 2012; Darabid et al., 2014). Developing motor endplates also undergo significant morphological remodeling, termed the plaque-to-pretzel transition, where the activity-dependent loss of postsynaptic AChRs at endplates leads to a highly-perforated appearance (Marques et al., 2000; Tintignac et al., 2015). We have found that motor endplates in the regenerating lizard tail reactivate a comparable process of morphological maturation between 30 DPA and 250 DPA.
Re-myelination of regenerated axons is critical for promoting appropriate nerve function and fast action potential propagation (Feltri et al., 2002; Chen and Strickland, 2003; Yu et al., 2005). We observed significant MBP expression along regenerating peripheral nerves in proximal regions near the breakpoint within 15 days of tail autotomy. However, MBP expression along peripheral axons within 1 mm of the distal tip was significant reduced relative to MBP expression in proximal nerves at both 15 and 30 DPA. These data confirm that Schwann cells are able to populate the regenerating nerve and rapidly remyelinate along the proximo-distal axis (Hughes and New, 1959; McPhilemy et al., 1990). Interestingly, presumptive oligodendrocytes within the cartilage tube expressed significantly higher levels of MBP in the distal tip relative to the peripheral axons, suggesting differences in the temporal regulation of differentiation in adult peripheral and central myelinating glia. Both oligodendrocytes and Schwann cells produce trophic molecules necessary for axonal regrowth and regeneration (Son and Thompson, 1995; Kumar et al., 2007; Pirotte et al., 2016). In regenerating rodent peripheral nerves, macrophage-derived VEGF stimulate blood vessel formation, which provide a requisite scaffold for Schwann cell dependent nerve regrowth (Cattin et al., 2015). Notably, the tip of the regenerating lizard tail is highly vascularized and expresses high levels of VEGF (Fisher et al., 2012; Hutchins et al., 2014; Payne et al., 2017). The lizard tail provides a novel model system to further examine poorly understood interactions between cellular subsystems in a regenerating appendage. In summary, our results indicate that during lizard tail regeneration, the neuromuscular system reactivates a coordinated process of axonal overgrowth and pruning, remyelination, and endplate morphological maturation that is analogous to a developmental program.
Adult-specific NMJ patterning in the regenerating lizard tail
During mammalian embryogenesis, NMJs primarily form on a restricted band along a narrow equatorial region of developing multinucleated muscle fibers. A refractory zone is thought to form around newly formed AChR clusters that inhibits the formation of more than one motor endplate per fiber in common parallel-fibered muscles (Bennett and Pettigrew, 1974; Frank et al., 1975; Tintignac et al., 2015). A variation on this theme has been observed in long serially-fibered muscles. In these muscles, some fibers terminate intrafascicularly forming multiple endplates per fascicle; however, individual intrafascicular fibers are still innervated at a single endplate (Paul et al., 2004; Harris, 2005). Regeneration following selective nerve injury in mammals typically leads to axonal reinnervation at the location of original NMJs without initiating major changes in synapse number (Rich and Lichtman, 1989; Li et al., 2011). In stark contrast, when nerves are artificially manipulated to cross-reinnervate a different denervated muscle, numerous ectopic NMJs form at sites outside of the original endplate band and many single muscle fibers are innervated at multiple endplates (Bennett and Pettigrew, 1974; Lømo, 2003; Bosse et al., 2006).
Here, we have noted a substantial and persistent increase in the density of BTX-labeled NMJs in regenerating lizard tail muscles at 120 DPA and 250 DPA relative to original tails. Furthermore, we observed numerous NMJs ectopically localized within the muscle body as opposed to along the muscle perimeter. These data suggest that regenerating muscle fibers are innervated at multiple sites. The increased length of regenerating muscle relative to embryonic muscle may contribute to enhanced NMJ density since the refractory zone between endplates allows for multiple NMJs in a single regenerated adult fiber (Lømo, 2003). It is also possible that regenerating axons exhibit enhanced secretion of synapse promoting cues, such as agrin, but a past transcriptomic analysis did not detect a significant change in agrin expression (Hutchins et al., 2014). The increased NMJ density also begs the question of whether this increase is necessary for adult tail functionality. Minimally, these findings show that de novo regeneration of the neuromuscular system employs some processes specific to adult regeneration.
One mechanistic question arising from this work is whether the increased NMJ density is regulated by molecular mediators from neurons or muscle. Neurons re-innervating the regenerating muscle are likely mismatched relative to the original pattern of neuromuscular innervation. In support of this, regenerating motor neurons in geckonid lizards undergo significant changes in size and expression of markers of neuronal identity during tail regeneration (Cristino et al., 2000a; Cristino et al., 2000b). Alterations in muscle redifferentiation also cannot be excluded. Our data provide additional support for past studies that have shown regenerated and original tail muscle both consist primarily of fast myosin expressing fibers (Supplemental Figure 2) (Alibardi, 1995; Meyer et al., 2002; Alibardi, 2015). Yet, the altered anatomy and organization of regenerated tail muscle, such as muscle groups attached to each other or the lack of a nested cone arrangement, points to key differences (Fisher et al., 2012; Ritzman et al., 2012; Alibardi, 2015). Regenerating muscle may even contain serially-fibered fascicles, which would promote an elevated NMJ density. In summary, lizard tail regeneration might be viewed as a naturally occurring cross-reinnervation experiment. Since many aspects of muscle patterning have initiated before NMJs become fully functional in the regenerating tail, it is possible early muscle abnormalities are less-dependent upon nerve derived cues. Future detailed studies of single muscle fiber morphology, differentiation, and endplate number in teased muscle preparations will be crucial for further mechanistic insight.
Few therapies exist for promoting regeneration of mammalian appendages such as digits or repairing volumetric muscle loss following severe, traumatic injury (Turner and Badylak, 2011). Directed skeletal muscle reinnervation is also important for treating long-term denervation and in a surgical technique for neural control of prosthetics, known as targeted muscle reinnervation. The regenerating lizard tail provides an accessible model to study nerve-muscle interactions and identify candidate molecules for enhancing de novo neuromuscular regeneration in mammalian systems.
Experimental Procedures
Animal care and tail collection
Adult Anolis carolinensis lizards were acquired from Charles D. Sullivan, Inc. (Nashville, TN) and maintained according to the guidelines of the Institutional Animal Care and Use Committee at Arizona State University. Sex-differences in Anolis neural circuits, which regulate copulatory behavior, have been defined (Wade, 2016). However, sexual dimorphism in the morphology of regenerated tails has not been detected (Ritzman et al., 2012). Thus, only female lizards were utilized in this study due to complications related to territorial aggression and fighting between co-housed males. The animals were housed in Percival incubators (Boone, IA), which maintained a constant 70% humidity and alternated between 14 hours of daylight at 28°C and 10 hours of darkness at 19°C. Anoles were fed crickets every 2 days, which were supplemented with Rep-cal calcium and Vitamin D (Repcal Research Labs, Los Gatos, CA) once weekly. Artificial plants inside each cage were misted daily as a water source. Animals were autotomized 2.5–3 cm from the vent, a region of the tail previously shown to contain autotomy planes (Ritzman et al., 2012). Tails were firmly pinched while lightly shaking the animal until the tail was released by stress-induced autotomy. Regenerated tails were collected 15, 30, 70, 90, 120 or 250 days post autotomy (DPA) by inducing autotomy just proximal to the regenerated portion of the tail.
Tissue processing and immunostaining
Tails were immersion fixed in 4% paraformaldehyde at 4°C with constant agitation for 24–48 hours, embedded in 5% agarose and sectioned on a vibrating microtome (Model 7000smz-2, Campden Instruments Ltd.). Transverse sections were collected 2–5 mm distal to the point of autotomy for regenerated tails and at a similar relative position for original tails, with the exception of the 15 DPA and 30 DPA time points, for which sections were collected from the entire length of the tail. Sagittal sections were also collected from the distal tips of 90 DPA tails. All sections were generated at a thickness of 50 µm.
Sections were permeabilized in PBS containing 0.2% Triton X-100 and then incubated in blocking solution (PBS, 5% normal donkey serum, 0.2% Triton X-100) for 30 minutes to 1 hour at room temperature. Sections were then incubated in a combination of primary antibodies diluted in blocking solution overnight at 4°C (Table 1). A mouse polyclonal antibody against β-III tubulin (TUJ1) was used to identify axons (Clone TUJ1, BioLegend, San Diego, CA). Myelination was detected by rat monoclonal antibody against myelin basic protein (MBP, Clone 12, Abcam, Cambridge, MA). A mouse monoclonal antibody against fast-twitch skeletal myosin (MY32) was used to detect skeletal muscle (Clone MY32, Sigma Aldrich, St. Louis, MO). Following 3 washes in PBS, sections were incubated in an appropriate combination of Alexa-fluor-conjugated secondary antibodies (Life Technologies), DAPI (1 ug/ml) and conjugated α-bungarotoxin (Thermo Fisher Scientific) diluted in blocking solution at 4°C overnight. Sections were rinsed 3 times in PBS and mounted.
Table 1.
List of antibodies used in this study
| Name | Target | Host & Isotype | Clonality | Dilution | Vendor | Catalog # |
|---|---|---|---|---|---|---|
| TUJ1 (TUBB3) | neuron specific βIII-tubulin | Mouse IgG2a | polyclonal | 1:1000 | BioLegend | 801202 |
| MBP | myelin basic protein | Rat IgG2a | monoclonal | 1:1000 | Abcam | ab7349 |
| MY-32 | myosin (skeletal, fast) | Mouse IgG1 | monoclonal | 1:500 | Sigma Aldrich | M4276 |
| Alexa 555 | Mouse IgG (H+L) | Donkey IgG | polyclonal | 1:1000 | Life Technologies | A31570 |
| Alexa 647 | Rat IgG (H+L) | Donkey IgG | polyclonal | 1:1000 | Jackson ImmunoResearch | 712-605-150 |
| Alexa 488 α-bungarotoxin | acetylcholine receptor | – | – | 1:1000 | Life Technologies | B13422 |
| DAPI | DNA/nuclei | – | – | 1:1000 | Roche Diagnostics | 10-236-276-001 |
Image collection and analysis
Immunolabeled sections were imaged on an Olympus Epifluorescent microscope with a Hamamatsu CCD camera, a Leica SP5, or a Zeiss LSM800 Confocal System. For MBP immunolabeled sections, laser intensity and gain settings were fixed when imaging along the proximal-distal axis of the regenerated tail. To determine total TUJ1+ pixel area, NMJ number, and cross-sectional muscle area, at least three images from three separate sections per animal were collected. Peripheral TUJ1+ pixels were measured in the muscle as defined by the region between the outer edge of the cartilage tube and the tail epidermis. Images were converted to 8-bit greyscale, manually thresholded, and measured in Fiji. Average TUJ1+ pixel and NMJ densities were calculated at each time point and then compared using a one-way ANOVA statistical test followed by Tukey’s post hoc test. The nonparametic Kruskal-Wallis rank test followed by the Dunn’s post-hoc test was also applied as an alternative assessment of relative density. Representative images have been cropped and adjusted for brightness and contrast in Photoshop.
Supplementary Material
Supplemental Figure 1. MBP expression is present in proximal nerves during early stages of lizard tail regeneration, but is reduced in distal nerves. MBP expression in (A–C) proximal and (D–F) distal sections of the regenerated tail at 15 DPA. Scale bar: 200 µm
Supplemental Figure 2. Skeletal muscle maturation begins during early stages of regeneration and expresses fast-twitch myosin. (A–C) Individual muscles exhibit significant expression of the mature muscle marker, MY32, at 30 DPA. Scale bar: 200 µm
Highlights.
Axon regrowth and de novo myelination occurs early during lizard tail regeneration.
NMJ distribution on de novo tail muscle is found at normal and ectopic locations.
The density of axons and NMJ’s in muscle was persistently higher in the regenerated tail.
The morphological maturation of regenerated NMJs mimics development.
Acknowledgments
We thank Michael Holter and Colton Smith for technical assistance; Joanna Palade for providing us with technical support in utilizing the MY32 antibody; the Department of Animal Care and Technologies at Arizona State University for support in lizard colony maintenance; and Terry Ritzman for helpful discussions. This work was supported by funding from the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) grant R21 RR031305, National Institute of Arthritis, Musculoskeletal, and Skin Diseases grant R21 AR064935 of the National Institutes of Health, and funding from the Arizona Biomedical Research Commission grant 1113 to KK and National Institute of Neurological Disorders and Stroke grant R01 NS097537 to JMN. Additional funding to KK was provided by the College of Liberal Arts and Sciences, Arizona State University.
References
- Alibardi L. Muscle differentiation and morphogenesis in the regenerating tail of lizards. J Anat. 1995;186:143–151. [PMC free article] [PubMed] [Google Scholar]
- Alibardi L, Gibbons J, Simpson SB. Fine structure of cells in the young regenerating spinal cord of the lizard Anolis carolinensis after H3-thymidine administration. Biol Struct Morphogen. 1992;4:45–52. [PubMed] [Google Scholar]
- Alibardi L. Regenerating tail muscles in lizard contain Fast but not Slow Myosin indicating that most myofibers belong to the fast twitch type for rapid contraction. Tissue Cell. 2015;47:533–540. doi: 10.1016/j.tice.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Alibardi L. Histochemical, Biochemical and Cell Biological aspects of tail regeneration in lizard, an amniote model for studies on tissue regeneration. Prog Histochem Cyto. 2014;48:143–244. doi: 10.1016/j.proghi.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Ballinger RE. Comparative demography of two viviparous iguanid lizards (Sceloporus jarrovi and Sceloporus poinsetti) Ecology. 1973;54:269–283. [Google Scholar]
- Bellairs AA, Bryant SV. Autotomy and regeneration in reptiles Biology of the Reptilia. John Wiley and Sons; New York: 1985. [Google Scholar]
- Bennett MR, Pettigrew AG. The formation of synapses in reinnervated and cross‐reinnervated striated muscle during development. J Physiol. 1974;241:547–573. doi: 10.1113/jphysiol.1974.sp010671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosse F, Hasenpusch-Theil K, Küry P, Müller HW. Gene expression profiling reveals that peripheral nerve regeneration is a consequence of both novel injury-dependent and reactivated developmental processes. J Neurochem. 2006;96:1441–1457. doi: 10.1111/j.1471-4159.2005.03635.x. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Bryant SV, Bellairs AD. Tail regeneration in the lizards Anguis fragilis and Lacerta dugesii. Zool J Linn Soc-London. 1967;46:297–305. [Google Scholar]
- Cattin AL, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJA, Calavia NG, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell. 2015;162:1127–1139. doi: 10.1016/j.cell.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Laminin γ1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon K, Silberstein L, Blau HM, Thompson WJ. Development of muscle fiber types in the prenatal rat hindlimb. Dev Biol. 1990;138:256–274. doi: 10.1016/0012-1606(90)90196-p. [DOI] [PubMed] [Google Scholar]
- Cox PG. Some aspects of tail regeneration in the lizard, Anolis carolinensis. I. A description based on histology and autoradiography. J Exp Zool Part A. 1969;171:127–149. [Google Scholar]
- Cristino L, Pica A, Corte, Della F, Bentivoglio M. Plastic changes and nitric oxide synthase induction in neurons that innervate the regenerated tail of the lizard Gekko gecko: I. Response of spinal motoneurons to tail amputation and regeneration. J Comp Neurol. 2000a;417:60–72. [PubMed] [Google Scholar]
- Cristino L, Pica A, Corte, Della F, Bentivoglio M. Plastic changes and nitric oxide synthase induction in neurons which innervate the regenerated tail of the lizard Gekko gecko: II. The response of dorsal root ganglion cells to tail amputation and regeneration. Brain Res. 2000b;871:83–93. doi: 10.1016/s0006-8993(00)02445-8. [DOI] [PubMed] [Google Scholar]
- Cristino L, Pica A, Corte, Della F, Bentivoglio M. Co-induction of nitric oxide synthase, bcl-2 and growth-associated protein-43 in spinal motoneurons during axon regeneration in the lizard tail. Neuroscience. 2000c;101:451–458. doi: 10.1016/s0306-4522(00)00393-6. [DOI] [PubMed] [Google Scholar]
- Darabid H, Perez-Gonzalez AP, Robitaille R. Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nat Rev Neurosci. 2014;15:703–718. [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Both myoblast lineage and innervation determine fiber type and are required for expression of the slow myosin heavy chain 2 gene. Dev Biol. 1997;188:167–180. doi: 10.1006/dbio.1997.8619. [DOI] [PubMed] [Google Scholar]
- Duffy MT, Liebich DR, Garner LK, Hawrych A, Simpson SB, Davis BM. Axonal sprouting and frank regeneration in the lizard tail spinal cord: correlation between changes in synaptic circuitry and axonal growth. J Comp Neurol. 1992;316:363–374. doi: 10.1002/cne.903160307. [DOI] [PubMed] [Google Scholar]
- Duffy MT, Simpson SB, Liebich DR. Origin of spinal cord axons in the lizard regenerated tail: supernormal projections from local spinal neurons. J Comp Neurol. 1990;293:208–222. doi: 10.1002/cne.902930205. [DOI] [PubMed] [Google Scholar]
- Egar M, Simpson SB, Singer M. The growth and differentiation of the regenerating spinal cord of the lizard Anolis carolinensis. J Morphol. 1970;131:131–152. doi: 10.1002/jmor.1051310202. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Porta DG, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, et al. Conditional disruption of β1 integrin in Schwann cells impedes interactions with axons. J Cell Biol. 2002;156:199–210. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RE, Geiger LA, Stroik LK, Hutchins ED, George RM, DeNardo DF, Kusumi K, Rawls JA, Wilson-Rawls J. A histological comparison of the original and regenerated tail in the green anole, Anolis carolinensis. Anat Rec. 2012;295:1609–1619. doi: 10.1002/ar.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank E, Jansen JK, Lono T, Westgaard RH. The interaction between foreign and original motor nerves innervating the soleus muscle of rats. J Physiol. 1975;247:725–743. doi: 10.1113/jphysiol.1975.sp010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CK, Quach ET, Krudy CA, Federici T, Kliem MA, Snyder BR, Raore B, Boulis NM. A Conditioning Lesion Provides Selective Protection in a Rat Model of Amyotrophic Lateral Sclerosis. PLoS ONE. 2009;4:e7357–8. doi: 10.1371/journal.pone.0007357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis GB, Bonvini LA, Irschick DJ. Losing stability: tail loss and jumping in the arboreal lizard Anolis carolinensis. J Exp Biol. 2009;212:604–609. doi: 10.1242/jeb.024349. [DOI] [PubMed] [Google Scholar]
- Harris AJ. Embryonic growth and innervation of rat skeletal muscles. I. Neural regulation of muscle fibre numbers. Philos Trans R Soc Lond, B, Biol Sci. 1981;293:257–277. doi: 10.1098/rstb.1981.0076. [DOI] [PubMed] [Google Scholar]
- Harris AJ. Muscle Fiber and Motor Unit Behavior in the Longest Human Skeletal Muscle. J Neurosci. 2005;25:8528–8533. doi: 10.1523/JNEUROSCI.0923-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman-Kim D, Mitchel JA, Bellamkonda RV. Topography, Cell Response, and Nerve Regeneration. Annu Rev Biomed Eng. 2010;12:203–231. doi: 10.1146/annurev-bioeng-070909-105351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, New D. Tail regeneration in the geckonid lizard, Sphaerodactylus. Development. 1959;7:281–302. [PubMed] [Google Scholar]
- Hughes SM, Salinas PC. Control of muscle fibre and motoneuron diversification. Curr Opin Neurobiol. 1999;9:54–64. doi: 10.1016/s0959-4388(99)80007-5. [DOI] [PubMed] [Google Scholar]
- Hutchins ED, Eckalbar WL, Wolter JM, Mangone M, Kusumi K. Differential expression of conserved and novel microRNAs during tail regeneration in the lizard Anolis carolinensis. BMC Genomics. 2016;17:339. doi: 10.1186/s12864-016-2640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchins ED, Markov GJ, Eckalbar WL, George RM, King JM, Tokuyama MA, Geiger LA, Emmert N, Ammar MJ, Allen AN, et al. Transcriptomic Analysis of Tail Regeneration in the Lizard Anolis carolinensis Reveals Activation of Conserved Vertebrate Developmental and Repair Mechanisms. PLoS ONE. 2014;9:e105004–12. doi: 10.1371/journal.pone.0105004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Lichtman JW. Motor Axon Regeneration and Muscle Reinnervation in Young Adult and Aged Animals. J Neurosci. 2013;33:19480–19491. doi: 10.1523/JNEUROSCI.4067-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamrin RP, Singer M. The influence of the spinal cord in regeneration of the tail of the lizard Anolis carolinensis. J Experimental Zoology. 1955;12:611–627. [Google Scholar]
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance-Jones C, Landmesser L. Motoneurone projection patterns in the chick hind limb following early partial reversals of the spinal cord. The J Physiol. 1980;302:581–602. doi: 10.1113/jphysiol.1980.sp013262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lee YI, Thompson WJ. Changes in aging mouse neuromuscular junctions are explained by degeneration and regeneration of muscle fiber segments at the synapse. J Neurosci. 2011;31:14910–14919. doi: 10.1523/JNEUROSCI.3590-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Burgess RW, Dominguez B, Pfaff SL, Sanes JR. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature. 2001;410:1057–1064. doi: 10.1038/35074025. [DOI] [PubMed] [Google Scholar]
- Lozito TP, Tuan RS. Lizard tail regeneration_ regulation of two distinct cartilage regions by Indian hedgehog. Dev Biol. 2015;399:249–262. doi: 10.1016/j.ydbio.2014.12.036. [DOI] [PubMed] [Google Scholar]
- Lozito TP, Tuan RS. Lizard tail skeletal regeneration combines aspects of fracture healing and blastema-based regeneration. Development. 2016;143:2946–2957. doi: 10.1242/dev.129585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lømo T. What controls the position, number, size, and distribution of neuromuscular junctions on rat muscle fibers? J Neurocytol. 2003;32:835. doi: 10.1023/B:NEUR.0000020627.18156.b1. [DOI] [PubMed] [Google Scholar]
- Maggs AM, Huxley C, Hughes SM. Nerve-dependent changes in skeletal muscle myosin heavy chain after experimental denervation and cross-reinnervation and in a demyelinating mouse model of Charcot-Marie-Tooth disease type 1A. Muscle Nerve. 2008;38:1572–1584. doi: 10.1002/mus.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques MJ, Conchello JA, Lichtman JW. From plaque to pretzel: fold formation and acetylcholine receptor loss at the developing neuromuscular junction. J Neurosci. 2000;20:3663–3675. doi: 10.1523/JNEUROSCI.20-10-03663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan IS. Differentiation of muscle fiber types in the chicken hindlimb. Dev Biol. 1983;97:222–228. doi: 10.1016/0012-1606(83)90079-9. [DOI] [PubMed] [Google Scholar]
- McLennan IS. Neurogenic and myogenic regulation of skeletal muscle formation: a critical re-evaluation. Prog Neurobio. 1994;44:119–140. doi: 10.1016/0301-0082(94)90035-3. [DOI] [PubMed] [Google Scholar]
- McPhilemy K, Mitchell LS, Griffiths IR, Morrison S, Deary AW, Sommer I, Kennedy PG. Effect of optic nerve transection upon myelin protein gene expression by oligodendrocytes: evidence for axonal influences on gene expression. J Neurocytol. 1990;19:494–503. doi: 10.1007/BF01257239. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Grafstein B. Axon outgrowth enhanced by a previous nerve injury. AMA Arch Neurol. 1973;29:53–55. doi: 10.1001/archneur.1973.00490250071008. [DOI] [PubMed] [Google Scholar]
- Maderson PFA, Salthe SN. Further Observations on Tail Regeneration in Anolis carolinensis (Iguanidae, Lacertilia) J Exp Zool. 1971;177:185–190. doi: 10.1002/jez.1401770206. [DOI] [PubMed] [Google Scholar]
- Meyer V, Preest MR, Lochetto SM. Physiology of original and regenerated lizard tails. Herpetologica. 2002;58:75–86. [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat Neurosci. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- Paul AC, Sheard PW, Duxson MJ. Development of a mammalian series-fibered muscle. Anat Rec. 2004;278A:571–578. doi: 10.1002/ar.a.20020. [DOI] [PubMed] [Google Scholar]
- Payne SL, Peacock HM, Vickaryous MK. Blood vessel formation during tail regeneration in the leopard gecko (Eublepharis macularius): The blastema is not avascular. J Morphol. 2017;278:380–389. doi: 10.1002/jmor.20648. [DOI] [PubMed] [Google Scholar]
- Pirotte N, Leynen N, Artois T, Smeets K. Do you have the nerves to regenerate? The importance of neural signalling in the regeneration process. Dev Biol. 2016;409:4–15. doi: 10.1016/j.ydbio.2015.09.025. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Tanaka EM. Editorial overview: Cell reprogramming, regeneration and repair. Curr Opin Genet Dev. 2016;40:iv–vi. doi: 10.1016/j.gde.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Rich MM, Lichtman JW. In vivo visualization of pre-and postsynaptic changes during synapse elimination in reinnervated mouse muscle. J Neurosci. 1989;9:1781–1805. doi: 10.1523/JNEUROSCI.09-05-01781.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzman TB, Stroik LK, Julik E, Hutchins ED, Lasku E, DeNardo DF, Wilson-Rawls J, Rawls JA, Kusumi K, Fisher RE. The Gross Anatomy of the Original and Regenerated Tail in the Green Anole (Anolis carolinensis) Anat Rec. 2012;295:1596–1608. doi: 10.1002/ar.22524. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Marshall LM, McMahan UJ. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978;78:176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma K, Belmonte JCI. Development of the limb neuromuscular system. Curr Opin Cell Biol. 2001;13:204–210. doi: 10.1016/s0955-0674(00)00198-8. [DOI] [PubMed] [Google Scholar]
- Simpson SB. Morphology of the regenerated spinal cord in the lizard, Anolis carolinensis. J Comp Neurol. 1968;134:193–210. doi: 10.1002/cne.901340207. [DOI] [PubMed] [Google Scholar]
- Singer M. Induction of Regeneration of Body Parts in the Lizard, Anolis. P Soc Exp Biol Med. 1961;107:106–108. [Google Scholar]
- Slater CR. Postnatal maturation of nerve-muscle junctions in hindlimb muscles of the mouse. Dev Biol. 1982;94:11–22. doi: 10.1016/0012-1606(82)90063-x. [DOI] [PubMed] [Google Scholar]
- Sleigh JN, Burgess RW, Gillingwater TH, Cader MZ. Morphological analysis of neuromuscular junction development and degeneration in rodent lumbrical muscles. J Neurosci Meth. 2014;227:159–165. doi: 10.1016/j.jneumeth.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YJ, Thompson WJ. Nerve sprouting in muscle is induced and guided by processes extended by Schwann cells. Neuron. 1995;14:133–141. doi: 10.1016/0896-6273(95)90247-3. [DOI] [PubMed] [Google Scholar]
- Tanaka EM. The Molecular and Cellular Choreography of Appendage Regeneration. Cell. 2016;165:1598–1608. doi: 10.1016/j.cell.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Tapia JC, Wylie JD, Kasthuri N, Hayworth KJ, Schalek R, Berger DR, Guatimosim C, Seung HS, Lichtman JW. Pervasive Synaptic Branch Removal in the Mammalian Neuromuscular System at Birth. Neuron. 2012;74:816–829. doi: 10.1016/j.neuron.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Tintignac LA, Brenner HR, Rüegg MA. Mechanisms Regulating Neuromuscular Junction Development and Function and Causes of Muscle Wasting. Physiol Rev. 2015;95:809–852. doi: 10.1152/physrev.00033.2014. [DOI] [PubMed] [Google Scholar]
- Tornini VA, Poss KD. Keeping at Arm’s Length during Regeneration. Dev Cell. 2014;29:139–145. doi: 10.1016/j.devcel.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsonis PA. Regeneration in Vertebrates. Dev Biol. 2000;221:273–284. doi: 10.1006/dbio.2000.9667. [DOI] [PubMed] [Google Scholar]
- Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2011;347:759–774. doi: 10.1007/s00441-011-1185-7. [DOI] [PubMed] [Google Scholar]
- Vogel M, Landmesser L. Distribution of fiber types in embryonic chick limb muscles innervated by foreign motoneurons. Dev Biol. 1987;119:481–495. doi: 10.1016/0012-1606(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Wade J. Genetic regulation of sex differences in songbirds and lizards. Philos Trans R Soc Lond, B, Biol Sci. 2016;371:20150112–10. doi: 10.1098/rstb.2015.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WM, Feltri ML, Wrabetz L, Strickland S, Chen ZL. Schwann Cell-Specific Ablation of Laminin 1 Causes Apoptosis and Prevents Proliferation. J Neurosci. 2005;25:4463–4472. doi: 10.1523/JNEUROSCI.5032-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. MBP expression is present in proximal nerves during early stages of lizard tail regeneration, but is reduced in distal nerves. MBP expression in (A–C) proximal and (D–F) distal sections of the regenerated tail at 15 DPA. Scale bar: 200 µm
Supplemental Figure 2. Skeletal muscle maturation begins during early stages of regeneration and expresses fast-twitch myosin. (A–C) Individual muscles exhibit significant expression of the mature muscle marker, MY32, at 30 DPA. Scale bar: 200 µm