Abstract
Vertebrates exhibit striking left-right (L-R) asymmetries in the structure and position of the internal organs. Symmetry is broken by motile cilia-generated asymmetric fluid flow, resulting in a signaling cascade — the Nodal-Pitx2 pathway — being robustly established within mesodermal tissue on the left side only. This pathway impinges upon various organ primordia to instruct their side-specific development. Recently, progress has been made in understanding both the breaking of embryonic L-R symmetry and how the Nodal-Pitx2 pathway controls lateralized cell differentiation, migration, and other aspects of cell behavior, as well as tissue-level mechanisms, that drive asymmetries in organ formation. Proper execution of asymmetric organogenesis is critical to health, making furthering our understanding of L-R development an important concern.
Keywords: Left-right asymmetry, cilia, Nodal, Pitx2, morphogenesis, cell migration
Left-right asymmetry in vertebrates: development and disease
Asymmetries between left and right are abundant in nature. Striking examples include fiddler crabs, with one large and one small claw, and flounders that lie permanently on one side. The narwhal’s tusk is a modified upper left incisor. In most vertebrates, however, left-right (L-R) asymmetries are hidden beneath the skin; the organs and vasculature are conspicuously L-R asymmetric in their position and pattern. The heart, for example, loops asymmetrically during development and ultimately acquires a leftward position in the chest cavity. The right and left lungs are comprised of distinct numbers of lobes. The stomach and pancreas sit to the left and the liver to the right in the abdomen. The gut coils asymmetrically, while the brain exhibits morphological and functional asymmetries. The consequences of aberrations to proper L-R patterning can be severe. Heterotaxy (see Glossary) is a heritable disorder present in approximately 1 in 10,000 births and is characterized by randomization of body situs (situs ambiguous) [1]. Notably, the incidence of congenital heart disease (CHD) is significantly increased in heterotaxy patients; transposition of the great arteries and ventricular septal defects are particularly prevalent [2]. Extracardiac malformations including multiple spleens (polysplenia), a midline liver, and extrahepatic biliary atresia may also present in heterotaxy patients [1]. In addition to being a complex genetic condition, model organism studies suggest that abnormalities of L-R patterning can also be induced by environmental factors [3]. Another disorder, primary ciliary dyskinesia (PCD), caused by defects in motile cilia, similarly results in L-R defects including situs ambiguous or the complete reversal of organ arrangement (situs inversus).
Early in development, an L-R asymmetric pathway (the ‘Nodal-Pitx2 pathway’) is established in lateral mesoderm tissue [4]. This pathway is active on the left side only, but how it informs the subsequent asymmetric morphogenesis of organs is only beginning to be understood. In this Review, we start with a brief discussion of the breaking of symmetry and the establishment of the Nodal-Pitx2 pathway. We then focus on how asymmetries in organ morphogenesis are instructed by the Nodal-Pitx2 pathway and downstream factors and processes. We shall see that although some distinct molecular, cellular, and tissue-level mechanisms are employed in different organs in response to Nodal signaling, common themes are also starting to emerge.
The origin of asymmetry and the conserved Nodal-Pitx2 pathway
In vertebrates, L-R asymmetry originates in transient midline structures known as Left-Right Organizers (LROs) that appear at the posterior end of the notochord during early somite stages. Shortly after LROs form, asymmetries in gene expression emerge in peripherally-located cells around LROs (Figure 1A). Though LROs are structurally diverse [5], the logic of how L-R symmetry is broken within them appears to be conserved across many vertebrates [6]. Cilia, microtubule-based organelles that protrude from the apical surface of cells, are found within LROs, one cilium per cell [7]. These cilia rotate and, as a result, generate fluid flow within the cavity of the LRO which is L-R asymmetric (Figure 1A) (Box 1). Asymmetric flows then drive asymmetries in gene expression around LROs; critically, the Nodal pathway repressor Dand5 is downregulated on the left side of LROs, resulting in an R>L Dand5 asymmetry that promotes activation of left-sided Nodal signaling (Figure 1A) [8–13]. How flow is sensed by flow-receiving cells is an intriguing question and is briefly discussed in Box 2.
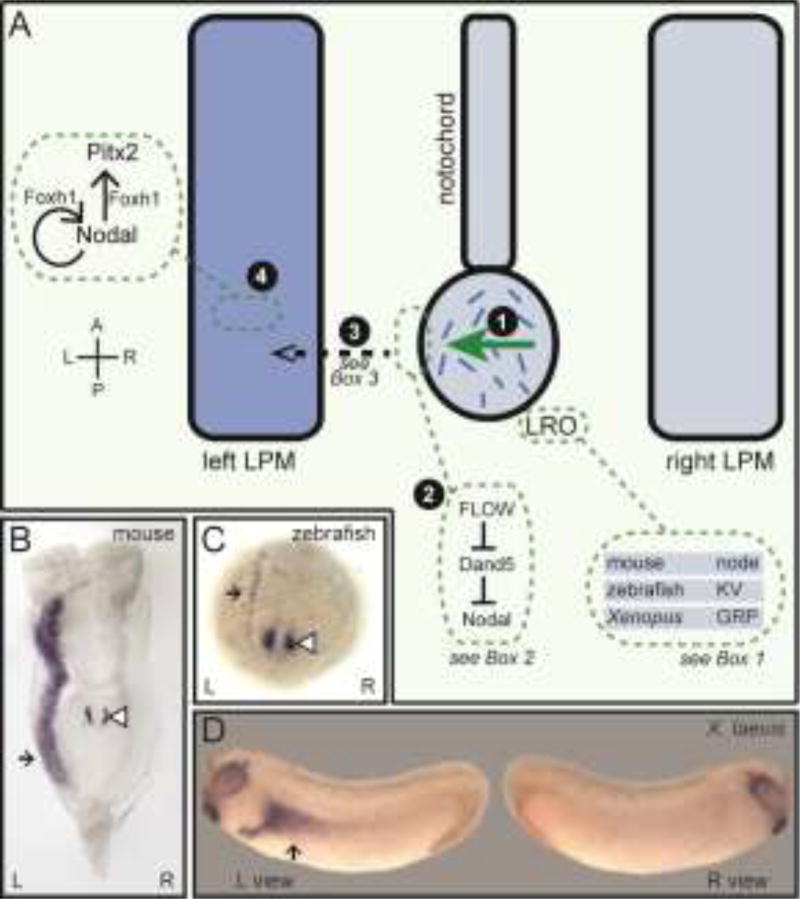
Figure 1. The origin of left-right asymmetry in vertebrates.
(A) Embryonic L-R symmetry is broken during somite stages when an asymmetric fluid flow is generated by motile cilia within midline L-R coordinators (LROs), transient structures at the posterior end of the notochord (1). This asymmetric flow is sensed at the periphery of LROs, resulting in repression of Dand5, and thereby the activation of Nodal, on the left side (2). Asymmetric signals, possibly Nodal itself, are then propagated to LPM tissue (3) where Nodal signals are established in the left but not right LPM; there they activate further Nodal expression and expression of the transcription factor Pitx2, requiring FoxH1 (4). This asymmetric Nodal-Pitx2 pathway is critical for downstream asymmetric organ morphogenesis and is conserved across species. (B–D) Whole mount in situ hybridization in different organisms using probes for Nodal (B–C) or Pitx2 (D). Black arrow denotes LPM expression while white arrowhead denotes LRO expression. A; anterior, GRP; gastroceol roof plate, KV; Kupffer's vesicle, L; left, LPM; lateral plate mesoderm, LRO; left-right coordinator, R; right, P; posterior.
Box 1. The generation of asymmetric flow.
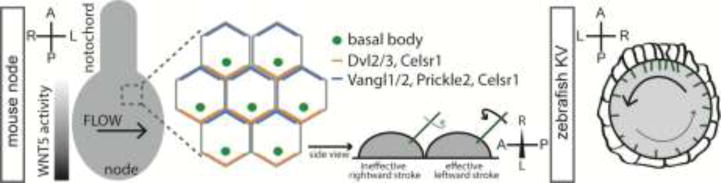
Motile cilia are cellular protrusions that beat or rotate to move extracellular fluid [57] in a variety of contexts including in mammalian airways, Xenopus larval skin and in the kidney and spinal canal of zebrafish [57–60]. A range of vertebrates also exhibit motile cilia within LROs where they generate L-R asymmetric fluid flows that drive asymmetries in signaling molecule activities around the periphery of LROs [58, 61–63]. In mouse and Xenopus, LRO cilia are polarized such that they are positioned to the posterior side of LRO cells, a process which depends on core planar cell polarity components [64] and, at least in mouse embryos, a gradient of WNT5 activity across the node in the A-P dimension [65]. This positioning imparts a posterior tilt to the cilia which, owing to the clockwise rotation of cilia when viewed ventrally, results in an ineffective rightward stroke near the cell surface and an effective leftward stroke that drives fluid flow across the node towards the left side (Figure I).
The LRO of zebrafish, Danio rerio, embryos is somewhat different. Rather than being a relatively flat ciliated surface like the mouse node, Kupffer's vesicle (KV) is an internal fluid-filled sphere with ciliated cells on all surfaces. This architecture results in circular counterclockwise flow of fluid within the lumen of KV. Cells at the anterior tip of KV are more columnar resulting in a higher density of cilia and thereby faster fluid flow in this region, an arrangement that results in L-R asymmetry in the strength of flow, with flow being fastest in the anterior left segment of the spherical KV (Figure I) [66, 67].
Thus, while species-specific differences in LRO architecture exist, the overall logic of cilia-driven asymmetric flow is conserved in many vertebrates. However, a motile ciliated LRO is absent in chicken and pig embryos [19]; we still don't understand the origin of asymmetry in these organisms.
Box 2. The sensation of asymmetric flow.
How asymmetric flow within LROs is sensed by the embryo to elicit changes in gene expression is not well understood. A small number of factors that act on a flow sensory pathway, by virtue of the fact that they have no role in flow generation but are required for asymmetries in Dand5 to manifest, have been discovered. These include Polycystin 1-like 1 (PKD1L1), a multi-pass transmembrane protein with a large extracellular N-terminus, and the transient receptor potential (TRP) non-selective cation channel PKD2 [68–72]. PKD1L1 and PKD2 physically interact, co-localize to LRO cilia and both are required for proper Dand5 asymmetry as well as the establishment of the Nodal-Pitx2 pathway [68– 72]. Moreover, the expression of Pkd1l1 is relatively restricted to LROs [68, 69]; while Pkd2 is expressed more broadly, it is required only in sensory cells surrounding the mouse node, which harbor immotile sensory cilia, for proper L-R patterning [13]. Finally, genetic experiments in mouse embryos revealed a pathway in which Pkd1l1 acts upstream of and genetically represses Pkd2 [32]. Thus, while the requirement for PKD1L1 and PKD2 is clear from genetic studies, further work is required to address how they act on the pathway between flow and gene expression asymmetries. One model argues that PKD2 responds to flow-based signals by eliciting an intra-ciliary Ca2+ signal, which may then spread to the cell body to activate downstream pathways [73]. In cell culture, PKD1L1, likely in combination with PKD2, can also act as part of a flow-responsive pathway that results in a Ca2+ signal [32]. But in the embryo there remains no obvious connection between Ca2+ asymmetries and asymmetric gene expression, while the idea that sensory cilia act as sensors of flow by initiating ciliary Ca2+ signals has recently come under scrutiny [74]. Thus, though Ca2+ asymmetries are likely germane to the flow response, exactly how and when they act on the pathway that leads to gene expression asymmetries still needs elucidating.
More broadly, it is also not understood what facet of asymmetric flow is sensed by cells. Different models have been proposed to explain this: (1) a mechanosensation model in which the force of flow itself is sensed [75, 76]; or (2) a chemosensation model in which a chemical determinant, or vesicle carrying determinants, is asymmetrically distributed by flow [77]. Various experimental and modeling approaches have been used to assess these possibilities [61, 78–80], but controversy remains about whether LRO cells sense the flow forces, a chemical determinant, or a combination of both.
Asymmetries that originate in and around LROs are then transferred to the lateral plate mesoderm (LPM) (Box 3), where expression of the gene encoding the signaling molecule NODAL, a transforming growth factor-beta (TGFβ) superfamily ligand, is activated in the posterior left LPM (Figure 1A–C). Once initiated, Nodal activity spreads throughout the entire left LPM by an auto-activation mechanism (Figure 1A), yet remains absent from the right side owing to antagonism primarily from LEFTY proteins [4]. Nodal signaling in the left LPM activates expression of the homeodomain transcription factor-encoding gene Pitx2 (Figure 1A, D). Pitx2 expression in the left LPM remains for many hours after Nodal signaling has ceased and was originally hypothesized to be the driving force for asymmetric organ morphogenesis downstream of NODAL. Indeed, the requirement for Pitx2 in L-R asymmetry is clear from loss-of-function studies in mouse embryos including mutant embryos in which the critical asymmetric enhancer was deleted resulting in loss of LPM Pitx2 expression. These mutants exhibit striking L-R defects in most organs including in the patterning of the heart vessels and lobation of the lungs [14]. However, some morphological asymmetries are not affected by Pitx2 loss including heart looping, axial rotation, and stomach sidedness, suggesting that PITX2-independent signals downstream of Nodal are important for some aspects of asymmetric morphogenesis. Such signals may be even more relevant in zebrafish, since loss of pitx2 in this model does not affect organ asymmetry [15].
Box 3. Transfer of asymmetric signals from LRO to LPM.
Asymmetric signaling events occurring in and around LROs must be transferred to the LPM where the Nodal-Pitx2 pathway is activated on the left but not right side. Various studies have suggested how this might occur. First, L>R Ca2+ signals spread laterally beyond the node in mouse embryos and may reach as far as the LPM (Figure III) [75]. This Ca2+, or other signals, might travel intracellularly through endodermal cells which are connected by gap junctions [81–83]. Second, active NODAL ligand itself, which is produced in greater amounts at the left side of LROs, might directly travel to the LPM and activate its own expression [84]. Indeed, LRO-Nodal is required for LPM-Nodal expression in mouse [85], though this is thought not be the case in zebrafish since mutants lacking either LRO-spaw expression or spaw mutants themselves still express spaw in the LPM [41, 86]. Regardless, sulfated glycosaminoglycans (sGAGs) are also required for LRO-to-LPM signal transfer [84, 87]. In mouse embryos, the sGAGs are located in a basement membrane between endoderm and the mesoderm [84]. These and other data led to an intriguing hypothesis being proposed: Ca2+ spreading through gap junctions in endodermal cells (potentially via purinergic wave propagation) results in increased secretion of sGAGs which then aids in the transfer of NODAL protein [81, 88]. Thus, the puzzle of how asymmetric signals that originate at LROs are transferred to the LPM is beginning to be unraveled.
To sum, the asymmetric expression of Nodal and Pitx2 is highly conserved in all vertebrates so far studied, as well as many invertebrates [16]; the Nodal-Pitx2 pathway encompasses a large asymmetric domain within the embryo and influences the asymmetric development of several organs (Figure 1B–D). Indeed, structures that retain symmetry, like the somites and developing limbs, must be actively protected from the effects of this asymmetric pathway [17, 18].
Asymmetric morphogenesis of the organs
A remaining major challenge is to discover how the Nodal-Pitx2 pathway, and potentially other lateralized signals, drive asymmetric organ morphogenesis. This will require a range of approaches and methodologies across different organisms. Such investigations are starting to give us answers in the case of the gut, heart, and brain.
The gut and its derivatives
It has been convincingly argued that the need for asymmetries within the gastrointestinal system drove the evolutionary adoption of handed asymmetry in internal organs [19, 20]. Though gut asymmetries have long fascinated embryologists, only recently have great strides been made in our understanding of the cellular, molecular, and mechanical basis of gut L-R patterning.
The early gut, divided into foregut, midgut and hindgut, is a rod-like tube of endodermal cells. Asymmetries first emerge when portions of the gut are displaced from the midline during a process known as gut looping. In zebrafish, the first looping event occurs at a particular position along the anterior-posterior extent when the region that will give rise to the liver and intestinal bulb moves to the left of the midline. Imaging the emergence of gut asymmetries revealed that epithelial cells of the LPM, which flanks the endoderm, exhibit striking morphological and migratory asymmetries that impinge upon the endoderm by pushing it to the left (Figure 2A, Key Figure) [21]. This asymmetric cell behavior results from ECM remodeling that ultimately occurs downstream of the Nodal-Pitx2 pathway [22]. Specifically, Laminin is depleted by the activity of matrix metalloproteinases (MMP) along the route of the migrating LPM cells. Upstream, MMP activity itself is regulated by the basic helix-loop-helix (bHLH) transcription factor HAND2, such that hand2 mutants fail to undergo looping morphogenesis [22]. Interestingly, both Laminin and HAND2 play a dual role in L-R patterning, being required for earlier steps in setting up the Nodal-Pitx2 pathway in addition to LPM migrations during gut looping [22, 23]. This demonstrates that regulators of asymmetry can have multiple roles at different stages of L-R patterning, something that can complicate analysis of some mutants and should thus be kept in mind.
Key Figure. Figure 2. L-R asymmetric organogenesis of the gut, heart, and brain.
(A) Upper panels: the endoderm (green) acquires an asymmetric morphology between 24 and 30 hpf. By 30 hpf, the liver (L) and pancreatic (P) buds have emerged and the intestine has looped asymmetrically. Lower panels: at 24 hpf, the gut endoderm exists as a central rod surrounded by LPM. The LPM (orange) migrates medially toward the endodermal rod, with the left LPM migrating dorsally and the right LPM ventro-laterally. By 30 hpf, the gut has shifted to the left as a result of these LPM migrations. (B) In amniotes, the gut tube is connected to the body wall by the dorsal mesentery. Cell shape, adhesion, and ECM changes downstream of the Nodal-Pitx2 pathway, with the right mesenchyme decondensing and the epithelium attaining a cuboidal architecture, result in the tilting of the gut tube towards the left, a critical first asymmetry in the gut looping process. Boxed panel: differential elongation of the DM and the gut tube cause compressive forces to be imparted on the gut (purple arrows), resulting in a buckling event that drives looping. (C) Schematic of mouse lungs showing four right-sided lobes and a single left-sided lung lobe. Bilateral Nodal-Pitx2 pathway activity in mutants generally leads to left lung isomerism whereas right isomerism occurs in mutants that fail to activate Nodal-Pitx2. Examples of mutants displaying these phenotypes are given. (D) Heart jogging in zebrafish occurs when a symmetrical cardiac cone, composed of atrial (light red) and ventricular (dark red) cells, a volcano-shaped structure (see side view in dotted box), becomes asymmetric to form the cardiac tube by 24 hpf. Spaw signals from the left LPM, which activates target genes in the left-sided cells of the cone only. Subsequently, the left cells migrate left and anterior at a faster rate than cells on the right. This results in the clockwise rotation and, owing to involution and extension extension of the cone, forms a linear tube that points to the left side. (E) Schematic of the epithalamic region of the zebrafish brain showing the left and right Hb, the PpO and the ep. The PpO migrates left towards the left Hb down an FGF gradient that increases from the midline to the lateral sides. Nodal, active on the left side only, promotes left-sided migration of the PpO. A; anterior, D; dorsal, ep; epiphysis, Hb; habenulae, IL; inferior lobe, L; left, LL; left lobe, LPM; lateral plate mesoderm, ML; middle lobe, P; posterior, PCL; post-caval lobe, PpO; parapineal organ, R; right, SL; superior lobe, V; ventral.
Gut asymmetries have also been studied in the context of the midgut in chicken and mouse embryos. The midgut, precursor of the small intestine, grows at a much faster rate than the trunk and therefore, as it exceeds the length of the body, it is forced to undergo looping. The stereotypical patterns of looping are conserved within mammalian species and are dependent upon the physical forces that arise between the gut tube and the associated dorsal mesentery (DM) [24], a ‘neck’ of mesoderm tissue that connects the gut tube to the body wall (Figure 2B). The initial chirality of these looping events depends, at least in amniotes, on asymmetries in cellular architecture within the DM itself [25]. The DM consists of four distinct cellular compartments (Figure 2B); a right epithelium and mesenchyme and a left epithelium and mesenchyme. Initially, the left and right epithelial layers are columnar while the internal mesenchyme is densely packed. Pitx2 is expressed in the left-sided cells of the DM (both epithelial and mesenchymal), since these are derivatives of left LPM. In a mechanism that is dependent on PITX2, the right-sided epithelium becomes cuboidal while the mesenchyme decondenses but the left side does not undergo these changes [25, 26]. The result is the tilting of the primitive gut tube to the left side (Figure 2B), thereby breaking the L-R symmetry and biasing gut rotation. Further experiments have provided insight into the signaling mechanisms that operate downstream of asymmetric PITX2 to drive these critical asymmetries in cellular architecture, including asymmetric alterations to cell adhesion, ECM properties, and Wnt signaling [25–27].
The initial leftward tilting of the gut — the direction of which is governed by the Nodal-Pitx2 pathway — may coordinate with forces imparted on the tube from the differential growth of the gut (fast elongation) and DM (slow elongation) to drive stereotypical looping patterns. Experimental separation of the gut tube from the DM causes the tube to straighten showing that when attached to the DM the gut tube is placed under compressive forces from the DM (Figure 2B) [24]. Thus, it could be that gut coiling is induced by compressive forces and that the sidedness of these coiling events is ultimately determined by gut tilting that occurs as a result of Nodal-Pitx2 pathway-induced L-R asymmetric cellular changes within the DM. The differential growth of the DM and the gut is in part controlled by a BMP2 signal, present in the DM and dorsal part of the gut tube, that represses DM elongation; experimental increase of this signal results in a larger disparity between the growth of the gut and the DM causing an increased number of intestinal loops [28]. Chick and zebra finch show different strengths of BMP2 and distinct looping patterns, suggesting that evolutionary alterations in the strength of this BMP signal might explain distinct intestinal looping patterns across species [28].
A comparable but distinct role to that played by the DM in intestinal looping is played by the splanchnic mesodermal plate (SMP), a transient structure that is also derived from the LPM, in the spleno-pancreatic region of the developing gut. Between E9.5–10.5 in mouse, the left side of the SMP proliferates more than the right side, resulting in an asymmetric outgrowth of the SMP [29]. Moreover, left-sided epithelial cells maintain a columnar appearance while the equivalent cells on the right attain mesenchymal characteristics. This depends on the Nodal-Pitx2 cascade and, in particular, the downstream asymmetrically expressed Nkx3-2/Bapx1 transcription factor-encoding gene. One model suggests that Nkx3-2 induces Fgf10 which signals to the dorsal pancreatic bud initiating its migration towards the FGF signal on the left side where the SMP outgrowth has arisen [20].
The examples in the previous section make clear the active and critical role of LPM tissue and its derivatives in gut asymmetry at different A-P levels along the gut tube. By contrast, some asymmetries at the level of the stomach appear to rely more on endoderm-intrinsic mechanisms. For instance stomach curvature in mouse and Xenopus relies on asymmetries of tissue architecture and epithelial polarity of cells of the stomach itself, where Pitx2 is expressed in the left stomach wall only [30].
The lungs of mammals, derived from the foregut, are incredibly complex tree-like branched networks often containing millions of airways. They also exhibit striking L-R asymmetry: the mouse lung consists of four right-sided lobes and a single large left lobe (Figure 2C). In humans, this ratio is three right and two left lobes. The lung develops by a branching program that is remarkably stereotyped [31] and, moreover, the asymmetric aspect of lung branching is under the overall control of the Nodal-Pitx2 pathway in so much as lungs develop in the complete L-R reverse configuration in some mouse mutants that randomly activate LPM Nodal [31]. Furthermore, loss of Pitx2 causes lung right isomerism, where four lobes are present on both the left and right sides [14], whereas mutations that result in bilateral activity of the Nodal-Pitx2 pathway generally cause left isomerism (Figure 2C), showing that presence or absence of Nodal-Pitx2 is instructive in defining lungs as 'left' or 'right', respectively. More complex lobation patterns (e.g. 3:2 or 1:3 lobe ratios, amongst others) are present in mutants that bilaterally activate the Nodal-Pitx2 pathway but where the strength of the signal is not equal on both sides [32]. However, the basis of asymmetric branching remains largely unexplored.
The heart and vessels
The heart is a strikingly asymmetrical organ, both in terms of its intricate patterning, connections to the vasculature, and its overall positioning in the chest cavity. Defects in the proper L-R patterning of the heart invariably result in CHD [33]; indeed, CHD is commonly associated with heterotaxy and PCD.
The process of symmetry breaking in the heart occurs in multiple stages [34]. The earliest of these in zebrafish is cardiac jogging, in which midline cardiac progenitor cells (CPCs) move to the left side of the embryo as they extend into a funnel-shaped structure called the cardiac tube (2D). By 22 hours post fertilization (hpf), the zebrafish heart consists of a midline positioned cone of myocardial cells with underlying endocardial cells [35]. Shortly thereafter, the cells on the left side of the cone migrate anteriorly more quickly than cells on the right side, resulting in a clockwise rotation and left-lateral movement of the cone, which then involutes and extends to produce a leftward pointing cardiac tube by 24–26 hpf [36–38] (Figure 2D).
How is this asymmetry regulated by the Nodal-Pitx2 pathway? At the symmetric cardiac cone stage, CPCs on the left side receive a Nodal signal from the anterior left LPM (Figure 2D) [36, 37]. This signal drives the fast migration of left-sided cells at around 0.5 µm per minute compared with 0.3 µm per minute for right-sided cells [39]. Embryos which lack the zebrafish NODAL homolog Southpaw/Spaw, exhibit bilaterally slow CPC migration whereas embryos expressing spaw in both the left and right LPM exhibit bilaterally fast CPC migration [39]. The mechanism by which Nodal signaling to the left side of the cone increase CPC migration rates is not well understood, although some targets of Spaw in this cell type have been identified [37, 40]. One is Hyaluronan synthase 2 (Has2), an ECM-modifying enzyme which is expressed in left-sided CPCs at a higher level than right-sided cells and might function to dampen BMP activity [40]. Another is a subunit of the nonmuscle myosin II (namely, myh9a/myh9l2), expression of which is repressed by Nodal activity; accordingly, myosin light chain II was found to be more active in right-sided CPCs at the cone stage and left side-specific misexpression of a constitutively active myosin light chain kinase resulted in reversal of, or midline (i.e. unbiased), cardiac jogging [40]. Thus, whilst we are beginning to understand the molecular signals that act downstream of Nodal in the zebrafish cardiac cone, there remains much to be elucidated. It seems likely that Nodal and downstream signals will converge on the control of cytoskeletal and extracellular matrix dynamics to promote asymmetric CPC migration which, ultimately, results in a lateralized heart tube. Additional TGFβ signals, including those of the bone morphogenetic protein (BMP) family, are also required for cardiac jogging, though their roles have been more controversial [37, 39, 40].
Cardiac looping is a highly conserved event in which a primitive heart tube bends in an L-R asymmetric fashion to form a coiled heart in which the various developing structures are brought into their approximate final configuration. The initial bend in the straight tube occurs towards the ventral side and a helical twist results in a kinked tube with the convex surface pointing towards the right. This is the first asymmetric event in looping, and is named the 'dextral (D) loop'. The execution of properly lateralized heart looping depends on the coordination of asymmetric gene expression/pathway activity and mechanical forces, potentially both intrinsic and extrinsic to the heart.
In zebrafish, loss of spaw does not prevent the morphogenetic process of cardiac looping but it does result in L-R reversed looping in approximately 25% of embryos [36]. However, the majority still loop correctly, while hearts cultured ex vivo, beyond the influence of asymmetric LPM Spaw, still loop dextrally most times [41]. Mechanistically, looping requires the action of actin and myosin; Spaw signals can drive asymmetric actin1b expression. Overall, this supports a model in which heart looping is governed by a heart-intrinsic mechanism that nevertheless can be influenced, in part, by Nodal signals from outside the heart, which perhaps serve to increase robustness [41]. Interestingly, Pitx2 is not required for heart looping in zebrafish or mouse, revealing that NODAL works independently of PITX2 in this aspect of asymmetric morphogenesis.
Physical simulations of chick heart looping have revealed the plausibility of a ‘growth-induced buckling model’. Placing a silicone rod under various compressive loads within a confined space, which models cardiac growth within the pericardial cavity, can generate the same changes of form as occurs in the looping embryonic heart [42]. Providing the model with no L-R bias results in a 50:50 split between D- and levo (L)-loops, whereas inputting only very subtle L-R asymmetric displacements of the caudal end (as occurs in embryos) results in consistently lateralized loops [42]. Thus, differential growth of the linear cardiac tube and the pericardial cavity resulting in compressive loads being placed on the heart can drive a buckling process which results in morphological changes in the tube that closely mimic looping. This potential mechanism awaits experimental validation but has clear parallels to intestinal coiling discussed earlier.
The heart is required to generate blood flow well before it has developed into its final intricate form. While contraction and blood flow forces are not required for cardiac looping, fluid flows do play a role in another aspect of asymmetric heart development, the development of the branchial arch arteries (BAAs). Mammalian embryos exhibit five BAAs, important structures through which blood flows out of the heart during development. Initially, the BAA system develops in an L-R symmetrical fashion. However, over the course of 24 hours in the mouse, from embryonic day (E) 10.5–11.5, or approximately five days in human embryos, the BAA system undergoes extensive L-R asymmetrical remodeling. The fourth and sixth arteries on the left form the aortic artery and pulmonary trunk, whereas the equivalent structures on the right side regress. In Pitx2 mutants, the right sixth arch artery persists rather than regresses, implicating the Nodal-Pitx2 pathway in these L-R asymmetries [43]. However, PITX2 is not itself present in asymmetrically remodeled arteries, leaving open the question of how PITX2 exerts its influence on the asymmetric persistence/regression of BAAs. In part, the answer appears to be that PITX2 governs morphogenesis in the second heart field (SHF), where it is present in the left myocardial layer of the outflow tract (OFT) [44]. Normally, the OFT spirals around 180° and then, slightly later, undergoes a 90° back-turn driven by rotation of the arterial pole which positions the entrance of the right sixth BAA adjacent to the aorta. These movements render the right sixth BAA narrower and longer than the equivalent structure on the left and do not occur properly in Pitx2 mutants. This lengthening and narrowing results in decreased blood flow through the right sixth BAA and was associated with decreased growth factor signaling of the platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) family. Experimental inhibition of these factors results in regression of both the left and right sixth BAAs [43]. Together, these results suggest a model in which PITX2 drives chiral rotations of the OFT that results in an L-R asymmetric distribution of blood flow; decreased circulation on the right causes decreased growth factor signaling and, as a result, the regression of the right sixth BAA. By contrast, on the left side, higher levels of blood flow promotes higher PDGF and VEGF signaling and therefore results in the persistence of the left sixth BAA. The use of asymmetries in the dynamics of blood flow (haemodynamics) driven by upstream L-R asymmetries in PITX2 represents a fascinating method of converting L-R genetic asymmetries into morphological asymmetries.
The brain
The brains of many animals are asymmetrically organized. In humans, structural and functional asymmetries exist between the hemispheres, while a small number of genes are known to be asymmetrically expressed [45]. Brain asymmetry in many animals is linked to Nodal signals, but mechanisms of how Nodal drives brain asymmetries are not well understood [46]. Currently, the majority of progress in our understanding of the origins of brain asymmetry comes from the zebrafish model [46, 47], in which reversing the side of Nodal activity by and large reverses morphological brain asymmetries as well as some lateralized behaviors [48].
In the zebrafish brain, asymmetries are most pronounced in the epithalamus, which consists of the left and right habenulae (Hb) and the pineal complex, itself composed of the midline-located epiphysis and the left-sided parapineal organ (PpO) (Figure 2E). The PpO attains its left-sided position by migrating from a midline position during development towards the left Hb [49, 50]. This process involves signaling by both Nodal, expressed in the left side of the epithalimus, and fibroblast growth factor (FGF), expressed symmetrically in the Hb. In fgf8 mutants, the PpO fails to migrate, instead remaining at the midline [51]. Conditions with symmetric Nodal (absent or bilaterally expressed), result in randomized PpO migration to the left or right, while an ectopic source of FGF can direct PpO migration, but only when Nodal is absent [51, 52]. These findings have been synthesized into a model in which the PpO is unstable at the midline owing to the bilateral sources of FGF. Under these conditions, asymmetric Nodal somehow biases FGF activity, allowing the PpO to migrate to the left [51]. One possibility is that Nodal increases the competence of left-sided PpO cells to respond to the FGF signal [53], but this has yet to be proven. When the influence of asymmetric Nodal is absent, small and stochastic deviations in FGF asymmetry can break the unstable midline position of the PpO, causing it to migrate left or right in a random fashion (Figure 2E).
Concluding remarks and future perspectives
The central goal of developmental biology is to understand the principles by which form, structure and pattern emerge during embryogenesis. This requires both a molecular understanding of the signals and interactions that exist between and within cells as well as an appreciation of the role of physical processes such as fluid flow and mechanical deformation; it is the interplay of these facets that sculpt the forming embryo [54, 55]. The field of L-R patterning aims to understand how L-R symmetries are first broken in the embryo, how lateralized signaling pathways are initiated and maintained, and to discover the molecular and cell biological principles of how organs and other structures become asymmetric in pattern and position. As the discussions in this review attest, these aims are beginning to be accomplished. However, there remains much to be learned about each of these phases of L-R patterning (see Outstanding Questions Box). One emerging theme is that the role of mechanical forces is critical in many aspects of L-R patterning, ranging from the asymmetric cilia-driven fluid flows that break symmetry within LROs to the deformations of entire tissues by growth and migratory processes. Tissue-scale buckling events may underlie looping morphogenesis - the bending and coiling of initially straight tubes - across distinct organs and species. Moreover, directed migrations that drive asymmetries can occur within the organ itself (e.g. CPC migration underlies asymmetric cardiac jogging in zebrafish) or neighboring tissue migrations/deformations can push or pull nearby or physically connected tubular structures to drive their asymmetry (e.g. mesodermal tissue interacting with the developing gut). These examples make clear the importance of a holistic view of developmental processes — in which understanding can only be achieved when many of the seemingly distinct working parts are considered together — to complement molecular- and cellular-level reductionist understanding.
Outstanding Questions Box.
What signaling pathways act downstream of the Nodal-Pitx2 pathway to drive asymmetric changes in cell morphology, adhesion, and migratory behavior in different contexts?
How is organ shape dictated by the physical properties of surrounding cells and extracellular matrix?
How is the movement of entire tissues coordinated by signaling between different layers and by intrinsic and extrinsic forces acting on the migrating cells?
What are the evolutionary pressures that drive asymmetries in organ placement and patterning?
Overall, a detailed appreciation of how L-R asymmetries originate and how they drive organ asymmetries will not only provide many new insights into developmental biology but also into human conditions such as heterotaxy. In the future, the mechanisms underlying asymmetry will need to be understood if we are to achieve the goal of using stem cells to generate or regenerate functional organs. Other related phenomena, such as the causes of the unexplained laterality of some cancers [56], are highly intriguing and await future investigation.
Trends Box.
Gut looping morphogenesis depends on Nodal-Pitx2 pathway-induced changes to the cellular architecture of the dorsal mesentery coupled with physical forces imparted on the gut by the dorsal mesentery.
In zebrafish, gut asymmetries are driven by asymmetric migration of the lateral plate mesoderm which pushes the gut tube away from the midline.
Early cardiac asymmetries depend on Nodal signaling-induced asymmetries in cardiac progenitor cell migration.
Cardiac looping asymmetry is driven by forces intrinsic to the heart but made robust by external Nodal pathway cues.
Asymmetric migration of the parapineal in the zebrafish brain is achieved by an integration of FGF and Nodal signals, which drive migration and bias the direction, respectively.
Acknowledgments
We thank José Pelliccia, Martin Blum, Dominic Norris, and Jennifer Keynton for supplying images of stained embryos. We also thank Martin Blum, Nicholas Morante and Charlotte Dean for insightful discussion and Alessandro Giammei for help with artwork. We apologize to those scientists whose work we couldn't discuss due to limited space. DTG is supported by an American Heart Association Founders Affiliate postdoctoral fellowship. Work in the Burdine laboratory is supported by the National Institute of Child Health and Development grant 2R01HD048584.
Glossary
- Cilia
cell surface projections built around a microtubule cytoskeleton that can be motile, generating fluid flows, and/or sensory, responding to multiple external inputs
- Heterotaxy
a complex genetic syndrome occurring in approximately 1 in 10, 000 people worldwide in which the organs are improperly lateralized and exhibit lack of concordance
- Lateral plate mesoderm
mesodermal tissue at the periphery of the embryo. The Nodal-Pitx2 pathway is activated exclusively in left lateral plate mesoderm, but remains switched off on the right side
- Left-right organizers
transient midline structures in which motile cilia generate asymmetric fluid flow that breaks embryonic L-R symmetry
- Nodal-Pitx2 pathway
refers to the highly conserved asymmetric expression of Nodal, which then induces Pitx2, in left LPM tissue
- Primary ciliary dyskinesia
a rare (1 in 15,000 to 1 in 30,000) autosomal recessive disorder caused by defects in cilia motility characterized by chronic respiratory tract infections, infertility, and abnormal organ asymmetry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shiraishi I, Ichikawa H. Human heterotaxy syndrome - from molecular genetics to clinical features, management, and prognosis. Circulation journal : official journal of the Japanese Circulation Society. 2012;76:2066–2075. doi: 10.1253/circj.cj-12-0957. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, Robinson BV, Minnix SL, Olbrich H, Severin T, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115:2814–2821. doi: 10.1161/CIRCULATIONAHA.106.649038. [DOI] [PubMed] [Google Scholar]
- 3.Hachisuga M, Oki S, Kitajima K, Ikuta S, Sumi T, Kato K, Wake N, Meno C. Hyperglycemia impairs left-right axis formation and thereby disturbs heart morphogenesis in mouse embryos. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E5300–5307. doi: 10.1073/pnas.1504529112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiratori H, Hamada H. TGFbeta signaling in establishing left-right asymmetry. Seminars in cell & developmental biology. 2014;32:80–84. doi: 10.1016/j.semcdb.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Amack JD. Salient features of the ciliated organ of asymmetry. Bioarchitecture. 2014;4:6–15. doi: 10.4161/bioa.28014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum M, Weber T, Beyer T, Vick P. Evolution of leftward flow. Seminars in cell & developmental biology. 2009;20:464–471. doi: 10.1016/j.semcdb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta A, Amack JD. Cilia in vertebrate left-right patterning. Philosophical transactions of the Royal Society of London. 2016;371 doi: 10.1098/rstb.2015.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marques S, Borges AC, Silva AC, Freitas S, Cordenonsi M, Belo JA. The activity of the Nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes & development. 2004;18:2342–2347. doi: 10.1101/gad.306504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura T, Saito D, Kawasumi A, Shinohara K, Asai Y, Takaoka K, Dong F, Takamatsu A, Belo JA, Mochizuki A, et al. Fluid flow and interlinked feedback loops establish left-right asymmetric decay of Cerl2 mRNA. Nature communications. 2012;3:1322. doi: 10.1038/ncomms2319. [DOI] [PubMed] [Google Scholar]
- 10.Schweickert A, Vick P, Getwan M, Weber T, Schneider I, Eberhardt M, Beyer T, Pachur A, Blum M. The nodal inhibitor Coco is a critical target of leftward flow in Xenopus. Curr Biol. 2010;20:738–743. doi: 10.1016/j.cub.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto H, Rebagliati M, Ahmad N, Muraoka O, Kurokawa T, Hibi M, Suzuki T. The Cerberus/Dan-family protein Charon is a negative regulator of Nodal signaling during left-right patterning in zebrafish. Development (Cambridge, England) 2004;131:1741–1753. doi: 10.1242/dev.01070. [DOI] [PubMed] [Google Scholar]
- 12.Hojo M, Takashima S, Kobayashi D, Sumeragi A, Shimada A, Tsukahara T, Yokoi H, Narita T, Jindo T, Kage T, et al. Right-elevated expression of charon is regulated by fluid flow in medaka Kupffer's vesicle. Development, growth & differentiation. 2007;49:395–405. doi: 10.1111/j.1440-169X.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, et al. Cilia at the Node of Mouse Embryos Sense Fluid Flow for Left-Right Determination via Pkd2. Science (New York, N.Y. 2012 doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shiratori H, Yashiro K, Shen MM, Hamada H. Conserved regulation and role of Pitx2 in situs-specific morphogenesis of visceral organs. Development (Cambridge, England) 2006;133:3015–3025. doi: 10.1242/dev.02470. [DOI] [PubMed] [Google Scholar]
- 15.Ji Y, Buel SM, Amack JD. Mutations in zebrafish pitx2 model congenital malformations in Axenfeld-Rieger syndrome but do not disrupt left-right placement of visceral organs. Developmental biology. 2016;416:69–81. doi: 10.1016/j.ydbio.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namigai EK, Kenny NJ, Shimeld SM. Right across the tree of life: the evolution of left-right asymmetry in the Bilateria. Genesis. 2014;52:458–470. doi: 10.1002/dvg.22748. [DOI] [PubMed] [Google Scholar]
- 17.Sulaiman FA, Nishimoto S, Murphy GR, Kucharska A, Butterfield NC, Newbury-Ecob R, Logan MP. Tbx5 Buffers Inherent Left/Right Asymmetry Ensuring Symmetric Forelimb Formation. PLoS genetics. 2016;12:e1006521. doi: 10.1371/journal.pgen.1006521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilhais-Neto GC, Maruhashi M, Smith KT, Vasseur-Cognet M, Peterson AS, Workman JL, Pourquie O. Rere controls retinoic acid signalling and somite bilateral symmetry. Nature. 2010;463:953–957. doi: 10.1038/nature08763. [DOI] [PubMed] [Google Scholar]
- 19.Blum M, Feistel K, Thumberger T, Schweickert A. The evolution and conservation of left-right patterning mechanisms. Development (Cambridge, England) 2014;141:1603–1613. doi: 10.1242/dev.100560. [DOI] [PubMed] [Google Scholar]
- 20.Burn SF, Hill RE. Left-right asymmetry in gut development: what happens next? Bioessays. 2009;31:1026–1037. doi: 10.1002/bies.200900056. [DOI] [PubMed] [Google Scholar]
- 21.Horne-Badovinac S, Rebagliati M, Stainier DY. A cellular framework for gut-looping morphogenesis in zebrafish. Science (New York, N.Y. 2003;302:662–665. doi: 10.1126/science.1085397. [DOI] [PubMed] [Google Scholar]
- 22.Yin C, Kikuchi K, Hochgreb T, Poss KD, Stainier DY. Hand2 regulates extracellular matrix remodeling essential for gut-looping morphogenesis in zebrafish. Developmental cell. 2010;18:973–984. doi: 10.1016/j.devcel.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochgreb-Hagele T, Yin C, Koo DE, Bronner ME, Stainier DY. Laminin beta1a controls distinct steps during the establishment of digestive organ laterality. Development (Cambridge, England) 2013;140:2734–2745. doi: 10.1242/dev.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savin T, Kurpios NA, Shyer AE, Florescu P, Liang H, Mahadevan L, Tabin CJ. On the growth and form of the gut. Nature. 2011;476:57–62. doi: 10.1038/nature10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis NM, Kurpios NA, Sun X, Gros J, Martin JF, Tabin CJ. The chirality of gut rotation derives from left-right asymmetric changes in the architecture of the dorsal mesentery. Developmental cell. 2008;15:134–145. doi: 10.1016/j.devcel.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurpios NA, Ibanes M, Davis NM, Lui W, Katz T, Martin JF, Izpisua Belmonte JC, Tabin CJ. The direction of gut looping is established by changes in the extracellular matrix and in cell:cell adhesion. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:8499–8506. doi: 10.1073/pnas.0803578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh IC, Thomsen M, Gludish DW, Alfonso-Parra C, Bai Y, Martin JF, Kurpios NA. Integration of left-right Pitx2 transcription and Wnt signaling drives asymmetric gut morphogenesis via Daam2. Developmental cell. 2013;26:629–644. doi: 10.1016/j.devcel.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nerurkar NL, Mahadevan L, Tabin CJ. BMP signaling controls buckling forces to modulate looping morphogenesis of the gut. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:2277–2282. doi: 10.1073/pnas.1700307114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecksher-Sorensen J, Watson RP, Lettice LA, Serup P, Eley L, De Angelis C, Ahlgren U, Hill RE. The splanchnic mesodermal plate directs spleen and pancreatic laterality, and is regulated by Bapx1/Nkx3.2. Development (Cambridge, England) 2004;131:4665–4675. doi: 10.1242/dev.01364. [DOI] [PubMed] [Google Scholar]
- 30.Davis A, Amin NM, Johnson C, Bagley K, Ghashghaei HT, Nascone-Yoder N. Stomach curvature is generated by left-right asymmetric gut morphogenesis. Development (Cambridge, England) 2017;144:1477–1483. doi: 10.1242/dev.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzger RJ, Klein OD, Martin GR, Krasnow MA. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grimes DT, Keynton JL, Buenavista MT, Jin X, Patel SH, Kyosuke S, Vibert J, Williams DJ, Hamada H, Hussain R, et al. Genetic Analysis Reveals a Hierarchy of Interactions between Polycystin-Encoding Genes and Genes Controlling Cilia Function during Left-Right Determination. PLoS genetics. 2016;12:e1006070. doi: 10.1371/journal.pgen.1006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramsdell AF. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Developmental biology. 2005;288:1–20. doi: 10.1016/j.ydbio.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 34.Grant MG, Patterson VL, Grimes DT, Burdine RD. Modeling Syndromic Congenital Heart Defects in Zebrafish. Current topics in developmental biology. 2017;124:1–40. doi: 10.1016/bs.ctdb.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Staudt D, Stainier D. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annual review of genetics. 2012;46:397–418. doi: 10.1146/annurev-genet-110711-155646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker K, Holtzman NG, Burdine RD. Direct and indirect roles for Nodal signaling in two axis conversions during asymmetric morphogenesis of the zebrafish heart. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13924–13929. doi: 10.1073/pnas.0802159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith KA, Chocron S, von der Hardt S, de Pater E, Soufan A, Bussmann J, Schulte-Merker S, Hammerschmidt M, Bakkers J. Rotation and asymmetric development of the zebrafish heart requires directed migration of cardiac progenitor cells. Developmental cell. 2008;14:287–297. doi: 10.1016/j.devcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 38.de Campos-Baptista MI, Holtzman NG, Yelon D, Schier AF. Nodal signaling promotes the speed and directional movement of cardiomyocytes in zebrafish. Dev Dyn. 2008;237:3624–3633. doi: 10.1002/dvdy.21777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenhart KF, Holtzman NG, Williams JR, Burdine RD. Integration of nodal and BMP signals in the heart requires FoxH1 to create left-right differences in cell migration rates that direct cardiac asymmetry. PLoS genetics. 2013;9:e1003109. doi: 10.1371/journal.pgen.1003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veerkamp J, Rudolph F, Cseresnyes Z, Priller F, Otten C, Renz M, Schaefer L, Abdelilah-Seyfried S. Unilateral dampening of Bmp activity by nodal generates cardiac left-right asymmetry. Developmental cell. 2013;24:660–667. doi: 10.1016/j.devcel.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 41.Noel ES, Verhoeven M, Lagendijk AK, Tessadori F, Smith K, Choorapoikayil S, den Hertog J, Bakkers J. A Nodal-independent and tissue-intrinsic mechanism controls heart-looping chirality. Nature communications. 2013;4:2754. doi: 10.1038/ncomms3754. [DOI] [PubMed] [Google Scholar]
- 42.Bayraktar M, Manner J. Cardiac looping may be driven by compressive loads resulting from unequal growth of the heart and pericardial cavity. Observations on a physical simulation model. Frontiers in physiology. 2014;5:112. doi: 10.3389/fphys.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yashiro K, Shiratori H, Hamada H. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature. 2007;450:285–288. doi: 10.1038/nature06254. [DOI] [PubMed] [Google Scholar]
- 44.Ai D, Liu W, Ma L, Dong F, Lu MF, Wang D, Verzi MP, Cai C, Gage PJ, Evans S, et al. Pitx2 regulates cardiac left-right asymmetry by patterning second cardiac lineage-derived myocardium. Developmental biology. 2006;296:437–449. doi: 10.1016/j.ydbio.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlebach G, Francks C. Lateralization of gene expression in human language cortex. Cortex; a journal devoted to the study of the nervous system and behavior. 2015;67:30–36. doi: 10.1016/j.cortex.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Concha ML, Bianco IH, Wilson SW. Encoding asymmetry within neural circuits. Nature reviews. Neuroscience. 2012;13:832–843. doi: 10.1038/nrn3371. [DOI] [PubMed] [Google Scholar]
- 47.Gunturkun O, Ocklenburg S. Ontogenesis of Lateralization. Neuron. 2017;94:249–263. doi: 10.1016/j.neuron.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 48.Barth KA, Miklosi A, Watkins J, Bianco IH, Wilson SW, Andrew RJ. fsi zebrafish show concordant reversal of laterality of viscera, neuroanatomy, and a subset of behavioral responses. Curr Biol. 2005;15:844–850. doi: 10.1016/j.cub.2005.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Snelson CD, Santhakumar K, Halpern ME, Gamse JT. Tbx2b is required for the development of the parapineal organ. Development (Cambridge, England) 2008;135:1693–1702. doi: 10.1242/dev.016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Concha ML, Russell C, Regan JC, Tawk M, Sidi S, Gilmour DT, Kapsimali M, Sumoy L, Goldstone K, Amaya E, et al. Local tissue interactions across the dorsal midline of the forebrain establish CNS laterality. Neuron. 2003;39:423–438. doi: 10.1016/s0896-6273(03)00437-9. [DOI] [PubMed] [Google Scholar]
- 51.Regan JC, Concha ML, Roussigne M, Russell C, Wilson SW. An Fgf8-dependent bistable cell migratory event establishes CNS asymmetry. Neuron. 2009;61:27–34. doi: 10.1016/j.neuron.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Concha ML, Burdine RD, Russell C, Schier AF, Wilson SW. A nodal signaling pathway regulates the laterality of neuroanatomical asymmetries in the zebrafish forebrain. Neuron. 2000;28:399–409. doi: 10.1016/s0896-6273(00)00120-3. [DOI] [PubMed] [Google Scholar]
- 53.Roussigne M, Blader P, Wilson SW. Breaking symmetry: the zebrafish as a model for understanding left-right asymmetry in the developing brain. Developmental neurobiology. 2012;72:269–281. doi: 10.1002/dneu.20885. [DOI] [PubMed] [Google Scholar]
- 54.LeGoff L, Lecuit T. Mechanical Forces and Growth in Animal Tissues. Cold Spring Harbor perspectives in biology. 2015;8:a019232. doi: 10.1101/cshperspect.a019232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamada H. Role of physical forces in embryonic development. Seminars in cell & developmental biology. 2015;47–48:88–91. doi: 10.1016/j.semcdb.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Wilting J, Hagedorn M. Left-right asymmetry in embryonic development and breast cancer: common molecular determinants? Current medicinal chemistry. 2011;18:5519–5527. doi: 10.2174/092986711798347252. [DOI] [PubMed] [Google Scholar]
- 57.Praveen K, Davis EE, Katsanis N. Unique among ciliopathies: primary ciliary dyskinesia, a motile cilia disorder. F1000prime reports. 2015;7:36. doi: 10.12703/P7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development (Cambridge, England) 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 59.Grimes DT, Boswell CW, Morante NF, Henkelman RM, Burdine RD, Ciruna B. Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science (New York, N.Y. 2016;352:1341–1344. doi: 10.1126/science.aaf6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wallingford JB, Mitchell B. Strange as it may seem: the many links between Wnt signaling, planar cell polarity, and cilia. Genes & development. 2011;25:201–213. doi: 10.1101/gad.2008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okada Y, Takeda S, Tanaka Y, Izpisua Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 63.Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 64.Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nature cell biology. 2010;12:170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- 65.Minegishi K, Hashimoto M, Ajima R, Takaoka K, Shinohara K, Ikawa Y, Nishimura H, McMahon AP, Willert K, Okada Y, et al. A Wnt5 Activity Asymmetry and Intercellular Signaling via PCP Proteins Polarize Node Cells for Left-Right Symmetry Breaking. Developmental cell. 2017;40:439–452. e434. doi: 10.1016/j.devcel.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang G, Cadwallader AB, Jang DS, Tsang M, Yost HJ, Amack JD. The Rho kinase Rock2b establishes anteroposterior asymmetry of the ciliated Kupffer's vesicle in zebrafish. Development (Cambridge, England) 2011;138:45–54. doi: 10.1242/dev.052985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang G, Manning ML, Amack JD. Regional cell shape changes control form and function of Kupffer's vesicle in the zebrafish embryo. Developmental biology. 2012;370:52–62. doi: 10.1016/j.ydbio.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Field S, Riley KL, Grimes DT, Hilton H, Simon M, Powles-Glover N, Siggers P, Bogani D, Greenfield A, Norris DP. Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development (Cambridge, England) 2011;138:1131–1142. doi: 10.1242/dev.058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kamura K, Kobayashi D, Uehara Y, Koshida S, Iijima N, Kudo A, Yokoyama T, Takeda H. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development (Cambridge, England) 2011;138:1121–1129. doi: 10.1242/dev.058271. [DOI] [PubMed] [Google Scholar]
- 70.Pennekamp P, Karcher C, Fischer A, Schweickert A, Skryabin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–943. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- 71.Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Developmental biology. 2005;287:274–288. doi: 10.1016/j.ydbio.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 72.Schottenfeld J, Sullivan-Brown J, Burdine RD. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development (Cambridge, England) 2007;134:1605–1615. doi: 10.1242/dev.02827. [DOI] [PubMed] [Google Scholar]
- 73.Yuan S, Zhao L, Brueckner M, Sun Z. Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr Biol. 2015;25:556–567. doi: 10.1016/j.cub.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE. Primary cilia are not calcium-responsive mechanosensors. Nature. 2016;531:656–660. doi: 10.1038/nature17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 76.Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes & development. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- 77.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 78.Shinohara K, Kawasumi A, Takamatsu A, Yoshiba S, Botilde Y, Motoyama N, Reith W, Durand B, Shiratori H, Hamada H. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nature communications. 2012;3:622. doi: 10.1038/ncomms1624. [DOI] [PubMed] [Google Scholar]
- 79.Chen D, Norris D, Ventikos Y. The active and passive ciliary motion in the embryo node: a computational fluid dynamics model. J Biomech. 2009;42:210–216. doi: 10.1016/j.jbiomech.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 80.Cartwright JH, Piro N, Piro O, Tuval I. Embryonic nodal flow and the dynamics of nodal vesicular parcels. J R Soc Interface. 2007;4:49–55. doi: 10.1098/rsif.2006.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beyer T, Thumberger T, Schweickert A, Blum M. Connexin26-mediated transfer of laterality cues in Xenopus. Biology Open. 2012;1:473–481. doi: 10.1242/bio.2012760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Viotti M, Niu L, Shi SH, Hadjantonakis AK. Role of the gut endoderm in relaying left-right patterning in mice. PLoS biology. 2012;10:e1001276. doi: 10.1371/journal.pbio.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saund RS, Kanai-Azuma M, Kanai Y, Kim I, Lucero MT, Saijoh Y. Gut endoderm is involved in the transfer of left-right asymmetry from the node to the lateral plate mesoderm in the mouse embryo. Development (Cambridge, England) 2012;139:2426–2435. doi: 10.1242/dev.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oki S, Hashimoto R, Okui Y, Shen MM, Mekada E, Otani H, Saijoh Y, Hamada H. Sulfated glycosaminoglycans are necessary for Nodal signal transmission from the node to the left lateral plate in the mouse embryo. Development (Cambridge, England) 2007;134:3893–3904. doi: 10.1242/dev.009464. [DOI] [PubMed] [Google Scholar]
- 85.Brennan J, Norris DP, Robertson EJ. Nodal activity in the node governs left-right asymmetry. Genes & development. 2002;16:2339–2344. doi: 10.1101/gad.1016202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Burdine RD, Grimes DT. Antagonistic interactions in the zebrafish midline prior to the emergence of asymmetric gene expression are important for left-right patterning. Philosophical transactions of the Royal Society of London. 2016;371 doi: 10.1098/rstb.2015.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marjoram L, Wright C. Rapid differential transport of Nodal and Lefty on sulfated proteoglycan-rich extracellular matrix regulates left-right asymmetry in Xenopus. Development (Cambridge, England) 2011;138:475–485. doi: 10.1242/dev.056010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Norris DP. Cilia, calcium and the basis of left-right asymmetry. BMC biology. 2012;10:102. doi: 10.1186/1741-7007-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]