Short abstract
Chemotherapy drugs such as oxaliplatin can increase nociceptive neuron excitability to result in neuropathic pain in orofacial and other regions in patients following chemotherapy. However, mechanisms underlying chemotherapy-induced increases of nociceptive neuron excitability are not fully understood. Kv4.3 channels are voltage-gated K+ channels mediating A-type K+ (IA) currents to control neuronal excitability. In the present study, we examined Kv4.3 channel expression on trigeminal neurons that innervate orofacial regions (V2 TG neurons) of rats using immunostaining method. We showed that strong Kv4.3 immunoreactivity (Kv4.3-ir) was present mainly in small-sized V2 TG neurons. The numbers of Kv4.3-ir positive V2 TG neurons were significantly reduced in oxaliplatin-treated rats, suggesting down-regulation of Kv4.3 channel expression on V2 TG neurons by the chemotherapy drug. Patch-clamp recordings from acutely dissociated rat V2 TG neurons showed that almost all nociceptive-like V2 TG neurons displayed IA currents with slow inactivation kinetics. The amplitudes of IA currents were significantly reduced in these nociceptive-like V2 TG neurons of oxaliplatin-treated group. Furthermore, we found that the excitability of nociceptive-like V2 TG neurons was significantly higher in the oxaliplatin-treated group than in the control group. These findings raise a possibility that down-regulation of Kv4.3 channels and IA currents in nociceptive V2 TG neurons is an underlying mechanism of oxaliplatin-induced orofacial neuropathic pain.
Keywords: Trigeminal ganglion neurons, chemotherapy-induced peripheral neuropathy, neuropathic pain, orofacial pain, Kv4.3 channels, oxaliplatin
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and dose-limiting side effect for several essential chemotherapy drugs including oxaliplatin.1 In patients with CIPN, neuropathic pain is often experienced in extremities,2 but it can also occur in proximal body parts including the orofacial regions.3,4 Neuropathic pain of CIPN is often manifested with exaggerated pain sensations triggered by innocuous mechanical stimuli (mechanical allodynia) and by innocuous or mild noxious cooling temperatures (cold allodynia/hyperalgesia). The neuropathic pain in orofacial regions is a significant clinical issue due to its special location, severity, and lack of effective treatment.5 Mechanisms underlying chemotherapy-induced neuropathic pain in orofacial regions are not fully understood. This lack of knowledge prevents us from identifying therapeutic targets for effective treatment of the neuropathic pain.
Orofacial regions are largely innervated by the maxillary (V2) branch of the trigeminal nerves. V2 trigeminal nerves of c- and Aδ-fibers convey most nociceptive signals from orofacial areas to the central nervous system. The excitability of these nociceptive nerve fibers may become abnormally increased by chemotherapy drugs resulting in exaggerated pain induced by innocuous thermal and mechanical stimuli. Since the excitability of peripheral afferents including trigeminal nerves is largely controlled by voltage-gated K+ channels,6 a reduction of the expression and/or functions of voltage-gated K+ channels in nociceptive V2 trigeminal neurons (V2 TG neurons) may lead to neuronal hyper-excitability and neuropathic pain such as orofacial mechanical and cold allodynia in CIPN.7
Previous patch-clamp recordings made from dorsal root ganglion (DRG) neurons have shown that membrane depolarization evokes two major types of K+ outward currents, an inactivating K+ outward current and a non-inactivating K+ outward current.8–10 The inactivating K+ outward current, also known as type-A current (IA), can be further classified into fast inactivating current (IAf) and slowly inactivating current (IAs) based on their inactivation kinetics.8,10,11 It has been shown that IA currents become reduced following chronic compression of the DRG.12 This change in IA currents is suggested to contribute to the enhanced neuronal excitability and the pain and hyperalgesia associated with chronic compression of the DRG.12 A recent study also shows that IA currents in joint sensory neurons are reduced and excitability of these neurons are enhanced in a murine model of antigen-induced arthritis.13 IA currents in DRG neurons are thought to be mediated by Kv1.4, Kv3.4, Kv4.2, and Kv4.3 channels.14 Kv4.3 channels are found to be expressed in nociceptive DRG neurons that also express TRPV1 receptors and NaV1.8 channels.15,16 Nociceptive DRG neurons also express Kv3.4 and Kv1.4 channels,15,17 but have negligible expression of Kv4.2.16 In a neuropathic pain model induced by spinal nerve ligation in rats, the protein levels of Kv3.4 and Kv4.3 in the DRG neurons are found to be greatly reduced.18 It is currently not known whether Kv4.3 channels are expressed on V2 TG neurons to mediate IA currents in these cells. Furthermore, it is also not known whether Kv4.3 channel expression and IA currents may be altered under pathological conditions including CIPN. In the present study, we addressed these questions by immunostaining and patch-clamp recordings on acutely dissociated V2 TG neurons of control and oxaliplatin-treated rats.
Materials and methods
Rat model of CIPN
Male Sprague-Dawley rats (300–450 g) were used. Animal care and use conformed to NIH guidelines for care and use of experimental animals. Experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. Animals were divided into two experimental groups, the CIPN group induced by oxaliplatin (oxaliplatin-treated group) and the control group. For the oxaliplatin-treated group,19 rats were injected with oxaliplatin intraperitoneally at a dose of 2 mg/kg (200 µl each rat) for five consecutive days (an accumulative dose of 10 mg/kg). The rats in the control group were injected with the same amount of saline.
Immunohistochemistry
TGs were harvested from animals 28 to 30 days following oxaliplatin or saline injections and used for immunostaining to determine Kv4.3 expression. In brief, animals were anesthetized with ketamine/xylazine (100 mg/kg:10 mg/kg, i.p.), transcardially exsanguinated with 0.1 M phosphate-buffered solution (PB), and perfused with 4% paraformaldehyde (PFA) in 0.1 M PB solution. TGs were removed and placed in 30% sucrose for cryoprotection for two nights. The TGs were then embedded in OCT® compound (Baxter Scientific) and cut on a cryostat (Leica Biosystems, Buffalo Grove, IL) into 10-μm sections. The sections of TGs were thaw-mounted onto slides and allowed to air-dry. For each slide, three TG sections from saline-injected animals (control) and three TG sections from oxaliplatin-treated animals were mounted on the same slide. These TG sections were then encircled together with hydrophobic resin (PAP Pen—The Binding Site) so that the two groups were paired in immunostaining. The slide-mounted sections were rinsed with the BupHTM-Modified Dulbecco’s Phosphate-Buffered Saline (PBS, ThermoFisher Scientific, Waltham, MA) three times, and then sequentially incubated at room temperature with ethanol solutions at the concentrations of 50% for 10 min, 70% for 10 min, and 50% for 10 min. The slides were rinsed three times with PBS and sections were incubated with 10% normal goat serum in PBS for 1 h at room temperature. The sections were incubated with a polyclonal rabbit anti-Kv4.3 antibody (1:2000 diluted with 5% normal goat serum in PBS; Abcam, Cambridge, MA) at 4°C for two nights. Following three rinses with PBS solution, the sections were incubated with a secondary antibody for one night at room temperature. The secondary antibody (1:1000 in 5% normal goat serum in PBS) was a goat anti-rabbit IgG conjugated with Alexa-594 (ThermoFisher Scientific, Waltham, MA). The sections were rinsed three times with PBS solution, cover-slipped with the Prolong Diamond Antifade Mountant medium (ThermoFisher Scientific). Slices were viewed under an upright fluorescent microscope (BX-43, Olympus, Tokyo, Japan) and images were captured using a CCD camera (DP80, Olympus, Tokyo, Japan).
Patch-clamp recordings
Animals were euthanized by overdose of isoflurane 28 to 30 days following oxaliplatin or saline injections. TGs were then harvested from each animal. Under a dissection microscope, V2 and V1 parts of TG (V2 TG and V1 TG) were identified and a cut along the midline of TG was made to separate V2 part from the neighboring V1 region of the TG. The V2 TG was dissected out and incubated with dispase II (5 mg/ml) plus type I collagenase (2 mg/ml) in 2 ml bath solution at 35°C for 45 min. The bath solution was the same one used for cell perfusion in electrophysiology experiments (see below). After a rinse, V2 TGs were triturated to dissociate the neurons in the bath solution, and the dissociated V2 TG neurons were plated on glass coverslips coated with poly-D-lysine and maintained at room temperature. Whole-cell patch-clamp recordings were performed within 1 to 4 h after cell plating.
The cells were perfused with normal bath solution flowing at 1 ml/min in a 0.5-ml recording chamber placed on the stage of an Olympus IX70 microscope. The bath solution contained (in mM) 150 NaCl, 5 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, 10 HEPES, with pH of 7.35 and osmolarity of 330 mosM. The internal solution of electrodes contained (in mM) 135 K-gluconate, 0.5 CaCl2, 2 MgCl2, 5 KCl, 5 EGTA, 10 HEPES, 2 Na2-ATP, and 0.5 Na2-GTP, with pH of 7.35 and osmolarity of 330–335 mOsm. Recording electrode resistance was ∼5 MΩ. The junction potential was −15 mV and was adjusted during data analysis. The series resistance of each recording was below 30 MΩ and was not compensated. Recording signals were amplified with Axopatch 200B (Axon Instruments), filtered at 2 kHz, and sampled at 5 kHz using pClamp 10 (Axon Instruments). Unless otherwise indicated, all reagents were purchased from Sigma.
To determine membrane and action potential properties of V2 TG cells, recordings were performed under the whole-cell current-clamp mode. Step current pulses were injected into cells through patch-clamp electrodes. The step currents were applied from −50 pA to 1975 pA in increments of 25 pA per step and the duration of each pulse was 1 s. To determine voltage-activated currents, recordings were performed under the whole-cell voltage-clamp mode. V2 TG neurons were held at −75 mV in voltage-clamp experiments, and voltage steps were applied from −125 mV to 35 mV with increments of 10 mV each step and step duration of 500 ms. IA current components, which were predominant at the initial phase of outward currents, were not isolated from total outward currents in the present study. All recordings were performed at the room temperature of 24°C.
Data analysis
For the data of immunostaining on TG sections, Kv4.3-immuno-positive and Kv4.3-immuno-negative neurons in V2 TG region were visually identified from the acquired fluorescent images. The percent of KV4.3-ir positive neurons was then calculated. For the data of electrophysiological recordings, membrane parameters and voltage-activated currents were analyzed using Clampfit 10 software. Unless otherwise indicated, data are reported as mean ± SEM, *p < 0.05, **p < 0.01, and ***p < 0.001, unpaired Student’s t test.
Results
We explored whether there was a down-regulation of Kv4.3 channel expression in V2 TG neurons of rats 28 to 30 days following oxaliplatin treatment. In our previous studies, oxaliplatin treatment resulted in orofacial neuropathic pain that was manifested by orofacial mechanical and cold allodynia.20 The reason for selecting the aforementioned time period in the present study was that our previous studies showed most prominent orofacial neuropathic pain during this time period. We focused on V2 TG neurons because orofacial regions are largely innervated by this branch of the trigeminal nerve system. In control animals, Kv4.3 immunoreactivity (Kv4.3-ir) positive V2 TG neurons were mostly small-sized neurons with mean diameter of 20 μm (20.5 ± 0.3 μm, 419 positive neurons out of 2083 total neurons in 21 TG sections of seven rats, Figure 1(a) and (c)). In contrast, Kv4.3-ir negative neurons from these TG sections of control animals showed broader cell size distribution with mean diameter of 26 μm (25.9 ± 0.2 μm, 1664 negative neurons out of 2083 total neurons, Figure 1(a) and (d)). In oxaliplatin-treated animals, Kv4.3-ir positive V2 TG neurons also were mostly small-sized neurons with mean diameter of 20 μm (19.7 ± 0.4 μm, 206 positive neurons out of 1937 total neurons in 20 TG sections of seven rats, Figure 1(b) and (e)). Kv4.3-ir negative V2 TG neurons in oxaliplatin-treated animals displayed wider cell size distribution with mean diameter of 24 μm (23.5 ± 0.2 μm, 1731 negative neurons out of 1937 total neurons, Figure 1(b) and (f)).
Figure 1.
Kv4.3-immunoreactivity in V2 trigeminal ganglion neurons of control and oxaliplatin-treated rats. (a) Image shows Kv4.3 immunoreactivity (Kv4.3-ir) in the V2 region of a trigeminal ganglion (V2 TG) section from a control rat. (b) Similar to (a) except the V2 TG was from a rat 28 days following oxaliplatin (Oxa) treatment. Arrows in both (a) and (b) indicate Kv4.3-ir positive neurons. An arrowhead in either (a) or (b) indicates a Kv4.3-ir negative neuron. (c and d) Histograms of Kv4.3-ir positive (c, mean diameter: 20.5 ± 6.5 μm, n = 419 cells) and negative (d, mean diameter: 25.9 ± 8.4 μm, n = 1664 cells) V2 TG neurons in control rats. (e and f) Histograms of Kv4.3-ir positive (e, mean diameter: 19.7 ± 5.2 μm, n = 206 cells) and negative (f, mean diameter: 23.5 ± 7.5 μm, n = 1731 cells) V2 TG neurons in oxaliplatin-treated rats. (g) Summary data of the percent of K4.3-ir positive V2 TG neurons in control group (Control) and oxaliplatin-treated group (Oxa). (h) Summary data of the numbers of V2 TG neuron in each field (under 20× objective) of control (open bar, n = 21 fields) and oxaliplatin-treated group (closed bar, n = 20 fields). Data in (g) and (h) represent mean ± SEM, ***p < 0.001, comparing with control group.
We compared the percentage of Kv4.3-ir positive V2 TG neurons between control and oxaliplatin-treated groups (Figure 1(g)). In control group, about 20% of V2 TG neurons (20.3 ± 1.1%, 2083 neurons in 21 TG sections from seven rats, n = 7) were Kv4.3-ir positive. In oxaliplatin-treated animals, about 10% V2 TG neurons (10.2 ± 0.6%, 1937 neurons in 20 TG sections from 7 rats, n = 7) were Kv4.3-ir positive, significantly less than that of the control group (p < 0.001, Figure 1(g)). We compared total numbers of V2 TG neurons between control and oxaliplatin-treated groups to see if there was a net loss of total V2 TG neurons following oxaliplatin treatment. In the oxilaplatin-treated group, the average numbers of V2 TG neurons in each field were 97 ± 3.9 (20 sections of seven rats, n = 7), not significantly different from those of control group (99 ± 4.2, 21 sections of seven rats, n = 7).
We performed patch-clamp recordings from small- to medium-sized V2 TG neurons (diameter: 24 to 42 µm, 31.61 ± 0.73 µm, n = 57) acutely dissociated from control and oxaliplatin-treated groups. Under the voltage-clamp mode, an outward current could be evoked when a depolarization step from −75 mV to −25 mV was applied to a V2 TG neuron (Figure 2(a) and (d)). In some V2 TG neurons, the voltage-activated outward current showed slow inactivation component (Figure 2(a)), consistent with IAs subtype of IA currents reported previously in DRG neurons.9,10 These cells had sizes of 29.7 ± 1.0 µm (n = 33) in diameter and membrane capacitance of 27.3 ± 3.0 pF (n = 33). In other V2 TG neurons, the outward current displayed fast inactivation component (Figure 2(d)), consistent with the IAf subtypes of IA currents reported previously in DRG neurons.9,10 These cells had sizes of 34.4 ± 0.8 µm (n = 24) in diameter and membrane capacitance of 37.5 ± 2.4 pF (n = 24), relatively larger than the cells showing IAs currents.
Figure 2.
IAs and IAf current components of voltage-activated K+ outward currents and action potential properties in V2 TG neurons. (a) Sample trace shows a voltage-activated K+ outward current with an IAs current component in a V2 TG neuron of a control animal. The current was evoked by a depolarizing step from the holding voltage of −75 mV to −25 mV in a duration of 500 ms. Decay time constant (τ) of 65 ms was obtained by fitting the decay phase (arrow indicated) to a simple exponential equation. (b) Sample trace shows multiple action potential firing in response to a supra threshold depolarizing current step in the same cell of (a). (c) Sample trace shows the first action potential in (b) at an expanded scale. The action potential has a broad width with a shoulder in its repolarization phase, a property of nociceptive-like TG neurons. (d) Sample trace shows an IAf current in a different V2 TG neuron of a control rat. The depolarizing step was the same as (a). τ = 3.8 ms. In both (a) and (d), voltage-activated inward currents (i.e., voltage-gated Na+ currents) at initial time were truncated. (e) Sample trace shows a single action potential firing in response to a supra threshold depolarizing current step in the same cell of (d). (f) Sample trace shows the action potential in (e) at an expanded scale. The action potential has a narrow width without a shoulder in repolarization phase, a property of non-nociceptive-like TG neurons. (g) Numbers of V2 TG neurons showing IAs (solid bar) and IAf (open bar) in control group (Control) and oxaliplatin-treated group (Oxa). Cell numbers are indicated in each bar. (h) V2 TG neurons with IAs currents are sub-classified based on their action potential properties. Top panel, numbers of the V2 TG neurons showing multiple AP firing (solid bar) and single AP firing (open bar). Bottom panel, numbers of the V2 TG neurons that are nociceptive-like (solid bar) and non-nociceptive-like (open bar). (i) V2 TG neurons with IAf currents are sub-classified based on their action potential properties. Top panel, numbers of the V2 TG neurons showing multiple AP firing (solid bar) and single AP firing (open bar). Bottom panel, numbers of the V2 TG neurons that are nociceptive-like (solid bar) and non-nociceptive-like (open bar). From (g) to (i), V2 TG neurons were obtained from control group (control) and oxaliplatin-treated group (Oxa). Data represent cell numbers in each sub-class, *p < 0.05, comparing between control group and Oxa-treated group, Chi-square test.
In the control group, most V2 TG neurons (21 of 29 cells, 72%) displayed IAs currents and 28% of the V2 TG neurons (8 out of 29 cells) showed IAf currents (Figure 2(g)). Interestingly, most V2 TG neurons that showed IAs currents fired multiple action potentials in response to membrane depolarization (Figure 2(b) and (h) top panel). Action potentials in all V2 TG neurons with IAs currents had broader width (3.69 ± 0.21 ms, n = 21) with a shoulder in their repolarization phase (Figure 2(c)), an electrophysiological property of nociceptive neurons. Accordingly, V2 TG neurons having this action potential feature are termed nociceptive-like V2 TG neurons in the present study (Figure 2(h) bottom panel). In contrast to the V2 TG neurons showing IAs currents, most V2 TG neurons displaying IAf currents fired single action potentials in response to supra threshold membrane depolarization (Figure 2(e) and (i) top panel). Action potentials in most cells with IAf had narrow width (1.63 ± 0.36 ms, n = 8, Figure 2(c)), an electrophysiological property of non-nociceptive neurons. Accordingly, V2 TG neurons having this action potential property are termed non-nociceptive-like V2 TG neurons in the present study (Figure 2(i) bottom panel).
For the oxaliplatin-treated group, IAs or IAf currents were also shown in V2 TG neurons (G–I). In contrast to the control group, significantly less V2 TG neurons (12 out of 28 cells) displayed IAs currents and majority of V2 TG neurons (16 out of 28 cells) showed IAf currents in oxaliplatin-treated group (Figure 2(g)). Similar to control group, IAs currents were associated with nociceptive-like and multiple action potential firing properties (Figure 2(h)) and IAf currents were associated with non-nociceptive and single action potential firing properties (Figure 2(i)).
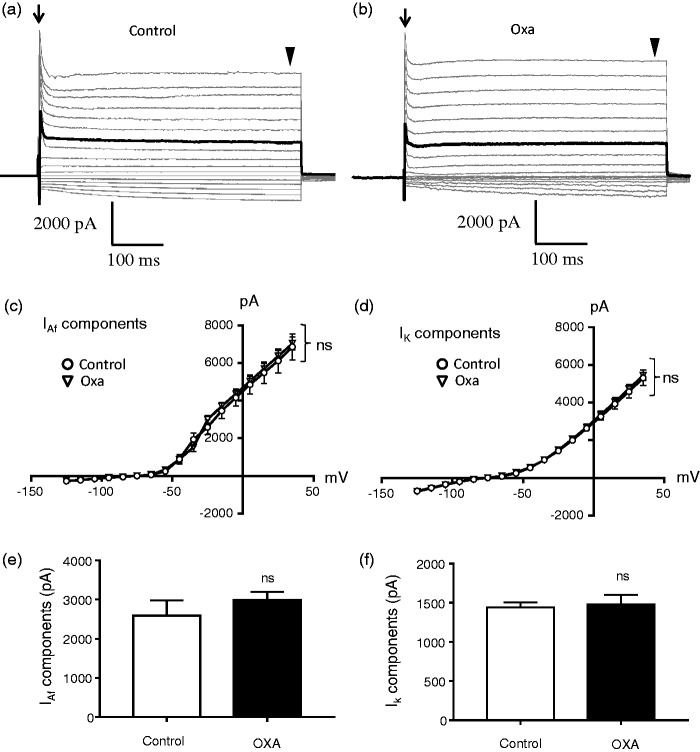
We examined voltage-activated K+ outward currents in non-nociceptive-like V2 TG neurons to determine whether these currents were changed following oxaliplatin treatment (Figure 3). V2 TG neurons in this set of experiments all had narrow action potentials and their outward currents showed IAf current components without detectable IAs current components. Current–voltage relationship (I–V curve) of voltage-activated K+ currents showed no significant difference in IAf current components between control group and oxaliplatin-treated group (Figure 3(a) to (c)). There was also no significant difference in non-inactivating outward current components (IK components) between the control group and the oxaliplatin-treated group (Figure 3(a), (b), and (d)). We compared IAf outward current components evoked at −25 mV between control and oxaliplatin-treated group. There was no significant difference in the amplitude between the two groups (Figure 3(e)). Similarly, amplitudes of IK current components evoked at −25 mV were also not significantly different between the two groups (Figure 3(f)).
Figure 3.
Lack of effects by oxaliplatin treatment on voltage-activated K+ currents in non-nociceptive-like V2 trigeminal ganglion neurons. (a) Sample traces show voltage-activated currents in a non-nociceptive-like V2 TG neuron of a control rat. Arrow indicates IAf current components at initial time of voltage-activated outward currents. Arrowhead indicates the non-inactivating components of voltage-activated outward currents. (b) Similar to (a) except the recording was made from a V2 TG neuron of an oxaliplatin-treated rat. In both (a) and (b), the dark traces highlight voltage-activated outward currents with IAf current components evoked at −25 mV. Voltage-activated inward Na+ currents at initial time were truncated in both (a) and (b). (c) I–V curve of voltage-activated currents with IAf current components at initial time. Open circles, control group (n = 8); open triangles, Oxa-treated group (n = 16). (d) I–V curve of voltage-activated currents with non-inactivation outward current components at later time. Open circles, control group (n = 8); open triangles, Oxa-treated group (n = 16). (e) Amplitudes of IAf outward current components evoked at the voltage step from −75 to −25 mV in non-nociceptive-like V2 TG neurons. Open bar, control group (n = 8); closed bar, Oxa-treated group (n = 18). (f) Amplitudes of non-inactivating outward current components evoked at the voltage step from −75 to −25 mV in non-nociceptive-like V2 trigeminal ganglion neurons. Open bar, control group (n = 8); closed bar, Oxa-treated group (n = 16). Data represent mean ± SEM, ns, not significantly different between control and Oxa-treated group, two-way ANOVA (c and d) or unpaired Student’s t test (e and f).
Voltage-activated K+ currents were examined in nociceptive-like V2 TG neurons of the control and oxaliplatin-treatment groups. V2 TG neurons in this set of experiments all had broad action potentials. These V2 TG neurons all showed IAs current components at initial time of voltage-activated outward currents. I–V curve showed a reduction of IAs current components following oxaliplatin treatment (Figure 4(a) to (c)). I–V curve also showed a reduction of non-inactivating components of voltage-activated outward currents following oxaliplatin treatment (Figure 4(a), (b), and (d)). We chose IAs current components evoked at −25 mV for a statistical comparison between the control and oxaliplatin-treated group. The amplitudes of IAs were significantly smaller (p < 0.01) in oxaliplatin-treated group (1402 ± 132 pA, n = 12) than in the control group (2050 ± 142 pA, n = 21, Figure 4(e)). Similarly, the amplitudes of non-inactivating components of voltage-activated outward currents evoked at −25 mV were significantly smaller (p < 0.01) in the oxaliplatin-treated group (415 ± 91 pA, n = 12) than in control group (824 ± 126 pA, n = 21, Figure 4(f)).
Figure 4.
Down-regulation of voltage-activated outward currents in nociceptive-like V2 trigeminal ganglion neurons following oxaliplatin treatment. (a) Sample traces show voltage-activated currents in a nociceptive-like V2 TG neuron of a control rat. Arrow indicates IAs current components of voltage-activated outward currents at initial time. Arrowhead indicates non-inactivating components of voltage-activated outward currents. (b) Similar to (a) except the recording was made from a nociceptive V2 TG neuron of an oxaliplatin-treated rat. In both (a) and (b), the dark traces highlight voltage-activated outward currents with IAs current components evoked at −25 mV. Voltage-activated inward Na+ currents at initial time were truncated in both (a) and (b). (c) I–V curve of voltage-activated currents with IAs components at initial time. Open circles, control group (n = 21); open triangles, Oxa-treated group (n = 12). (d) I–V curve of voltage-activated currents with non-inactivation outward current components at later time. Open circles, control group (n = 21); open triangles, Oxa-treated group (n = 12). (e) Amplitudes of IAs outward current components evoked by a voltage step from −75 to −25 mV in nociceptive-like V2 TG neurons. Open bar, control group (n = 21); closed bar, Oxa-treated group (n = 12). (f) Amplitudes of non-inactivating outward current components evoked by a voltage step from −75 to −25 mV in nociceptive-like V2 TG neurons. Open bar, control group (n = 21); closed bar, Oxa-treated group (n = 12). Data represent mean ± SEM, *p < 0.05, **p < 0.01, comparing between control group and oxaliplatin-treated group, two-way ANOVA (c and d), or unpaired Student’s t test (e and f).
We determined whether membrane excitability of nociceptive-like V2 TG neurons was increased following oxaliplatin treatment. V2 TG neurons in this set of experiments all had broad width of action potentials, multiple action potential firing pattern, and IAs current components in voltage-activated outward K+ currents. Rheobase for initiating action potential firing in these nociceptive-like V2 TG neurons was significantly lower (p < 0.05) in oxaliplatin-treated group (108 ± 11 pA, n = 9) than in control group (175 ± 21 pA, n = 14, Figure 5(a) to (c)). Resting membrane potentials (Figure 5(d)) were slightly less negative in oxaliplatin-treated group (55.4 ± 1.0 mV, n = 9) than in control group (52.2 ± 1.2 mV, n = 14, p = 0.055). Input resistance (Figure 5(e)), action potential threshold (Figure 5(f)), and action potential numbers at 2× rheobase (Figure 5(g)) were not found to be significantly different between the control and oxaliplatin-treated groups.
Figure 5.
Increases of membrane excitability in nociceptive-like V2 trigeminal ganglion neurons following oxaliplatin treatment. (a) Sample traces show action potential firing in responses to rheobase (dark) and 2× rheobase (gray) depolarizing current steps in a nociceptive-like V2 TG neuron of control group. The rheobase is 200 pA. Resting membrane potential is −61 mV. (b) Similar to (a) except the recording was made from a nociceptive-like V2 TG neuron of oxaliplatin-treated group. The rheobase is 75 pA. Resting membrane potential is −54 mV. (c to g) Summary data of rheobase (c), resting membrane potential (d, RMP), input resistance (e), action potential threshold (f), and action potential numbers at 2× rheobase (g) of nociceptive-like V2 TG neurons. Open bars, control group (n = 14, c to g); solid bars, oxilaplatin-treated group (n = 9, c to g). Data represent mean ± SEM, *p < 0.05, comparing between control group and oxaliplatin-treated group.
Discussion
In the present study, we have demonstrated that Kv4.3 channels are expressed mainly in small-sized V2 TG neurons of rats. We show that treatment of animals with the chemotherapy drug oxaliplatin leads to a significant down regulation of Kv4.3 channel expression in V2 TG neurons. We have further found that oxaliplatin treatment results in a significant reduction of IAs components of voltage-activated K+ outward currents. These changes in Kv4.3 channels and IAs currents are accompanied by an increase of membrane excitability in nociceptive-like V2 TG neurons. To our knowledge, this is the first report of such changes in V2 TG neurons. These findings may provide new insights into mechanisms underlying chemotherapy-induced neuropathic pain in orofacial regions.
The oxaliplatin treatment regimen used in the present study has been demonstrated to be a reliable rat model of CIPN in our previously studies.20,21 We have previously shown that the oxaliplatin treatment regimen leads to mechanical and cold allodynia in orofacial regions.20,21 Oxaliplatin is the first line chemotherapy drug for advanced colorectal cancer and it is also an essential chemotherapy drug for several other types of cancers.1 Most cancer patients treated with oxaliplatin develop CIPN with symptoms including mechanical allodynia and cold allodynia/hyperalgesia.22 Clinically, CIPN can occur in proximal body parts including the orofacial regions3,4 in addition to the extremities.2 Thus, our study with V2 TG neurons in the rat model of CIPN is of clinical relevance to orofacial pain in CIPN patients.
In the present study, strong Kv4.3 immunoreactivity is observed in small-sized V2 TG neurons in control group. Consistently, previous studies in DRG neurons have shown that Kv4.3 channels are expressed on small-sized nociceptive DRG neurons that also express TRV1 receptors and NaV1.8 channels.15,16 Thus, Kv4.3-expressing V2 TG neurons may be also primarily nociceptive neurons. Our immunostaining results show a significant reduction of Kv4.3-ir positive TG neurons in animals following oxaliplatin treatment. These results suggest that Kv4.3 channel expression undergoes down-regulation following oxaliplatin treatment. An alternative interpretation is that oxaliplatin might result in the loss of Kv4.3-expressing TG neurons, but this is not the case as V2 TG neuron numbers were not significantly different between the control and oxaliplatin-treated groups. Down-regulation of Kv4.3 channel expression has been observed in nociceptive DRG neurons in a neuropathic pain model induced by spinal nerve ligation in rats.18 This change in Kv4.3 expression in nociceptive DRG neurons has been suggested to be an underlying mechanism leading to hyper-excitability of nociceptive DRG neurons and pathological pain.18 Thus, down-regulation of Kv4.3 channel expression in V2 TG neurons may also lead to hyper-excitability of nociceptive V2 TG neurons and pathological pain in orofacial regions.
Our patch-clamp recordings from acutely dissociated V2 TG neurons show two subtypes of IA currents, IAs and IAf currents, in V2 TG neurons. This is consistent with previous observations of the two subtypes of IA currents in DRG neurons.8–10 V2 TG neurons with IAs currents appear to be mostly nociceptive neurons since their action potential width was broad. Previous studies in DRG neurons have shown that some cells co-express both IAs and IAf currents,8,10 raising a possibility that some of our nociceptive-like V2 TG neurons with IAs currents may also contain some degree of IAf currents. In contrast to V2 TG neurons with IAs currents (or IAs plus IAf currents), V2 TG neurons with only IAf currents fire narrow-width action potentials, a non-nociceptive neuron property. This is consistent with a previous study in DRG neurons showing that DRG neurons with IAf currents are usually the neurons without an action-potential shoulder.8
We show that IAs current components, but not IAf current components, are significantly reduced in the oxaliplatin-treated group. The reduction of IAs currents in nociceptive-like V2 TG neurons is consistent with the down-regulation of Kv4.3 expression shown in our immunostaining results. Thus, down-regulation of Kv4.3 expression should at least partially account for the reduction of IAs currents in our V2 TG neurons following oxaliplatin treatment. In nociceptive DRG neurons, previous studies have shown that a number of other voltage-gated K+ channels including Kv3.4, Kv1.4, and Kv4.2 channels are also expressed on nociceptive DRG neurons.15,17 Therefore, it will be interesting to determine in the future whether these IA types of K+ channels may be expressed on V2 TG neurons and undergo down-regulation following oxaliplatin treatment. In our nociceptive-like V2 TG neurons, we have found that the non-inactivating components of voltage-activated outward currents were also significantly reduced. These non-inactivating current components could be part of IAs currents and become down-regulated. Alternatively, the non-inactivating outward current components could be mediated by other voltage-gated K+ channels and down-regulated in nociceptive-like V2 TG neurons following oxaliplatin treatment.
Since IAs currents are activated at voltages near resting membrane potentials, these low-threshold voltage-activated K+ currents would serve as a brake to counteract membrane depolarization and thereby control neuronal excitability. The reduction of IAs currents in nociceptive-like V2 TG neurons in the oxaliplatin-treated group would compromise this excitability-controlling mechanism. Consistently, we have found that a reduction of IAs currents is accompanied by an increase of excitability in nociceptive-like V2 TG neurons in the oxaliplatin-treated group. In our recent study, we have shown that controlling V2 TG neuron excitability is also mediated by Im currents of KCNQ2/3 channels, another type of low-threshold voltage-activated K+ currents.20,21 Furthermore, our recent study has found that oxaliplatin treatment leads to down-regulation of KCNQ2/3 channels in V2 TG neurons and orofacial mechanical allodynia.20 Thus, V2 TG neuron excitability is controlled by different types of low-threshold voltage-gated K+ channels and their down-regulation by chemotherapy drugs may be a common mechanism underlying chemotherapy-induced orofacial neuropathic pain.
Author contributions
JGG conceived and designed the experiments and wrote the paper. VV performed electrophysiology experiments, and JL performed immunostaining experiments.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by NIH grants DE018661 and DE023090 to J.G.G.
References
- 1.Cavaletti G and, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol 2010; 6: 657–666. [DOI] [PubMed] [Google Scholar]
- 2.Banach M Juranek JK andZygulska AL.. Chemotherapy-induced neuropathies-a growing problem for patients and health care providers. Brain Behav 2017; 7: e00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton RB, Apfel SC, Dutcher JP, et al. Taxol produces a predominantly sensory neuropathy. Neurology 1989; 39: 368–373. [DOI] [PubMed] [Google Scholar]
- 4.Iniguez C, Larrode P, Mayordomo JI, et al. Reversible peripheral neuropathy induced by a single administration of high-dose paclitaxel. Neurology 1998; 51: 868–870. [DOI] [PubMed] [Google Scholar]
- 5.Baron R Binder A andWasner G.. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010; 9: 807–819. [DOI] [PubMed] [Google Scholar]
- 6.Waxman SG and, Zamponi GW. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 2014; 17: 153–163. [DOI] [PubMed] [Google Scholar]
- 7.Busserolles J, Tsantoulas C, Eschalier A, et al. Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain 2016; 157: S7–S14. [DOI] [PubMed] [Google Scholar]
- 8.Gold MS, Shuster MJ and, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol 1996; 75: 2629–2646. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura N, White G, Weight FF, et al. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J Physiol 1996; 494: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarria I, Ling J and, Gu JG. Thermal sensitivity of voltage-gated Na+ channels and A-type K+ channels contributes to somatosensory neuron excitability at cooling temperatures. J Neurochem 2012; 122: 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rola R, Witkowski G and, Szulczyk PJ. Voltage-dependent K+ currents in rat cardiac dorsal root ganglion neurons. Neuroscience 2003; 119: 181–191. [DOI] [PubMed] [Google Scholar]
- 12.Tan ZY, Donnelly DF and, LaMotte RH. Effects of a chronic compression of the dorsal root ganglion on voltage-gated Na+ and K+ currents in cutaneous afferent neurons. J Neurophysiol 2006; 95: 1115–1123. [DOI] [PubMed] [Google Scholar]
- 13.Qu L and, Caterina MJ. Enhanced excitability and suppression of A-type K(+) currents in joint sensory neurons in a murine model of antigen-induced arthritis. Sci Rep 2016; 6: 28899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao XH, Byun HS, Chen SR, et al. Reduction in voltage-gated K+ channel activity in primary sensory neurons in painful diabetic neuropathy: role of brain-derived neurotrophic factor. J Neurochem 2010; 114: 1460–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang HY, Cheng JK, Shih YH, et al. Expression of A-type K channel alpha subunits Kv 4.2 and Kv 4.3 in rat spinal lamina II excitatory interneurons and colocalization with pain-modulating molecules. Eur J Neurosci 2005; 22: 1149–1157. [DOI] [PubMed] [Google Scholar]
- 16.Phuket TR andCovarrubias M.. Kv4 channels underlie the subthreshold-operating A-type K-current in nociceptive dorsal root ganglion neurons. Front Mol Neurosci 2009; 2: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasband MN, Park EW, Vanderah TW, et al. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A 2001; 98: 13373–13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chien LY, Cheng JK, Chu D, et al. Reduced expression of A-type potassium channels in primary sensory neurons induces mechanical hypersensitivity. J Neurosci 2007; 27: 9855–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling B, Coudore-Civiale MA, Balayssac D, et al. Behavioral and immunohistological assessment of painful neuropathy induced by a single oxaliplatin injection in the rat. Toxicology 2007; 234: 176–184. [DOI] [PubMed] [Google Scholar]
- 20.Ling J, Erol F, Viatchenko-Karpinski V, et al. Orofacial neuropathic pain induced by oxaliplatin: downregulation of KCNQ2 channels in V2 trigeminal ganglion neurons and treatment by the KCNQ2 channel potentiator retigabine. Mol Pain 2017; 13: 1744806917724715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abd-Elsayed AA, Ikeda R, Jia Z, et al. KCNQ channels in nociceptive cold-sensing trigeminal ganglion neurons as therapeutic targets for treating orofacial cold hyperalgesia. Mol Pain 2015; 11: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cersosimo RJ. Oxaliplatin-associated neuropathy: a review. Ann Pharmacother 2005; 39: 128–135. [DOI] [PubMed] [Google Scholar]