Abstract
Background
In the present study we retrospectively evaluated the results of outpatients who had an HPV analysis, and present objective evidence for the administration of preventive inoculation in our area.
Material/Methods
We retrospectively reviewed 532 outpatients who visited a single center between 2012 and 2016 and had an HPV infection analysis. The criteria for inclusion of patients with unhealthy cervix in the study were: erosion, chronic cervicitis, healed lacerations, hypertrophied cervix, and abnormal discharges from the cervix.
Results
We found that 122 out of 532 patients were infected with HPV, and the rate of multiple infections was 59.0% (72/122). HR-HPV (group 1 carcinogens HPV-16 (18.9%, 23/122), HPV-18 (13.1%, 16/122), HPV- 31 (4.9%, 6/122), HPV-33 (3.3%, 4/122), HPV-35 (7.4.9%/122), HPV-39 (5.7%, 7/122), HPV-45 (5.7%, 7/122), HPV-51 (11.5%, 15/122); Group 3 LR-HPV; HPV-6 (31.1%, 38/122), HPV-11 (26.2%, 32/122), HPV-42 (9.0%, 11/122) and HPV-43 (4.9%, 6/122). In terms of linear-by-linear association test, no significant statistical difference was identified between years. The P value for HPV infection rate on year basis was P>0.05.
Conclusions
In this hospital-based retrospective analysis, HPV types were found to be similar to HPV types reported in developed countries. We firmly suggest that patients should be informed about the risk of HPV infection at early ages.
MeSH Keywords: Epidemiology; Genotype; Human Papillomavirus DNA Tests; Hybridization, Genetic
Background
HPV genotypes are divided into 2 groups – high risk or low risk – in terms of their carcinogenicity. There are approximately one dozen high-risk HPV genotypes, which account for the generation of malignant lesions, whereas the low-risk genotypes can cause the introduction of low-grade carcinoma [1–4].
Worldwide, the most widespread HR-HPV genotypes are 16 and 18, which are responsible for 70% of CC, while 31, 33, 35, 52, 58, and other HR-HPV genotypes are seen with a lower frequency, accounting for 20% [5–8].
Cervical cancer is ranked as the second most widespread cancers in women. It is also the fifth most common type of deadly cancer [9]. Similarly, HPV is the most common sexually transmitted virus. It is a harmless, resilient virus which is resistant to widely used disinfectants. Therefore, it can reproduce in healthcare centers and be directly transmitted through nosocomial means not related to sexual intercourse [10].
Human papillomavirus (HPV) infection is one of the most common risk factors for cervical cancer development [11]. Cervical cancer is the fourth most widespread illness, and the majority (90%) of cervical cancer-related mortality is in developed countries [12]. Worldwide, the incidence of cervical cancer is ranked as the second most common woman illness, and it has now a new trend towards young ages [13]. Over 200 strains of HPV have been identified, and of these, 60 are determined to infect the human genital system. HPV strains can be divided into low-risk and high-risk groups according to their oncogenous potency. While low-risk strains are mostly associated with genital warts and low-grade squamous intraepithelial lesions (LSIL), high-risk strains are mainly related to high-grade squamous intraepithelial lesions (HSIL) and cervical cancer [14,15]. HPV 6 and 11 are the low-risk strains that are observed in genital warts, while HPV16 and HPV18 are the most common types of high-risk strains that are observed in malign lesions. They are the main cause (70%) of cervical cancer cases worldwide. Apart from HPV16 and 18, the most common types of HPV strains that cause cervical cancer are 31, 33, 35, 45, 52, and 58. These are also responsible for 30% of cervical cancer cases around the world [16].
The genotype range of HPV infection changes by geographic area. Statistical data provided by the International Agency for Research on Cancer (IARC, 2007) shows that the leading 5 HPV subtypes by infection incidence are: HPV-16, -53, -52, -18, and -39 for North America; HPV- 16, -18, -31, -33, and -58 for Europe; and HPV-16, -52, -58, -18, and -56 for Asia. Recent studies show that HPV-52, -16, and -58 are the most common HPV subtypes that cause infection in China [17].
A study conducted by Zulqarnain Baloch et al. in China, titled “Ethnic and Geographic Variations in HPV Prevalence and Genotype Distribution in North-Western Yunnan, China”, analyzed 522 women. According to the HPV PCR and genotyping result, 18.4% (96/522) of the women were HPV-positive, with 10.9% (57/522) of the women infected with a single HPV genotype, and 6.5% (39/522) were infected with multiple genotypes [18]. Another study by Zulqarnain Baloch et al. in China, and titled “Epidemiologic characterization of human papillomavirus (HPV) infection in various regions of Yunnan Province of China, China”, determined that the prevalence of HPV-16 (5.9%) and HPV-58 (3.5%) were significantly (P=0.001, P=0.001) higher in the southwest region compared with those in the central, north-east, south-east, and northwest regions [19].
In previous studies, the HPV prevalence among Turkish women is reported to be 25% [20]. Similarly, our study has also analyzed the cervical HPV infection ratio of outpatients who visited the gynecology unit of HRS Ankara hospital within the last 4 years. In addition, we have also compared age groups with the HPV subtypes. Therefore, our study is important in determining an efficient prevention strategy that includes screening programs, public health training, and inoculation.
Material and Methods
Study subjects
The participants of this study are 532 outpatient women who visited the Department of Obstetrics and Gynecology, Health Research System In Vitro Fertilization (HRS IVF) Centre, Ankara, Turkey between February 2012 and December 2016. These patients are divided into 4 age groups: 20–23, 24–26, 27–30, and >30 years. According to their socio-demographic features, the age ratio of the participants was 27.88±4.77. Socioeconomic status (SES) is an important social determinant that can have profound impacts on women’s health. In the last decades, populations of urban and rural regions have been categorized by various means of measuring SES. The modified Kuppuswamy’s SES Scale was used in this study. Kuppuswamy’s scale yields a composite score of 3 variables: education, occupation of the head of the family, and monthly household income. We carried out the study after consent by the Harran university Medicine Ethics Committee was obtained. Also, an informed consent was received from the study subjects. The inclusion criteria for the study were: having no pregnancy during the study, being sexually active, having no hysterectomy application, and not being diagnosed with cervical cancer in the past. Exclusion criteria were: women with cervix carcinoma, pregnant females, patients with vaginal bleeding during examination and those who used vaginal medications, vaginal contraceptives, or douches in the last 48 hours before the examination.
Repeat samples from the same patients were not included in the study. Only 1 sample was received from every patient, and was transferred to our laboratory for HPV DNA examination. For the HPV DNA test, these cervical samples were analyzed by using the HPV High-Risk Screen Real-TM (Sacace, Como, Italy) [21].
HPV sample retrieval
We opened the cervix with a vaginal speculum. Excess secretions during the opening were cleaned by a sponge. A cervical brush was penetrated into the opening of the cervix and rotated clockwise 4–6 times to collect exfoliated cervical cells. These cells were put into a tube that contains cell protection liquid. These tubes were stored in a refrigerator at 4°C and examined within 4 days of collection.
DNA isolation and HPV genotyping
Cervical samples were retained in 500 μL of Tris-EDTA (TE) buffer (10 mmol/L Tris hydrochloride, 1 mmol/L EDTA, pH 8), and made homogenous via efficient mixing on a vortex. We put 100 μL of mixed specimen in 10 μL of protease solution (65 mg/mL) (Sigma-Aldrich Corp, St. Louis, MO, USA) and 250 μL of K buffer for 60 minutes at 45°C. After centrifugation at 10,000 g for 10 minutes at 12°C, DNA was removed from the supernatant via a mixture of 250 μL alkali phenol and 250-μL chloroform-isoamyl alcohol (24: 1), and later precipitated with 500-μL isopropyl alcohol. DNA was washed in 75% ethyl alcohol at 10,000 g for 5 minutes at 4°C, air-dried at 37°C, and dissolved in 100 μL of distilled water [22].
Type-specific real-time PCR reactions
With TaqMan-based type-specific real-time PCR method, all cervical samples were examined to determine whether HPV types 6, 11, 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, or 82 were present [22,23]. The genotype spectrum of these techniques was extended to contain all high-risk HPVs (Table 1).
Table 1.
List of Probe Sequence used in real -time PCR assays.
| HPV subtype | Probe sequence |
|---|---|
| HPV-6 | 5′-cataagaagataccttaggacttg-3′ |
| HPV-11 | 5′-acttagcagtaacgtctcagatgt-3′ |
| HPV-42 | 5′-gctatcgtcatgaactatgctaga-3′ |
| HPV-43 | 5′-actgtgtcatgacacgtatcaagt-3′ |
| HPV-16 | 5′-ctatacaagtacgtcgtcgatatg-3′ |
| HPV-52 | 5′-cagagctcgagacacgtatcaact-3′ |
| HPV-58 | 5′-agcaaatgaaacagttgcagga-3′ |
| HPV-53 | 5′-ttctatcatgacacgtatcactgg-3′ |
| HPV-18 | 5′-gtatcactaagtactcgtcgaatg-3′ |
| HPV-39 | 5′-cctaagtgtagacacgtatcagtt-3′ |
| HPV-33 | 5′-ctgatcatgaactacgtcatggat-3′ |
| HPV-31 | 5′-gacttcaatagtactagtcatcgg-3′ |
| HPV-51 | 5′-tctgaatgaaacagttgctaca-3′ |
| HPV-68 | 5′-atgtgtcatgagtatcatagc-3′ |
| HPV-66 | 5′-gcatctatagtatcagttagacg-3′ |
| HPV-56 | 5′-aatggtcatgacacgtatcaagga-3′ |
| HPV-59 | 5′-gcctaatgaaacagttgcaccc-3′ |
| HPV-45 | 5′-atgtgtcatgacacgtatcatagc-3′ |
| HPV-35 | 5′-ctagtgtcatgacacgtatcatag-3′ |
| HPV-73 | 5′-actgcaacaccatccagttgtg-3′ |
| HPV-82 | 5′-gttatacaaggtggggattacta-3′ |
The TaqMan probes were labelled with fluorescent reporter dyes (FAM; 6-carboxy fluorescein and JOE; 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxy-fluorescein) at the 5′ end and with a black hole quencher (BHQ) as the non-fluorescent quencher at the 3′ end. The human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was employed as an internal control, and the PCR mixture without template DNA was used as a negative control in all PCR reactions.
HPV DNA amplification and detection
HPV DNA was amplified by the SPF10 PCR primer set, and each run was supported with many quality control samples. In the event of each PCR run, 18 samples were tested, together with 1 negative control (water) and 1 positive control (HPV 18-containing cells). Amplification products were first tested with probe hybridization in a microtiter plate assay to detect the presence of HPV DNA, as described earlier, and this assay also contained proper negative and positive controls. SFP10-amplimers from HPV-positive samples were later analyzed by reverse hybridization on the HPV reverse hybridization line probe assay (LiPA). PCR products are hybridized at high stringency to these probes, producing a type-specific hybridization pattern. The HPV-LiPA enables specific detection of 25 HPV genotypes, HPV 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74. HPV 6, 11, 34, 40, 42, 43, 44, 53, 54, 70, and 74 were regarded as low-risk types, while HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were regarded as high-risk types. Part of the beta-globin gene was amplified from each sample as a positive control for DNA isolation. Proper negative and positive controls were employed to control the performance of the technique [24,25].
Genomic analyses (using databases and software)
The primers and probes were designated using the OligoYap 4.0 software program [26]. They were also confirmed with a novel software program designed in our laboratory for molecular biology researches (OligoBee 1.0).
Statistical analysis
The findings were analyzed using SPSS 22.0 statistical software. Positive infection rate was calculated as percentage. For the study subjects that had more than 1 infection, the positive HPV rate was calculated for each genotype. With the chi-square test, the infection rate was determined for each group. P-values were two-sided, and statistical significance was set at P<0.05. Linear-by-linear association test and Gamma Value were used to evaluate changes in HPV prevalence by calendar year group and age group.
Results
The findings of 532 outpatient women who visited our gynecology unit between 2012 and 2016 were retrospectively examined. Their socio-demographic features were also analyzed year by year. The subtypes of HPV infection were analyzed for each patient. According to their socio-demographic features, the age ratio of the participants was 27.88±4.77. The age spectrum was 20–23, 24–26, 27–30, and >30 years. The results for each age group were 40.2% (49/122), 4.1% (5/122), 18.9% (23/122), and 36.9% (45/122), respectively. The distribution by years was 18.0% (22/122) in 2012, 19.7% (24/122) in 2013, 16.4% (20/122) in 2014, 24.6% (30/122) in 2015, and 21.3% (26/122) in 2016.
The relation between HPV infection rates and age
The relation between HPV DNA-positive infection rates and patient ages was: 40.2% (49/122), 4.1% (5/122), 18.9% (23/122), and 36.9% (45/122) for 20–23, 24–26, 27–30, and >30 age groups (Table 2, Figures 1, 2).
Table 2.
Percentage distributions of HPV types detected in single and multiple HPV infections.
| Positive | ||
|---|---|---|
| Frequency | Percentage | |
| HPV 6 | 38 | 31.1 |
| HPV 11 | 32 | 26.2 |
| HPV 16 | 23 | 18.9 |
| HPV 18 | 16 | 13.1 |
| HPV 31 | 6 | 4.9 |
| HPV 35 | 9 | 7.4 |
| HPV 39 | 7 | 5.7 |
| HPV 42 | 11 | 9.0 |
| HPV 43 | 6 | 4.9 |
| HPV 45 | 7 | 5.7 |
| HPV 52 | 13 | 10.7 |
| HPV 53 | 14 | 11.5 |
| HPV 56 | 9 | 7.4 |
| HPV 58 | 6 | 4.9 |
| HPV 59 | 10 | 8.2 |
| HPV 66 | 8 | 6.6 |
| HPV 68 | 7 | 5.7 |
| HPV 82 | 8 | 6.6 |
| HPV 73 | 4 | 3.3 |
| HPV 33 | 4 | 3.3 |
| HPV 51 | 14 | 11.5 |
Figure 1.
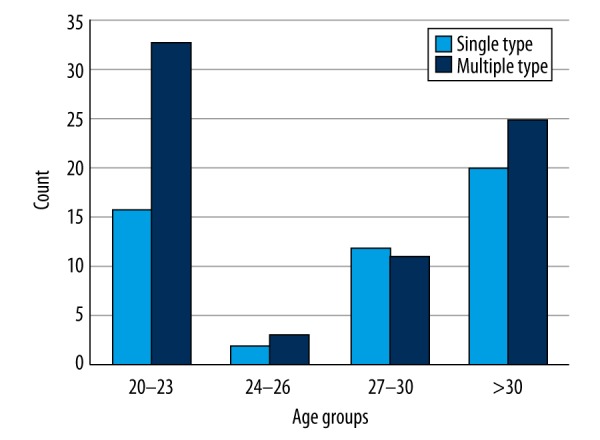
Age groups and HPV count.
Figure 2.
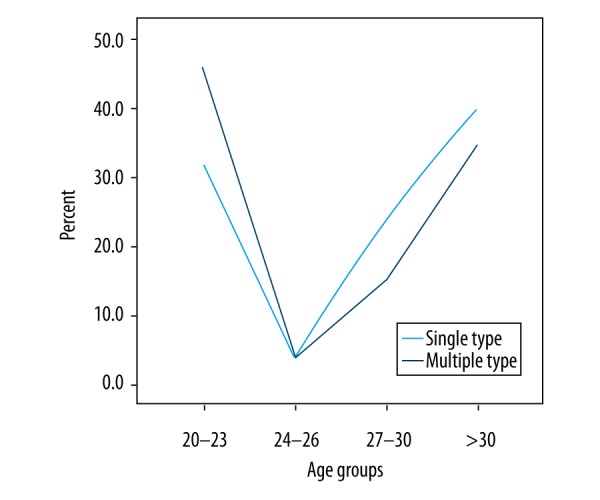
Age groups and HPV prevalence %.
The relation between HPV infection rates and year
The relation between HPV DNA-positive infection rates and year was: (18.0%, 22/122) in 2012, (19.7%, 24/122) in 2013, (16.4%, 20/122) in 2014, (24.6%, 30/122) in 2015, and (21.3%, 26/122) in 2016 (Table 3).
Table 3.
Distribution infection of gynaecological outpatients with 21 subtypes of human papillomavirus (HPV) in different years.
| Positive case | 2012 (n=22) | 2013 (n=24) | 2014 (n=20) | 2015 (n=30) | 2016 (n=26) | χ2 | p | Linear-by-linear association | p | Gamma value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV6 | 38 | 6 (27.3%) | 7 (29.2%) | 8 (40.0%) | 12 (40.0%) | 5 (19.2%) | 3.747 | .441 | .035 | .852 | −.038 |
| HPV 11 | 32 | 9 (40.9%) | 7 (29.2%) | 4 (20.0%) | 10 (33.3%) | 2 (7.7%) | 8.358 | .079 | 4.515 | .034* | −.311 |
| HPV 16 | 23 | 9 (40.9%) | 4 (16.7%) | 2 (10.0%) | 2 (6.7%) | 6 (23.1%) | 11.311 | .023* | 3.003 | .083 | −.257 |
| HPV 18 | 16 | 2 (9.1%) | 1 (4.2%) | 2 (10.0%) | 5 (16.7%) | 6 (23.1%) | 4.766 | .312 | 3.679 | 0.055 | .371 |
| HPV 31 | 6 | 1 (4.5%) | 1 (4.2%) | 1 (5.0%) | 2 (6.7%) | 1 (3.8%) | .296 | .990 | .008 | .927 | .022 |
| HPV 35 | 9 | 1 (4.5%) | 2 (8.3%) | 1 (5.0%) | 1 (3.3%) | 4 (15.4%) | 3.614 | .461 | .935 | .334 | .252 |
| HPV 39 | 7 | 0 (0.0%) | 1 (4.2%) | 1 (5.0%) | 2 (6.7%) | 3 (11.5%) | 3.134 | .536 | 2.881 | .090 | .476 |
| HPV 42 | 11 | 4 (18.2%) | 2 (8.3%) | 2 (10.0%) | 2 (6.7%) | 1 (3.8%) | 3.339 | .503 | 2.609 | .106 | −.356 |
| HPV43 | 6 | 1 (4.5%) | 0 (0.0%) | 2 (10.0%) | 2 (6.7%) | 1 (3.8%) | 2.613 | .625 | .149 | .699 | .100 |
| HPV 45 | 7 | 1 (4.5%) | 2 (8.3%) | 1 (5.0%) | 1 (3.3%) | 2 (7.7%) | .881 | .927 | .003 | .957 | .022 |
| HPV 52 | 13 | 1 (4.5%) | 3 (12.5%) | 2 (10.0%) | 4 (13.3%) | 3 (11.5%) | 1.205 | .877 | .525 | .469 | .148 |
| HPV 53 | 14 | 2 (9.1%) | 4 (16.7%) | 0 (0.0%) | 3 (10.0%) | 5 (19.2%) | 4.956 | .292 | .460 | .498 | .153 |
| HPV 56 | 9 | 1 (4.5%) | 3 (12.5%) | 3 (15.0%) | 0 (0.0%) | 2 (7.7%) | 5.274 | .260 | .245 | .620 | −.120 |
| HPV 58 | 6 | 1 (4.5%) | 0 (0.0%) | 2 (10.0%) | 1 (3.3%) | 2 (7.7%) | 2.942 | .568 | .464 | .496 | .199 |
| HPV 59 | 10 | 2 (9.1%) | 1 (4.2%) | 1 (5.0%) | 3 (10.0%) | 3 (11.5%) | 1.328 | .857 | .439 | .508 | .162 |
| HPV 66 | 8 | 4 (18.2%) | 1 (4.2%) | 1 (5.0%) | 1 (3.3%) | 1 (3.8%) | 5.976 | .201 | 3.170 | .075 | −.441 |
| HPV 68 | 7 | 2 (9.1%) | 1 (4.2%) | 1 (5.0%) | 3 (10.0%) | 0 (0.0%) | 3.177 | .529 | .590 | .443 | −.224 |
| HPV 82 | 8 | 3 (13.6%) | 0 (0.0%) | 2 (10.0%) | 3 (10.0%) | 0 (0.0%) | 6.275 | .180 | 1.017 | .313 | −.271 |
| HPV 73 | 4 | 1 (4.5%) | 1 (4.2%) | 1 (5.0%) | 1 (3.3%) | 0 (0.0%) | 1.240 | .872 | .774 | .379 | −.326 |
| HPV 33 | 4 | 1 (4.5%) | 0 (0.0%) | 1 (5.0%) | 1 (3.3%) | 1 (3.8%) | 1.138 | .888 | .037 | .847 | .069 |
| HPV 51 | 15 | 3 (13.6%) | 4 (16.7%) | 2 (10.0%) | 3 (10.0%) | 2 (7.7%) | 1.211 | .876 | .848 | .357 | −.185 |
Linear-by -linear Association test and Gamma Value for trend to evaluate changes in HPV prevalence by calendar year group.
p<0.05.
HPV infection rates
We found that 122 out of 532 patients were infected with HPV. Therefore, the infection ratio is 22.9%. The rate of multiple infections was 59.0% (72/122). HR-HPV (group 1 carcinogens HPV-16 (% 18.9, 23/122), HPV-18 (% 13.1, 16/122), HPV-31 (% 4.9, 6/122), HPV-33 (% 3.3, 4/122), HPV-35 (% 7.4, 9/122), HPV-39 (5.7%, 7/122), HPV-45 (% 5.7, 7/122), HPV-51 (% 11.5, 15/122), HPV-52 (% 10.7, 13/122), HPV-56 (% 7.4, 9/122), HPV-58 (4.9%, 6/122), HPV-59 (% 8.2, 10/122); Group 2A Probable carcinogens HPV-68 (% 5.7, 7/122); and Group 2B Possible carcinogens HPV-66 (% 6.6, 8/122), HPV-53 (% 11.5, 14/122), HPV-73 (3.3%, 4/122), HPV-82 (% 6.6, 8/122) and Group 3 LR-HPV; HPV-6 (% 31.1, 38/122), HPV-11 (% 26.2, 32/122), HPV-42 (% 9.0, 11/122) and HPV-43 (% 4.9, 6/122). The positive HPV DNA infection rate was 18.0% (22/122) in 2012, 19.7% (24/122) in 2013, 16.4% (20/122) in 2014, 24.6% (30/122) in 2015, and 21.3% (26/122) in 2016 (Table 4). In terms of linear-by-linear association test, no significant statistical difference was identified between years. The P value for HPV infection rate on a year basis was P>0.05.
Table 4.
Distribution of individual HPV genotypes according to age.
| Positive case | 20–23 age (n=192) | 24–26 age (n=29) | 27–30 age (n=84) | >30 age (n=227) | χ2 | p | Linear-by-linear association | p | Gamma value | |
|---|---|---|---|---|---|---|---|---|---|---|
| HPV 6 | 38 | 18 (36.7%) | 1 (20.0%) | 8 (34.8%) | 11 (24.4%) | 2.087 | .554 | 1.333 | .248 | −.194 |
| HPV 11 | 32 | 12 (24.5%) | 0 (0.0%) | 9 (39.1%) | 11 (24.4%) | 3.907 | .272 | .115 | .735 | .027 |
| HPV 16 | 23 | 10 (20.4%) | 2 (40.0%) | 3 (13.0%) | 8 (17.8%) | 2.080 | .556 | .279 | .579 | −.082 |
| HPV 18 | 16 | 8 (16.3%) | 1 (20.0%) | 2 (8.7%) | 5 (11.1%) | 1.204 | .752 | .769 | .380 | −.178 |
| HPV 31 | 6 | 2 (4.1%) | 0 (0.0%) | 1 (4.3%) | 3 (6.7%) | .642 | .887 | .333 | .564 | .207 |
| HPV 35 | 9 | 1 (2.0%) | 2 (40.0%) | 2 (8.7%) | 4 (8.9%) | 10.039 | .018* | 1.217 | .270 | .284 |
| HPV 39 | 7 | 2 (4.1%) | 0 (0.0%) | 0 (0.0%) | 5 (11.1%) | 4.355 | .226 | 1.573 | .210 | .468 |
| HPV 42 | 11 | 8 (16.3%) | 0 (0.0%) | 1 (4.3%) | 2 (4.4%) | 5.445 | .142 | 4.258 | .039* | −536 |
| HPV 43 | 6 | 2 (4.1%) | 0 (0.0%) | 1 (4.3%) | 3 (6.7%) | .642 | .887 | .333 | .564 | .207 |
| HPV 45 | 7 | 2 (4.1%) | 0 (0.0%) | 0 (0.0%) | 5 (11.1%) | 4.355 | .226 | 1.573 | .210 | .468 |
| HPV 52 | 13 | 9 (18.4%) | 0 (0.0%) | 1 (4.3%) | 3 (6.7%) | 5.371 | .147 | 3.710 | .054 | −.459 |
| HPV 53 | 14 | 7 (14.3%) | 1 (20.0%) | 2 (8.7%) | 4 (8.9%) | 1.210 | .751 | .844 | .358 | −.202 |
| HPV 56 | 9 | 3 (6.1%) | 0 (0.0%) | 2 (8.7%) | 4 (8.9%) | .720 | .868 | .345 | .557 | .159 |
| HPV 58 | 6 | 4 (8.2%) | 1 (20.0%) | 1 (4.3%) | 0 (0.0%) | 5.879 | .118 | 3.670 | .055 | −.616 |
| HPV 59 | 10 | 3 (6.1%) | 0 (0.0%) | 3 (13.0%) | 4 (8.9%) | 1.473 | .688 | .458 | .499 | .153 |
| HPV 66 | 8 | 6 (12.2%) | 0 (0.0%) | 1 (4.3%) | 1 (2.2%) | 4.501 | .212 | 3.838 | .050 | −.599 |
| HPV 68 | 7 | 5 (10.0%) | 0 (0.0%) | 2 (8.7%) | 0 (0.0%) | 5.223 | .156 | 3.737 | .053 | −.639 |
| HPV 82 | 8 | 4 (8.2%) | 0 (0.0%) | 2 (8.7%) | 2 (4.4%) | 1.057 | .788 | .358 | .550 | −.199 |
| HPV 73 | 4 | 2 (4.1%) | 0 (0.0%) | 0 (0.0%) | 2 (4.4%) | 1.242 | .743 | 0.01 | .970 | .027 |
| HPV 33 | 4 | 3 (6.1%) | 0 (0.0%) | 0 (0.0%) | 1 (2.2%) | 2.357 | .502 | 1.375 | .241 | −.490 |
| HPV 51 | 15 | 7 (14.3%) | 0 (0.0%) | 3 (13.0%) | 4 (8.9%) | 1.381 | .710 | .500 | .480 | −.177 |
Linear-by -linear association test and Gamma Value for trend to evaluate changes in HPV prevalence by age group.
p<0.05.
Discussion
HPV is a highly contaminating virus. It is one of the many DNA viruses that might particularly harm human skin and mucosal epithelium. Therefore, it may lead to an excessive proliferation of human skin and mucosal epithelium. Since HPV infection leads to an abnormal reproduction in skin and mucosal epithelium cells, it might cause verrucose lesions and papillomas in host tissue. The infection incidence increases rapidly after a woman becomes sexually active.
After a woman is infected with HPV, there are some risk factors that might help cervical cancer develop. Human papilloma virus (HPV) has been established as a necessary cause of cervical cancer. Therefore, detection of high-risk HPV is becoming increasingly attractive as a primary screening tool.
In the present study, we aimed to retrospectively evaluate the results of outpatients who had an HPV analysis, and to present objective evidence for the administration of preventive inoculation in our area. We have identified that the ratio of HPV infection for those under the age of 30 years is increasing ever year. Therefore, HPV infection is more common in young peoples, and those who are 20–24 years old are more susceptible to HPV infection. These results present clear evidence for choosing groups of young patients for a preventive inoculation study in our region.
One of the main aims of a gynecological examination is to detect neoplasms in the genital area as early as possible. Since 1944, when Papanicolaou and Traut introduced cytology into clinical applications, it has been possible to determine cervical cancer at pre-invasive phases, and decrease morbidity and mortality that results from this malignancy.
Similarly, early diagnosis and treatment of HPV infection has an important role in preventing cervical cancer. Epidemiologic and biological research demonstrate that the statistical distribution of HPV infection and its subtypes varies by region, age, and some other demographic features of the population [27,28]. According to the statistical data provided by the IARC, 5 HPV subtypes with highest infection incidence are HPV-16, -18, -58, -52, and -31.
Although a quadrivalent HPV vaccine for HPV 16, 18, 6, and 11 subtypes was developed in 2007, and a bivalent vaccine for HPV 16 and 18 was developed in 2008, the prevalence of current HPV subtypes are still quite high. In the last 40 years, HPV incidence has been dramatically increasing with changes in sexual behavioral. Therefore, it is suggested that HPV infection is probably an epidemic that results from such changes in sexual habits [29–31]. In a study carried out by Nah E-T et al., 15,426 women were analyzed. The most common high-risk HPV subtype among these patients was determined to be HPV 52 (1.9%) [32]. In some other previous studies, 100,000 women in 5 continents who have normal cytology records were examined.
The positive HPV rate was found to be 11.7% (range: 6.1–33.5%) [33,34]. In another study, performed by Zhang et al. in the suburbs of Shanghai, China, 33,562 women participants were analyzed. Their human papilloma virus (HPV) prevalence and genotype distribution were examined. The HPV prevalence was 18.98%, and the 10 leading genotypes were HPV 16 (3.36%), HPV 58 (2.65%), HPV 52 (2.48%), HPV 51 (1.58%), HPV 54 (1.40%), HPV 68 (1.32%), HPV 18 (1.23%), HPV 6 (1.15%), HPV 56 (1.10%), and HPV 33 (1.07%). Important differences were revealed among age groups and month groups regarding the prevalence of single and multiple infection of high-risk and low-risk HPV (P<0.05) [35]. In another study, conducted by Guzhalinuer Abulizi et al. in China, risk factors for human papilloma virus infection among Uyghur women from Xinjiang, China were analyzed. Of 6000 women participants, 505 (8.42%) patients were identified as HPV DNA-positive [36]. In 2 different regions of the same country, the HPV prevalence results of large-scale studies were determined to be different. Therefore, as many studies in the literature suggest, we can conclude that socioeconomic and socio-cultural features of the population and effective prevention methods such as public health training and vaccination could influence the prevalence of HPV infection.
Epidemiologic and biological research demonstrates that the statistical distribution of HPV infection and its subtypes depends on region, age, and some other demographic features of the population [37,38]. Our study was conducted in Ankara, Turkey, so the patients were from a region where the socioeconomic and cultural level is high. According to previous studies, the HPV prevalence for Turkish women ranges from 2% to 25% depending on the analysis method, the number of patients examined, and the HPV types researched [20,39,40]. In these contexts, it is important to determine the infection rate, genetic profile, and epidemiological patterns of HPV in Ankara so that HPV infection can be controlled and even prevented in this region. We believe that, with certain different screening, diagnosis, and treatment methods, an efficient prevention method could be developed. A previous study by our group found that women with clinical diagnosis of unhealthy cervix should be evaluated by cytology to detect any premalignant or malignant lesions, and that Pap smear, colposcopy, and histopathology should be collectively used to evaluate cervical findings [41]. The results in our study show that 122 out of 532 patients were infected with HPV. Therefore, the infection ratio was 22.9%. While the single-infection rate was 41.0% (50/122), the rate of multiple infections was 59.0% (72/122) (Figure 3). Among 21 HPV subtypes, infection rates of the 2 main low-risk HPV subtypes are HPV-6 (31.1%, 38/122) and HPV-11 (26.2%, 32/122).
Figure 3.
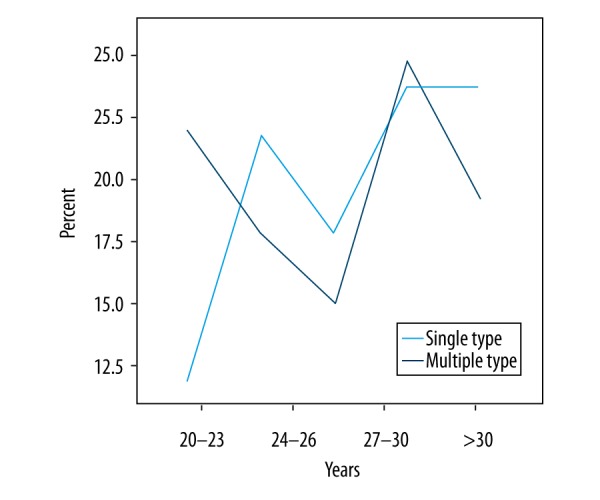
Changes in the rates of single and multiple human papillomavirus infections in gynecological outpatients, 2012–2016.
The leading 5 HPV subtypes were HPV-16 (18.9%, 23/122), HPV-18 (13.1%, 16/122), HPV-31 (4.9%, 6/122), HPV-35 (7.4%, 9/122), and HPV-39 (5.7%, 7/122). The positive HPV DNA infection rate was 18.0% (22/122) in 2012, 19.7% (24/122) in 2013, 16.4% (20/122) in 2014, 24.6% (30/122) in 2015, and 21.3% (26/122) in 2016 (Table 4). In terms of linear-by-linear association test, no significant statistical difference was identified between years. The P value for HPV infection rate on a yearly basis was P>0.05.
Our findings suggest that the HPV infection ratio of outpatients in the last 5 years was 22.9%. This figure is similar to the findings of some other studies. For instance, in a study by Lili Qian et al., the HPV infection rate was 22.3% [27]. In a study conducted by Nah et al. in Korea, 18,815 women were examined; 5227 (27.8%) of them were identified to have a high-risk type of HPV infection, and of these 5227 patients, 1994 (38.1%) women were determined to have multiple infections [20]. The relationship between HPV infection and age is also a significant factor. There are some studies in the field which claim that young women are more sexually active and they are more prone to have sex with multiple partners [42]. In addition, they are more vulnerable to HPV infection because their immune systems are not sensitized. As a result, the HPV infection rate is higher in women 16–24 years old [43,44].
In another study, it is claimed that the HPV infection ratio is also high in menopausal women since their immune functions are deteriorating. Therefore, it is suggested that, in terms of age, there 2 peak periods in HPV infection ratio [45]. In this study, the HPV infection rate for the 20–23 age group was 40.2% (49/122), and for the >30 age group it was 36.9% (45/122).
In short, these findings clearly demonstrate that young women and menopausal women are the high-risk groups. Therefore, a clinical HPV test is important for them. In addition, it is crucial that these women should be provided with preventive vaccines at early ages.
Limitations
The present study had some limitations. First, the number of participants included in this study was relatively low. Second, this was a single-center pilot study including data from only from a private hospital. Thirdly, women with low socioeconomic status were not included in this study. Therefore, age group comparisons could not be carried out on a large scale. In this respect, multi-center studies with higher numbers of participants might be useful to elucidate this area of research.
Strength
Our study has some advantages. First, the data analyzed in the were obtained from a private hospital, which has a strong network and database. Moreover, it is the first study conducted with such a large group of women with high socioeconomic status.
Conclusions
The present study provided invaluable results about HPV and genotype distribution in women with high socioeconomic status in Ankara. In this particular hospital-based retrospective analysis, HPV types were found to be similar to HPV types reported in developed countries. The results demonstrate the real population-based HPV prevalence in our country. However, a serially-sequenced study with large numbers of patients from multiple centers is needed to determine the distribution of HPV types. We firmly suggest that patients should be informed about the risk of HPV infection at early ages, and preventive inoculation methods should be applied for them.
Footnotes
Source of support: Departmental sources
References
- 1.Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: A pooled analysis. Lancet. 2005;366:991–98. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 2.Li N, Franceschi S, Howell-Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: Variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–35. doi: 10.1002/ijc.25396. [DOI] [PubMed] [Google Scholar]
- 3.Crow JM. HPV: The global burden. Nature. 2012;488:S2–3. doi: 10.1038/488S2a. [DOI] [PubMed] [Google Scholar]
- 4.Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of noncancerous HPV-related conditions: HPV-6/11 disease. Vaccine. 2006;24:S35–41. doi: 10.1016/j.vaccine.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Clifford G, Franceschi S, Diaz M, et al. Chapter 3: HPV type distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24:26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Chacón J, Sanz I, Rubio MD, et al. Detection and genotyping of high-risk human papillomavirus in cervical specimens. Enferm Infecc Microbiol Clin. 2007;25:311–16. doi: 10.1157/13102266. [DOI] [PubMed] [Google Scholar]
- 7.Cuschieri KS, Whitley MJ, Cubie HA. Human papillomavirus type specific DNA and RNA persistence-implications for cervical disease progression and monitoring. J Med Virol. 2004;73:65–70. doi: 10.1002/jmv.20062. [DOI] [PubMed] [Google Scholar]
- 8.Del Mistro A, Salamanca HF, Trevisan R, et al. Human papillomavirus typing of invasive cervical cancers in Italy. Infect Agent Cancer. 2006;1:9. doi: 10.1186/1750-9378-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong EP. Prophylaxis of cervical cancer and related cervical disease: A review of the cost-effectiveness of vaccination against oncogenic HPV types. J Manag Care Pharm. 2010;16:217–30. doi: 10.18553/jmcp.2010.16.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryndock EJ, Meyers C. A risk for non-sexual transmission of human papillomavirus? Expert Rev Anti Infect Ther. 2014;12:1165–70. doi: 10.1586/14787210.2014.959497. [DOI] [PubMed] [Google Scholar]
- 11.zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res. 1976;36(2):794. [PubMed] [Google Scholar]
- 12.Globocan. Estimated cancer incidence, mortality and prevalence worldwide in 2012. Updated 2017. [Google Scholar]
- 13.den Boon JA, Pyeon D, Wang SS, et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. Proc Natl Acad Sci USA. 2015;112(25):E3255–64. doi: 10.1073/pnas.1509322112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard HU. Gene expression of genital human papillomavirus and considerations on potential antiviral approaches. Antivir Ther. 2002;7(4):219–37. [PubMed] [Google Scholar]
- 15.Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 16.de Sanjosé S, Quint WG, Alemany L, et al. Retrospective international survey and HPV time trends study group human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11(11):1048–56. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 17.Zeng Z, Yang H, Li Z, et al. Prevalence and genotype distribution of HPV infection in China: analysis of 51,345 HPV genotyping results from China’s largest CAP certified Laboratory. J Cancer. 2016;7(9):1037–43. doi: 10.7150/jca.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baloch Z, Yuan T, Wang B, et al. Ethnic and geographic variations in HPV prevalence and genotype distribution in North-Western Yunnan, China. J Med Virol. 2016;88:532–40. doi: 10.1002/jmv.24352. [DOI] [PubMed] [Google Scholar]
- 19.Baloch Z, Li Y, Yuan T, et al. Epidemiologic characterization of human papillomavirus (HPV) infection in various regions of Yunnan Province of China. BMC Infect Dis. 2016;16:228. doi: 10.1186/s12879-016-1562-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dursun P, Ayhan A, Mutlu L, et al. HPV types in turkey: multicenter hospital based evaluation of 6388 patients in Turkish Gynecologic Oncology Group Centers. Turk Patoloji Derg. 2013;29(3):210–16. doi: 10.5146/tjpath.2013.01188. [DOI] [PubMed] [Google Scholar]
- 21.Sacace Biotechnologies. HPV High Risk Screen Real-TM Quant. Como, Italy: Sacace Biotechnologies; 2009. [Google Scholar]
- 22.Şahiner F, Kubar A, Yapar M, et al. Detection of major HPVs by a new multiplex real-time PCR assay using type-specific primers. J Microbiol Methods. 2014;97:44–50. doi: 10.1016/j.mimet.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Şahiner F, Gümral R, Şener K, et al. Investigation of HPVDNA in cervical smear samples by two different methods: MY09/11 consensus PCR and type-specific real-time PCR. Mikrobiyol Bul. 2012;46(4):624–36. [PubMed] [Google Scholar]
- 24.Kleter B, van Doorn LJ, Schrauwen L, et al. Development and clinical evaluation of a highly sensitive PCR-reverse hybridization line probe assay for detection and identification of anogenital human papillomavirus. J Clin Microbiol. 1999;37:2508–17. doi: 10.1128/jcm.37.8.2508-2517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleter B, van Doorn LJ, ter Schegget J, et al. A novel short-fragment PCR assay for highly sensitive broad-spectrum detection of anogenital human papillomaviruses. Am J Pathol. 1998;153:1731–39. doi: 10.1016/S0002-9440(10)65688-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yapar M, Aydogan H, Pahsa A, et al. Rapid and quantitative detection of Crimean Congo haemorrhagic fever virus by one-step real-time reverse transcriptase-PCR. Jpn J Infect Dis. 2005;58:358–62. [PubMed] [Google Scholar]
- 27.Lee EH1, Um TH, Chi HS, et al. Prevalence and distribution of human papillomavirus infection in Korean women as determined by restriction fragment mass polymorphism assay. J Korean Med Sci. 2012;27(9):1091–97. doi: 10.3346/jkms.2012.27.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rey-Ares L, Ciapponi A, Pichon-Riviere A. Efficacy and safety of human papilloma virus vaccine in cervical cancer prevention: Systematic review and meta-analysis. Arch Argent Pediatr. 2012;110(6):483–89. doi: 10.5546/aap.2012.eng.483. [DOI] [PubMed] [Google Scholar]
- 29.Brewster DH, Bhatti LA. Increasing incidence of squamous cell carcinoma of the anus in Scotland, 1975–2002. Br J Cancer. 2006;95:87–90. doi: 10.1038/sj.bjc.6603175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasman A, Attner P, Hammarstedt L, et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: An epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–66. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 31.de Sanjose S, Cortes X, Mendez C, et al. Age at sexual initiation and number of sexual partners in the female Spanish population results from the AFRODITA survey. Eur J Obstet Gynecol Reprod Biol. 2008;140:234–40. doi: 10.1016/j.ejogrb.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Nah EH, Cho S, Kim S, Cho HI. Human papillomavirus genotype distribution among 18,815 women in 13 Korean cities and relationship with cervical cytology findings. Ann Lab Med. 2017;37(5):426–33. doi: 10.3343/alm.2017.37.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: A pooled analysis. Lancet. 2005;366:991–98. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 34.Bruni L, Diaz M, Castellsagué X, et al. Cervical humanpapillomavirus prevalence in 5 continents: Meta-analysis of 1million women with normal cytological findings. J Infect Dis. 2010;202:1789–99. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C, Zhang C, Huang J, et al. Prevalence and genotype distribution of human papillomavirus among females in the suburb of Shanghai, China. J Med Virol. 2017 doi: 10.1002/jmv.24899. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Abulizi G, Li H, Mijiti P, et al. Risk factors for human papillomavirus infection prevalent among Uyghur women from Xinjiang, China. Oncotarget. 2017 doi: 10.18632/oncotarget.18901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee EH1, Um TH, Chi HS, et al. Prevalence and distribution of human papillomavirus infection in Korean women as determined by restriction fragment mass polymorphism assay. J Korean Med Sci. 2012;27(9):1091–97. doi: 10.3346/jkms.2012.27.9.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian L, Zhang Y, Cui D, et al. Analysis of epidemiological trends in human papillomavirus infection among gynecological outpatients in Hangzhou, China, 2011–2015. BMC Infect Dis. 2017;17:393. doi: 10.1186/s12879-017-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inal MM, Köse S, Yildirim Y, et al. The relationship between human papillomavirus infection and cervical intraepithelial neoplasia in Turkish women. Int J Gynecol Cancer. 2007;7:1266–70. doi: 10.1111/j.1525-1438.2007.00944.x. [DOI] [PubMed] [Google Scholar]
- 40.Ozcelik B, Serin IS, Gokahmetoglu S, et al. Human papillomavirus frequency of women at low risk of developing cervical cancer: A preliminary study from a Turkish university hospital. Eur J Gynaecol Oncol. 2003;24:157–59. [PubMed] [Google Scholar]
- 41.Barut MU, Kale A, Kuyumcuoğlu U, et al. Analysis of sensitivity, specificity, and positive and negative predictive values of smear and colposcopy in diagnosis of premalignant and malignant cervical lesions. Med Sci Monit. 2015;21:3860–67. doi: 10.12659/MSM.895227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao FH, Tiggelaar SM, Hu SY, et al. A multi-center survey of age of sexual debut and sexual behavior in Chinese women: Suggestions for optimal age of human papillomavirus vaccination in China. Cancer Epidemiol. 2012;36(4):384–90. doi: 10.1016/j.canep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slama J, Drazdakova M, Dunder P, et al. High-risk human papillomavirus DNA in the primary tumor, sentinel, and nonsentinel pelvic lymph nodes in patients with early-stage cervical cancer: A correlation with histopathology. Int J Gynecol Cancer. 2009;19(4):703–7. doi: 10.1111/IGC.0b013e3181a13186. [DOI] [PubMed] [Google Scholar]
- 44.Sherpa AT, Clifford GM, Vaccarella S, et al. Human papillomavirus infection in women with and without cervical cancer in Nepal. Cancer Causes Control. 2010;21:323–30. doi: 10.1007/s10552-009-9467-z. [DOI] [PubMed] [Google Scholar]
- 45.Kim MJ, Kim JJ, Kim S. Type-specific prevalence of high-risk human papillomavirus by cervical cytology and age: data from the health check-ups of 7,014 Korean women. Obstet Gynecol Sci. 2013;56(2):110–20. doi: 10.5468/OGS.2013.56.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]


