Abstract
Intraventricular hemorrhage (IVH) is a devastating morbidity in preterm infants and can result in poor neurodevelopmental outcomes. Intraventricular hemorrhage usually occurs within 72 hours after birth; post–acute-phase IVH (>1 week after birth) is uncommon. Development of the hemostatic system in fetuses and neonates is an age-dependent evolving process, and the neonatal hemostatic system is characterized by low levels of vitamin K–dependent factors, with further reduction caused by prematurity. Importantly, a severe coagulation deficiency can be a major contributing factor of IVH. Active maternal Crohn disease (CD) during pregnancy causes malnutrition via enteral malabsorption; this may include vitamin K deficiency, resulting in fetal vitamin K deficiency. We herein describe a preterm infant who was born to a mother with CD and developed post–acute-phase IVH due to coagulopathy despite vitamin K administration.
Keywords: Intraventricular hemorrhage, preterm infant, Crohn disease, coagulopathy
Introduction
Intraventricular hemorrhage (IVH) is a devastating morbidity in preterm infants and can result in poor neurodevelopmental outcomes.1 Intraventricular hemorrhage usually occurs within 72 hours after birth; post–acute-phase IVH (>1 week after birth) is uncommon.2,3 Development of the hemostatic system in fetuses and neonates is an age-dependent evolving process, and the neonatal hemostatic system is characterized by low levels of vitamin K–dependent factors, with further reduction caused by prematurity. Importantly, a severe coagulation deficiency can be a major contributing factor of IVH.4 Active maternal Crohn disease (CD) during pregnancy causes malnutrition via enteral malabsorption; this may include vitamin K deficiency, resulting in fetal vitamin K deficiency.5 We herein describe a preterm infant who was born to a mother with CD and developed post–acute-phase IVH due to coagulopathy despite vitamin K administration.
Case Presentation
A 34-year-old woman with CD (gravida 1, para 1) who had been diagnosed with pregnancy-induced hypertension (PIH) and marginal placenta previa at 20 weeks of pregnancy was referred to our center because of fetal growth restriction (FGR). She exhibited intermittent genital bleeding after admission, and her pregnancy was interrupted due to massive bleeding at 26 weeks 6 days of gestation. The history of the mother such as PIH, thrombocytopenia, low antithrombin III, and FGR suggested a minor form of HELLP syndrome; however, her liver enzyme levels (aspartate aminotransferase [AST]: 34 IU/L, alanine aminotransferase [ALT]: 20 IU/L) did not meet the criteria. A female infant was born via emergent cesarean section (birth weight: 666 g [−1.7 SD]; Apgar scores of 3 and 5 at 1 and 5 minutes, respIectively).
The mother had developed CD at 25 years of age; however, she had not been diagnosed with vitamin K deficiency before pregnancy. The disease deteriorated during her first pregnancy (28 years of age) with the symptoms of intractable diarrhea and coagulopathy (prothrombin time: 57%) since 33 weeks of gestation. Based on the findings of reduced prothrombin time, she was diagnosed with vitamin K deficiency and received intravenous vitamin K administration on the day before delivery. Her first child, a male infant, was delivered at 33 weeks of gestation 6 days at regional hospital by emergent cesarean section and found to have third-grade IVH at birth; this proceeded to posthemorrhagic hydrocephalus. At 29 years of age, the mother underwent surgical repair of an anal fistula and required an elemental diet and parenteral nutrition with daily prednisolone (40 mg/d). At 31 years of age, infliximab and oral mesalazine were administered for treatment of an ileum-skin fistula. She achieved remission and required no steroid treatment thereafter. Her coagulation status at delivery was normal (Table 1).
Table 1.
Laboratory data.
| Mother |
Patient |
Institutional reference range | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 1 | Day 2 | Day 8 | Day 9 | Day 10 | Day 11 | Day 12 | Day 13 | Day 15 | Day 18 | ||
| WBC count/µL | 7800 | 3100 | 4600 | 4300 | 3500 | 2800 | 4100 | 5800 | 4500 | 6700 | 10,800 | 4000-8500 |
| Hemoglobin, g/dL | 10.6 | 13.5 | 13.6 | 14.0 | 13.5 | 16.2 | 16.9 | 16.7 | 16.3 | 14.1 | 12.4 | 11.8-15.0 |
| Platelets, ×104/µL | 11.5 | 17.4 | 17.2 | 11.0 | 9.8 | 9.0 | 8.3 | 8.1 | 7.7 | 6.4 | 14.4 | 13-30 |
| APTT, s | 29.0 | >180 | 63.7 | >180 | 113 | 88 | 76.1 | 56.9 | 44.6 | 74.7 | 69 | 25-40 |
| PT (INR) | 0.83 | 1.66 | 1.18 | 1.72 | 1.33 | 1.27 | 1.2 | 1.11 | 1.08 | 1.20 | 1.28 | 0.8-1.15 |
| Thrombotest (INR) | 1.24 | 1.27 | 1.25 | 1.21 | 1.06 | 0.9-1.1 | ||||||
| HPT (INR) | 1.45 | 1.45 | 1.28 | 1.26 | 1.15 | 0.8-1.15 | ||||||
| Antithrombin, % | 54 | 26 | 35 | 13 | 69 | 47 | 54 | 43 | 47 | 44 | 34 | 80-120 |
| Fibrinogen, mg/dL | 299 | 80 | 199 | 95 | 135 | 188 | 223 | 229 | 232 | 180 | 240 | 170-350 |
| D-dimer, μg/mL | 7.7 | 34.6 | 2.0 | 4.8 | 5.7 | 5.2 | 4.4 | 4.9 | 3.4 | 1 | 1.1 | 0-1 |
| FDP, μg/mL | 41.1 | <5 | 6.6 | 8.1 | 8.0 | 6.9 | 7.6 | 6.3 | <5 | <5 | 0-5 | |
Abbreviations: APTT, activated partial thromboplastin time; FDP, fibrin degradation products; HPT, hepaplastin test; INR, international normalized ratio; PT, prothrombin time; WBC, white blood cell.
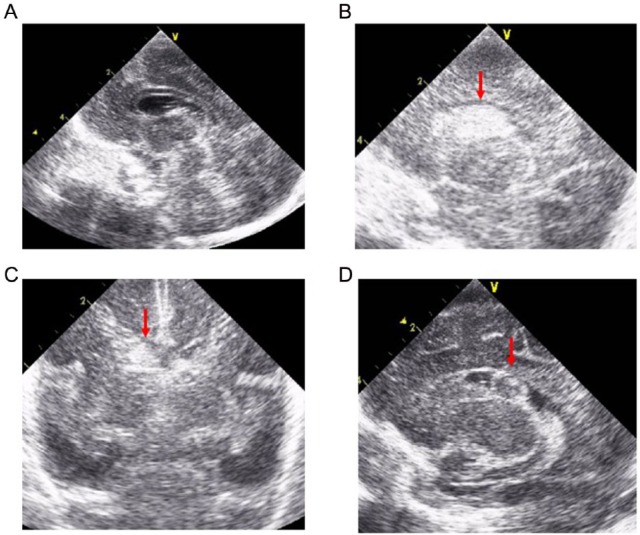
The infant was tracheally intubated soon after birth, received an artificial pulmonary surfactant for respiratory distress syndrome, and was mechanically ventilated on synchronized intermittent mandatory ventilation mode. She was hypotensive on the neonatal intensive care unit (NICU) admission and required inotropes and volume expanders including albumin, packed red blood cells, fresh frozen plasma (FFP), and hydrocortisone via umbilical venous catheters to maintain her blood pressure. She also received a dose of indomethacin at 15 hours, and patent ductus arteriosus was closed at 36 hours after birth. Initial blood tests revealed leukocytopenia and massive coagulopathy (Table 1). Initial brain ultrasound (Figure 1A) and echocardiography revealed no abnormalities. Her coagulopathy was recovered on day 2 (Table 1) after intravenous administration of vitamin K2 (menatetrenone, 1 mg) at the admission to NICU and FFP. She required 2 additional doses of surfactant on days 2 and 4 of life for respiratory distress. Her cardiorespiratory condition was maintained under mechanical ventilation and inotrope management in the postacute phase; however, enteral nutrition could not be increased (<20 mL/kg/d) because of feeding intolerance. In addition, following brain ultrasound performed at 8, 27, 36, and 46 hours and 4 days after birth revealed no abnormality. On day 8 of life, pulmonary hemorrhage occurred unexpectedly, and ultrasound examination revealed grade 2 IVH (Figure 1B and C) and relapse of the coagulopathy without signs of disseminated intravascular coagulation (Table 1). The coagulopathy gradually improved after multiple intravenous administrations of vitamin K2 (1 mg on days 8 and 9 and 2 mg on day 14), packed red blood cells (days 9 and 18), and FFP (days 8-12, 15, and 18); the IVH did not progress thereafter. She has never received fat-soluble vitamins with parenteral nutrition. Tests for lupus anticoagulant (day 10) and protein induced by vitamin K absence or antagonist II (PIVKA-II, day 14) were negative. She had no signs of infections throughout the course. The coagulopathy did not relapse, and the absorption of hemorrhage and the formation of subependymal cysts were confirmed by brain ultrasound on day 34 (Figure 1D). Brain magnetic resonance imaging performed on day 115 did not reveal any abnormal findings except right obsolete hemorrhage. She was discharged on day 121 of life. She recently turned 7 years of age with no episodes of bleeding tendency or developmental delay (deviation quotient assessed by Wechsler Preschool and Primary Scale of Intelligence at 6 years of age: 90).
Figure 1.
Brain ultrasound images. (A) Brain ultrasound revealed no abnormality on admission. (B, C) Grade 2 intraventricular hemorrhage was detected (arrows) in the right lateral ventricle on day 8. (D) The formation of subependymal cysts was confirmed by brain ultrasound at day 34.
Discussion
Crohn disease is an inflammatory bowel disease that typically causes transmural inflammation with skip lesions. The incidence of CD has increased during the past few decades.6 A large cohort study suggested that pregnancy in patients with CD is associated with increased risks of low birth weight, preterm birth, and small for gestational age6,7; however, no evidence is available regarding the bleeding risk in newborns. The maternal medications for CD in the present case showed no evidence of contributing to the neonatal coagulopathy.8 And, the maternal vitamin K deficiency may have been responsible for the bleeding tendency at birth, as occurred with the mother’s first child, but not for the coagulopathy after postnatal vitamin K administration.5 In addition, it is reported that only a little amount of vitamin K crosses placenta during delivery.9 Theoretically, vitamin K deficiency is not associated with decreased fibrinogen levels as seen in our case.10
The limitation of our case is that the timing we measured PIVKA-II was too late to prove that the cause of coagulopathy was not vitamin K deficiency because PIVKA-II was already negative from 24 hours to 4 days after vitamin K administration in previous report about normal newborns, although PIVKA-II values could be dependent on vitamin K preparation used.11–13 Multiple predisposing factors, including maternal bleeding, birth asphyxia, prematurity, and FGR, in addition to maternal CD might be responsible for the cardiopulmonary instability and hematologic disturbances including leukocytopenia, anemia, and long-lasting thrombocytopenia in this case. A possible mechanism of coagulopathy is poor synthetic ability of clotting factor in liver in critically ill very premature infant, although the data of hepatic function of this patient were within normal range (AST: 15 IU/L, ALT: 3 IU/L),9 in addition to insufficient placental transport of nutrients due to maternal CD. And, it is noteworthy that vitamin K is necessary not only for the synthesis of 4 plasma clotting factors but also for the production of 2 anticlotting factors of protein C and protein S.14 Thus, vitamin K administration could result in unfavorable if the reactivity of the vitamin K–dependent clotting factors and anticlotting factors were altered. However, it should be emphasized that this is not the typical clinical course of a preterm infant of a mother with CD.
In conclusion, we believe that careful observation for coagulation is warranted even after vitamin K administration in preterm infants born of a mother with CD.
Acknowledgments
The authors gratefully acknowledge the staff of their institution for contributing to patient care. They thank Angela Morben, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially supported by grants for Scientific Research from the JSPS KAKENHI (K.F. and I.M.).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: KF and KN managed the patient and contributed to the conception of the manuscript; KF and SF drafted the manuscript; and KI and IM critically reviewed the manuscript. All authors read and approved the final manuscript.
References
- 1. Volpe JJ. Intraventricular hemorrhage and brain injury in the premature infant. Diagnosis, prognosis, and prevention. Clin Perinatol. 1989;16:387–411. [PubMed] [Google Scholar]
- 2. Perlman JM, Volpe JJ. Intraventricular hemorrhage in extremely small premature infants. Am J Dis Child. 1986;140:1122–1124. [DOI] [PubMed] [Google Scholar]
- 3. Whitelaw A, Odd D. Postnatal phenobarbital for the prevention of intraventricular hemorrhage in preterm infants. Cochrane Database Syst Rev. 2007;4:CD001691. [DOI] [PubMed] [Google Scholar]
- 4. Kuperman AA, Brenner B, Kenet G. Intraventricular hemorrhage in preterm infants and coagulation—ambivalent perspectives? Thromb Res. 2013;131:S35–S38. [DOI] [PubMed] [Google Scholar]
- 5. Ohishi A, Nakashima S, Ogata T, et al. Early vitamin K deficiency bleeding in a neonate associated with maternal Crohn’s disease. J Perinatol. 2014;34:636–639. [DOI] [PubMed] [Google Scholar]
- 6. Getahun D, Fassett MJ, Longstreth GF, et al. Association between maternal inflammatory bowel disease and adverse perinatal outcomes. J Perinatol. 2014;34:435–440. [DOI] [PubMed] [Google Scholar]
- 7. Fonager K, Sorensen HT, Olsen J, Dahlerup JF, Rasmussen SN. Pregnancy outcome for women with Crohn’s disease: a follow-up study based on linkage between national registries. Am J Gastroenterol. 1998;93:2426–2430. [DOI] [PubMed] [Google Scholar]
- 8. Katz JA, Antoni C, Keenan GF, Smith DE, Jacobs SJ, Lichtenstein GR. Outcome of pregnancy in women receiving infliximab for the treatment of Crohn’s disease and rheumatoid arthritis. Am J Gastroenterol. 2004;99:2385–2392. [DOI] [PubMed] [Google Scholar]
- 9. Lippi G, Franchini M. Vitamin K in neonates: facts and myths. Blood Transfus. 2011;9:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sutor AH, von Kries R, Cornelissen EA, McNinch AW, Andrew M. Vitamin K deficiency bleeding (VKDB) in infancy. ISTH Pediatric/Perinatal Subcommittee. International Society on Thrombosis and Haemostasis. Thromb Haemost. 1999;81:456–461. [PubMed] [Google Scholar]
- 11. Schubiger G, Gruter J, Shearer MJ. Plasma vitamin K1 and PIVKA-II after oral administration of mixed-micellar or cremophor EL-solubilized preparations of vitamin K1 to normal breast-fed newborns. J Pediatr Gastroenterol Nutr. 1997;24:280–284. [DOI] [PubMed] [Google Scholar]
- 12. Greer FR, Marshall SP, Severson RR, et al. A new mixed micellar preparation for oral vitamin K prophylaxis: randomised controlled comparison with an intramuscular formulation in breast fed infants. Arch Dis Child. 1998;79:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pereira SP, Shearer MJ, Williams R, Mieli-Vergani G. Intestinal absorption of mixed micellar phylloquinone (vitamin K1) is unreliable in infants with conjugated hyperbilirubinaemia: implications for oral prophylaxis of vitamin K deficiency bleeding. Arch Dis Child. 2003;88:F113–F118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shirakawa Y, Shirahata A, Fukuda M. Differences in reactivity to vitamin K administration of the vitamin K-dependent procoagulant factors, protein C and S, and osteocalcin. Semin Thromb Hemost. 2000;26:119–126. [DOI] [PubMed] [Google Scholar]