Abstract
IFN-γ activation of mononuclear phagocytes significantly increases indoleamine 2,3-dioxygenase (IDO) and flux through the kynurenine pathway (KP). However, the effect of IDO on NAD+ synthesis, the end product of KP metabolism, is unknown. To investigate this, primary human peripheral blood mononuclear cells were cultured up to 10 days and activated with IFN-γ in the presence or absence of a poly(ADP-ribose) polymerase (PARP) inhibitor. Day 10 macrophages had significantly higher NAD+ levels compared with monocytes. IFN-γ activation of macrophages resulted in the highest induction of IDO but decreased intracellular NAD+ concentrations at both 24 and 48 hours. However, IFN-γ activation of both day 6 and day 10 macrophages in the presence of a PARP inhibitor resulted in significantly higher intracellular NAD+ levels at 24 hours. This study provides evidence for the first time that an immune-mediated increase in IDO activity increases NAD+ biosynthesis concomitantly with an increase in NAD+ catabolism in primary human macrophages.
Keywords: monocyte; macrophage; tryptophan; NAD+; indoleamine 2,3-dioxygenase; PARP
Introduction
Mononuclear phagocytes (ie, monocyte/macrophages) are an important part of the bodies’ phagocytic and cell defence system and are found in virtually all tissues of the body with large numbers in the lymphoid organs, the liver, lungs, and digestive tract.1 These cells, influenced by the microenvironment, mount specific defence processes. Circulating monocytes will differentiate into tissue macrophages which can be broadly classified in 2 groups: M1 macrophages, following activation by IFN-γ or lipopolysaccharides (LPS) or M2-activated macrophages which are further subdivided in M2a (stimulated by interleukin 4 [IL]-4 or IL-13), M2b (immune complexes in combination with IL-1β or LPS), and M2c (IL-10, transforming growth factor β or glucocorticoids).2 Activation of macrophages with IFN-γ, tumour necrosis factor α and IL-1β significantly upregulates the first and rate-limiting enzyme of the kynurenine pathway (KP), indoleamine 2,3-dioxygenase (IDO). The reasons for why the inflammatory response increases IDO activation, and subsequent increased flux through the KP, are not fully understood. Although previous research has shown a role for reducing foetal tissue rejection in allogeneic pregnancy3 and certain upstream KP metabolites have antimicrobial activity, the reasons for its increase in downstream KP metabolism during normal macrophage activation is still unknown.
Previous research using the murine monocyte/macrophage cell line RAW264.74 and human astroglial cell line HTB-1385 suggest a link between the IFN-γ–mediated actions of increased free radical synthesis and IDO induction. However, these immortalised cell lines are physiologically different from the nontransformed cells. Being essentially phenotypically stable, these cells do not undergo differentiation (ie, maturation), although they can be activated by an appropriate stimulus such as IFN-γ. Nontransformed mononuclear phagocytes, however, can undergo both differentiation and activation.
After migrating from the bloodstream, monocytes undergo irreversible maturation (differentiation) into macrophages and tissue-specific cells. The process of proliferation and differentiation is mediated by a variety of specific glycoproteins including colony-stimulating factor (CSF-1), granulocyte-macrophage colony-stimulating factor, IL-3, and others.1 Adhesion to the vascular endothelium is also an early signal for monocyte differentiation in vivo.6 In a similar way, adherence to the synthetic substratum of a tissue culture dish in vitro has also been shown to facilitate the differentiation of isolated monocytes into mature macrophages.7
As the potential for localised DNA damage and subsequent poly(ADP-ribose) polymerase (PARP) activation, NAD depletion is high in mononuclear phagocytic cells following IFN-γ activation.8 The aim of this study was to investigate the effect of IFN-γ treatment on intracellular NAD concentration and IDO activity in human peripheral blood mononuclear cells at different stages of differentiation.
Materials and Methods
Materials
RPMI 1640 was purchased from Life Technologies (Sydney, Australia). 3-Aminobenzamide (3ABA), phenazine methosulfate, alcohol dehydrogenase (baker’s yeast), bicine, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, catalase (3200 U/mg solid, bovine liver), tryptophan, and p-dimethylamino-benzaldehyde (Ehrlich reagent) were purchased from Sigma-Aldrich (Sydney, Australia). Human recombinant IFN-γ was purchased from Australian Laboratory Services (Sydney, Australia). Bovine calf serum was purchased from Commonwealth Serum Laboratories (Sydney, Australia).
Monocyte isolation
Human peripheral blood monocytes were extracted from 400 mL of whole blood from healthy human immunodeficiency virus 1–seronegative volunteers as described by Kazazi et al.9 Briefly, blood mononuclear cells were obtained by differential centrifugation in Ficoll-Hypaque (Pharmacia-AMRAD, Sydney, Australia). Monocytes were separated from other mononuclear cells by counter-current elutriation (Beckman centrifuge J-6M/E fitted with a JE-5.0 Elutriation Rotor; Beckman Coulter, Sydney, Australia) followed by plastic adherence. Cells were plated at a density of 7 × 105 cells/mL of medium which consisted of RPMI 1640 supplemented with 10% heat-inactivated foetal bovine serum, 10% heat-inactivated pooled AB+ human serum, and 50 μM tryptophan (RF10/10) in a 48-well tissue culture plate (Nunc, Sydney, Australia). Cells were then placed immediately in a 37°C incubator in a humidified atmosphere of 5% CO2.
NAD analysis
The cellular pyridine nucleotide content (NAD+ + NADH) was measured by the Thiazolyl blue microcycling assay of Bernofsky and Swan adapted to 96-well plate format.10
Quinolinic acid assay
Quinolinic acid (QUIN) was measured by gas chromatography-electron capture ion mass spectrometry as described previously.11
IDO activity
The IDO activity was determined in the cell homogenate by a slight modification of the method of Hara et al.12 Briefly, 100 μL of 30% trichloroacetic acid (TCA) was added to 200 μL of the culture supernatant, vortexed, and then spun at 10 000 rpm for 5 minutes. About 125 μL of the supernatant was added to 125 μL of Ehrlich reagent (100 mg/5 mL glacial acetic acid) in a microtitre plate well (96-well format). Samples were read against a reagent blank with a 492-nm filter in a Multiskan MS (Labsystems, Vantaa, Finland) microplate reader. The change in kynurenine concentration was obtained by subtracting the kynurenine content of fresh uncultured media from the value derived from the cultured supernatant.
The kynurenine concentration of samples containing 3ABA, which produces spectral interference with the Takikawa method (unpublished observations), was obtained using high-performance liquid chromatography (HPLC). Briefly, samples for HPLC analysis were prepared by precipitating the protein in 200 μL of sample with 100 μL of 30% TCA. The sample was then centrifuged at 15 000 rpm for 5 minutes. About 50 μL of the resultant supernatant was injected onto a C18 reverse-phase column (250 mm × 6 mm). The mobile phase was ammonium acetate buffer (0.1 M, pH 4.65) with 4% acetonitrile. Flow rate was 0.6 mL/min (GBC LC1150 HPLC pump). Sample was detected by UV at 365 nM using a (GBC LC1250 UV/VIS detector).
Experimental procedure
A total of 700 000 freshly elutriated cells were seeded into each well of a 24-well culture plate containing 1 mL of culture media. IFN-γ (500-600 U/mL) and/or the PARP inhibitor 3ABA (≥5 mM) was then added to selected wells for 24 or 48 hours on days 0, 6, and 10 in culture; cells were equilibrated for ~30 minutes at 37°C in 5% CO2 before the addition of 3ABA and/or IFN-γ (Figure 1). At the end of the experimental period, the supernatants were aspirated and stored at −80°C until analysis for QUIN. Intracellular NAD concentration and IDO activity were performed as soon as possible on the sonicated whole-cell homogenate, stored at −80°C immediately following the experimental period. Remaining cell cultures were fed with fresh medium every 3 or 4 days.
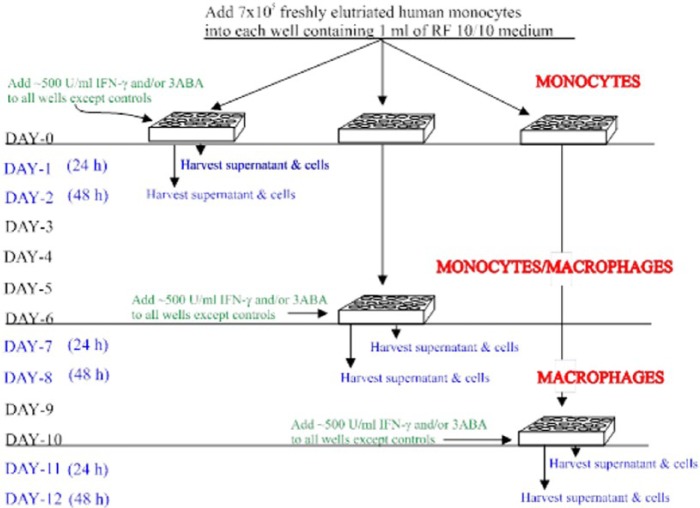
Figure 1.
Experimental procedure. Human peripheral blood monocytes were maintained in culture for up to 12 days. Cells at different stages of cell maturation (differentiation) were treated with IFN-γ and/or 3ABA for 24 or 48 hours before analysis of intracellular NAD and IDO and selected tryptophan metabolites in the supernatant. 3ABA indicates 3-aminobenzamide; IDO, indoleamine 2,3-dioxygenase.
Statistical analysis
Results are expressed as the mean ± SEM. Significant differences between treatment groups was determined using the 2-tailed t test or, where indicated, analysis of variance with the post hoc Tukey-Kramer multiple comparisons test. Differences between treatment groups was considered significant if P < .05.
Results
Effect of IFN-γ concentration and exposure time on IDO activity in monocytes and macrophages
In this study, we investigated the effect of exposing human mononuclear phagocytes at different stages of maturation to increasing concentrations of IFN-γ for 24 and 48 hours.
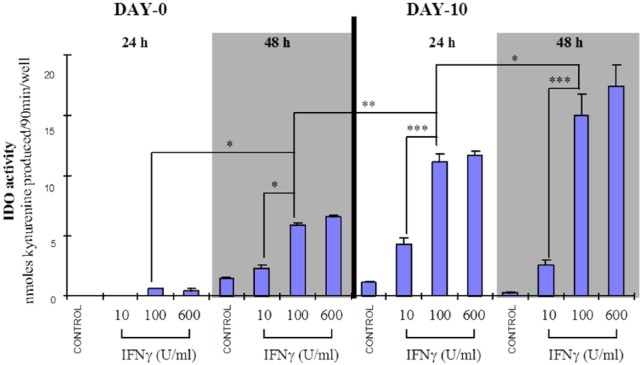
Figure 2 shows that the IFN-γ–mediated increase in IDO activity is dependent on both the concentration of IFN-γ, where a maximum response is reached at 100 U/mL, and the time in culture, which corresponds to the level of cell differentiation.
Figure 2.
Maturation-dependent increase in IDO activity in IFN-γ–activated human monocyte/macrophages from a single donor. Human mononuclear phagocytic cells from a single donor cultured for 0 or 10 days were exposed to IFN-γ at a final concentration of 10, 100, or 600 U/mL for either 24 or 48 hours. IDO activity was determined on the cell homogenate. A dose- (IFN-γ), time-, and maturation-dependent increase in IDO activity was observed. *P < .05; **P < .01; ***P < .001 (n = 4-12). Statistics used was the Tukey-Kramer analysis of variance. IDO indicates indoleamine 2,3-dioxygenase.
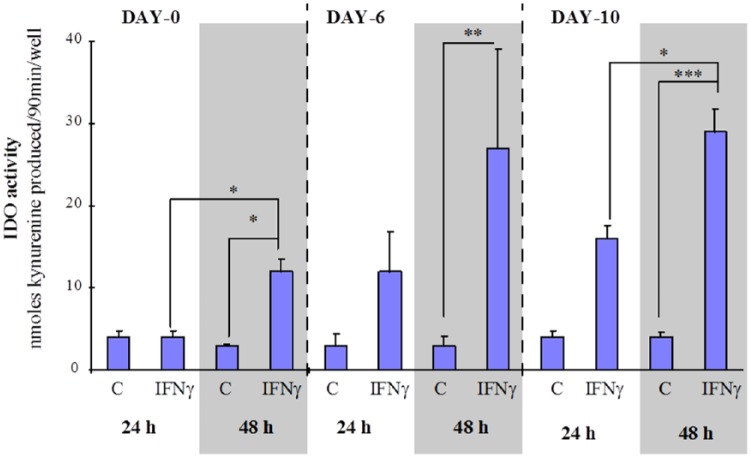
This same pattern of ‘cell differentiation and exposure time’ dependent increase in IDO activity was observed for multiple donors (Figure 3), where the IDO activity from cells by day 6 in culture exposed to IFN-γ (600 U/mL) for 24 or 48 hours was not significantly different from the respective IDO activities measured for IFN-γ–treated (24 or 48 hours) day 10 macrophages (Figure 3).
Figure 3.
Average, maturation-dependent increase in IDO activity in IFN-γ–activated monocyte/macrophages from multiple donors. Human mononuclear phagocytic cells from multiple donors were cultured for 0, 6, or 10 days, then either left untreated (C) or treated with IFN-γ (600 U/mL) for 24 or 48 hours. IDO activity was determined on the cell homogenate. ‘A statistically significant time-dependent (24-48 hours) increase in IDO activity was observed for day 0 and day 10 cultures’. The response to IFN-γ–induced IDO activity was significantly greater in macrophages (day 10) compared with monocytes (day 0). *P < .001; **P < .05; ***P < .001 compared with IFN-γ day 0, 48 hours (n = 7-24) separate cultures from 4 different donors. Statistics used was the Tukey-Kramer analysis of variance. IDO indicates indoleamine 2,3-dioxygenase.
One incongruent result for day 0 monocytes (48-hour IFN-γ treatment) from one donor was not included in Figure 3. These monocytes produced a very high IDO activity (57 ± 2 nmol kynurenine/90 min/well, n = 11) compared with the mean activity of other donors cells (12 ± 2 nmol kynurenine/90 min/well, n = 15), whereas IFN-γ activation of day 10 macrophages from this same donor produced an IDO activity within the normal range (~30 nmol kynurenine/90 min/well) (see Figure 2).
Effect of cell maturation on intracellular NAD concentration
The intracellular NAD concentration of untreated cells increased significantly as cells matured (ie, differentiated) from monocytes at day 0 to macrophages by day 10 in culture (Figure 4).
Figure 4.
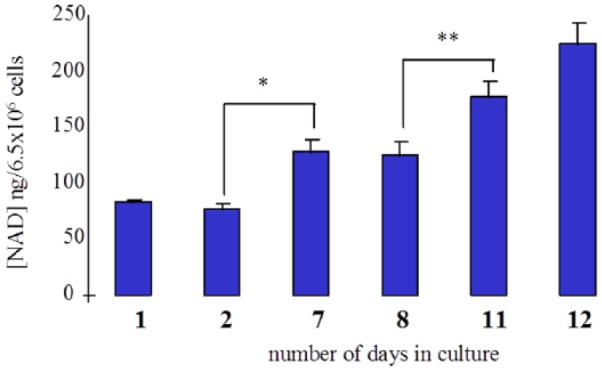
Intracellular NAD concentrations increase in unstimulated human monocyte/macrophages with the degree of differentiation. Human monocyte/macrophages from a single donor were cultured from 1 to 12 days. Intracellular NAD concentration was determined on the cell homogenate. Cellular NAD concentration increased significantly with time corresponding with the stage of cell differentiation from monocyte to macrophage. *P < .01; **P < .05 (n = 3-8).
Effect of IFN-γ in the absence and presence of a PARP inhibitor on monocytes and monocyte-derived macrophages
After 0, 6, or 10 days in culture, human recombinant IFN-γ (600 U/mL) ± the PARP inhibitor (3ABA) was added to selected wells for either 24 or 48 hours. Cells were then sonicated and assayed for NAD concentration in the cell homogenate. Exposure to IFN-γ for 24 hours decreased intracellular NAD in cultures at all stages of differentiation (days 0-6, Figure 5A to C). Coadministration of IFN-γ with 3ABA for 24 hours resulted in a significant increase in cellular NAD levels above treatment with 3ABA alone in both day 6 and day 10 cultures (Figure 5B and C).
Figure 5.
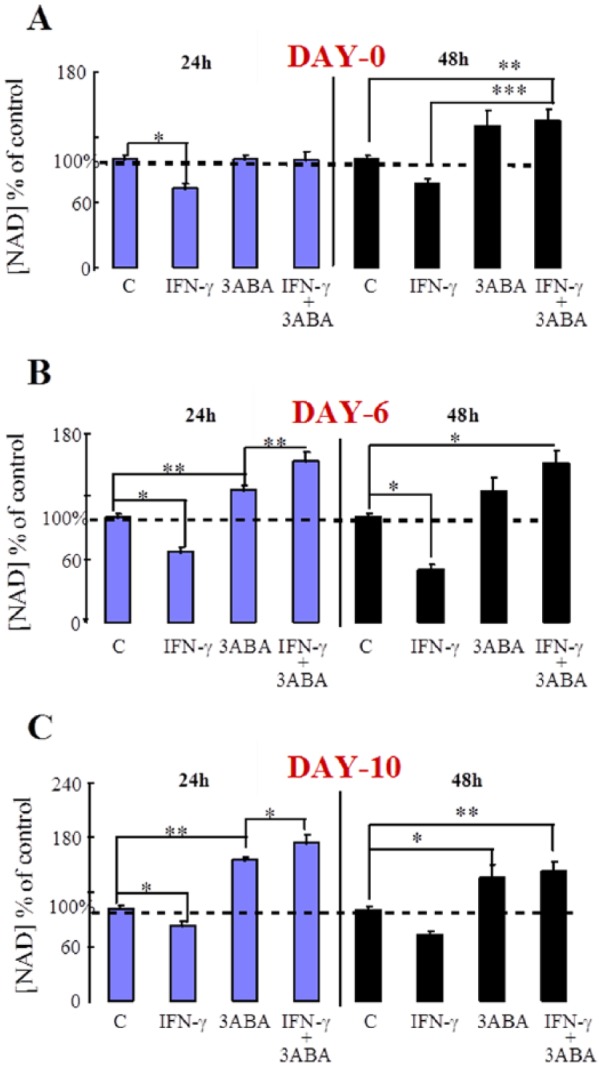
Effect of IFN-γ and 3ABA on intracellular NAD concentration in monocytes (A): 6-day-old MDM and 10-day-old MDM. Human MDMs from 4 different donors were cultured for the times indicated and then either left untreated (C) or treated with IFN-γ (600 U/mL) and/or 3ABA (≥5 mM) for 24 or 48 hours. NAD concentration was determined on the cell homogenate. (A) *P < .01; **P < .05; ***P < .001 (n = 3-23). (B) *P < .001; **P < .05 (n = 3-10). (C) *P < .05; **P < .01 (n = 8-19). Statistics used was the Tukey-Kramer analysis of variance. 3ABA indicates 3-aminobenzamide; MDMs, monocyte-derived macrophages.
Effect of IFN-γ on QUIN production in monocytes and macrophages
Supernatant from selected cultures was analysed for QUIN, the final KP intermediate before phosphoribosylation.
Quinolinic acid concentrations were significantly increased in the culture supernatant after exposure to IFN-γ for 48 hours in both monocytes and macrophages. Quinolinic acid production from macrophages was significantly greater than for undifferentiated monocytes (Figure 6).
Figure 6.
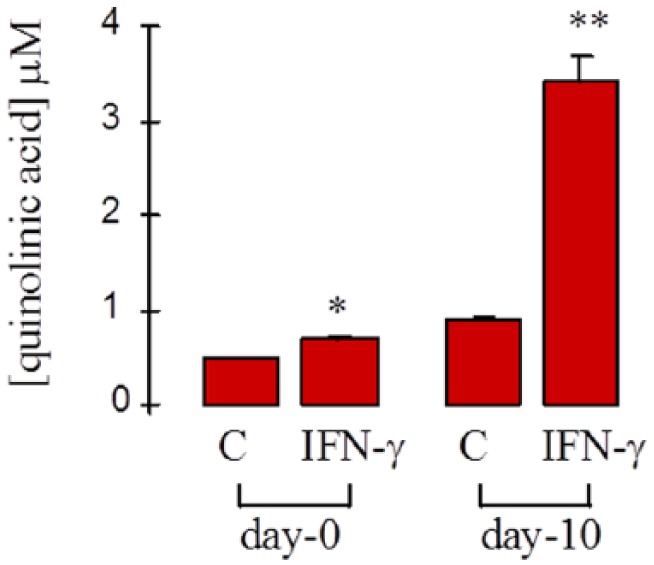
Effect of IFN-γ on quinolinic acid production in human monocyte/macrophages. Human monocyte/macrophage cells from 3 different donors were cultured for either 0 or 10 days and then either left untreated (C) or treated with IFN-γ (600 U/mL) for 48 hours. Quinolinic acid concentration was determined in the culture supernatant. *P < .0001 compared with (C) day 0; **P < .0001 compared with (C) day 10; ***P < .0001 compared with IFN-γ day 0 (n = 4-7).
Effect of 3ABA on IDO activity and supernatant kynurenine and QUIN concentration in monocytes and macrophages
The PARP inhibitor 3ABA was used throughout this study as an effective inhibitor of the NAD catabolic enzyme PARP. We sought to determine whether 3ABA had any non-specific effects on the activity of the KP in either IFN-γ–activated or untreated control day 10 macrophages. Figure 7 shows that both IDO activity and supernatant kynurenine concentration were marginally reduced in IFN-γ–activated macrophages treated with 3ABA. Quinolinic acid levels in the supernatant were reduced following 3ABA treatment in unactivated macrophages. Although the absolute value for QUIN concentration was lower in activated macrophage cultures in the presence of 3ABA, this did not reach statistical significance (Figure 7).
Figure 7.
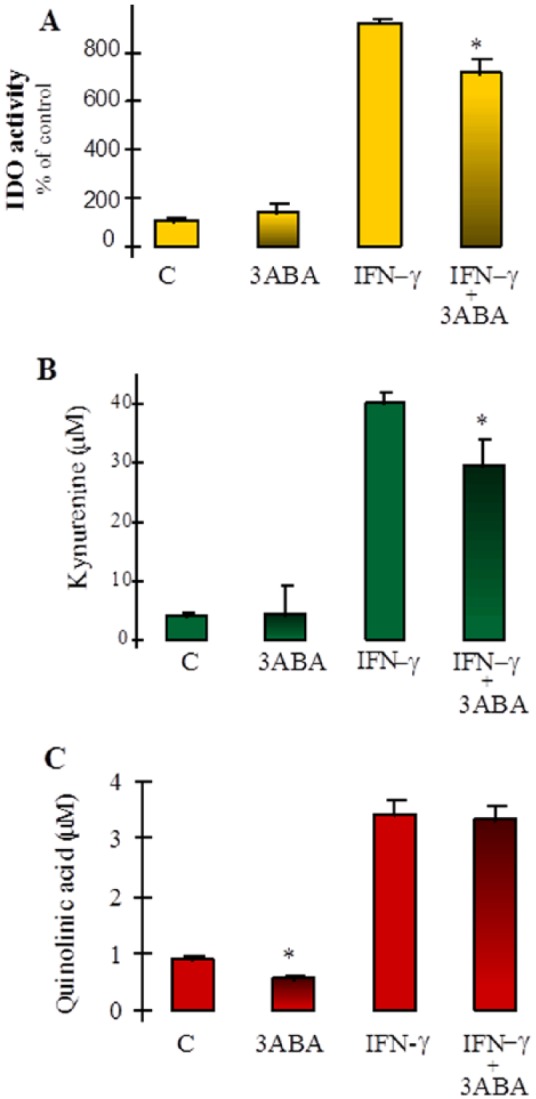
The effect of 3ABA on kynurenine pathway metabolism: 10-day-old macrophages were either left untreated or treated with 3ABA or IFN-γ or 3ABA + IFN-γ. After 48 hours, samples were taken and analysed for (A) IDO activity, determined from the cell homogenate. Results are presented as a percentage of untreated control cultures (C), *P < .005 (n = 5-6) compared with IFN-γ. (B) Kynurenine concentration in the culture supernatant, *P < .05 (n = 5-6) compared with IFN-γ. (C) Quinolinic acid concentration in the culture supernatant, *P < .001 (n = 5-6) compared with control (C). 3ABA indicates 3-aminobenzamide; IDO, indoleamine 2,3-dioxygenase.
Discussion
Various KP metabolites have been detected in the extracellular fluid of immune-activated cells.13,14 However, the relationship between increased oxidative tryptophan catabolism and de novo NAD synthesis has not been investigated.
Although previous studies using a macrophage cell line15 strongly suggest an increase in NAD synthesis and catabolism following IDO induction by IFN-γ, a repeat of these studies was needed in nontransformed primary cells.
The effect of IFN-γ on IDO induction and intracellular NAD concentration was investigated in human monocyte-derived macrophages (MDM) at 3 stages of differentiation:
Undifferentiated (fresh monocytes);
Partially differentiated (after day 6 in culture);
Fully differentiated (day 10 in culture) MDMs.
Monocyte to macrophage differentiation
Mononuclear phagocytes play an important role in host cell–mediated immunity. Undifferentiated monocytes are released continually into the bloodstream from the bone marrow. These cells then migrate to the viscera where local factors initiate the process of differentiation into specialised resident macrophages. During inflammation, the release of chemotactic cytokines increases the migration of monocytes to the site of injury. These infiltrating monocytes along with the resident macrophages are then activated by soluble factors, in particular, IFN-γ.16
In this study, it was found that KP metabolism was increased to a much greater extent in differentiated compared with undifferentiated cells in response to IFN-γ, as determined by IDO activity, kynurenine, QUIN, and NAD assays.
IDO induction by IFN-γ in MDMs
Consistent with previous reports, IFN-γ effectively increased IDO activity in these cultured monocyte/macrophages14 at all stages of differentiation in a dose-dependent fashion (Figures 2 and 3).
In this study, we found that, in agreement with previous investigations,17 exposure to the same concentration of IFN-γ produced a much greater increase in IDO activity in differentiated macrophages compared with undifferentiated monocytes (Figure 2 & 3). This is consistent with an overall greater metabolic activity and response to immune modulators in macrophages compared with monocytes.
Effect of IFN-γ activation at different stages of cell differentiation on QUIN production
Consistent with the pattern shown for IDO activity (Figures 2 and 3), QUIN production was much greater in IFN-γ–activated macrophages compared with monocytes (Figure 6).
This demonstrates an increased flux through this pathway at least as far as QUIN under these conditions.
Quinolinic acid concentration in the extracellular fluid, however, was approximately an order of magnitude lower than that for kynurenine (Figure 7B and C), indicating that only a relatively small percentage of degraded tryptophan is secreted as QUIN under these conditions. The reason for this is not clear. Kynurenine can be converted to at least 2 other metabolites besides 3-hydroxykynurenine, kynurenic acid, and anthranilic acid. Although MDMs do not appear to secrete kynurenic acid15 unless stimulated by TLR-3,18 the kynurenine excreted by these cells may be taken up by other cells within the vicinity of the inflammation site and used for either de novo production of NAD or synthesis of either/or both of these 2 alternative compounds.
Interestingly, the mild inhibitory effect of 3ABA on IDO did not significantly affect the level of QUIN secretion indicating that kynurenine production at maximum activation may be saturating downstream enzymes with substrate.
A more extensive investigation using coculture techniques is needed to provide further insight into the fate of kynurenine under these conditions.
Effect of IFN-γ activation, in the presence or absence of 3ABA, at different stages of cell differentiation on intracellular NAD concentration
The intracellular NAD concentration in unactivated cells increased with the amount of time spent in culture. This corresponded to the level of cell differentiation from monocyte (lowest NAD content) to macrophage (highest NAD content) (Figure 4).
This is consistent with the increase in morphological size, and we assume metabolic requirements, of the cell in direct relation to the degree of differentiation.
Following 24-hour treatment with 3ABA (PARP inhibition), intracellular NAD increased in unactivated cells at days 6 and 10 but not day 0 (Figure 5A to C), suggesting that NAD catabolism due to PARP activity increases as a consequence of in vitro cell differentiation.
It has been known for some time that activation of human MDM with IFN-γ also increases PARP activity.19 Following IFN-γ activation, intracellular NAD concentration decreased significantly in cells at all 3 stages of differentiation (Figure 5A to C), indicating that the catabolism of NAD in these activated cells was greater than its synthesis.
NAD levels, however, in these IFN-γ–activated cells in the presence of the PARP inhibitor, 3ABA, was either retained (Figure 5A) or significantly increased (Figure 5B and C). Increased de novo synthesis of NAD in undifferentiated cells (day 0) is not expected as induction of IDO by IFN-γ in monocytes is negligible at 24 hours (Figure 2). The significant increase in NAD levels after activation and PARP inhibition on days 6 and 10 (Figure 5B and C), however, appears to reflect the corresponding significant increase in IDO activity (Figure 3).
Although 24-hour exposure to IFN-γ in the presence of a PARP inhibitor resulted in a significant increase in cellular NAD coinciding with increased IDO activity, 48-hour exposure to IFN-γ and 3ABA did not reproduce this result (Figure 5A and C). The reasons for this are not clear. Figure 7 shows that treatment with 3ABA for 48 hours reduces both IDO activity and supernatant kynurenine concentration in IFN-γ–activated cells, whereas QUIN levels were marginally reduced in 3ABA-treated unactivated cells only. This suggests that exposure to 3ABA for longer periods may reduce flux through the KP to some degree. However, as the production of the immediate NAD precursor QUIN was not significantly reduced, NAD synthesis may not be affected. It is possible, though, that longer term activation of these cells (ie, 48 hours) may result in either an accumulation of cytotoxic products in the static culture medium or a decrease in essential metabolic cofactors, both of which may affect enzyme involved in the synthesis of NAD.
Conversion of QUIN into NAD requires cofactors, such as phosphoribosyl pyrophosphate, adenosine triphosphate, and glutamine, any of which may become depleted over time and thereby rate limiting in the synthesis of NAD. Further studies are required to test this hypothesis. In addition, it has been suggested by others that expression of the NAD synthetic enzyme quinolinate phosphoribosyl transferase in macrophages may be dependent on specific stimulatory signals20 which may be disrupted in the presence of 3ABA.
Conclusions
In conclusion, this study using human MDMs has provided evidence which indicates that an immune-mediated increase in IDO activity does increase NAD biosynthesis concomitantly with an increase in NAD catabolism. This phenomenon was clearly observed in differentiated cells after 24-hour exposure to maximal activating doses of IFN-γ but was less obvious after 48 hours.
These results are consistent with data from a previous study using the RAW264.7 monocyte/macrophage cell line.15 However, the process of NAD synthesis in the human primary cells following IDO induction by IFN-γ appears to be affected by the degree of cell differentiation at the time of immune activation.
These results do support a role for IDO induction in increased NAD synthesis which may provide substrate for continuing PARP activity facilitating nuclear repair and hence long-term cell (ie, monocyte/macrophage) viability. As inhibitors for IDO have been developed for potential clinical use in inflammatory-associated conditions,21 recognition of the role of IDO induction and its role in maintaining NAD+ levels in front-line immune cells may be relevant to developing final effective treatment regimens.
Acknowledgments
The author would like to acknowledge the kind assistance of Dr G Smythe for his assistance with the GC-MS assays, Dr H Naif for his advice on monocyte isolation and culture, and Dr V Kapoor for reading early drafts of this manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was part funded by grants from the National Health and Medical Research Council of Australia.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: RSG conceived and designed the experiments, analysed the data, wrote the first draft of the manuscript, and reviewed and approved the final manuscript.
Disclosures and Ethics: As a requirement of publication, author has provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality, and (where applicable) protection of human and animal research subjects. The author has read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The author has also confirmed that this article is unique and not under consideration or published in any other publication and that has permission from rights holders to reproduce any copyrighted material. The external blind peer reviewers report no conflicts of interest.
References
- 1. Papadimitriou JM, Ashman RB. Macrophages: current views on their differentiation, structure and function. Ultrastruct Pathol. 1989;13:343–372. [DOI] [PubMed] [Google Scholar]
- 2. Martinez F, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. [DOI] [PubMed] [Google Scholar]
- 3. MacKenzie CR, Heseler K, Müller A, Däubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–244. [DOI] [PubMed] [Google Scholar]
- 4. Grant RS, Passey R, Matanovic G, Smythe G, Kapoor V. Evidence for increased de novo synthesis of NAD in immune-activated RAW264.7 macrophages: a self-protective mechanism? Arch Biochem Biophys. 1999;72:1–7. [DOI] [PubMed] [Google Scholar]
- 5. Grant RS, Naif H, Espinosa M, Kapoor V. IDO induction in IFN-gamma activated astroglia: a role in improving cell viability during oxidative stress. Redox Rep. 2000;5:101–104. [DOI] [PubMed] [Google Scholar]
- 6. Wallis W, Beatty P, Ochs H, Harlan J. Human monocyte adherence to cultured vascular endothelium: monoclonal antibody-defined mechanisms. J Immunol. 1985;135:2323–2330. [PubMed] [Google Scholar]
- 7. Naif HM, Li S, Hoshon M, Mathijs JM, Williamson P, Cunningham AL. The state of maturation of monocytes into macrophages determines the effects of IL-4 and IL-13 on HIV replication. J Immunol. 1997;158:501–511. [PubMed] [Google Scholar]
- 8. Melo ES, Barbeiro DF, Gorjão R, et al. Gene expression reprogramming protects macrophage from septic-induced cell death. Mol Immunol. 2010;47:2587–2593. [DOI] [PubMed] [Google Scholar]
- 9. Kazazi F, Mathijs JM, Foley P, Cunningham AL. Variations in CD4 expression by human monocytes and macrophages and their relationships to infection with the human immunodeficiency virus. J General Virol. 1989;70:2661–2672. [DOI] [PubMed] [Google Scholar]
- 10. Grant RS, Kapoor V. Murine glial cells regenerate NAD, after peroxide-induced depletion, using either nicotinic acid, nicotinamide, or quinolinic acid as substrates. J Neurochem. 1998;70:1759–1763. [DOI] [PubMed] [Google Scholar]
- 11. Kerr SJ, Armati PJ, Pemberton LA, Smythe G, Tattam B, Brew BJ. Kynurenine pathway inhibition reduces neurotoxicity of HIV-1-infected macrophages. Neurology. 1997;49:1671–1681. [DOI] [PubMed] [Google Scholar]
- 12. Hara T, Yamakura F, Takikawa O, et al. Diazotization of kynurenine by acidified nitrite secreted from indoleamine 2,3-dioxygenase-expressing myeloid dendritic cells. J Immunol Methods. 2008;332:162–169. [DOI] [PubMed] [Google Scholar]
- 13. Saito K, Seishima M, Noma A, Markey SP, Heyes MP. Cytokine and drug modulation of kynurenine pathway metabolism by blood mononuclear cells. Adv Exper Med Biol. 1996:398:161–165. [DOI] [PubMed] [Google Scholar]
- 14. Werner-Felmeyer G, Werner E, Fuchs D, Hausen A, Reibnegger G, Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in-vitro. Biochimica et Biophysica Acta. 1989;1012:140–147. [DOI] [PubMed] [Google Scholar]
- 15. Grant RS, Passey R, Matanovic G, Smythe G, Kapoor V. Evidence for increased de novo synthesis of NAD in immune-activated RAW264.7 macrophages: a self-protective mechanism? Arch Biochem Biophys. 1999;372:1–7. [DOI] [PubMed] [Google Scholar]
- 16. Nathan CF, Prendergast TJ, Wiebe ME, et al. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984;160:600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carlin JN, Borden EC, Sondel PM, Byrne GI. Interferon-induced indoleamine 2,3-dioxygenase activity in human mononuclear phagocytes. J Leukocyte Biol. 1989;45:29–34. [DOI] [PubMed] [Google Scholar]
- 18. Orhan F, Bhat M, Sandberg K, et al. Tryptophan metabolism along the Kynurenine pathway downstream of toll-like receptor stimulation in peripheral monocytes. Scand J Immunol. 2016;84:262–271. [DOI] [PubMed] [Google Scholar]
- 19. Berton G, Sorio C, Laudanna C, Menegazzi M, De Prati A, Suzuki H. Activation of human monocyte-derived macrophages by interferon g is accompanied by increase of poly(ADP-ribose) polymerase activity. Biochim Biophys Acta. 1991;1091:101–109. [DOI] [PubMed] [Google Scholar]
- 20. Moffett J, Namboodiri M. Tryptophan and the immune response. Immunol Cell Biol. 2003;81:247–265. [DOI] [PubMed] [Google Scholar]
- 21. Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2014;3:e957994. [DOI] [PMC free article] [PubMed] [Google Scholar]