Abstract
Previous studies showed good agreement between pacemaker respiratory disturbance index (RDI) and polysomnography for diagnosis of severe sleep apnea (SA). The aim of this study is to investigate the diagnostic accuracy of RDI compared with apnea-hypopnea index (AHI) from a cardiorespiratory sleep study for the diagnosis of severe SA within patients requiring a pacemaker and meeting diastolic dysfunction criteria. Secondary objectives are as follows: correlation between plasma aldosterone level and SA severity, diagnostic accuracy of RDI for moderate SA, prevalence of SA among patients with diastolic dysfunction, occurrence of arrhythmias, and improvement of RDI with continuous positive airway pressure therapy. We designed a monocentric prospective nonrandomized study of prevalent cases to include 68 patients with a 6-month follow-up. Both RDI and AHI will be compared 2 months after implantation and after 1 month of continuous positive airway pressure treatment in patients with severe SA. This is the first study that examines diagnostic accuracy of pacemaker algorithm for the diagnosis of SA and correlation with plasma aldosterone levels in patients with diastolic dysfunction.
Protocol version: V04. 04/04/2017 Trial registration: ClinicalTrials.gov NCT02751021
Keywords: sleep apnea, diagnosis, attended cardiorespiratory sleep study, diastolic dysfunction, aldosterone
Introduction
Sleep apnea, impaired diastolic function, and hyperaldosteronism
Sleep apnea (SA) affects 5% to 7% of the general population and reaches 15% of people more than 70 years.1,2 Its prevalence is very high among patients with hypertension or other cardiovascular diseases such as coronary artery disease, heart failure (HF), stroke, and atrial fibrillation (AF).3 It has been shown that SA is a risk factor for these conditions. Underlying mechanism is mostly obstructive resulting in collapse of the upper airways at pharyngeal level during sleep. Conversely, central SA is less frequent and often associated with HF.4,5 Sleep apnea has a particularly high prevalence among patients who have indication for cardiac pacing reaching 60%.6 It has also been associated with ventricular arrhythmias and nocturnal sudden cardiac death.7 Sleep-related breathing disorders result in nocturnal intermittent hypoxemia. This leads to chronic reflex activation of the sympathetic nervous system, vascular vasoconstriction, production of vasoactive substances such as endothelin, oxidative stress, and, finally, endothelial dysfunction.4
Heart failure with preserved left ventricular ejection fraction (LVEF) accounts for almost 50% of HF cases. It is defined by clinical signs of HF, with a normal LVEF associated with other features such as abnormal relaxation, filling, and diastolic stiffness.8 Diastolic dysfunction appears as the first stage of this particular type of HF but can also be found in patients with no clinical sign of HF. Relaxation abnormalities increase with age, diabetes, hypertension, cardiomyopathy, and impaired systolic function.9 Until now, there are no published data about the prevalence of obstructive SA in this specific subgroup of patients presenting a diastolic dysfunction. However, SA has been recognized as an independent risk factor for hypertension,10 and several studies have shown a very high prevalence of obstructive SA in patients with resistant hypertension and associated hyperaldosteronism.11
SA diagnosis
Obstructive SA is often underdiagnosed by clinical examination due to numerous and nonspecific symptoms. Moreover, better related symptoms such as snoring, apneas, and excessive daytime sleepiness are often lacking in patients with cardiovascular disorders. Confirmation tests are expensive and their access is limited with significant waiting lists. The gold standard remains the polysomnography (PSG) but type 3 portable monitors exhibit good diagnostic performance.12 The recording is performed at home or during attended cardiorespiratory sleep study (ACSS). Apnea-hypopnea index (AHI) represents the sum of apnea and hypopnea episodes per hour of sleep. It is considered abnormal if AHI is greater than or equal to 5 per hour.
SA diagnosis with pacemakers
The Sleep Apnea Monitoring (SAM) algorithm uses a rate-responsive pacemaker (PM) sensor, providing an index of minute ventilation derived from measurements of real-time transthoracic impedance variation.13 The SAM algorithm thus detects 2 types of events: apneas (absence of significant respiratory cycle for more than 10 seconds) and severe hypopneas (50% or more decrease in minute ventilation compared with the mean value over the last breathing period sustained for more than 10 seconds) as shown in Figure 1. These events are then reported with the respiratory disturbance index (RDI) which is the average number of events (apnea or hypopnea) detected per hour of estimated sleep (see Figure, additional file 1, which illustrates SAM data available during PM interrogation). The new version of the SAM algorithm was developed with better management of noise.
Figure 1.
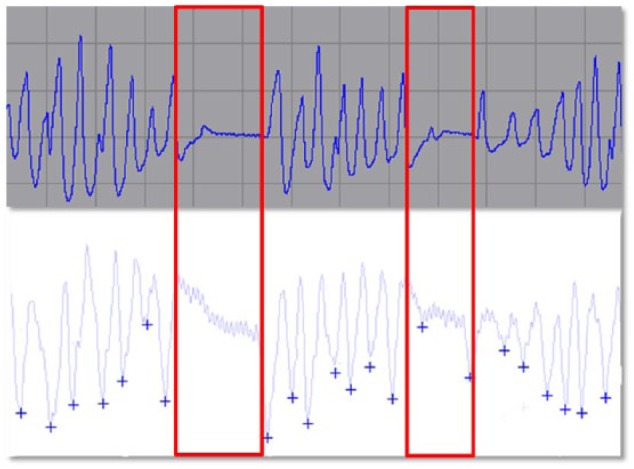
Nasal flow measured during polysomnography (top window) and corresponding recording of transthoracic impedance signal by the minute ventilation sensor (bottom window). Two apneas are recorded with the fall of the nasal pressure for more than 90% and the concurrent decreasing in the transthoracic impedance signal from the pacemaker.
Reprinted with permission from Defaye et al.14
We performed a review of published studies in English on PubMed database using the following key words: “sleep apnea” and “pacemaker.” This research provided 87 citations. After exclusion of those that did not assess diagnostic accuracy of PM, 7 citations remained. Major findings are summarized in Table 1. Two were performed with PM that did not allow automatic treatment of the transthoracic impedance15,16 and the signal was processed and analyzed using a dedicated software. All studies13–18 except one19 used PSG as reference test but with different AHI threshold values according to SA severity required for diagnosis. Designs were different and therefore diagnostic accuracy statistics are difficult to compare. Padeletti et al19 did not report if patients were correctly classified for SA based on PM index but only correlation between mean values of AHI from PM and in-home respiratory monitoring. In the study by Aimé et al,17 only apneic patients based on PM index (40/61 patients, 65.6%) were proposed to undergo PSG. Only 65% of them realized the reference test and the positive predictive value of PM index could be overestimated. Despite these discrepancies, these studies exhibit a good agreement between the PM-derived RDI and AHI from PSG.13–16 Correlation coefficient between the 2 tests was found to be similar to other home-based PSG recording systems.15,18 Nevertheless, it was shown that systematic bias between the 2 methods was higher in patients with severe SA.14,15,18 The recently published report from Dias et al18 has also shown that despite good correlation between RDI and AHI values, the agreement between SAM algorithm and PSG was poor regarding SA diagnosis and severity assessment (κ coefficient = 0.167). All studies showed good diagnostic accuracy of PM indexes. Defaye et al reported in the most recent prospective multicenter study a sensibility (Se) of 88.9% and a specificity (Sp) of 84.6% compared with PSG for the diagnosis of severe SA (AHI ≥ 30/h). The best threshold value of RDI to predict patients with severe SA was 20 events per hour.14 The same team found a different cutoff with a previous version of the algorithm.13 Likewise, the cutoff value was also different when using another type of PM without automatic signal processing.16 The work by Aimé et al is also interesting as they investigated diagnostic accuracy of both short-term RDI from last 24 hours and the “smoothed” index calculated by averaging the overall number of apneas and hypopneas over the period between 2 follow-up visits. There was a good correlation between these indexes and, moreover, the smoothed index had a better sensitivity to detect SA than the short-term index.17 Among these studies, only 1 reported data about arrhythmias. The incidence of AF or other rhythm disorders was similar in patients with and without SA, based on in-home respiratory monitoring (AHI ≥ 10/h).19
Table 1.
Review of published studies comparing PM algorithm and sleep studies for SA diagnosis.
| Author | No. of patients (M/F) | Age, y | BMI, kg/m2 | Device | TTI signal analysis | Reference test (AHI threshold) | Prognostic value | ROC curve (AUC, cutoff value) | Agreement (Bland and Altman/Pearson correlation coefficient r) |
|---|---|---|---|---|---|---|---|---|---|
| Defaye et al13 | 42 (30/12) | 70 ± 8 | 26 ± 4 | Talent 3 (ELA Medical) | Automatic RDI during estimated sleep | PSG AHI ≥ 30/h |
Se = 75% Sp = 94% PPV = 75% NPV = 94% |
AUC = 0.75 (95% CI: 0.6-0.87) for AHI ≥ 30 Cutoff = 30.6 |
MD = 0.9 (95% CI: −3.6 to 5.4) P > .2 2SD = 30 Pearson r = NA |
| Scharf et al14 | 22 (14/8) | 65.6 ± 13.4 | 28 ± 5.8 | Kappa 400 (Medtronic) | No automatic signal treatment Manual scoring by investigators |
PSG AHI > 20/h |
Se = 100% Sp = 100% |
AUC = 1.0 for AHI ≥ 20 AUC = 0.966 ± 0.37 for AHI ≥ 15 AUC = 0.95 ± 0.05 for AHI ≥ 5 |
MD = −1.5 ± 10.6 P < .05 2SD = 20.8 Pearson r = .869 P < .001 |
| Shalaby et al15 | 60 (45/15) | 69 ± 12 | 30 ± 6 | Pulsar Max Pulsar Max II Insignia Plus (Guidant) |
No automatic signal treatment Automatic algorithm developed by the investigators |
PSG AHI ≥ 30/h |
Se = 82% Sp = 88% |
AUC = 0.91 for AHI ≥ 30 cutoff = 39 AUC = 0.85 for IAH ≥ 15 cutoff = 37 |
MD = 2.5 2SD = 34.2 Pearson r = 0.8 P < .01 |
| Padeletti et al18 | 20 (15/5) | 78 | 25.5 | Talent 3 DR (ELA Medical) | Automatic RDI during estimated sleep | In-home respiratory monitoring AHI > 10/h |
NA | NA | Pearson r = NA AHI = 27 ± 14 PM RDI = 16 ± 13 P = .15 |
| Defaye et al16 | 40 (27/13) | 73.8 ± 19 | 27.7 ± 4.4 | REPLY 200 SR/DR (Sorin CRM) | Automatic RDI during estimated sleep | PSG AHI ≥ 30/h |
Se = 88.9% Sp = 84.6% PPV = 88.9% NPV = 84.6% |
AUC = 0.91 (95% CI: 0.8-1.0; P < .001) for AHI ≥ 30 cutoff = 20 |
MD = 9.2 ± 11.6 2SD = (−14 to 32.4) P = NA Pearson r = NA |
| Aimé et al17 | 61 (50%) | 71.4 ± 10.9 | 26.6 ± 4.1 | Talent 3 DR (ELA Medical) | Automatic RDI during estimated sleep | PSG AHI ≥ 15 |
PPV = 84.6% | NA | NA |
| Dias et al18 | 54 (31/23) | 77 (69-81) | 27 (23-30) | REPLY 200 SR/DR (LivaNova) | Automatic RDI during estimated sleep | PSG AHI ≥ 5 AHI ≥ 15 |
Se = 80% Sp = 79% Se = 100% Sp = 70% |
AHI ≥ 5; cutoff = 10 AUC = 0.81 (95% CI: 0.68-0.95) AHI ≥ 15; cutoff = 13 AUC = 0.86 (95% CI:0.76-0.95) |
MD = 0.7 ± 14.7 2SD = 33.3 Pearson r = .522 (P < .001) |
Abbreviations: 95% CI, confidence interval at 95%; AHI, apnea-hypopnea index (events per hour); AUC, area under curve; BMI, body mass index; F, female; M, male; MD, mean difference or systematic bias (events per hour); NA, not available; NPV, negative predictive value; PPV, positive predictive value; PSG, polysomnography; RDI, respiratory disturbance index (events per hour); ROC, receiver operating curve; 2SD, 2 standard deviations or limit of agreement; Se, sensibility; Sp, specificity; TTI, transthoracic impedance.
Data not available were calculated if possible, otherwise NA. Values are mean ± SD. In the study by Dias et al, 60 patients were recruited but 6 excluded for nonavailable RDI; age and BMI are the median and interquartile range.
Aim of the study
This study investigates the diagnostic performance of the RDI given by the PM compared with the result of ACSS realized in the sleep laboratory for the diagnosis of severe SA and the correlation with plasma aldosterone levels in a population of patients presenting with diastolic dysfunction.
Methods
Study design and ethics
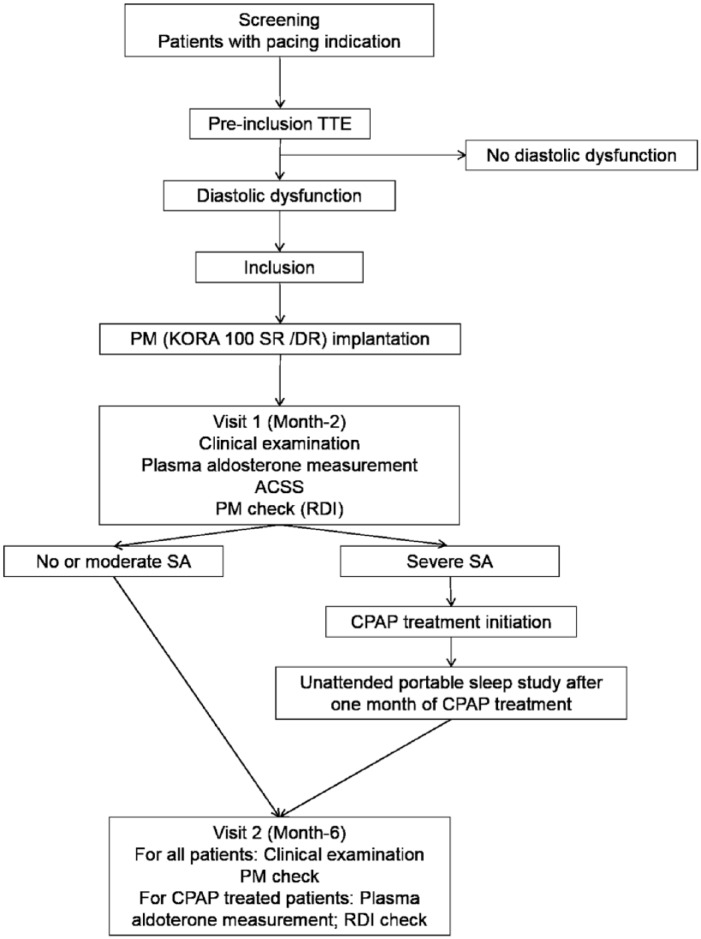
The SAPAAD (Sleep Apnea Diagnosis Using a Novel Pacemaker Algorithm and Link With Aldosterone Plasma Level in Patients Presenting With Diastolic Dysfunction) study is a prospective, single-center, diagnostic cohort study. The institutional review board of the University Hospital of Caen (November 19, 2015) and the ethics committee (CPP Nord Ouest III, March 5, 2016) approved the study as well as the Agence Nationale de Sécurité du Médicament (register number: 2016-A00034-47; March 4, 2016). The SAPAAD study was registered on April 25, 2016, on the ClinicalTrials.gov Web site with trial identification number NCT02751021. The SAPAAD study follows the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines.20 The flowchart of the protocol is shown in Figure 2. All patients provided written informed consent prior to their enrollment for the study participation, use, and disclosure of their information in publications.
Figure 2.
Study flowchart. ACSS indicates attended cardiorespiratory sleep study; CPAP, continuous positive airway pressure pocket; DR, double room; PM, pacemaker; RDI, respiratory disturbance index; SA, sleep apnea; SR, single room; TTE, transthoracic echocardiography.
Study population
Consecutive patients with clinical and electrical indication for permanent cardiac pacing, scheduled or not, will be screened. According to current European Guidelines,21 patients are presenting with third-degree atrioventricular block, second-degree atrioventricular block mobitz 2 type, bundle branch block associated with syncope and infra-Hisian block at electrophysiological study, or symptomatic sinus node dysfunction including brady-tachy form of sick sinus syndrome or bradyarrhythmia. Patients with diastolic dysfunction diagnosed at the transthoracic echocardiography (TTE) are eligible for the study. Patients fulfilling one of these criteria will not be included: younger than 18 years old, lack of informed consent form, impossibility to fit in the scheduled study plan, indication for cardiac resynchronization or LVEF lower than 45%, indication for epicardial PM, and known severe SA treated by continuous positive airway pressure (CPAP).
Standard procedures
Transthoracic echocardiography
According to our standard of care, all patients undergo a TTE before a PM implantation. This examination is conducted at the echocardiography laboratory in Caen University Hospital, using Epic 7 (Philips Healthcare, Amsterdam, Netherlands), during left lateral decubitus, with simultaneous electrocardiographic (ECG) monitoring.
PM implantation
Implantation is realized at the Heart Rhythm Unit of Caen University Hospital according to current guidelines and local standard of care. The procedure is performed under local anesthesia, and implantation is preferentially performed in left prepectoral subcutaneous pocket, with puncture of the left cephalic vein or if necessary the left subclavian vein, then positioning the right ventricular lead apical or septal, and an atrial lead in the right appendage or on the side wall of the right atrium if required. Choice of the leads is at the discretion of the operator, and the device is a KORA 100 or KORA 250, single or double room (Sorin Livanova, Clamart, France). On the second day after implantation, we perform clinical examination (in particular, the wound), ECG with and without magnet, device checking and programming, and a chest X-ray. Patient is discharged if there is no complication.
PM follow-up
After 2 months of hospital discharge, all patients undergo a follow-up visit with clinical examination and device control. Impedance, detection, and stimulation thresholds are measured and PM memories are read. Arrhythmias, mean heart rate, stimulation rate, and RDI are collected. The next follow-up is scheduled 6 months after implantation. If appropriate, data are collected for the first month of CPAP treatment.
Interventions
Clinical examination
Special concerns are taken during clinical examination to detect physical signs or symptoms that might be related to SA. Weight, height, and body mass index are systematically reported. Patients are asked about daytime sleepiness, drowsiness, snoring, and other symptoms less frequent such as nocturia, nocturnal choking, and gasping. We use the Epworth clinical scale (see table, additional file 2, which reports items and scoring of the scale) and the Berlin questionnaire (see table, additional file 3, which reports items and scoring of the questionnaire) to estimate a pretest probability of SA.
Attended cardiorespiratory sleep study
The ACSS is conducted in the sleep laboratory of Caen University Hospital. Monitoring is performed by usual methods of the center with a type 3 portable monitor (CID 102L; Cidelec, France). Recorded signals are as follows: nasal flow, pulse oxymetry, respiratory inductance plethysmography, brightness, suprasternal pressure, body position, actimetry, and analysis of respiratory sounds. The recording will be analyzed using Cidelec software by a practitioner with a board certification in sleep medicine to characterize nocturnal events and calculate the AHI of the study night. Mild SA is defined by an AHI between 5 and 15 events per hour, moderate SA by an AHI between 15 and 30 events per hour, and severe SA if AHI is greater than or equal to 30 events per hour.
A self-questionnaire is given to the patient about the quality of sleep during the night recorded (estimated latency of sleep and awakening, number of nocturnal arousals and pattern, presence of dream activity, and restful sleep or not). A blood punction is realized previous to the ACSS for blood gas measures. This sample will also be used for aldosterone test.
Plasma aldosterone level measurement
Blood samples are centrifuged within 30 minutes, and 1-mL plasma aliquots are immediately frozen and stored at −80°C. Plasma aldosterone concentrations are measured with a commercially available radioimmunoassay kit (Beckman Coulter Immunotech, Marseille, France).
SA treatment
A specific treatment with nocturnal CPAP ventilation is proposed to all patients with a severe obstructive SA diagnosed (AHI ≥ 30/h). If central mechanism is mainly responsible for SA, the type of ventilation can be different, for example, adaptive servo-ventilation. The CPAP device and settings are decided by the sleep physician according to patients’ profile. Device is provided by the AIR de Basse Normandie association. After 1 month of the beginning of CPAP therapy, patients will have a control unattended sleep study with a portable monitor. Compliance with ventilation is evaluated from nightly duration of CPAP use. Effectiveness is assessed using clinical evaluation of sleepiness and the decrease in AHI.
Study end points
The primary objective is the diagnostic accuracy of RDI compared with AHI for the diagnosis of severe SA. Secondary objectives are correlation between plasma aldosterone level, SA severity and its improvement with specific treatment, diagnostic accuracy of RDI compared with AHI for the diagnosis of moderate SA, prevalence of SA among patients with diastolic dysfunction, occurrence of atrial and ventricular arrhythmias, and improvement of RDI with CPAP. Judgment criteria for all the objectives are listed in Table 2.
Table 2.
Study end point measures.
| End point | Judgment criteria | |
|---|---|---|
| Primary end point measure | Diagnostic accuracy of RDI compared with AHI for the diagnosis of severe sleep apnea (AHI greater than 30 events/h) | Comparison between RDI (value of the sleep study recording night and mean RDI of the previous month) and AHI from the ACCS for the diagnosis of severe SA |
| Secondary end point measures | Correlation between plasma aldosterone levels, sleep apnea severity, and its evolution with specific treatment | Measurement of plasma aldosterone 2 months after PM implantation and 1 month after CPAP in apneic patients and correlation with AHI |
| Diagnostic accuracy of RDI compared with AHI for the diagnosis of moderate sleep apnea (AHI between 15 and 30 events/h) | Comparison between RDI (value of the sleep study recording night and mean RDI of the previous month) and AHI from the ACSS for the diagnosis of moderate SA | |
| Prevalence study of sleep apnea among patients with diastolic dysfunction | No. of patients with mild SA (AHI between 5 and 15 events/h), moderate (AHI between 15 and 30 events/h), and severe (AHI greater than 30 events/h), no. of subjects with obstructive or central SA based on the ACSS results | |
| Occurrence of atrial and ventricular arrhythmias | AF burden, mean duration of AF episodes, percentage of atrial and ventricular stimulation, episodes of ventricular arrhythmias | |
| Improvement of RDI with CPAP | Comparison between RDI and AHI after CPAP for patients with severe SA |
Abbreviations: ACSS, attended cardiorespiratory sleep study; AF, atrial fibrillation; AHI, apnea-hypopnea index; CPAP, continuous positive airway pressure; PM, pacemaker; RDI, respiratory disturbance index; SA, sleep apnea.
Sample size estimation
Data from the literature on the diagnostic performance of type 3 portable monitors compared with the PSG show variable results with a sensitivity between 61% and 91%.22,23 To highlight an agreement (measured by the intraclass correlation coefficient) between the RDI and AHI of at least 0.80, for an alpha risk equal to 5% and 90% power, 63 patients must be recruited.24 We plan therefore to recruit 68 patients, considering that there is a risk that for about 5% of patients to have data not fully exploitable (lost sight of missing data, etc).
Statistical plan
The continuous quantitative variables are expressed as mean ± SD, and qualitative variables are the number of patients and percentages. The occurrence and severity of SA are evaluated only in patients who have undergone ACSS. The diagnostic performance of the SAM algorithm for screening moderate (15 ≤ AHI ≤ 30 events per hour) and severe SA (AHI ≥ 30 events per hour) is evaluated by calculating the area under the receiver operator characteristic curve, with (1-Sp) on x-axis and Se on the y-axis. The best threshold value of RDI to discriminate populations (patients without moderate SA and patients with moderate to severe SA, patients without severe SA and patients with severe SA) is the one that gives the best compromise between Se and Sp, corresponding to the area under the curve nearest from 1. The 95% confidence intervals for both Se and Sp and positive and negative predictive values as well as positive and negative likelihood ratios of the RDI are calculated. The agreement between RDI and AHI for the detection of moderate and severe SAs is evaluated first by the Bland and Altman method and also by the intraclass correlation coefficient. The significance level used is P < 0.05. Statistical analyses are performed with IBM-SPSS Statistics (IBM Corp. Released 2013. IBM-SPSS Statistics for Windows, Version 22.0; Armonk, NY, USA: IBM Corp.). Data are analyzed in intention to treat.
Feasibility
We implant in our center almost 300 single-chamber and dual-chamber PMs each year. The prevalence of diastolic dysfunction (stage I to III) is about 45% in patients more than 65 years and reaches almost 70% in patients more than 75 years.9 Taking into account patients’ exclusions either for refusal or if already under CPAP, we can assume recruiting all patients required in the study within 18 months.
Registration
The data will be collected and registered using electronic case report forms (e-CRFs) by a dedicated local technical research team using the OpenClinica open source software, version 3.13 (OpenClinica, LLC and collaborators, Waltham, MA, USA: www.OpenClinica.com).
Data collected
Baseline characteristics will be collected: age, sex, weight, height, body mass index, smoking status, caffeine and alcohol consumption, history and type of diabetes mellitus, history of cardiovascular disease (hypertension, coronary artery disease, valvular heart disease, arrhythmias, cardiac medications), PM indication, heart rhythm (sinus or AF), heart rate, Berlin questionnaire result, and Epworth score. During follow-up visits (months 2 and 6), clinical variables, and Epworth and Berlin scores will be recorded.
During screening TTE, the following will be recorded: in 2-dimensional mode, systolic and diastolic diameter of the left ventricle, interventricular septal wall thickness, left atrium diameter and volume, LVEF with Simpson method, left ventricular outflow track (LVOT) diameter; in pulse-wave mode, LVOT flow, left ventricle filling with E and A waves velocity, A wave duration, E/A ratio; study of pulmonary vein flow with S/D waves ratio and A reverse wave duration; in continuous-wave mode, transaortic flow, aortic valve area; in color M mode, slope of left ventricle filling flow; and in tissue Doppler imaging (TDI) mode, early diastolic velocity (Ea) wave at lateral and septal mitral annulus, E/Ea ratio at lateral and septal mitral annulus.
Data from PM implantation will be recorded: type of PM (single or double room), implantation site (left or right and pre- or retropectoral), position of the atrial and ventricular leads, intraoperative complications (tamponade, pneumothorax, excessive blood loss, life-threatening arrhythmias), and device programming.
During ACSS, different data will be collected: duration of recording, mean and lowest Oxi-Pulse oxymetry, duration with oximetry below 90% and below 85%, snoring index, and the AHI.
Blood samples will be taken during month 2 follow-up visit and at month 6 follow-up visit in case of CPAP treatment (arterial blood gas and plasma aldosterone).
Data monitoring will be performed by Clinical Research Unit of the Caen University Hospital.
Adverse events
Security of patients will be routinely assessed according to the center’s standard of care and current guidelines. Serious adverse events will be reported in a dedicated form in the e-CRF and immediately declared to authorities by study promoter. Expected adverse events, related to associate drugs, disease progression, or standard procedures listed in the study protocol, will be reported in the e-CRF and declared at the end of the study in the security final report.
Study organization
The study promotion is performed by the University Hospital of Caen, France. The study is funded, thanks to the financial support from both the University Hospital of Caen and Sorin LivaNova (Sorin Group France SAS, Clamart).
Duration and time line
Patients from the University Hospital of Caen will be included during 18 months. The protocol, approval from the ethical committee, financial support, and e-CRFs were developed in 2015 and 2016. Inclusion and follow-up of patients are planned until first semester of 2018. The database will be closed in 2018, after which data analysis, manuscript writing, and submission for publication will follow.
Discussion
Sleep apnea is frequent especially in patients with heart disease and is underdiagnosed due to poor clinical presentation. Obstructive SA is associated with a poor quality of life and represents a major health problem, acting as an independent risk factor for cardiovascular morbidity and mortality.25 Continuous positive airway pressure can improve both respiratory parameters and prognosis of related comorbidities.26,27
Diastolic dysfunction is a common finding in the elderly, especially in case of diabetes or hypertension, and represents a first step toward HF with preserved LVEF. All-cause mortality in a cohort study from general population was higher among participants who had diastolic dysfunction after adjustment for age, sex, and LVEF.9 Despite some conflicting data, it now seems fairly clear that patients with moderate to severe SA have a high prevalence of relaxation abnormalities. The severity of obstructive SA expressed by AHI is correlated with the Ea by TDI after adjustment for other known variables.28 Furthermore, the effective treatment of obstructive SA with CPAP ventilation prevents the worsening of diastolic function echocardiographic parameters and even reverses them in early stages.29,30 However, little is known about SA prevalence among patients presenting with diastolic dysfunction, although it is a frequent finding among PM recipients and is probably correlated with high plasma aldosterone levels. It has already been observed that primary aldosteronism is associated with left ventricular remodeling and diastolic function impairment which is reversible with adrenalectomy.31,32 Therefore, it seems very important to improve SA detection because SA may worsen prognosis in this specific population.
The SAM algorithm developed by LivaNova is a new screening tool for SA diagnosis. There are several data on its accuracy13,14,17,19 and a prospective study already showed that the RDI was well correlated with PSG.14 The interest of our study is to validate the diagnostic accuracy of the SAM algorithm within a particular subgroup of patients. We assume that in this population, this algorithm could be an effective screening tool allowing to detect suspected high-risk patients for SA and to address them to a sleep specialist. In addition, if the RDI is low, it would be possible to rule out SA with no need of a time-consuming, expensive test such as a PSG.
Unlike Defaye et al,14 we chose attended sleep study with type 3 portable monitor as reference test. Diagnostic accuracy of ACSS is lower than complete PSG but it can be used as an alternative for the diagnosis of SA12 especially in patients with high pretest probability of moderate to severe SA. With lower cost and easier implementation than PSG, ACSS represents a more “real-life” gold standard.
The choice of the primary judgment criteria was also challenging. We want to take into account 2 values of RDI: the one of the sleep study night and the mean value over the previous month. It is probably methodologically better to compare 2 variables recorded at the same time. However, we know that one limiting factor of sleep studies is sleep quality and reproducibility of AHI measured on a single night resulting in false-negative results. As the algorithm provides RDI values over a 6-month period, we assume that average RDI from previous month might be a more relevant data than a single night value. This feature may compensate the weakness of a single-signal recording by the SAM algorithm compared with ACSS result based on several signals. Aimé et al17 suggested already that a “smoothed” index should be more sensitive to detect SA. Finally, the decrease of the RDI, compared to the AHI, after CPAP treatment will also test the agreement between these 2 values. The follow-up of RDI after CPAP therapy could be an alternative to a control sleep study.
Among hypertensive patients, SA prevalence is increased in patients with hyperaldosteronism.33 Moreover, the severity of obstructive SA is correlated with the level of plasma aldosterone.33–35 Recently, it has been shown that plasma aldosterone levels are significantly higher in apneic patients and that CPAP treatment reduces plasma aldosterone levels.36 Our study is the first one to examine the relationship between SA and plasma aldosterone level in a specific population of patients presenting with diastolic dysfunction related to systemic hypertension or not. We aim to better understand the role of hyperaldosteronism in this particular situation and the beneficial effect of CPAP treatment. It could support the use of renin-angiotensin-aldosterone system blockers in case of systemic hypertension requiring drug therapy in this particular group of patients.
The SAM algorithm has been exclusively studied for diagnosis of severe SA. As a secondary objective, we aim to show the level of agreement between RDI and AHI for diagnosis of moderate SA. Even these patients do not require CPAP treatment, they have to be closely followed by a sleep specialist and they can benefit from non-CPAP treatment.
As SA is a well-known risk factor for arrhythmias, PM memories are very useful to diagnose nocturnal arrhythmias related to SA and the potential improvement with CPAP treatment. Padeletti et al19 did not find any difference in arrhythmias incidence between patients with and without SA but their patients were probably less severe than our patients because their diagnostic cutoff AHI was 10 events per hour. Severe obstructive SA can also influence atrioventricular conduction abnormalities, and evolution of pacing rates after CPAP treatment could be relevant.
Some comments could be addressed concerning the limitations of the study. First, the study population is limited to patients with diastolic dysfunction to observe high prevalence of obstructive SA without a control group of patients with no diastolic dysfunction. Further studies may be conducted to evaluate the diagnostic accuracy of the SAM in a population with a lower prevalence of obstructive SA. The echocardiographic analyses of diastolic dysfunction can also be challenging due to the initial bradycardia requiring PM implantation. The choice of ACSS with type III portable monitor is also a limit of our study as it is not the gold standard for SA diagnosis. Past studies have shown a good diagnostic accuracy of this test compared with PSG in patients with high pretest probability and for the diagnosis of moderate to severe SA. Especially, in patients with other types of sleep-related breathing disorders, ACSS could underestimate SA diagnosis. Considering our population with a high probability of SA, we assume that ACSS could represent a reliable standard test. As we discussed earlier, it is the most widespread test to confirm SA diagnosis in daily practice. We chose to evaluate the benefit of CPAP therapy at 1 month. Improvement of both indexes is supposed to happen as soon as CPAP is used; arrhythmias and heart rate should also decrease quickly if they were related to severe SA. However, this treatment period could be too short to observe changes in plasmatic aldosterone levels.
Conclusions
The SAPAAD study is a prospective diagnostic cohort study powered to assess the diagnostic accuracy of a specific algorithm provided by a new generation of PM for the diagnosis of severe SA. This study aims to highlight not only a correlation between plasma aldosterone level and SA occurrence and severity but also improvement after treatment. The study also evaluates diagnostic accuracy of the algorithm for the diagnosis of moderate SA, characteristics of SA among patients with diastolic dysfunction, related arrhythmias, and the potential use of the PM algorithm as a monitoring test for CPAP-treated patients.
Acknowledgments
The authors thank Fabien Chaillot, Cathy Gaillard, François Fournel, and Flavien Robion, from the Clinical Research Unit of the Caen University Hospital, for their practical help to the study; Patricia Akan from AIR de Basse Normandie; Anne Rousseau-Plasse, Amel Amblard, and Jude Cadet from LivaNova; and Pascal Defaye from Grenoble University Hospital. Caen University Hospital funded half of the SAPAAD study. The study is ongoing. A total of 30 patients have been recruited since June 2016.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study is half-funded by Sorin LivaNova.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors Contributions: LC-R wrote the manuscript. LC-R designed the study with PUM, VF, J-NP, PM, JA, AP, and ES. RM performed the statistical plan. All authors read and approved the final manuscript.
References
- 1. Giordanella J. Rapport sur le thème du sommeil; 2006. http://solidarites-sante.gouv.fr/IMG/pdf/rapport-5.pdf.
- 2. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. [DOI] [PubMed] [Google Scholar]
- 3. Stevenson IH, Teichtahl H, Cunnington D, Ciavarella S, Gordon I, Kalman JM. Prevalence of sleep disordered breathing in paroxysmal and persistent atrial fibrillation patients with normal left ventricular function. Eur Heart J. 2008;29:1662–1669. doi: 10.1093/eurheartj/ehn214. [DOI] [PubMed] [Google Scholar]
- 4. Somers VK, White DP, Amin R, et al. ; American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College. Sleep apnea and cardiovascular disease: An American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee; Council on Clinical Cardiology, Stroke Council and Council on Cardiovascular Nursing In Collaboration With the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). J Am Coll Cardiol. 2008;52:686–717. doi: 10.1016/j.jacc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 5. Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160:1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 6. Garrigue S, Pépin J-L, Defaye P, et al. High prevalence of sleep apnea syndrome in patients with long-term pacing: the European Multicenter Polysomnographic Study. Circulation. 2007;115:1703–1709. doi: 10.1161/CIRCULATIONAHA.106.659706. [DOI] [PubMed] [Google Scholar]
- 7. Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med. 2005;352:1206–1214. doi: 10.1056/NEJMoa041832. [DOI] [PubMed] [Google Scholar]
- 8. Sharma K, Kass DA. Heart failure with preserved ejection fraction: mechanisms, clinical features, and therapies. Circ Res. 2014;115:79–96. doi: 10.1161/CIRCRESAHA.115.302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 10. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 11. Muxfeldt ES, Margallo VS, Guimarães GM, Salles GF. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am J Hypertens. 2014;27:1069–1078. doi: 10.1093/ajh/hpu023. [DOI] [PubMed] [Google Scholar]
- 12. Collop NA, Anderson WM, Boehlecke B, et al. ; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 13. Defaye P, Pépin J-L, Poezevara Y, et al. Automatic recognition of abnormal respiratory events during sleep by a pacemaker transthoracic impedance sensor. J Cardiovasc Electrophysiol. 2004;15:1034–1040. doi: 10.1046/j.1540-8167.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- 14. Defaye P, de la Cruz I, Martí-Almor J, et al. A pacemaker transthoracic impedance sensor with an advanced algorithm to identify severe sleep apnea: the DREAM European study. Heart Rhythm Off J Heart Rhythm Soc. 2014;11:842–848. doi: 10.1016/j.hrthm.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 15. Scharf C, Cho YK, Bloch KE, et al. Diagnosis of sleep-related breathing disorders by visual analysis of transthoracic impedance signals in pacemakers. Circulation. 2004;110:2562–2567. doi: 10.1161/01.CIR.0000145540.36097.EB. [DOI] [PubMed] [Google Scholar]
- 16. Shalaby A, Atwood C, Hansen C, et al. Feasibility of automated detection of advanced sleep disordered breathing utilizing an implantable pacemaker ventilation sensor. Pacing Clin Electrophysiol. 2006;29:1036–1043. doi: 10.1111/j.1540-8159.2006.00496.x. [DOI] [PubMed] [Google Scholar]
- 17. Aimé E, Rovida M, Contardi D, et al. Long-term screening for sleep apnoea in paced patients: preliminary assessment of a novel patient management flowchart by using automatic pacemaker indexes and sleep lab polygraphy. Heart Lung Circ. 2014;23:943–950. doi: 10.1016/j.hlc.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 18. Dias M, Gonçalves I, Amann B, et al. Utility of new-generation pacemakers in sleep apnea screening. Sleep Med. 2017;37:27–31. doi: 10.1016/j.sleep.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 19. Padeletti M, Vignini S, Ricciardi G, et al. Sleep disordered breathing and arrhythmia burden in pacemaker recipients. Pacing Clin Electrophysiol. 2010;33:1462–1466. doi: 10.1111/j.1540-8159.2010.02881.x. [DOI] [PubMed] [Google Scholar]
- 20. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brignole M, Auricchio A, Baron-Esquivias G, et al. ; European Society of Cardiology (ESC). European Heart Rhythm Association (EHRA). 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eurospace. 2013;15:1070–1118. doi: 10.1093/europace/eut206. [DOI] [PubMed] [Google Scholar]
- 22. Escourrou P, Meslier N, Raffestin B, et al. Which clinical approach and which diagnostic procedures for obstructive sleep apnea syndrome? Rev Mal Respir. 2010;27:S115–S123. doi: 10.1016/S0761-8425(10)70017-6. [DOI] [PubMed] [Google Scholar]
- 23. Pietzsch JB, Garner A, Cipriano LE, Linehan JH. An integrated health-economic analysis of diagnostic and therapeutic strategies in the treatment of moderate-to-severe obstructive sleep apnea. Sleep. 2011;34:695–709. doi: 10.5665/SLEEP.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zou GY. Sample size formulas for estimating intraclass correlation coefficients with precision and assurance. Stat Med. 2012;31:3972–3981. doi: 10.1002/sim.5466. [DOI] [PubMed] [Google Scholar]
- 25. Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med. 2014;11:e1001599. doi: 10.1371/journal.pmed.1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 27. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353:2025–2033. doi: 10.1056/NEJMoa051001. [DOI] [PubMed] [Google Scholar]
- 28. Kim SH, Cho GY, Shin C, et al. Impact of obstructive sleep apnea on left ventricular diastolic function. Am J Cardiol. 2008;101:1663–1668. doi: 10.1016/j.amjcard.2008.01.056. [DOI] [PubMed] [Google Scholar]
- 29. Arias MA, García-Río F, Alonso-Fernández A, Mediano O, Martínez I, Villamor J. Obstructive sleep apnea syndrome affects left ventricular diastolic function: effects of nasal continuous positive airway pressure in men. Circulation. 2005;112:375–383. doi: 10.1161/CIRCULATIONAHA.104.501841. [DOI] [PubMed] [Google Scholar]
- 30. Butt M, Dwivedi G, Shantsila A, Khair OA, Lip GY. Left ventricular systolic and diastolic function in obstructive sleep apnea: impact of continuous positive airway pressure therapy. Circ Heart Fail. 2012;5:226–233. doi: 10.1161/CIRCHEARTFAILURE.111.964106. [DOI] [PubMed] [Google Scholar]
- 31. Lin YH, Wang SM, Wu VC, et al. The association of serum potassium level with left ventricular mass in patients with primary aldosteronism. Eur J Clin Invest. 2011;41:743–750. doi: 10.1111/j.1365-2362.2010.02462.x. [DOI] [PubMed] [Google Scholar]
- 32. Hung C-S, Chou C-H, Wu X-M, et al. Circulating tissue inhibitor of matrix metalloproteinase-1 is associated with aldosterone-induced diastolic dysfunction. J Hypertens. 2015;33:1922–1930 (discussion 1930). doi: 10.1097/HJH.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 33. Sim JJ, Yan EH, Liu IL, et al. Positive relationship of sleep apnea to hyperaldosteronism in an ethnically diverse population. J Hypertens. 2011;29:1553–1559. doi: 10.1097/HJH.0b013e3283492219. [DOI] [PubMed] [Google Scholar]
- 34. Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest. 2007;131:453–459. doi: 10.1378/chest.06-1442. [DOI] [PubMed] [Google Scholar]
- 35. Gonzaga CC, Gaddam KK, Ahmed MI, et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–368. [PMC free article] [PubMed] [Google Scholar]
- 36. Barceló A, Piérola J, Esquinas C, et al. Relationship between aldosterone and the metabolic syndrome in patients with obstructive sleep apnea hypopnea syndrome: effect of continuous positive airway pressure treatment. PLoS ONE. 2014;9:e84362. doi: 10.1371/journal.pone.0084362. [DOI] [PMC free article] [PubMed] [Google Scholar]