Abstract
Calcium phosphate cements (CPCs) are frequently used to repair bone defects. Since their discovery in the 1980s, extensive research has been conducted to improve their properties, and emerging evidence supports their increased application in bone tissue engineering. Much effort has been made to enhance the biological performance of CPCs, including their biocompatibility, osteoconductivity, osteoinductivity, biodegradability, bioactivity, and interactions with cells. This review article focuses on the major recent developments in CPCs, including 3D printing, injectability, stem cell delivery, growth factor and drug delivery, and pre-vascularization of CPC scaffolds via co-culture and tri-culture techniques to enhance angiogenesis and osteogenesis.
Introduction
There has been a continuous and fast-paced emergence of new synthetic biomaterials developed for bone repair and regeneration over the past several decades. These biomaterials include metals, polymers, ceramics, bioactive glasses, calcium sulfates, calcium carbonates and calcium phosphates (CaPs). Among them, calcium phosphate cements (CPCs) are promising for clinical applications due to their advantageous properties including bioactivity, osteoconductivity, injectability and moldability. The discovery of the first CPC occurred inadvertently via the observation of calcium phosphate solubility behavior.1–3 Brown and Chow found that the solubilities of tetracalcium phosphate [TTCP: Ca4(PO4)2O], dicalcium phosphate (DCPA: CaHPO4) and dicalcium phosphate dehydrate (DCPD: CaHPO4 2H2O) were much greater than that of hydroxyapatite (HA) under neutral pH conditions.4 A slurry containing appropriate amounts of TTCP and DCPD (or DCPA) led to HA precipitation as an end product and was capable of self-setting to form a hard mass.2,3 In the decade following this first discovery, CPCs were approved by the Food and Drug Administration (FDA) and were introduced into clinical practice for the treatment of craniofacial defects5 and bone fractures.6 Since then, other CPC formulations have been developed, and a large amount of research has been conducted.7–18 Currently, CPCs are defined as a combination of one or more calcium phosphate powders which, upon mixing with a liquid phase, form a paste able to self-set and harden in situ in the bone defect site to form a scaffold.19
One of the most important characteristics of CPCs is their ability to form in situ through a body-temperature dissolution-precipitation reaction.19 This feature gives rise to other beneficial properties such as molding capability upon mixing,20 injectability that enables minimally invasive application,21 and the ability to serve as a carrier for drug and biological molecule delivery.22 Early research on CPCs primarily focused on improved setting, handling and mechanical properties of CPCs through the tailoring of many processing parameters such as cement composition, additives, porogens, and particle size.23–28 In recent years, in addition to the development of new processing technologies in CPC manufacturing, the paradigm has shifted toward biological responses by emphasizing the enhancement of biological interactions of CPCs with cells and tissues as well as their applications in bone tissue engineering.29–33 Biological responses of scaffolds are a key factor in the translational application of biomaterials and their commercialization for clinic applications. Several meritorious reviews on CPCs have described their mechanical properties,34–36 processing approaches,37,38 drug delivery,19,22,39,40 and functional enhancement by polymeric additives,41 which will not be repeated here. The present article reviews the major new developments in CPC processing technologies in recent years and focuses on novel biological interactions of CPCs, particularly in the context of stem cell responses and delivery as well as in vivo bone regeneration. The various CPC categories described in this article and their major biological properties are summarized in the diagram in Figure 1.
Figure 1.
Schematic diagram summarizing the various CPC categories described in this article and their major biological properties.
Pre-fabricated CPC scaffolds and 3D printing
Although injectability is one of the advantages of CPCs, pre-fabricated CPC scaffolds are often prepared for two reasons: (1) To ensure a complete setting reaction because only fully set CPCs demonstrate excellent tissue responses. When CPCs fail to set, they cause inflammatory reactions.42 Therefore, manufacturing pre-fabricated CPCs ensures complete setting prior to in vivo application. (2) To facilitate the creation of interconnected macroporous structures into CPCs. Self-setting CPC scaffolds without any modification are microporous but not macroporous and have limited pore interconnections.43 To promote tissue in-growth and accelerate the CPC degradation rate and subsequent replacement by bone, macropores were incorporated into CPCs via two methods: particle leaching (the addition of water-soluble particles, such as sodium bicarbonate, mannitol, salt or glucose, that dissolve or degrade after setting) and gas-foaming (the formation of air bubbles during the setting period).37,44 In situ setting with particle leaching has several disadvantages. First, because the porogens inside the cement have limited exposure to body fluids, the degradation or solubility of the particles may be compromised, which leads to limited porosity.45 Second, the in vivo dissolution of some particles may result in hyperosmosis.46 Third, some porogens may increase the paste viscosity and impede the injectability of CPC. The major drawback of in situ application of the gas-foaming method is the risk of air emboli or emphysema. Therefore, pre-fabricated CPC scaffolds have been developed to allow more delicate control of the setting process and macroporous architecture of the scaffolds before in vivo implantation.
Recently, three-dimensional (3D) printing has rapidly developed to allow the fabrication of pre-set CPC scaffolds. 3D printing is an additive manufacturing process in which geometrical data are used to produce 3D structures by depositing materials layer by layer.47 3D-printed CPC scaffolds are favored over customization to meet the specific needs of each patient/defect. The benefits for clinical applications include easy adaptation and fixation, reduced surgical time, favorable esthetic results and minimal waste products. There are several different techniques for 3D printing, including direct 3D printing (direct ink writing), fused deposition modeling (FDM), stereolithography (SLA), and selective laser sintering (SLS). For a detailed description of each technique, readers are encouraged to read previous review papers on this topic.48,49 For CPC scaffolds, binder jetting is the most commonly employed 3D printing technique.50 Briefly, one or several print heads spray a binder solution (for example, an aqueous solution) precisely onto a bed layer of CPC powder. The binder locally joins adjacent powder particles together and hardens the wetted areas through the dissolution-precipitation reaction. The process repeats by spreading another layer of powder and ejecting binders according to a pass designed by the computer. This continues until the complete 3D structure is formed.48 The printability of the material is related to many parameters such as particle size and size distribution, morphology and surface area of the powder, roughness and flowability of the powders, the solubility/wettability/reactivity of the powder with the binder, and binder drop size.51 A study investigating beta-tricalcium phosphate powder suggested that 3D printing was not feasible with particles either too small (with a mean particle size of 7 μm) or too large (with a mean particle size of 51 μm), while mean particle sizes in the range of 20–35 μm resulted in good printing accuracy.51 Small particles tend to agglomerate under the influence of van der Waals forces. Very fine or porous particles exhibit low flowability and high surface roughness. Therefore, these factors greatly affect the smoothness and homogeneity of the powder bed, resulting in smearing and poor resolution.51 However, although large particles have better flowability, they tend to yield layer displacements due to low powder bed stability and low accuracy because the resolution is at least twice the particle size.52 Flowability was shown to be significantly reduced by decreasing the HA granule size.53 To work with small particle sizes to achieve a high resolution, strategies such as plasma coating51 and moisture application54 were attempted to stabilize the top layer surface and allow particle rearrangement and wetting while avoiding particle ejection out of the powder bed. Furthermore, by adding reactive minerals such as calcium sulfates into calcium phosphate, significant improvements to 3D printing parameters are achieved.55 The dimensional accuracy of printed CPC scaffolds (powder: alfa-TCP; liquid: Na2HPO4) is ~200·μm, which indicates a good degree of fitting to craniofacial defects in anatomical models.56 A critical step for powder-based 3D printing is the removal of the loose powder inside the pores of the printed scaffold after printing, a process known as depowdering. Depowdering is especially challenging when the pores and pore interconnections are small and found in the innermost parts of the scaffolds with large dimensions. One possible solution may be the use of depowdering-friendly designs with large windows and free-to-move fillers.57 In addition, layer thickness and printing orientations (parallel to the X, Y and Z directions) are important for depowdering.58 Shear forces at the powder bed increase with reduced layer thickness, which leads to the deterioration of the final printed samples upon depowdering. Depowdering is easier in scaffolds printed in the X and Y directions than that in scaffolds printed in the Z direction because of the distortion in samples printed in the Z direction.58 However, the relationship between 3D printing parameters and CPC scaffold quality and performance has yet to be established and warrants further study.
3D plotting (direct ink writing, direct write assembly, material extrusion) is another common technique for CPC 3D printing.59 This is an extrusion-based printing technology in which a paste or viscous materials, instead of powders, are used as the starting form and deposited as strands via a nozzle in a layer-by-layer fashion based on predesigned structures.60 For 3D plotting, the printability is dependent on even dispersion, viscosity, fluidity, extrusion performance, setting time of the paste, and the shape stability of the printed strands to withstand the weight of the structure during assembly. The setting time for CPCs plays an important role in controlling the printable time period of the paste. One study reported the printable time of a CPC (powder: TECP:DCPA=1:1 molar ratio, liquid/binder: polyvinyl alcohol) as only 10·min, which makes printing difficult.61 With the addition of a mesoporous calcium silicate, the printable time was increased to approximately 120·min.61 Other optimizations of the direct printing ink formulation have included the addition of gelatin to introduce an induction time for the onset of the CPC setting reaction.62 Specifically, this formula includes Targon 1128 as the dispersant, hydroxypropyl methylcellulose (HPMC) as the thickening agent, polyethylenimine (PEI) as the jellifying agent,63 and a ready-to-use oil-based CPC paste that sets only upon contact with water and thus has no time limit for printing.59
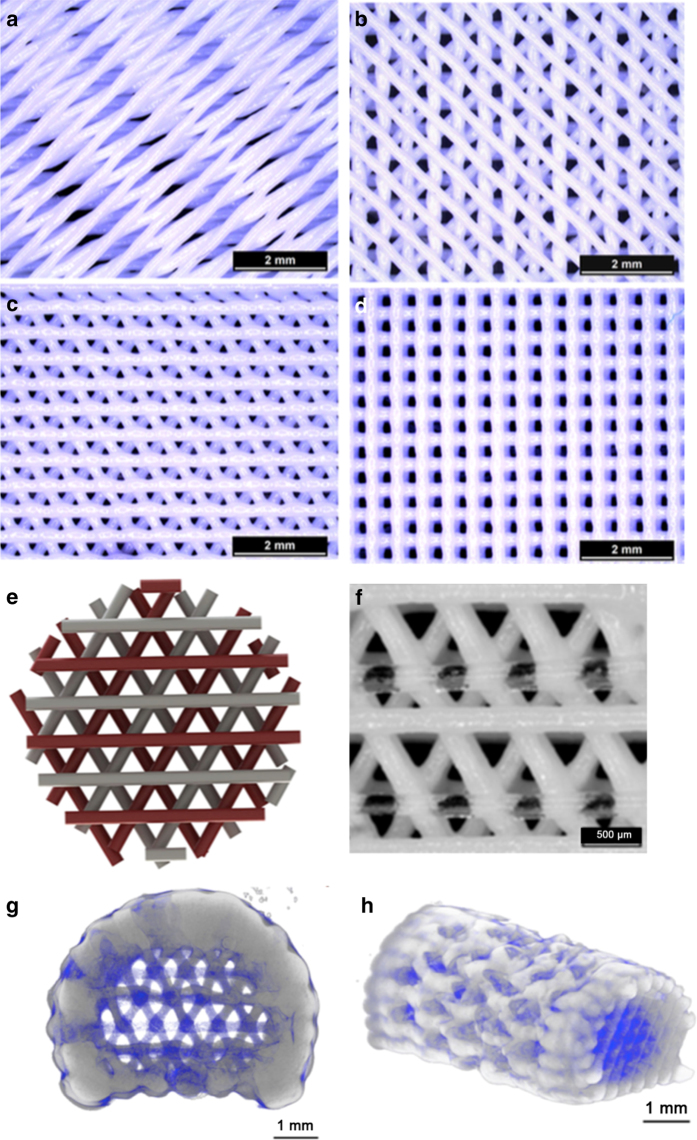
A critical issue for printing resolution is nozzle diameter and the stability of the extruded strands.50 3D plotting has two advantages: (1) it enables easy printing of a combination of different materials,64 and (2) due to the mild conditions, it allows simultaneous cell or growth factor plotting, known as bioprinting.64,65 Using a two-channel plotting method, a scaffold with the combination of an oil-based CPC and an alginate-gellan hydrogel was fabricated and laden with growth factor VEGF, involving a highly sophisticated strand arrangement, pore structure and geometry (Figure 2).64 In another study, a bone morphogenetic protein 2 (BMP2)-loaded mesoporous silica/CPC porous scaffold was 3D-plotted and tested in in vitro cell culture and in a rabbit femur defect model.66 The scaffold promoted the osteogenic differentiation of human bone marrow stromal cells (hBMSCs) and enhanced vascularization and osteogenesis compared to the CPC control.66 In terms of cell-containing bioprinting, hydrogels such as alginate,67 collagen,68 synthetic polymers such as PLGA, and PEG69 are primarily used as bioinks due to their resemblance to the extracellular matrix (ECM) and good printability. In some cases, calcium phosphates are added to enhance cell attachment and osteogenic differentiation, thus favoring the use of bioink for bone tissue engineering applications.67
Figure 2.
Highly sophisticated CPC scaffold structures via 3D plotting. Stereomicroscopic images of CPC scaffolds plotted with 15° (a), 45° (b), 60° (c) and 90° (d) configurations (change in orientation relative to the layer underneath). Design and printing of a CPC-hydrogel biphasic scaffold: model of biphasic scaffolds with CPC (white) and a growth factor-loaded hydrogel (red) (e); the printed scaffold (f); 3D reconstructions from micro-CT data of the biphasic scaffold (g, h). CPC is grayish white. Alginate-gellan hydrogel is blue. (Adapted from Ahlfeld et al.64 with permission.)
In general, due to the incremental addition of materials, 3D printing allows for not only the easy control of scaffold shape and geometry but also the control of fine features such as interconnected porosity, pore size and distribution, and complex spatial heterogeneity, which are not achievable with traditional strategies.50 The possibility of manufacturing customized implants with almost no design limitations makes 3D printing highly valuable in reconstructive surgery. However, more extensive research is needed to optimize the key parameters for successful 3D printing of CPC scaffolds.
Injectable CPC scaffolds
Traditional bone grafting requires an open surgical approach to graft application sites and may be associated with complications such as a large surgical scar, increased pain and a longer post-operative recovery. To overcome these drawbacks, injectable bone graft substitutes are used for minimally invasive surgery. Two main obstacles that inhibit CPC injectability are liquid-solid phase separation during injection70 and paste disintegration upon contact with blood or body fluids.71 Phase separation leads to not only the presence of non-extrudable paste left in the syringe but also extravasation at the injection site and a decrease in the viscosity and mechanical strength of CPCs. The disintegration of CPCs in the body causes inflammatory responses and even severe consequences such as cement embolism and cardiovascular deterioration by simulating blood coagulation.72 Therefore, efforts have been made to improve CPC injectability. These strategies include the following: (1) increasing the viscosity of the liquid phase by adding viscous binders such as chitosan,24 gelatin,73 hyaluronic acid,74 methylcellulose,75 and others; (2) optimizing the CPC powder in terms of the particle size, particle size distribution, particle shape, and particle-particle interactions;76 (3) regulating the setting reaction;77 and (4) modifying the extrusion parameters such as CPC mixing and the sizes of the syringes and/or needles.78 All of these factors were discussed in detail in a recent review on CPC injectability.70
Recently, many studies have applied various injectable CPC formulations into animal models for bone regeneration.79,80 Injectable CPCs containing 50% (volume ratio) microspheres (poly(lactic-co-glycolic acid) (PLGA), gelatin (GEL) or poly(trimethylene carbonate) (PTMC)) were implanted into rabbit femoral bone defects. CPC/GEL had a significantly lower score than all other groups at the cement-bone interface. Both CPC and CPC/PLGA showed a better response than CPC/PTMC at 4 weeks, but there were no significant differences among these three groups at 8 and 12 weeks.79 A recent study applied a commercially injectable CPC (Calcibon) with platelet lysates in bilateral calvarial defects in rats.81 The delivery of the platelet lysate enhanced bone healing with an injectable CPC at early healing times. In large animal models, injectable CPCs have also shown promise for bone regeneration. For example, injectable CPC/PLGA composites demonstrated biocompatibility and direct bone contact for sinus floor augmentation procedures in a sheep model.82 Another study evaluated the efficiency of local bisphosphonate delivery via injectable CPC in vertebral bodies of the lumbar spine of an osteoporotic sheep model where the consequences of osteoporotic fractures were highly deleterious in patients. The bisphosphonate-combined cement in vertebral body bone defects had a beneficial impact on both bone content and the micro-architectural properties of the trabecular bone surrounding the implant.83 These animal studies demonstrated the promise of using injectable CPCs for bone repair and regeneration.
Indeed, CPCs have gained clinical acceptance as valuable bone substitution biomaterials for over 20 years, and several CPCs are commercially available. Injectable CPCs were used to repair human periodontal intrabony defects and showed favorable radiographic results.84 CPCs were also used in young patients for balloon kyphoplasty instead of polymethylmethacrylate cement. In most cases, good integration of CPCs in the vertebra was observed with no radiological signs of osteolysis or osteonecrosis. Only a few patients showed demineralization in follow-up CT scans.85 Several papers reviewing the properties of injectable CPCs are available for readers who want additional detail.86–88 The present review focuses on new developments in CPCs with an emphasis on their biological interactions and cell delivery as detailed in subsequent sections.
Biological requirements and biological responses of CPCs
Biocompatibility
Biocompatibility is defined as the property of a material being compatible with living tissues. Biocompatible materials do not induce a toxic response when implanted in the body.89 Biocompatibility is an essential requirement for tissue-engineered products to support cellular activities and optimize tissue regeneration without eliciting a cytotoxic effect in those cells or causing undesirable local or systemic responses in the host. The end products of the dissolution-precipitation reactions for CPCs include brushite (DCPD) and apatite (HA or calcium deficient HA (CDHA)), which are known to be biocompatible.90 Pre-set CPCs exhibit favorable short-term and long-term biocompatibility, as evidenced by many studies evaluating tissue responses in rats,91,92 rabbits,93 dogs,94 sheep,16,32 and goats,95 as well as various types of cultured cells.24,93,96 However, injectable CPCs require the completion of the setting reaction to avoid cytotoxicity, as unset or disintegrated CPCs cause severe inflammatory responses, blood clotting, and cement embolism.72,97 Incorporating polymers into CPCs is a strategy used to improve CPC properties.41 In a recent study, an injectable macroporous CPC was prepared by the syringe-foaming method using a hydrophilic viscous polymeric solution known as silanized-hydroxypropyl methylcellulose (Si-HPMC).98 Si-HPMC not only acts as a foaming agent to create macroporous structures inside CPCs but also endows the CPC paste with an appealing rheological behavior at the early stage of setting due to its self-crosslinking properties, thus improving its injectability and cohesion.98 Indeed, when this CPC was injected into defective rabbit femurs, no adverse foreign body reaction was observed at 1 week and 6 weeks post-implantation.98
Bioactivity
Bioactivity refers to the ability of bone scaffolds to bind directly to the surrounding bone without the formation of fibrous tissue.99 Bioactivity is often evaluated by examining the ability to form apatite on the biomaterial in a simulated body fluid (SBF) with ion concentrations close to those in human blood plasma.100 A bioactive material is defined as one that accelerates apatite crystallization in a solution supersaturated with respect to hydroxyapatite.100 However, the validity of using an in vitro SBF test to predict the in vivo bioactivity of a material has been questioned.101 For example, Bohner and Lemaitre showed that a bioactivity test with SBF may not only give false-positive results but also false-negative results.101 The authors concluded that “in vitro bioactivity tests in SBF solutions cannot be used to predict the in vivo bone bonding ability of a material”. With some improvements to the protocol, these tests may be used for initial screening. However, the most reliable evaluation method remains in vivo implantation in a bone defect.
Bioactivity is one of the most important properties of CPCs.19 To further enhance CPC bioactivity, bioactive glass, which is known for its bioactivity, was incorporated into CPCs.102,103 The bioactive glass acted as a source of calcium and phosphate ions in the cement setting reaction. With this addition, increasing apatite formation was detected on the surface of the CaP compound after soaking in SBF for 7 days.103 In vivo examination of samples implanted into rabbit femoral bones indeed showed a better healing process and more bone growth with the addition of bioactive glass.103
Osteoconductivity
Osteoconductivity is defined as a biomaterial property that facilitates the in-growth of new bone into a surface or a volume in which the biomaterial serves as a scaffold to guide new bone formation.104 CPCs are osteoconductive because they permit the attachment, proliferation, migration and phenotypic expression of bone cells, leading to the formation of new bone.105,106 Osteoconduction is related to the architectural geometry of the scaffold.106 Intimate adaptation, fixation and stability of the implant to the defect site are of critical importance to facilitate the in-growth of bone tissue. In addition, the scaffold should have high porosity and interconnectivity with optimal pore sizes to ensure cell penetration, nutrient exchange and waste elimination. For bone tissue engineering, an ideal scaffold should have 60%–80% interconnected porosity with pore sizes ranging from 150 to 500 μm.107
Osteoconduction also depends on the chemical composition of the scaffold. The incorporation of several types of ions benefit CPC osteoconductivity. For instance, a silicon CPC (Si-CPC) was developed,108 and the cytocompatibility of the Si-doped cement was tested with a human osteoblast-like cell line (MG-63), which showed enhanced cell proliferation (up to threefold) over that without Si. When implanted in a rabbit parietal bone defect model, significantly greater amounts of new bone were detected in the 10% Si-CPC group compared to that in the CPC control group.108 In another study, strontium was incorporated into CPC (Sr-CPC) to enhance its osteoconductivity and accelerate its degradation.109 In vitro studies showed higher osteoblastic cell proliferation rates in Sr-CPC groups. In vivo studies demonstrated more rapid degradation and advanced osteoconductivity in the 10% Sr-CPC group compared to those in the CPC control at 2, 4, 8, 16, and 32 weeks after the operation.109
Osteoinductivity
Osteoinduction is defined as the recruitment and stimulation of progenitor cells to differentiate toward the osteoblastic lineage.104 CPCs are generally osteoconductive but not osteoinductive.20 However, several CPCs reportedly have the ability to form bone in nonosseous sites in vivo without the addition of osteogenic factors.110 Since this osteoinductive property is observed for some CPCs but not others, these materials are described as having “intrinsic” osteoinductivity.111 This inductive phenomenon is likely attributable to the combined effects of topography, composition, and micro and macroporosity of the CPC scaffolds.111 It is likely that the intricate architecture of the scaffold permits the entrapment and concentration of circulating growth factors, such as BMPs and osteoprogenitor cells, in vivo thus conferring osteoinduction capability upon the CPCs.111 In addition, CPCs serve as calcium and phosphate ion sources in vivo. Ca2+, PO43- and HPO42- ions are released into the surrounding tissues, regulate osteoblast functions112 and induce localized ion supersaturation, which causes the reprecipitation of carbonated apatite on the scaffold.113,114 A previous study proposed a new strategy to regulate bone marrow mesenchymal stem cell (BMSC) adhesion and osteogenic differentiation by adding magnesium into the CPC, thus improving its osteoinductivity.115 A CPC containing 5 wt% and 10 wt% magnesium not only enhanced BMSC adhesion but also upregulated osteogenic gene and protein expression in vitro. An in vivo study demonstrated that CPC with 5 wt% magnesium achieved the greatest bone volume at 2 and 8 weeks, confirming its beneficial osteogenesis effects via the addition of magnesium.115 To gain or enhance CPC osteoinductivity, novel strategies such as the addition of osteoprogenitor cells,116,117 growth factors,118,119 bioactive proteins120,121 or peptides122,123 into CPCs have exhibited favorable effects. Therefore, novel CPC compositions with intrinsic and engineered osteoinductivity are highly promising to enhance bone regeneration.
Biodegradability
Ideally, a CPC scaffold should degrade at the same rate that new bone forms. CPCs biodegrade primarily via two mechanisms: a passive resorption process via chemical dissolution and an active resorption through a cell-mediated process.124 The degradation of CPCs is tailored by controlling several factors: (1) physical factors such as the physical form of the CPC (particulate or bulk), porosity, surface area, and crystallinity (crystal size, crystal perfection, and grain size), and so on; (2) chemical factors such as the composition and ionic substitutions; and (3) biological factors such as the activation of macrophages or osteoclasts.125 Enhancing CPC degradation is achieved by adding rapidly degradable porogens such as PLGA to generate macropores upon PLGA degradation. PLGA degrades hydrolytically, leading to the production of lactic and glycolic acid monomers. The acidic nature of the resulting byproducts is an additional advantage of PLGAs in combination with poorly degradable CPCs because CPCs degrade by acid dissolution.126 After being injected into a rabbit femoral bone defect model, CPC-PLGA exhibited favorable bone responses with >55% degradation and >13% bone formation at 6 weeks and >90% degradation and >40% bone formation at 26 weeks postoperation.127 Based on this same mechanism, glucono delta-lactone (GDL), which has a faster degradation rate than PLGA, was incorporated into CPCs as acid-producing microparticles to accelerate CPC degradation.128 Indeed, histomorphometrical evaluation revealed that CPCs containing 10% of GDL degraded more rapidly and were replaced by more bone tissue (32.8%) than CPC-PLGA at 2 weeks after implantation in a rabbit femoral bone defect.128
CPC scaffold constructs for bone tissue engineering
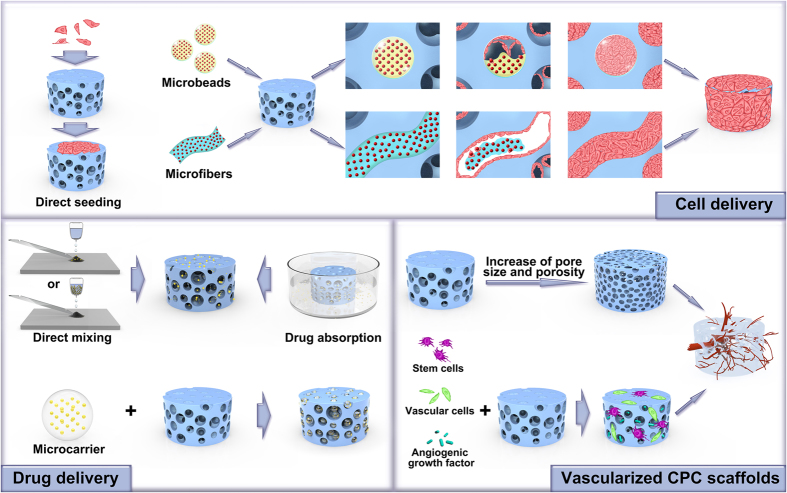
Cell delivery
Recent advancements in tissue engineering and regenerative medicine have indicated that cell-based therapeutics achieve robust regeneration with greater efficacy and better predictability than methods that do not involve cell seeding.129 These novel approaches employ scaffold constructs in combination with living cells to generate cell-driven, functional tissue rather than filling a defect with a nonliving scaffold. A tissue-engineered construct acts both as a scaffold to bridge the defect and as a cell delivery vehicle. The biomaterial-cell interactions of CPCs with various types of stem cells, such as BMSCs, umbilical cord mesenchymal stem cells (UCMSCs), embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), were previously reviewed.130,131 The present article specifically explores recent advances in strategies for cell delivery, specifically highlighting the design of CPC-based scaffolds.
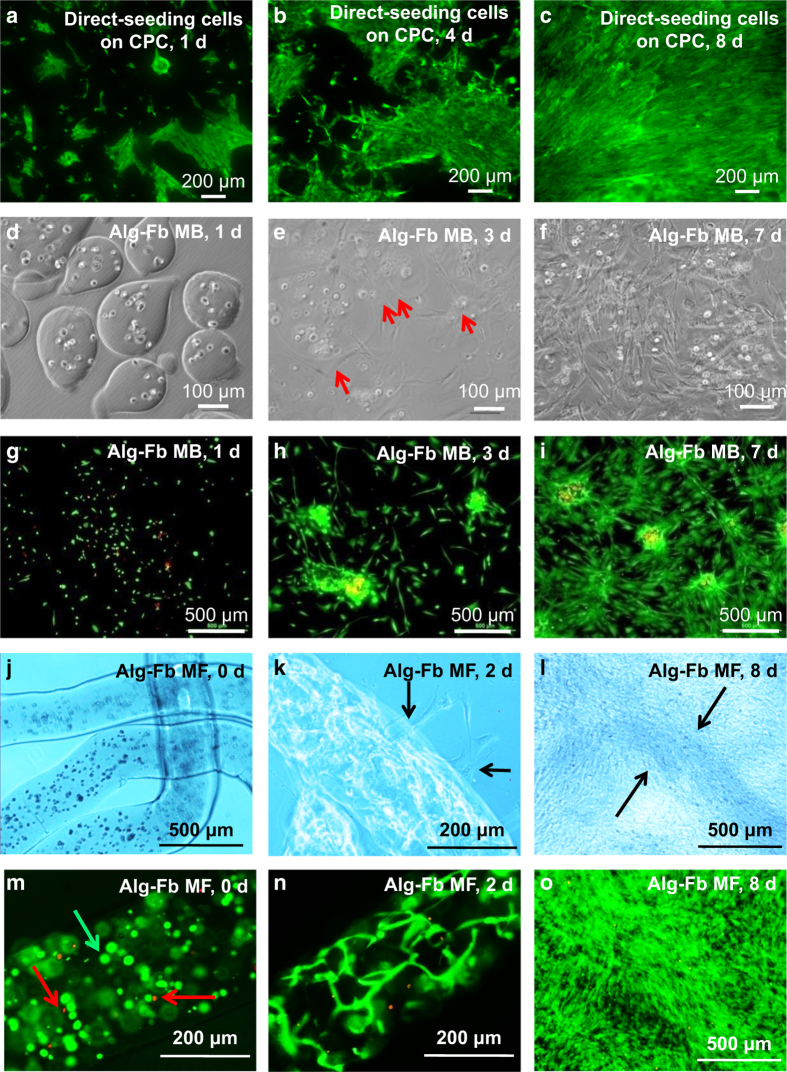
Direct cell seeding onto the porous surfaces of preformed CPC scaffolds is a common approach due to its simplicity. However, this type of static cell seeding has limitations, including low seeding efficiency and minimal cell penetration into the scaffold, leading to non-uniform cell distribution.132 It is not feasible to directly mix cells into the CPC paste because the mixing forces, ionic exchanges and pH fluctuation during CPC setting are detrimental to cell viability. To address this problem, cell encapsulation has been proposed to protect cells during CPC mixing and injection (Figure 3). In a recent study, human iPSC-derived MSCs (hiPSC-MSCs) were either pre-osteoinduced for 2 weeks (OS-hiPSC-MSCs) or transduced with BMP2 (BMP2-hiPSC-MSCs) to enhance their osteogenic capacity.133 The cells were then encapsulated in rapidly degradable alginate microbeads. The microbeads were mixed with CPC paste at a ratio of 1:1 and filled into cranial defects in nude rats.133 The results showed that the cells maintained good viability inside the microbeads after injection. Once the CPC set to form a scaffold, the cells were released as early as 3 days and demonstrated the up-regulation of osteogenic markers and bone mineral deposition. Cell-encapsulated groups produced greater amounts of new bone area in vivo, with 22.5%±7.6%, 38.9%±18.4%, and 44.7%±22.8% for the CPC-hiPSC-MSC, CPC-OS-hiPSC-MSC, and CPC-BMP2-hiPSC-MSC groups, respectively, compared to that for the non-cell CPC control group (15.6%±11.2%) at 12 weeks.133 Furthermore, the incorporation of cells accelerated the resorption of the CPC scaffold. The amount of residual CPC in the CPC-BMP2-hiPSC-MSC group was sevenfold less than that in the CPC control.133
Figure 3.
Methods of cell delivery via CPCs. Live-dead staining of (1) direct cell seeding on CPC surfaces (a–c); (2) cell encapsulation in alginate–fibrin microbeads (Alg-Fb MB) (d-i); (3) cell encapsulation in alginate–fibrin microfibers (Alg-Fb MaF) (j–o). (Adapted from Wang et al.133, and Song et al.139 with permission.)
Recently, rapidly degradable hydrogel fibers were developed for cell encapsulation and delivery.134 Encapsulation of cells inside microfibers possesses several advantages over microbeads. (1) Microfibers are easily fabricated by using a simple needle extrusion/external gelation method. To generate microbeads, air injection and electronic injection are needed to break up alginate droplets to form microbeads in sizes of several hundred microns.135 The air flow or electrostatic force during microbead formation may impose harsh shearing forces on the cells. Furthermore, the air flow forms “tails” on the microbeads, which may cause an immune response in vivo.135 (2) Microfibers with diameters of several hundred microns and millimeter-scale lengths are relatively easy to handle. (3) Microfibers provide more space for cellular self-assembly, through which living cells organize into functional units, allowing cells to grow, migrate and differentiate in the extracellular matrix.136 (4) Long microfibers form long macroporous channels with interconnectivity upon alginate degradation inside CPCs, while microbeads only form spherical pores with limited interconnectivity. These long channels improve osteoconductivity and nutrient and waste exchange of the scaffold. (5) Long microfibers potentially facilitate the formation of blood vessels in CPCs for bone engineering via co-seeding of endothelial cells and osteoblasts.
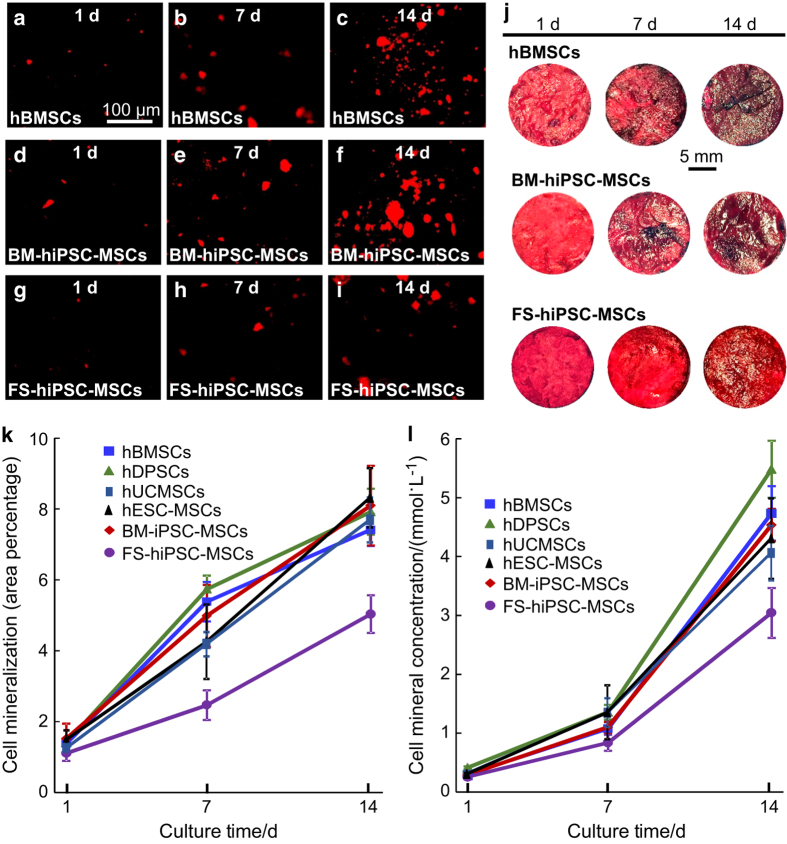
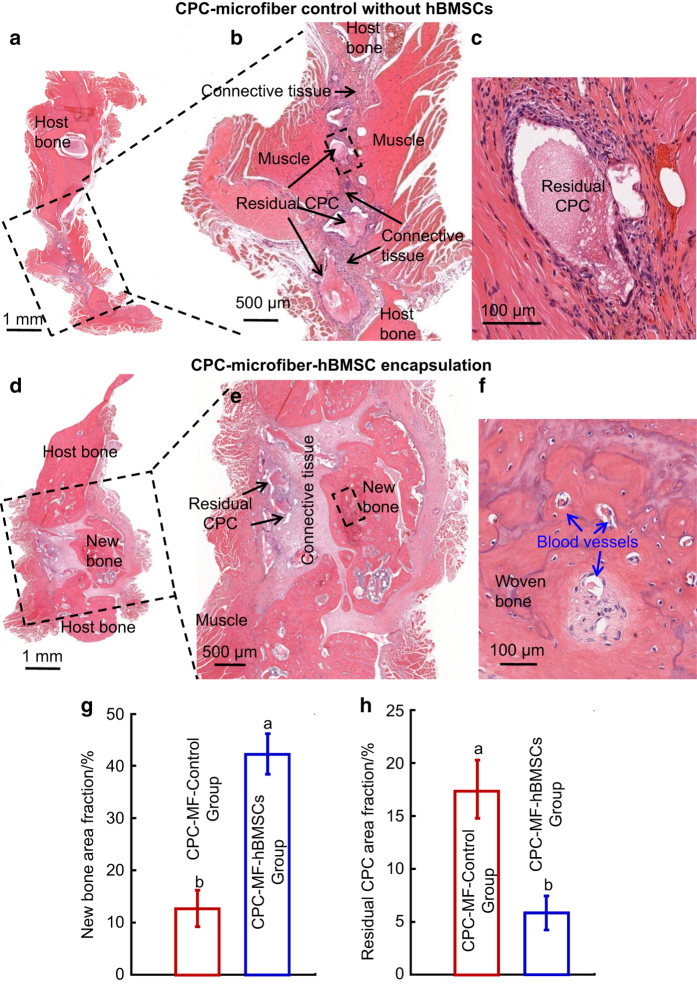
Recent studies have encapsulated six types of stem cells, specifically hBMSCs, human dental pulp stem cells (hDPSCs), hUCMSCs, hESC-MSCs, and hiPSC-MSCs derived from bone marrow (BM-hiPSC-MSCs) and foreskin (FS-hiPSC-MSCs), in hydrogel microfibers and then delivered them inside an injectable CPC.126,127 The CPC paste encapsulating the stem cells was fully injectable under a small injection force, and the injection exerted no harmful effects on cell viability.137 The porosity of the microfiber-CPC construct was 62%.138 All six types of cells proliferated well and differentiated down the osteogenic lineage. hUCMSCs, hESC-MSCs, hDPSCs, BM-hiPSC-MSCs and hBMSCs exhibited high ALP, RUNX2, COL1A1, and OC gene expression. Cell-synthesized bone minerals increased with time, with no significant differences among hUCMSCs, hESC-MSCs, hDPSCs, BM-hiPSC-MSCs and hBMSCs, indicating good bone regeneration potential similar to gold-standard hBMSCs.137,138 However, FS-hiPSC-MSCs were inferior in terms of osteogenic differentiation compared to other cell types (Figure 4).138 In another in vivo study, an hBMSC-encapsulated microfiber-CPC paste was applied to repair rat cranial defects,138 and the hBMSC-encapsulated microfiber-CPC tissue engineering construct exhibited a robust capacity for bone regeneration. At 12 weeks, an osseous bridge in the rat mandibular defect was observed in the CPC-microfiber-hBMSCs group with a new bone area fraction of 42.1%±7.8%, which was threefold greater than that of the control group (Figure 5).139 Therefore, these results demonstrate that injectable hydrogel microfiber-CPC paste is a promising carrier for cell delivery and greatly enhances bone regeneration in vivo.
Figure 4.
Synthesis of bone minerals by encapsulated stem cells. Images of (a–c) hBMSCs, (d–f) BM-hiPSC-MSCs, and (g–i) FS-hiPSC-MSCs stained with Xylenol orange (images of hESC-MSCs, hUCMSCs, and hDPSCs are similar to those of hBMSCs). (j) ARS staining of hBMSCs, BM-hiPSC-MSCs and FS-hiPSC-MSCs in CPC-CAF (images of hESC-MSCs, hUCMSCs, hDPSCs are similar to those of hBMSCs). (k) Xylenol orange mineral staining area (mean±s.d.; n=6). (l) ARS mineral concentration synthesized by cells in CPC-CAF (mean±s.d.; n=6). ARS: Alizarin red S, CAF: cell-encapsulating alginate–fibrin fibers. (Adapted from Wang et al.137–138 with permission.)
Figure 5.
Representative h&e images at 12 weeks after surgery with the CPC-microfiber control group (a–c) and the CPC-microfiber-hBMSCs group (d–f) as well as quantification of the new bone area fraction (g) and residual CPC area fraction (h). Bone bridging was achieved in rat critical-sized mandibular defects in the CPC-microfiber-hBMSC group. The defect was closed with newly formed bone. (b) and (c), (e) and (f) are high-magnification images. Bars with dissimilar letters indicate significantly different values (P<0.05). Each value is the mean±SD (n=6). MF: microfibers. (Adapted from Song et al.139 with permission.)
Drug delivery
The non-exothermic setting reaction and the intrinsic porosity of CPCs allow the incorporation of drugs and biologically active molecules with low risk of thermal denaturalization or loss of activity during preparation or implantation.19 For drug incorporation into CPCs, the drug is simply mixed with either the liquid or solid components of the cement.140 Alternatively, it is added by adsorption onto the pre-set scaffold141 or incorporated into polymeric microspheres or microfibers before blending with CPC paste.142 Several factors influence the loading and release of therapeutic substances. These include the microstructure, porosity and surface area of the CPCs, the way in which the drug is incorporated into the CPCs, and the interaction between the drug and the CPC matrix.19,143 CPCs have been used as drug carriers for antibiotics144 as well as anti-cancer,145 anti-inflammatory,146 and anti-resorptive (anti-osteoporotic) drugs.147 CPCs have also been used as drug carriers for therapeutically active proteins or growth factors that foster local bone generation.148 Recently, ionically modified CPCs (for example, with Sr2+, SiO44−, Zn2+, Mg2+) with the capability of influencing bone modeling and remodeling processes were investigated.115,149,150 For additional details, readers are referred to a review on the use of CPCs for drug delivery.19 Of note, the incorporation of the second phase of a degradable carrier into CPCs for drug delivery is beneficial for a more sustained release than directly loading the drugs into CPCs.148 For this purpose, gelatin microspheres,151 PLGA microparticles,152 bioactive glass,148 and chitosan/dextran sulfate microparticles153 have been used in CPCs to deliver drugs with tailored degradation rates to control the release profiles.
Vascularized CPC scaffolds
Adequate and rapid vascularization is essential for successful bone regeneration. Failure of the bone healing process, including delayed healing or non-unions, is often attributable to a lack of adequate vascularization.154 Furthermore, vascularization is critical for the viability of seeded cells in the scaffold. If the distance between cells and the nearest capillary network is greater than 100–200·μm, which exceeds the diffusion or perfusion limits of nutrients and oxygen, the viability of the seeded cells is compromised.89
Improvement in CPC vascularization is stimulated by modifications to the material itself. Physical features such as porosity and pore sizes are known to impact vascularization.155,156 To this end, a study fabricated a self-setting CPC composite with gelatin fibers to create interconnected hollow channels in the CPC after dissolution of the gelatin fibers.157 In vivo subcutaneous implantation showed that the resulting channels in CPC indeed facilitated vascular infiltration into the construct.157 In addition, different channel sizes induced different vascularization behaviors in vivo. Channels with a 250-μm diameter increased the expression of the representative angiogenic factors HIF1α, PLGF and migration factor CXCR4, which induce the formation of small vessels. Channels with a larger diameter of 500 μm enhanced VEGF expression, which induces the development of large vessels. More HIF1α-positive cells were found in the interconnected intersections of several channels, indicating high levels of sprouting and vasculogenesis potential under hypoxic conditions.157 While the majority of research has focused on modifying the physical features of CPCs to improve vascularization, chemical features, such as the release of ionic calcium and phosphate, have also been suggested to play a role in regulating vascularization.158 In a recent study, CPCs were coated with a graphene oxide-copper nanocomposite with the rationale that the oxygen-containing functional groups in graphene oxide would provide more binding sites for serum proteins and thereby enhance initial cell adhesion and other bioactivities.159 When incubated with rat BMSCs, CPCs with the novel graphene oxide-copper nanocomposite coating activated Hif-1α and further enhanced the expression of VEGF and BMP-2 via the Erk1/2 signaling pathway. Indeed, an in vivo study found more blood vessel volume and bone regeneration in the coated-CPC group.159 However, the mechanism underlying vascularization and the impact on bone regeneration efficacy via CPCs require additional experiments, particularly in vivo studies.
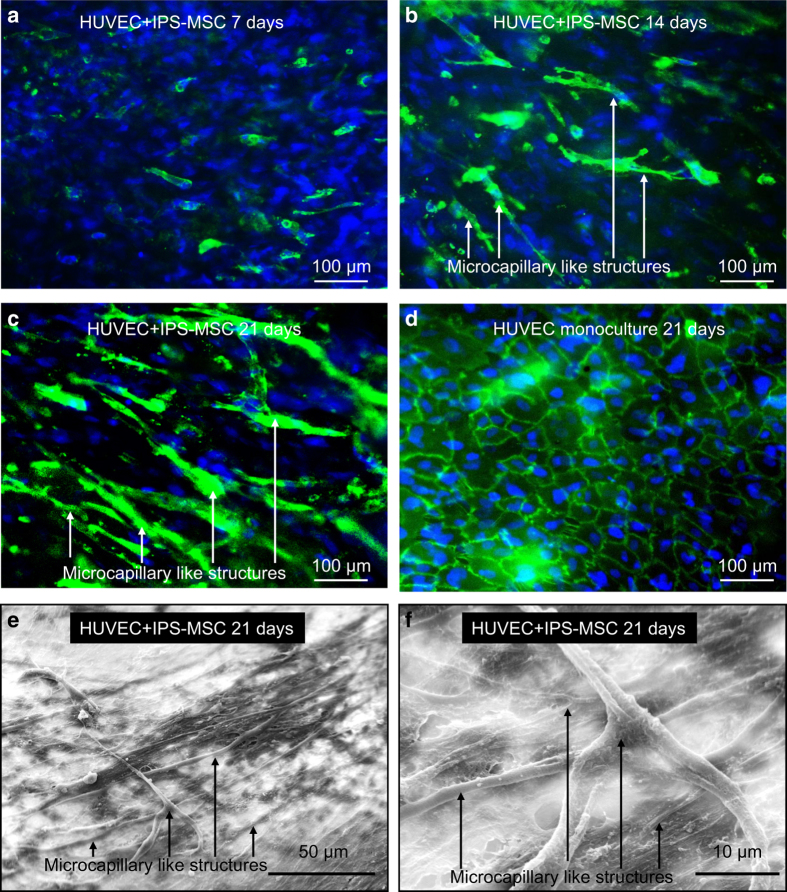
From a biological point of view, angiogenic growth factors, stem cells and vessel-forming cells are highly promising approaches to promote vascularization. A recent study investigated the use of autologous BMSCs in combination with autologous platelet-rich plasma (PRP) delivered via a macroporous CPC to regenerate large bone defects in minipigs.160 The CPC-BMSC-PRP group generated twofold more new bone and twofold higher blood vessel density compared to those of the macroporous CPC control at 12 weeks.160 In addition, recombinant growth factors and cell signaling molecules are alternatives to autologous growth factors that provide more flexible and delicate control over the dose and factors to be incorporated. Several studies have loaded dual agents, specifically BMPs and VEGF, in a single CPC scaffold, which demonstrated excellent angiogenic activity in vitro and in vivo.161,162 In addition to using growth factors, CPC pre-vascularization in vitro was investigated.163 In this method, vessel-forming cells were co-seeded with bone-forming cells on the engineered tissue construct to form microvascular structures before implantation in vivo. The co-culture of human osteoblasts and human umbilical vein endothelial cells (HUVECs) on gas-foaming macroporous CPCs in vitro successfully generated microcapillary-like structures and elevated the expression of angiogenic and osteogenic markers.163 Furthermore, the beneficial effects of co-culture were amplified by using an Arg–Gly–Asp (RGD) modification for the CPC scaffold.164 Similarly, the co-culture of hiPSC-MSCs and HUVECs on a macroporous CPC in vitro also generated microcapillary-like structures (Figure 6).165 In an animal study, HUVECs were co-cultured with four types of stem cells, specifically hUCMSCs, hBMSCs, hiPSC-MSCs and hESC-MSCs, on CPCs and then implanted in an 8-mm critical cranial bone defect in rats for 12 weeks.166 Microcapillary-like structures were successfully formed on CPCs in vitro in all four co-culture groups. New bone formation and the blood vessel densities of the co-cultured groups in vivo were much greater than that of the CPC control without cell seeding or the CPC-BMSCs group without co-culture (P<0.05).166 These results demonstrated the promise of co-culture and CPC pre-vascularization to greatly enhance osteogenesis and angiogenesis in vivo.
Figure 6.
Formation of microcapillary-like structures by HUVECs and hiPSC-MSCs co-cultured on CPC scaffolds at 21 days (a-c). HUVECs were identified by immunostaining with the endothelial marker PECAM1 in green on the cell membrane, and nuclei were stained with DAPI in blue. hiPSC-MSCs were identified by nuclei counterstained with DAPI in blue but lacking green staining on the cell membrane. Microcapillary-like structures increased with culture time. d shows the HUVEC monoculture control group, which exhibited no evidence of vascular-like structures. Representative SEM images of microcapillary-like structures via the co-culture system (e,f). (f) A higher magnification image of the image in e. (Adapted from Liu et al.165 with permission.)
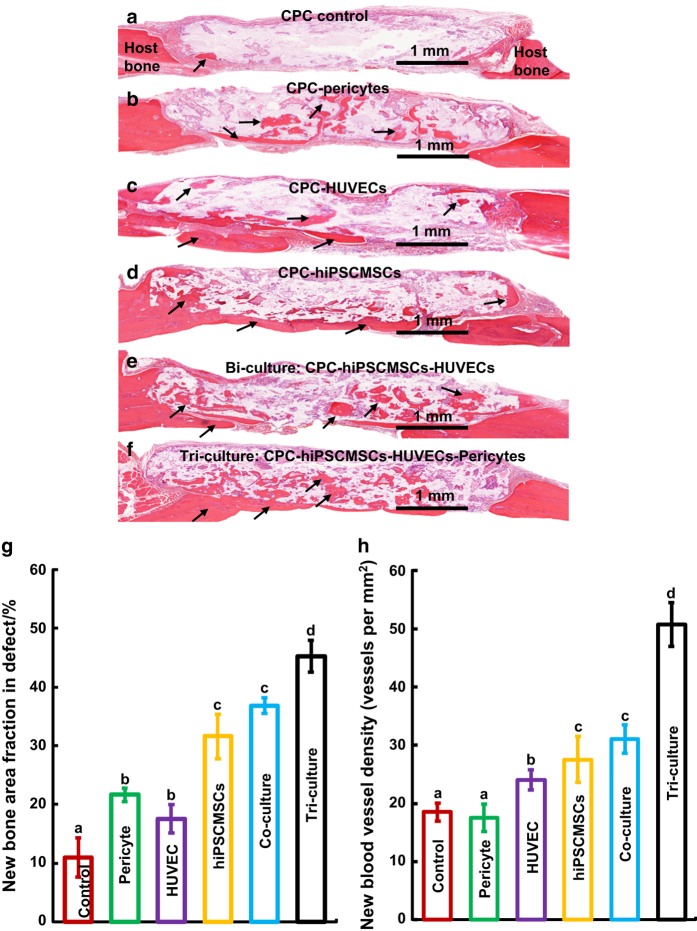
For successful bone regeneration, it is important to establish vascularization in a timely manner, but the stabilization of such a vascular network is of similar importance, although it is often neglected. Angiogenesis without vessel maturation produces abnormal, defective blood vessels that are prone to regression.167 Perivascular cells such as pericytes play important roles in the stabilization and maturation of blood vessels by guiding the developing vessels to respond to angiogenic stimuli.168 Enlightened by this fact, further improvement of the pre-vascularization strategy with the addition of pericytes was attempted.169 A tri-culture system comprising hiPSC-MSCs, HUVECs and pericytes was developed to pre-vascularize the CPC scaffolds.169 Both the bi-culture and tri-culture groups exhibited the formation of vessel-like structures in vitro, greatly elevated levels of angiogenic and osteogenic markers, and bone matrix mineralization. After implantation in a rat model with a cranial bone defect for 12 weeks, the tri-culture group demonstrated much higher amounts of new bone than the bi-culture and monoculture groups and the CPC control (Figure 7).169 The substantial increase in bone formation in the tri-culture group was likely related to enhanced vascularization and the stabilization and maturation of blood vessels.
Figure 7.
Representative h&e images at 12 weeks after the implantation of CPC scaffolds generated utilizing different pre-vascularization strategies in rat cranial bone defects. Mineralized new bone is stained in red (black arrows). The white area is attributable to slight detachment of the tissue. The dura is at the bottom. Cell-seeded groups had more new bone than the CPC control. Much higher amounts of new bone formed in the tri-culture group. Histomorphometric analysis of the fraction of new bone (g) and new blood vessel density (h). The tri-culture group had the greatest amount of new bone and new blood vessel density among all groups (P<0.05). Each value represents the mean±sd (n=6). Dissimilar letters indicated significantly different values (P<0.05). (Adapted from Zhang et al.169 with permission.)
In vivo pre-vascularization is also achieved using a surgical method involving the implantation of a scaffold into a well-vascularized and easily accessible body tissue such as a subcutaneous pocket or a muscle pouch. Microvascular structures are formed as a result of invasion and outgrowth of the surrounding host microvasculature.170,171 After the completion of pre-vascularization, the tissue construct is harvested and grafted into the defect site, where the preformed microvessels inside the construct inosculate and anastomose with the host blood vessels. The disadvantages of this approach are obvious: the invasive nature of the surgery, higher cost, and a relatively longer treatment process. Therefore, new tissue engineering methods utilizing CPC scaffolds with co-culture and tri-culture represent exciting alternative strategies that warrant further research for continued improvement to achieve wide clinical applications.
Conclusions
Due to their injectability, bioactivity and biocompatibility, CPCs are highly promising for bone tissue engineering applications and are used as scaffolds and carriers to deliver stem cells, drugs and growth factors. CPCs are either used as pre-set scaffolds or injectable pastes. 3D printing is a promising technology for fabricating CPC scaffolds with a high degree of accuracy and is used to develop intricately detailed biomimetic structures that are not achievable via traditional manufacturing methods. 3D printing has the potential to facilitate the next generation of smart and functional CPCs. Furthermore, with recent advances in tissue engineering, a new emphasis on “tissue regeneration by natural tissues” instead of “tissue replacement by biomaterials” has been proposed. Thus, CPCs with excellent biological interactions, such as osteoconductivity, osteoinductivity, biodegradability and bioactivity, are promising to meet this need. CPC composite constructs and hybrid systems involving the incorporation of cells, growth factors, bioactive molecules, bioinorganics, polymers, and bioactive glass are likely to yield favorable bone regenerative outcomes and greatly widen the clinical applications of CPCs. In addition, the co-culture and tri-culture of various tailored cell types with CPC scaffolds offer exciting potential for vascularization in bone tissue regeneration, which is especially important for treating large-sized bone defects. Further studies are needed to realize these promises and understand the underlying mechanisms to further the development of tissue engineering and regenerative medicine.
Acknowledgments
This study was supported by NIH R01 DE14190 and R21 DE22625 (HX), the National Science Foundation of China 81401794 (PW) and 81400487 (LW), the Youth Fund of Science and Technology of Jilin Province 20150520043JH (LW), the China Postdoctoral Science Foundation 2015M581405 (LW) and the University of Maryland School of Dentistry bridge fund (HX).
Footnotes
The authors declare no conflict of interest.
References
- Chow LC, Takagi S. A natural bone cement-A laboratory novelty led to the development of revolutionary new biomaterials. J Res Natl Inst Stand Technol 2001; 106: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WE, Chow LC. A new calcium phosphate, water setting cement. Brown PW (Ed). Cements Res Progress. Westerville: American Ceramic Society. 1986, 352–379. [Google Scholar]
- Brown W, Chow LC. A new calcium phosphate setting cement. J Dent Res 1983; 63: 672–679. [Google Scholar]
- Brown WE. Solubilities of phosphates and other sparingly soluble compounds. Griffith EJ, Beeton A, Spencer JM (eds). Environmental phosphorus handbook. New York: John Wiley & Sons. 1973, 203–239. [Google Scholar]
- Friedman CD, Costantino PD, Takagi S et al. Bone Source hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res 1998; 43: 428–432. [DOI] [PubMed] [Google Scholar]
- Constantz BR, Ison IC, Fulmer MT et al. Skeletal repair by in situ formation of the mineral phase of bone. Science 1995; 267: 1796–1799. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Fernandez E, De Maeyer EA et al. Setting reaction and hardening of an apatitic calcium phosphate cement. J Dent Res 1997; 76: 905–912. [DOI] [PubMed] [Google Scholar]
- Constantz BR, Barr BM, Ison IC et al. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J Biomed Mater Res A 1998; 43: 451–461. [DOI] [PubMed] [Google Scholar]
- Knaack D, Goad MEP, Aiolova M et al. Resorbable calcium phosphate bone substitute. J Biomed Mater Res A 1998; 43: 399–409. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Ishikawa K, Takechi M et al. Histological and compositional evaluations of three types of calcium phosphate cements when implanted in subcutaneous tissue immediately after mixing. J Biomed Mater Res A 1999; 48: 36–42. [DOI] [PubMed] [Google Scholar]
- Barralet JE, Gaunt T, Wright AJ et al. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J Biomed Mater Res 2002; 63: 1–9. [DOI] [PubMed] [Google Scholar]
- Yokoyama A, Yamamoto S, Kawasaki T et al. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials 2002; 23: 1091–1101. [DOI] [PubMed] [Google Scholar]
- Gisep A, Wieling R, Bohner M et al. Resorption patterns of calcium-phosphate cements in bone. J Biomed Mater Res A 2003; 66: 532–540. [DOI] [PubMed] [Google Scholar]
- Ehara A, Ogata K, Imazato S et al. Effects of alpha-TCP and TetCP on MC3T3-E1 proliferation, differentiation and mineralization. Biomaterials 2003; 24: 831–836. [DOI] [PubMed] [Google Scholar]
- Yuasa T, Miyamoto Y, Ishikawa K et al. Effects of apatite cements on proliferation and differentiation of human osteoblasts in vitro. Biomaterials 2004; 25: 1159–1166. [DOI] [PubMed] [Google Scholar]
- Apelt D, Theiss F, El-Warrak AO et al. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004; 25: 1439–1451. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Vlad MD, Gel MM et al. Modulation of porosity in apatitic cements by the use of alpha-tricalcium phosphate-calcium sulphate dihydrate mixtures. Biomaterials 2005; 26: 3395–3404. [DOI] [PubMed] [Google Scholar]
- del Real RP, Wolke JG, Vallet-Regi M, Jansen JA. A new method to produce macropores in calcium phosphate cements. Biomaterials 2002; 23: 3673–3680. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Canal C, Espanol M et al. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev 2012; 64: 1090–1110. [DOI] [PubMed] [Google Scholar]
- Chow LC. Calcium phosphate cements: chemistry, properties, and applications. MRS Proc 1999; 599: 27. [Google Scholar]
- Xu HH, Weir MD, Burguera EF et al. Injectable and macroporous calcium phosphate cement scaffold. Biomaterials 2006; 27: 4279–4287. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements as bone drug delivery systems: a review. J Control Release 2006; 113: 102–110. [DOI] [PubMed] [Google Scholar]
- An J, Wolke JG, Jansen JA et al. Influence of polymeric additives on the cohesion and mechanical properties of calcium phosphate cements. J Mater Sci Mater Med 2016; 27: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu HH, Simon CG Jr. Fast setting calcium phosphate-chitosan scaffold: mechanical properties and biocompatibility. Biomaterials 2005; 26: 1337–1348. [DOI] [PubMed] [Google Scholar]
- Cherng A, Takagi S, Chow LC. Effects of hydroxypropyl methylcellulose and other gelling agents on the handling properties of calcium phosphate cement. J Biomed Mater Res A 1997; 35: 273–277. [DOI] [PubMed] [Google Scholar]
- Fukase Y, Eanes ED, Takagi S et al. Setting reactions and compressive strengths of calcium phosphate cements. J Dent Res 1990; 69: 1852–1856. [DOI] [PubMed] [Google Scholar]
- Hesaraki S, Zamanian A, Moztarzadeh F. The influence of the acidic component of the gas-foaming porogen used in preparing an injectable porous calcium phosphate cement on its properties: acetic acid versus citric acid. J Biomed Mater Res B Appl Biomater 2008; 86: 208–216. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Driessens FC, Planell JA. Effect of the particle size on the micro and nanostructural features of a calcium phosphate cement: a kinetic analysis. Biomaterials 2004; 25: 3453–3462. [DOI] [PubMed] [Google Scholar]
- Weir MD, Xu HH. Osteoblastic induction on calcium phosphate cement-chitosan constructs for bone tissue engineering. J Biomed Mater Res A 2010; 94: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestres G, Le Van C, Ginebra MP. Silicon-stabilized α-tricalcium phosphate and its use in a calcium phosphate cement: characterization and cell response. Acta Biomater 2012; 8: 1169–1179. [DOI] [PubMed] [Google Scholar]
- Lanao RPF, Leeuwenburgh SC, Wolke JG et al. Bone response to fast-degrading, injectable calcium phosphate cements containing PLGA microparticles. Biomaterials 2011; 32: 8839–8847. [DOI] [PubMed] [Google Scholar]
- Theiss F, Apelt D, Brand B et al. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 2005; 26: 4383–4394. [DOI] [PubMed] [Google Scholar]
- Noetzel J, Özer K, Reisshauer B-H et al. Tissue responses to an experimental calcium phosphate cement and mineral trioxide aggregate as materials for furcation perforation repair: a histological study in dogs. Clin Oral Investig 2006; 10: 77. [DOI] [PubMed] [Google Scholar]
- Ambard AJ, Mueninghoff L. Calcium phosphate cement: review of mechanical and biological properties. J Prosthodont 2006; 15: 321–328. [DOI] [PubMed] [Google Scholar]
- Canal C, Ginebra MP. Fibre-reinforced calcium phosphate cements: a review. J Mech Behav Biomed Mater 2011; 4: 1658–1671. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu W, Schnitzler V et al. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater 2014; 10: 1035–1049. [DOI] [PubMed] [Google Scholar]
- Ginebra MP, Espanol M, Montufar EB et al. New processing approaches in calcium phosphate cements and their applications in regenerative medicine. Acta Biomater 2010; 6: 2863–2873. [DOI] [PubMed] [Google Scholar]
- Bohner M, Gbureck U, Barralet JE. Technological issues for the development of more efficient calcium phosphate bone cements: a critical assessment. Biomaterials 2005; 26: 6423–6429. [DOI] [PubMed] [Google Scholar]
- Verron E, Khairoun I, Guicheux J et al. Calcium phosphate biomaterials as bone drug delivery systems: a review. Drug Discov Today 2010; 15: 547–552. [DOI] [PubMed] [Google Scholar]
- Ginebra M-P, Traykova T, Planell JA. Calcium phosphate cements: competitive drug carriers for the musculoskeletal system? Biomaterials 2006; 27: 2171–2177. [DOI] [PubMed] [Google Scholar]
- Perez RA, Kim HW, Ginebra MP. Polymeric additives to enhance the functional properties of calcium phosphate cements. J Tissue Eng 2012; 3: 2041731412439555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K. Calcium phosphate cement. Ben-Nissan B (ed.). Advances in Calcium Phosphate Biomaterials. Berlin: Springer. 2014, 199–227. [Google Scholar]
- Chow LC. Next generation calcium phosphate-based biomaterials. Dent Mater J 2009; 28: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WL, Dennis RG, Kileny JL et al. Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng 2002; 8: 43–52. [DOI] [PubMed] [Google Scholar]
- Smith BT, Santoro M, Grosfeld EC et al. Incorporation of fast dissolving glucose porogens into an injectable calcium phosphate cement for bone tissue engineering. Acta Biomater 2017; 50: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaad E, Maire M, Lerouge S. Injectable thermosensitive chitosan hydrogels with controlled gelation kinetics and enhanced mechanical resistance. Carbohydr Polym 2015; 130: 87–96. [DOI] [PubMed] [Google Scholar]
- Guvendiren M, Molde J, Soares RMD et al. Designing biomaterials for 3D printing. ACS Biomater Sci Eng 2016; 2: 1679–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do AV, Khorsand B, Geary SM et al. 3D printing of scaffolds for tissue regeneration applications. Adv Healthc Mater 2015; 4: 1742–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today 2013; 16: 496–504. [Google Scholar]
- Trombetta R, Inzana JA, Schwarz EM et al. 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann Biomed Eng 2017; 45: 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butscher A, Bohner M, Roth C et al. Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater 2012; 8: 373–385. [DOI] [PubMed] [Google Scholar]
- Vacanti JP, Cima LG, Cima MJ. Vascularized tissue regeneration matrices formed by solid free form fabrication techniques. Google Patents. 2000; US Patent 6139574.
- Spath S, Seitz H. Influence of grain size and grain-size distribution on workability of granules with 3D printing. Int J Adv Manuf Technol 2014; 70: 135–144. [Google Scholar]
- Butscher A, Bohner M, Doebelin N et al. Moisture based three-dimensional printing of calcium phosphate structures for scaffold engineering. Acta Biomater 2013; 9: 5369–5378. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Buchanan F, Mitchell C et al. Printability of calcium phosphate: calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater Sci Eng C Mater Biol Appl 2014; 38: 1–10. [DOI] [PubMed] [Google Scholar]
- Bertol LS, Schabbach R, dos Santos LAL. Dimensional evaluation of patient-specific 3D printing using calcium phosphate cement for craniofacial bone reconstruction. J Biomater Appl 2017; 31: 799–806. [DOI] [PubMed] [Google Scholar]
- Butscher A, Bohner M, Doebelin N et al. New depowdering-friendly designs for three-dimensional printing of calcium phosphate bone substitutes. Acta Biomater 2013; 9: 9149–9158. [DOI] [PubMed] [Google Scholar]
- Farzadi A, Solati-Hashjin M, Asadi-Eydivand M et al. Effect of layer thickness and printing orientation on mechanical properties and dimensional accuracy of 3D printed porous samples for bone tissue engineering. PLoS ONE 2014; 9: e108252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lode A, Meissner K, Luo Y et al. Fabrication of porous scaffolds by three-dimensional plotting of a pasty calcium phosphate bone cement under mild conditions. J Tissue Eng Regen Med 2014; 8: 682–693. [DOI] [PubMed] [Google Scholar]
- Luo Y, Lode A, Wu C, Gelinsky M. 3D plotting of bioceramic scaffolds under physiological conditions for bone tissue engineering. In: Wu C, Chang J, Xiao Y (eds.). Advanced Bioactive Inorganic Materials for Bone Regeneration and Drug Delivery. Boca Raton: CRC Press. 2013, 83–103. [Google Scholar]
- Li C, Gao L, Chen F et al. Fabrication of mesoporous calcium silicate/calcium phosphate cement scaffolds with high mechanical strength by freeform fabrication system with micro-droplet jetting. Journal of Materials Science 2015; 50: 7182–7191. [Google Scholar]
- Maazouz Y, Montufar EB, Guillem-Marti J et al. Robocasting of biomimetic hydroxyapatite scaffolds using self-setting inks. J Mater Chem B 2014; 2: 5378–5386. [DOI] [PubMed] [Google Scholar]
- Marques CF, Perera FH, Marote A et al. Biphasic calcium phosphate scaffolds fabricated by direct write assembly: mechanical, anti-microbial and osteoblastic properties. J Eur Ceram Soc 2017; 37: 359–368. [Google Scholar]
- Ahlfeld T, Akkineni AR, Forster Y et al. Design and fabrication of complex scaffolds for bone defect healing: combined 3D plotting of a calcium phosphate cement and a growth factor-loaded hydrogel. Ann Biomed Eng 2017; 45: 224–236. [DOI] [PubMed] [Google Scholar]
- Billiet T, Gevaert E, De Schryver T et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials 2014; 35: 49–62. [DOI] [PubMed] [Google Scholar]
- Li C, Jiang C, Deng Y et al. RhBMP-2 loaded 3D-printed mesoporous silica/calcium phosphate cement porous scaffolds with enhanced vascularization and osteogenesis properties. Sci Rep 2017; 7: 41331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust S, Godla ME, Muller R et al. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomater 2014; 10: 630–640. [DOI] [PubMed] [Google Scholar]
- Park JY, Choi JC, Shim JH et al. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication 2014; 6: 035004. [DOI] [PubMed] [Google Scholar]
- Sawkins MJ, Mistry P, Brown BN et al. Cell and protein compatible 3D bioprinting of mechanically strong constructs for bone repair. Biofabrication 2015; 7: 035004. [DOI] [PubMed] [Google Scholar]
- O'Neill R, McCarthy HO, Montufar EB et al. Critical review: injectability of calcium phosphate pastes and cements. Acta Biomater 2017; 50: 1–19. [DOI] [PubMed] [Google Scholar]
- Khairoun I, Driessens FC, Boltong MG et al. Addition of cohesion promotors to calcium phosphate cements. Biomaterials 1999; 20: 393–398. [DOI] [PubMed] [Google Scholar]
- Krebs J, Aebli N, Goss BG et al. Cardiovascular changes after pulmonary embolism from injecting calcium phosphate cement. J Biomed Mater Res B Appl Biomater 2007; 82: 526–532. [DOI] [PubMed] [Google Scholar]
- Bigi A, Bracci B, Panzavolta S. Effect of added gelatin on the properties of calcium phosphate cement. Biomaterials 2004; 25: 2893–2899. [DOI] [PubMed] [Google Scholar]
- Alkhraisat MH, Rueda C, Marino FT et al. The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater 2009; 5: 3150–3156. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhang J, Weiss P et al. The influence of different cellulose ethers on both the handling and mechanical properties of calcium phosphate cements for bone substitution. Acta Biomater 2013; 9: 5740–5750. [DOI] [PubMed] [Google Scholar]
- O'Hara RM, Dunne NJ, Orr JF et al. Optimisation of the mechanical and handling properties of an injectable calcium phosphate cement. J Mater Sci Mater Med 2010; 21: 2299–2305. [DOI] [PubMed] [Google Scholar]
- Khairoun I, Boltong MG, Driessens FCM et al. Some factors controlling the injectability of calcium phosphate bone cements. J Mater Sci Mater Med 1998; 9: 425–428. [DOI] [PubMed] [Google Scholar]
- Burguera EF, Xu HH, Sun L. Injectable calcium phosphate cement: effects of powder-to-liquid ratio and needle size. J Biomed Mater Res B Appl Biomater 2008; 84: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Walboomers XF, Habraken WJ et al. Injectable calcium phosphate cement with PLGA, gelatin and PTMC microspheres in a rabbit femoral defect. Acta Biomater 2011; 7: 1752–1759. [DOI] [PubMed] [Google Scholar]
- Yang HL, Zhu XS, Chen L et al. Bone healing response to a synthetic calcium sulfate/beta-tricalcium phosphate graft material in a sheep vertebral body defect model. J Biomed Mater Res B Appl Biomater 2012; 100: 1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babo PS, Carvalho PP, Santo VE et al. Assessment of bone healing ability of calcium phosphate cements loaded with platelet lysate in rat calvarial defects. J Biomater Appl 2016; 31: 637–649. [DOI] [PubMed] [Google Scholar]
- Hoekstra JW, Klijn RJ, Meijer GJ et al. Maxillary sinus floor augmentation with injectable calcium phosphate cements: a pre-clinical study in sheep. Clin Oral Implants Res 2013; 24: 210–216. [DOI] [PubMed] [Google Scholar]
- Verron E, Pissonnier ML, Lesoeur J et al. Vertebroplasty using bisphosphonate-loaded calcium phosphate cement in a standardized vertebral body bone defect in an osteoporotic sheep model. Acta Biomater 2014; 10: 4887–4895. [DOI] [PubMed] [Google Scholar]
- Shirakata Y, Setoguchi T, Machigashira M et al. Comparison of injectable calcium phosphate bone cement grafting and open flap debridement in periodontal intrabony defects: a randomized clinical trial. J Periodontol 2008; 79: 25–32. [DOI] [PubMed] [Google Scholar]
- Gumpert R, Bodo K, Spuller E et al. Demineralization after balloon kyphoplasty with calcium phosphate cement: a histological evaluation in ten patients. Eur Spine J 2014; 23: 1361–1368. [DOI] [PubMed] [Google Scholar]
- Larsson S, Bauer TW. Use of injectable calcium phosphate cement for fracture fixation: a review. Clin Orthop Relat Res 2002; 395: 23–32. [DOI] [PubMed] [Google Scholar]
- Jansen J, Ooms E, Verdonschot N et al. Injectable calcium phosphate cement for bone repair and implant fixation. Orthop Clin North Am 2005; 36: 89–95. [DOI] [PubMed] [Google Scholar]
- Lewis G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. J Biomed Mater Res B Appl Biomater 2006; 76: 456–468. [DOI] [PubMed] [Google Scholar]
- Williams DF. On the mechanisms of biocompatibility. Biomaterials 2008; 29: 2941–2953. [DOI] [PubMed] [Google Scholar]
- Bohner M. Reactivity of calcium phosphate cements. J Mater Chem 2007; 17: 3980–3986. [Google Scholar]
- Ruhe PQ, Hedberg-Dirk EL, Padron NT et al. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng 2006; 12: 789–800. [DOI] [PubMed] [Google Scholar]
- Takechi M, Miyamoto Y, Ishikawa K et al. Initial histological evaluation of anti-washout type fast-setting calcium phosphate cement following subcutaneous implantation. Biomaterials 1998; 19: 2057–2063. [DOI] [PubMed] [Google Scholar]
- Guo H, Su J, Wei J et al. Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater 2009; 5: 268–278. [DOI] [PubMed] [Google Scholar]
- Lanao RF, Hoekstra J, Wolke J et al. Porous calcium phosphate cement for alveolar bone regeneration. J Tissue Eng Regen Med 2014; 8: 473–482. [DOI] [PubMed] [Google Scholar]
- del Real RP, Ooms E, Wolke JG et al. In vivo bone response to porous calcium phosphate cement. J Biomed Mater Res A 2003; 65: 30–36. [DOI] [PubMed] [Google Scholar]
- Xu HHK, Carey LE, Simon CG et al. Premixed calcium phosphate cements: synthesis, physical properties, and cell cytotoxicity. Dent Mater 2007; 23: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama Y, Ishikawa K, Mano T et al. Initial tissue response to anti-washout apatite cement in the rat palatal region: comparison with conventional apatite cement. J Biomed Mater Res A 2001; 55: 652–660. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu W, Gauthier O et al. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater 2016; 31: 326–338. [DOI] [PubMed] [Google Scholar]
- Hench LL, Splinter RJ, Allen WC et al. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res A 1971; 5: 117–141. [Google Scholar]
- Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006; 27: 2907–2915. [DOI] [PubMed] [Google Scholar]
- Bohner M, Lemaitre J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009; 30: 2175–2179. [DOI] [PubMed] [Google Scholar]
- D'Onofrio A, Kent NW, Shahdad SA et al. Development of novel strontium containing bioactive glass based calcium phosphate cement. Dent Mater 2016; 32: 703–712. [DOI] [PubMed] [Google Scholar]
- Sadiasa A, Sarkar SK, Franco RA et al. Bioactive glass incorporation in calcium phosphate cement-based injectable bone substitute for improved in vitro biocompatibility and in vivo bone regeneration. J Biomater Appl 2014; 28: 739–756. [DOI] [PubMed] [Google Scholar]
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001; 10: S96–S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau JL, Xu HH. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan composite scaffold. Biomaterials 2009; 30: 2675–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 2002; 395: 81–98. [DOI] [PubMed] [Google Scholar]
- Denry I, Kuhn LT. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dent Mater 2016; 32: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio JL, Rueda C, Manchon A et al. Effect of physicochemical properties of a cement based on silicocarnotite/calcium silicate on in vitro cell adhesion and in vivo cement degradation. Biomed Mater 2016; 11: 045005. [DOI] [PubMed] [Google Scholar]
- Kuang GM, Yau WP, Wu J et al. Strontium exerts dual effects on calcium phosphate cement: accelerating the degradation and enhancing the osteoconductivity both in vitro and in vivo. J Biomed Mater Res A 2015; 103: 1613–1621. [DOI] [PubMed] [Google Scholar]
- Yuan H, Li Y, de Bruijn JD et al. Tissue responses of calcium phosphate cement: a study in dogs. Biomaterials 2000; 21: 1283–1290. [DOI] [PubMed] [Google Scholar]
- LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev 2008; 108: 4742–4753. [DOI] [PubMed] [Google Scholar]
- Wu X, Itoh N, Taniguchi T et al. Requirement of calcium and phosphate ions in expression of sodium-dependent vitamin C transporter 2 and osteopontin in MC3T3-E1 osteoblastic cells. Biochim Biophys Acta 2003; 1641: 65–70. [DOI] [PubMed] [Google Scholar]
- Barradas AM, Yuan H, van Blitterswijk CA et al. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater 2011; 21: 407–429. [DOI] [PubMed] [Google Scholar]
- Habibovic P, Bassett DC, Doillon CJ et al. Collagen biomineralization in vivo by sustained release of inorganic phosphate ions. Adv Mater 2010; 22: 1858–1862. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ma X, Lin D et al. Magnesium modification of a calcium phosphate cement alters bone marrow stromal cell behavior via an integrin-mediated mechanism. Biomaterials 2015; 53: 251–264. [DOI] [PubMed] [Google Scholar]
- Thein-Han W, Liu J, Tang M et al. Induced pluripotent stem cell-derived mesenchymal stem cell seeding on biofunctionalized calcium phosphate cements. Bone Res 2013; 4: 371–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Habibovic P, Bao C et al. The homing of bone marrow MSCs to non-osseous sites for ectopic bone formation induced by osteoinductive calcium phosphate. Biomaterials 2013; 34: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhou H, Yang K et al. RhBMP-2-loaded calcium silicate/calcium phosphate cement scaffold with hierarchically porous structure for enhanced bone tissue regeneration. Biomaterials 2013; 34: 9381–9392. [DOI] [PubMed] [Google Scholar]
- Lee GH, Makkar P, Paul K et al. Incorporation of BMP-2 loaded collagen conjugated BCP granules in calcium phosphate cement based injectable bone substitutes for improved bone regeneration. Mater Sci Eng C 2017; 77: 713–724. [DOI] [PubMed] [Google Scholar]
- Dong J, Cui G, Bi L et al. The mechanical and biological studies of calcium phosphate cement-fibrin glue for bone reconstruction of rabbit femoral defects. Int J Nanomedicine 2013; 8: 1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takechi M, Ninomiya Y, Ohta K et al. Effects of apatite cement containing atelocollagen on attachment to and proliferation and differentiation of MC3T3-E1 osteoblastic cells. Materials 2016; 9: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wu X, Li J et al. The promotion of bone tissue regeneration by BMP2-derived peptide P24-loaded calcium phosphate cement microspheres. Ceram Int 2016; 42: 3177–3189. [Google Scholar]
- Wang T, Wu D, Li Y et al. Substance P incorporation in calcium phosphate cement for dental alveolar bone defect restoration. Mater Sci Eng C Mater Biol Appl 2016; 69: 546–553. [DOI] [PubMed] [Google Scholar]
- Sheikh Z, Abdallah MN, Hanafi AA et al. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials 2015; 8: 7913–7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Descamps M, Dejou J et al. The biodegradation mechanism of calcium phosphate biomaterials in bone. J Biomed Mater Res A 2002; 63: 408–412. [DOI] [PubMed] [Google Scholar]
- Lanao RPF, Leeuwenburgh SC, Wolke JG et al. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater 2011; 7: 3459–3468. [DOI] [PubMed] [Google Scholar]
- Grosfeld EC, Hoekstra JW, Herber RP et al. Long-term biological performance of injectable and degradable calcium phosphate cement. Biomed Mater 2016; 12: 015009. [DOI] [PubMed] [Google Scholar]
- Lanao RPF, Sariibrahimoglu K, Wang H et al. Accelerated calcium phosphate cement degradation due to incorporation of glucono-delta-lactone microparticles. Tissue Eng A 2014; 20: 378–388. [DOI] [PubMed] [Google Scholar]
- Schwarz KA, Leonard JN. Engineering cell-based therapies to interface robustly with host physiology. Adv Drug Deliv Rev 2016; 105: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhao L, Liu J et al. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res 2014; 2: 14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhao L, Chen W et al. Stem cells and calcium phosphate cement scaffolds for bone regeneration. J Dent Res 2014; 93: 618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalona GA, Udelsman B, Duncan DR et al. Cell-seeding techniques in vascular tissue engineering. Tissue Eng Part B Rev 2010; 16: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Song Y, Weir MD et al. A self-setting iPSMSC-alginate-calcium phosphate paste for bone tissue engineering. Dent Mater 2016; 32: 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaila SM, Popa EG, Reis RL et al. Fabrication of endothelial cell-laden carrageenan microfibers for microvascularized bone tissue engineering applications. Biomacromolecules 2014; 15: 2849–2860. [DOI] [PubMed] [Google Scholar]
- Kang A, Park J, Ju J et al. Cell encapsulation via microtechnologies. Biomaterials 2014; 35: 2651–2663. [DOI] [PubMed] [Google Scholar]
- Raof NA, Padgen MR, Gracias AR et al. One-dimensional self-assembly of mouse embryonic stem cells using an array of hydrogel microstrands. Biomaterials 2011; 32: 4498–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang P, Weir MD et al. Hydrogel fibers encapsulating human stem cells in an injectable calcium phosphate scaffold for bone tissue engineering. Biomed Mater 2016; 11: 065008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang C, Li C et al. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater Sci Eng C Mater Biol Appl 2016; 69: 1125–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhang C, Wang P et al. Engineering bone regeneration with novel cell-laden hydrogel microfiber-injectable calcium phosphate scaffold. Mater Sci Eng C Mater Biol Appl 2017; 75: 895–905. [DOI] [PubMed] [Google Scholar]
- Bohner M, Lemaitre J, Van Landuyt P et al. Gentamicin-loaded hydraulic calcium phosphate bone cement as antibiotic delivery system. J Pharm Sci 1997; 86: 565–572. [DOI] [PubMed] [Google Scholar]
- Le Nihouannen D, Hacking SA, Gbureck U et al. The use of RANKL-coated brushite cement to stimulate bone remodelling. Biomaterials 2008; 29: 3253–3259. [DOI] [PubMed] [Google Scholar]
- Farokhi M, Mottaghitalab F, Ai J et al. Sustained release of platelet-derived growth factor and vascular endothelial growth factor from silk/calcium phosphate/PLGA based nanocomposite scaffold. Int J Pharm 2013; 454: 216–225. [DOI] [PubMed] [Google Scholar]
- Parent M, Baradari H, Champion E et al. Design of calcium phosphate ceramics for drug delivery applications in bone diseases: a review of the parameters affecting the loading and release of the therapeutic substance. J Control Release 2017; 252: 1–17. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Wu V, Pernal S et al. Self-setting calcium phosphate cements with tunable antibiotic release rates for advanced antimicrobial applications. ACS Appl Mater Interfaces 2016; 8: 7691–7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Heredia MA, Kamphuis GJ, Thune PC et al. An injectable calcium phosphate cement for the local delivery of paclitaxel to bone. Biomaterials 2011; 32: 5411–5416. [DOI] [PubMed] [Google Scholar]
- Renaudin G, Laquerriere P, Filinchuk Y et al. Structural characterization of sol-gel derived Sr-substituted calcium phosphates with anti-osteoporotic and anti-inflammatory properties. J Mater Chem 2008; 18: 3593–3600. [Google Scholar]
- Gong T, Chen Y, Zhang Y et al. Osteogenic and anti-osteoporotic effects of risedronate-added calcium phosphate silicate cement. Biomed Mater 2016; 11: 045002. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Reither L, Thomas J et al. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater Sci 2017; 5: 578–588. [DOI] [PubMed] [Google Scholar]
- Zhu H, Guo D, Qi W et al. Development of Sr-incorporated biphasic calcium phosphate bone cement. Biomed Mater 2017; 12: 015016. [DOI] [PubMed] [Google Scholar]
- Li X, Sogo Y, Ito A et al. The optimum zinc content in set calcium phosphate cement for promoting bone formation in vivo. Mater Sci Eng C Mater Biol Appl 2009; 29: 969–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Liu X, Liu X et al. Calcium phosphate cement with BMP-2-loaded gelatin microspheres enhances bone healing in osteoporosis: a pilot study. Clin Orthop Relat Res 2010; 468: 1978–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhe PQ, Boerman OC, Russel FG et al. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J Control Release 2005; 106: 162–171. [DOI] [PubMed] [Google Scholar]
- Akkineni AR, Luo Y, Schumacher M et al. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomater 2015; 27: 264–274. [DOI] [PubMed] [Google Scholar]
- Schmidt-Bleek K, Schell H, Schulz N et al. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res 2012; 347: 567–573. [DOI] [PubMed] [Google Scholar]
- Feng B, Jinkang Z, Zhen W et al. The effect of pore size on tissue ingrowth and neovascularization in porous bioceramics of controlled architecture in vivo. Biomed Mater 2011; 6: 015007. [DOI] [PubMed] [Google Scholar]
- Xiao X, Wang W, Liu D et al. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci Rep 2015; 5: 9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Dong C, Shen Z et al. Vascularization of plastic calcium phosphate cement in vivo induced by in-situ-generated hollow channels. Mater Sci Eng C Mater Biol Appl 2016; 68: 153–162. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Habibovic P. Calcium phosphates and angiogenesis: implications and advances for bone regeneration. Trends Biotechnol 2016; 34: 983–992. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chang Q, Xu L et al. Graphene oxide-copper nanocomposite-coated porous CaP scaffold for vascularized bone regeneration via activation of hif-1alpha. Adv Healthc Mater 2016; 5: 1299–1309. [DOI] [PubMed] [Google Scholar]
- Qiu G, Shi Z, Xu HHK et al. Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J Tissue Eng Regen Med 2017. [Epub ahead of print]. [DOI] [PubMed]
- Patel ZS, Young S, Tabata Y et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008; 43: 931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HX, Zhang XP, Xiao GY et al. In vitro and in vivo evaluation of calcium phosphate composite scaffolds containing BMP-VEGF loaded PLGA microspheres for the treatment of avascular necrosis of the femoral head. Mater Sci Eng C Mater Biol Appl 2016; 60: 298–307. [DOI] [PubMed] [Google Scholar]
- Thein-Han W, Xu HH. Prevascularization of a gas-foaming macroporous calcium phosphate cement scaffold via coculture of human umbilical vein endothelial cells and osteoblasts. Tissue Eng Part A 2013; 19: 1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Thein-Han W, Weir MD et al. Prevascularization of biofunctional calcium phosphate cement for dental and craniofacial repairs. Dent Mater 2014; 30: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Chen W, Zhang C et al. Co-seeding human endothelial cells with human-induced pluripotent stem cell-derived mesenchymal stem cells on calcium phosphate scaffold enhances osteogenesis and vascularization in rats. Tissue Eng Part A 2017; 23: 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Liu X, Chen Q et al. Angiogenic and osteogenic regeneration in rats via calcium phosphate scaffold and endothelial cell co-culture with human bone marrow mesenchymal stem cells (MSCs), human umbilical cord MSCs, human induced pluripotent stem cell-derived MSCs and human embryonic stem cell-derived MSCs. J Tissue Eng Regen Med 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Jain RK, Au P, Tam J, Duda DG, Fukumura D. Engineering vascularized tissue. Nat Biotechnol 2005; 23: 821–823. [DOI] [PubMed] [Google Scholar]
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005; 7: 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Hu K, Liu X et al. Novel hiPSC-based tri-culture for pre-vascularization of calcium phosphate scaffold to enhance bone and vessel formation. Mater Sci Eng C Mater Biol Appl 2017; 79: 296–304. [DOI] [PubMed] [Google Scholar]
- Laschke MW, Vollmar B, Menger MD. Inosculation: connecting the life-sustaining pipelines. Tissue Eng Part B Rev 2009; 15: 455–465. [DOI] [PubMed] [Google Scholar]
- Beier JP, Horch RE, Hess A et al. Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J Tissue Eng Regen Med 2010; 4: 216–223. [DOI] [PubMed] [Google Scholar]