Cadotte and Tucker, in the current issue of TREE (pp. 429-437) ask the question whether the concept of environmental filtering should be abandoned. Environmental filtering has been defined as the set of abiotic environmental conditions that select for the establishment of a group of species in a given habitat [1,2]. The concept has been popular in community assembly research, particularly for plants [3]. Specifically, Cadotte and Tucker argue that the criteria to determine environmental filtering should include the co-variation of traits and demographic parameters along abiotic gradients, expanding on previous work that narrowed the criterion exclusively to mortality rates [2]. Here we want to use the momentum created by the Cadotte and Tucker piece to expand and update the concept and use of environmental filtering in community ecology.
We completely agree with Cadotte and Tucker that correlations of demographic parameters of macroorganisms should be studied along environmental gradients. We argue, however, that this information should be better used to infer how biotic (and specifically microbial-macrobial) interactions are modified, instead of pursuing to infer a “pure” environmental filter. This is because macroorganisms never interact with the environment alone: interactions between macroorganisms and microorganisms (e.g. fungi, bacteria, archaea, protozoa) are constant and “omnipresent” in all habitats and cannot be separated from the abiotic environment [4].
For example, experimental measures of demographic parameters (like mortality rates or intrinsic population growth) in the absence of competitors are considered the strongest evidence for detecting environmental filtering [2,5]. This interpretation is misleading for three reasons. First, it ignores the huge mortality impacts of microbe-mediated diseases on juvenile establishment [6]. This is not only true for “pathogen outbreaks” but also for the many host-microbe interactions that can turn negative at the earlier life-stages of the host. Second, in many cases macroorganisms are only able to establish, grow and reproduce in stressful environments via interactions with mutualistic microbes [7]. Third, recently, it has been shown that whole sets of microorganisms can be transferred between macroorganisms generations, making the combination of the microorganisms and host the unit of evolutionary selection [8]. All this evidence showcases that microorganisms cannot be separated from their host and are inherently present when they interact with their environment.
Community assembly should focus on biotic filters modified by an environmental background
Although Cadotte and Tucker briefly mention that pathogens or mutualists can also give rise to patterns consistent to environmental filtering, trying to separate these microorganism interactions from the environment is an unrealistic goal. Observational data can never effectively disentangle between the two. Further, experimentally attempting to doing so requires measuring macroorganism demographic parameters under sterile conditions (the only way to ensure absence of biotic interactions) along manipulated abiotic conditions. This test is not only expensive and extremely unpractical to carry out, but also not that informative to ecology.
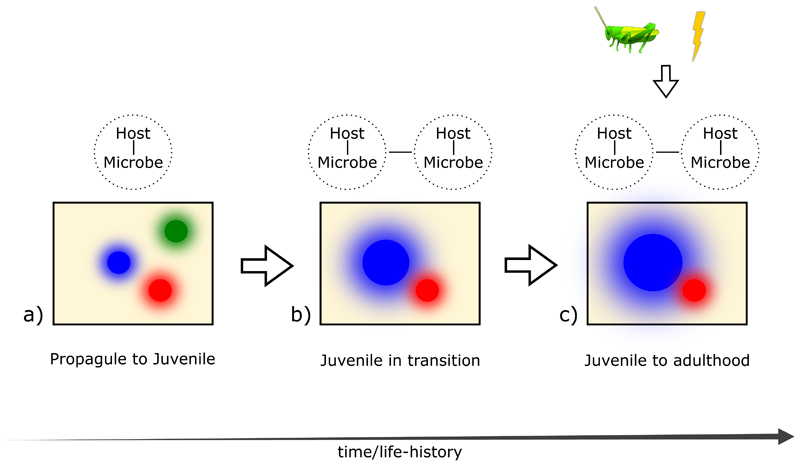
We think that shifting the environment from a “filter” to a “background” in front of which biotic interactions occur will lead to a more fruitful line of research than keeping the current paradigm. For instance, this change allows for a framework that better recognizes the role of microbial interactions during the assembly of macroorganism communities. In this framework macroorganisms can be better portrayed as host, where the environment determines the microbial community composition [9] and the outcomes of host-microbe interactions (Fig. 1).
Fig. 1.
Host-microbe interactions are ubiquitous throughout the hosts life-cyle. Proposed framework, portraying macroorganisms as “hosts” and community assembly as a progressive series of host-microbe interactions. The framework also includes the microbial interactions that shape microbial community structure before the arrival of any host in a given environment. a) After propagule arrival, we emphasize that mainly interactions between propagules/juvenile individuals and the local microbial community determine early host establishment in a given habitat. Propagules or juveniles (red, blue and green) colonize a new environment with defined abiotic conditions (yellow background). The microbiome of the introduced propagules (halos around circles) interacts with the already present microbiome of the environment. b) Competition among the surviving juvenile/adult species will happen within this host-microbe interactions matrix and will thus always entails a microbial component. Host-host competition should therefore be seen as an ecological interaction mediated by the respective microorganisms associated with each host. Possible outcomes of early host interactions would range from exclusion (green circle disappears) due to diseases and ultimate death; to survival (red circle stays at the same size) and growth (size of blue circle increases) when host-microbe interactions become commensalistic or mutualistic. While the juvenile transitions its microbiome coalesces with the environmental microbiome, and the other microbiomes of the developing macroorganismic community (overlapping halos around the circles) c) Finally, other higher-level trophic interactions that are more prominent between late juveniles to adult individuals will take place. As previously mentioned, the exact outcome from this spectrum of interactions would depend on the environmental background in front of which they occur. Here, further facilitative effects (blue circle size further increases) might occur. At this point, higher trophic-interactions or disturbances can further influence the community composition.
Final remarks
We believe that the time has come to update the way we see the environment during community assembly in the light of recent advances of microbial ecology. When the concept of environmental filtering was developed [1] the discipline of microbial ecology was still relatively young and the term is thus a “relic” of a time in which microbial ecology has not been well integrated with ecological theory.
Environmental filtering is a concept that has been developed and applied with macrobial systems in mind. In this letter, we hope we made the case that from the macrobial perspective, detecting the effects of a “pure” environmental filter is not a productive research goal given the omnipresence of microbial interactions. Ironically, free-living microbial communities represent, perhaps, the only cases where such a pure filter could be detected. At the micro scale, microbial communities exhibit a patchy distribution [10] leaving many potential habitats empty. Colonization of those habitats might entirely depend on abiotic factors. Thus, measuring an environmental filter in microbial systems is not only achievable but it advances our understanding of microbial community assembly. For the “macrobial world” the metaphor of environmental filter makes intuitive sense but without the recognition of microbial interactions it represents a misleading guide for empirical research.
Literature cited
- 1.Keddy PA. Assembly and Response Rules - 2 Goals for Predictive Community Ecology. J Veg Sci. 1992;3:157–164. [Google Scholar]
- 2.Kraft NJB, et al. Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol. 2015;29:592–599. [Google Scholar]
- 3.HilleRisLambers J, et al. Rethinking Community Assembly through the Lens of Coexistence Theory. Annu Rev Ecol Evol Syst. 2012;43:227–248. [Google Scholar]
- 4.Dubilier N, et al. Create a global microbiome effort. Nature. 2015;526:631–634. doi: 10.1038/526631a. [DOI] [PubMed] [Google Scholar]
- 5.Cadotte MW, Tucker CM. Should Environmental Filtering be Abandoned? Trends Ecol Evol. 2017;32:429–437. doi: 10.1016/j.tree.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Moles AT, Westoby M. What do seedlings die from and what are the implications for evolution of seed size? Oikos. 2004;106:193–199. [Google Scholar]
- 7.Friesen ML, et al. Microbially Mediated Plant Functional Traits. Annu Rev Ecol Evol Syst. 2011;42:23–46. [Google Scholar]
- 8.Wei Z, Jousset A. Plant breeding goes microbial. Trends Plant Sci. 2017 doi: 10.1016/j.tplants.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Pineda A, et al. Beneficial microbes in a changing environment: are they always helping plants to deal with insects? Funct Ecol. 2013;27:574–586. [Google Scholar]
- 10.Vos M, et al. Micro-scale determinants of bacterial diversity in soil. FEMS Microbiol Rev. 2013;37:936–954. doi: 10.1111/1574-6976.12023. [DOI] [PubMed] [Google Scholar]