Abstract
Heart failure is highly prevalent with more than 50% of cases being patients with a preserved ejection fraction (HFPEF), a figure that is projected to increase due to the changing risk factor landscape, in particular the ageing population. Overall mortality is similar to patients with heart failure with reduced ejection fraction (HFREF), as are the rates of hospitalisation. Patients with HFPEF have more comorbid conditions with fewer therapeutic options available. In this review, we explore the epidemiology of hospitalisation of HFPEF, the impact of current treatment modalities, and the potential of future therapies.
Keywords: Heart failure, admission, readmission, mortality, treatment
Introduction
Heart failure (HF) is highly prevalent, affecting 2% of the developed world’s population.1 It is the commonest cause of hospitalisation in individuals aged above 65 years and contributes to 1 in 9 deaths.2 Half of all HF occurs in patients with a preserved ejection fraction (HFPEF). The primary risk factors of HFPEF include ageing, obesity, diabetes, and hypertension – all factors that have become more prevalent – and consequently, HFPEF is projected to become the most common cause of hospitalisation in older adults over the coming years. There are numerous pharmacological and non-pharmacological therapies proven to improve survival and reduce HF hospitalisation in patients with heart failure and reduced ejection fraction (HFREF). Conversely, there are no proven therapies that have consistently demonstrated a reduction in morbidity or mortality for patients with HFPEF, highlighting a group with significant unmet clinical need.3 Heart failure readmission is common in HFPEF, similar to HFREF,4 due to a combination of disease complexity, limited therapeutic options, and a narrower therapeutic window in the elderly patient. In this review, we discuss the epidemiology and impact of readmission in HFPEF with a view to therapy and future research.
Pathophysiology
Part of the failing of therapeutic options in HFPEF occurs as a result of a complex interaction between multiple pathophysiologic entities, including myocardial stiffness, impaired calcium handling and nitric oxide-cyclic guanosine monophosphate pathway, endothelial dysfunction, microvascular rarefaction, large artery stiffening with altered wave reflection,5 and abnormalities in peripheral skeletal muscle oxygen delivery and metabolism.6 Beyond the pleiotropic effects of ageing on the cardiovascular system,7 obesity induces inflammatory changes and hypertension and impairs diastolic function centrally and further impairs skeletal muscle and physical function. Furthermore, increased paracardiac fat deposition is associated with myocardial dysfunction and the development of atrial fibrillation.8
Establishing the Diagnosis
Heart failure with preserved EF is a heterogeneous disorder with a definition that has evolved over the past decade as the pathophysiology has become increasingly well understood. The variability in previous epidemiological data regarding readmission in HFPEF must be viewed in the context of this changing definition. Although the clinical diagnosis of HF has been well defined based on previous criteria,9 many of these were developed based on HFREF, and signs such as a third heart sound are less relevant in patients with HFPEF. The current descriptions are consequently deliberately broad. The European Society of Cardiology (ESC) guidelines10 have defined it as ‘as a syndrome in which patients have typical symptoms (eg, breathlessness, ankle swelling, and fatigue) and signs (eg, elevated jugular venous pressure, pulmonary crackles, and displaced apex beat) resulting from an abnormality of cardiac structure or function’. The American Heart Association (AHA)/American College of Cardiology (ACC) guidelines give a similar broad definition: ‘Heart failure is a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood’.11
Clinical features alone are not sufficient to secure a diagnosis of HF as they are often non-specific, particularly in patients with HFPEF with several comorbid conditions, and may be attributed to other disease processes, eg, severe lung disease (breathlessness, pulmonary crackles) or renal failure (ankle swelling, fatigue, elevated jugular venous pressure). Unlike HFREF, where imaging has been focused on the evaluation of systolic function, the diagnosis of HFPEF is more difficult as abnormal diastology is less well understood.
Though broadly similar, the international guidelines for the diagnosis of HFPEF have developed over time (Table 1). They all mandate symptoms and signs of HF and a preserved EF (≥50%); importantly, many previous trials included patients with an EF greater than 40%; however, more recent guidelines define an EF between 40% and 50% as ‘mid-range’, in an attempt to drive research into patients with mild dysfunction. Key shared features of HFPEF include evidence of elevated filling pressure as suggested by E/e′ or dilated left atrium, or structural abnormality with an increased left ventricular mass index. The guidelines emphasise the exclusion of patients with non-cardiac causes of symptoms (eg, obesity, lung disease, anaemia). In practice, non-cardiac causes of symptoms often co-exist in patients with HFPEF and may even be a driving factor of the HF process, which adds to the difficulty in making a diagnosis.14 It is important to emphasise that uncorrected primary cardiac valve disease, isolated right ventricular failure, pericardial disease, and rarer diagnosed cardiomyopathies (infiltrative, inflammatory, viral, hereditary) should not be classified as HFPEF, although they are associated with HF symptoms in the presence of a ‘normal’ EF.
Table 1.
Three current international guidelines for the diagnosis of heart failure with preserved EF.
| ESC: Consensus for heart failure with normal EF12 | ESC: Guidelines for heart failure10 | ACC/AHA: Guidelines for heart failure13 | |
|---|---|---|---|
| Published | 2007 | 2016 | 2013 |
| Clinical | Signs and symptoms | Signs and symptoms | Signs and symptoms |
| LV function | Normal or mildly reduced systolic function or EF >50% | EF ≥50%; EF 40%-49% classified as heart failure with mid-range ejection fraction (HFmrEF) | Normal or preserved systolic function or EF ≥50%; EF 41%-49% classified as borderline HFPEF |
| LV size | Non-dilated: LVEDVI <97 mL/m2 or LVESVI <49 mL/m2 |
No requirement of LV to be dilated | No requirement of LV to be dilated |
| Natriuretic peptide | See below | BNP >35 pg/mL NT-proBNP >125 pg/mL |
No requirement of an elevated NP level |
| Diastolic dysfunction | CWP >12 mm Hg or LVEDP >16 mm Hg or E/e′>15 or E/e′ >8 and <15 with: BNP >200 pg/mL AF LAVI >40 mL/m2 Increased LVMI, or Abnormal diastology |
Dilated left atrium (LAVI >34 mL/m2) and/or left ventricular hypertrophy (LVMI >95 g/m2 F, >115 g/m2 M) and/or abnormal diastology (eg, mean E/e′ >13) | Structural remodelling (not specified) No requirement of abnormal diastology |
Abbreviations: AF, atrial fibrillation; BNP, B-type natriuretic peptide; CWF, coronary wedge pressure; EF, ejection fraction; LV, left ventricle; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LAVI, left atrial volume index; LVMI, left ventricular mass index; NP, natriuretic peptide; NT-pro BNP, N-terminal prohormone of brain natriuretic peptide.
Importantly, there are several limitations to the echocardiographic diagnosis of HFPEF. E/e′, a non-physiologic construct designed to estimate filling pressure in those with systolic dysfunction, demonstrates a modest correlation with left ventricular end-diastolic pressure at rest15; the relationship is stronger in those with reduced rather than preserved EF. Importantly, many patients with HFPEF have normal filling pressures at rest (measured either invasively or non-invasively).16,17 As such, exercise right heart catheterisation is a valuable tool in the evaluation of patients with dyspnoea to determine a cardiac or non-cardiac origin,18 confirming the diagnosis with a measure of pulmonary capillary wedge pressure and also providing important physiologic information on cardiac output, oxygen extraction, and lactate production.
Natriuretic peptides (NPs) are also commonly used for the diagnosis of HF, primarily based on data in patients with HFREF. Natriuretic peptides are increased in the setting of increased wall stress, and in patients with HFPEF, this may only occur with exertion as filling pressure at rest can be normal. Up to 30% of patients with HFPEF have a normal B-type natriuretic peptide (BNP) level at rest19; current ESC guidelines recommend that the diagnosis of HFPEF be made if patients have NP levels outside the normal range; however, there remain discrepancies between the guidelines in this regard. None of these guideline-endorsed diagnostic criteria have been validated prospectively for their diagnostic utility on an unselected population.20 This may explain the varying definitions (and inclusion criteria)3 used in observational and clinical studies.21,22
Risk Factors for Pathogenesis and Hospitalisation
The changing landscape of cardiovascular risk factors has led to an increased prevalence of HFPEF in the context of ageing and greater co-morbidity. Age, female sex, hypertension, and obesity are all associated with HFPEF, and these factors have been implicated mechanistically as causative factors.23 Heart failure with preserved EF is frequently associated with comorbidities, including hypertension (60%-80%), ischaemic heart disease (35%-70%), diabetes (20%-45%), and atrial fibrillation (15%-40%). There are ethnic differences in readmission rates for HFPEF, with higher rates of readmission among black patients, despite lower mortality rates – the so-called mortality readmission paradox.24
Arora et al25 analysed 30-day readmissions in HFPEF in the United States using the National Readmission Database, a large inpatient care database including around half of all US hospitalisation. Heart failure with preserved EF was defined as patients given a primary diagnosis code of ‘Diastolic Heart Failure’; importantly, echocardiographic and NP data were not available. In this cohort of 192,394 patients, 21% were readmitted within 30 days, with half of those occurring within 14 days and 28% (the largest group) due to acute HF. Age and comorbidities were the most significant predictors of readmission, in particular renal failure (odds ratio [OR], 1.21; confidence interval [CI], 1.17-1.25, P < .001) and chronic pulmonary disease (OR, 1.18; CI, 1.15-1.21, P < .001). Other studies have also demonstrated predictors, including a previous history of hospitalisation for HF, impaired functional status (New York Heart Association [NYHA] classes III-IV), and elevated NPs.26,27 Left atrial function has been implicated in the prediction of HF hospitalisation,28 as have QRS width and morphology.29,30
Treatment
Clinical trials in HFPEF have often used composite end points, including readmission by analysing the time to the first event; however, little emphasis is placed on readmissions, and in particular the cause of readmission. Furthermore, the definition of readmission is varied; HF hospitalisations can be defined according to the therapy provided, such as intravenous diuretics, or at clinician’s discretion if HF is considered a ‘major’ component of admission. Patients with HFPEF have a similar incidence rate of hospitalisation to those with HFREF; however, they incur significantly more annualised outpatient visits.31 Mortality rates of patients with HFPEF in community studies and registries are greater than those of patients with HFPEF in clinical trials. This is a common observation in HF trials, perhaps explained by the fact that registries often find patients based on previous admissions to hospital, whereas trial patients are often stable patients who are sought via specific screening programmes to enhance recruitment.32,33
Regardless of the type of HF, treatment of elevated filling pressures to reduce fluid accumulation has been demonstrated to prevent HF decompensation and is recommended in the guidelines.10 The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial studied the role of an implantable haemodynamic monitor to measure pulmonary artery pressure in patients with NYHA class III symptoms and recent HF hospitalisation. Patients were randomised to care, either standard care or treatment, based on investigator knowledge of pulmonary pressure. In the cohort of patients with an EF ≥50% (n = 66, mean EF = 50.6%), pulmonary artery pressure–guided therapy was associated with a reduced rate of HF hospitalisation than those who did not (0.41 events/patient-year vs 1.39 events/patient-year; P < .0001). Device-guided therapy was associated with more changes to medical therapy, particularly diuretics.
Several studies have investigated the role of renin-angiotensin blockade in HFPEF, in view of the marked positive findings in patients with HFREF. The Perindopril in Elderly People with Chronic Heart Failure (PEP-CHF) trial randomised patients to either perindopril or placebo, and although the overall trial was negative, there was a reduction in hospitalisation for HF at 12 months (hazard ratio [HR], 0.63; CI, 0.41-0.97, P = .03); however, these findings were not sustained at 3-year follow-up.34 The CHARM-Preserved trial demonstrated a mild reduction in admission rates in patients treated with candesartan; importantly, patients with an EF as low as 40% were included, and consequently, results are not specific to the HFPEF population. The irbesartan in patients with heart failure and preserved systolic function (I-PRESERVE) trial enrolled 4128 patients with symptoms of HF and an EF greater than 45% to either irbesartan or placebo.35 Patients were included if they had been hospitalised for HF within the previous 6 months and at minimum reported NYHA class II symptoms.
Carson et al32 analysed rehospitalisation in detail in the I-PRESERVE cohort. A total of 5863 hospitalisations occurred in 2278 patients during a mean follow-up of 49.5 months. Of those undergoing first hospitalisation, the majority were cardiovascular (54%), and HF hospitalisation made up the largest proportion (18%). For those with an initial HF admission, worsening HF was the reason for hospitalisation in a greater proportion (43%). Patients who were rehospitalised were older, more likely to have ischaemic heart disease, more commonly diabetic, had higher resting heart rates, and had lower EFs and lower estimated glomerular filtration rates. Hospitalisation was associated with a significant mortality risk. Overall, in hospitalised patients, the unadjusted HR for all-cause mortality was 8.53 (CI, 7.06-10.31) compared with patients who were never hospitalised. In those hospitalised for HF, the HR was 4.86 (CI, 4.19-5.76) following adjustment for baseline predictors of mortality.
Spironolactone, an aldosterone antagonist, received significant attention following the publication of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial, randomising 3445 patients from 6 countries.36 Patients were included based on 2 strata, either hospitalisation for HF or an elevated BNP. Overall, the trial was neutral for a composite primary end point; however, significant regional heterogeneity was noted and patients from the Americas subgroup treated with spironolactone did have fewer hospitalisations in post hoc analyses.37 Patients with lower NPs at enrolment appeared to benefit more from spironolactone treatment, perhaps suggestive that earlier therapy can slow progression and prevent worsening of HF.
Overall, the 6 phase 3 clinical trials in HFPEF all failed to meet their primary end point (Figure 1); however, it must be noted that the inclusion criteria for these trials varied substantially. While some studies have shown evidence of a reduction in HF hospitalisation, these were secondary end points and in the absence of a significant primary end point can only be considered as hypothesis generating. As such, both angiotensin II receptor blockers and aldosterone receptor antagonists have a class IIb indication to reduce the risk of HF hospitalisation.11
Figure 1.
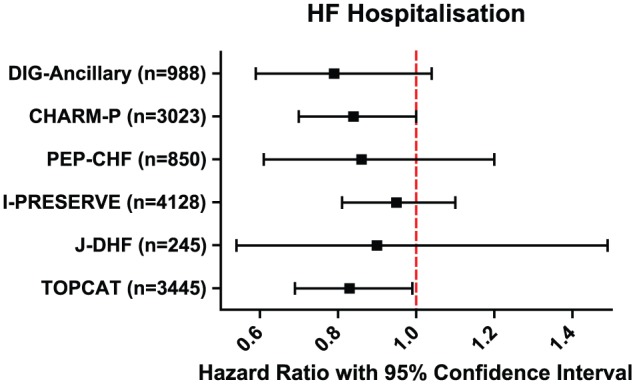
Heart failure (HF) hospitalisation across 6 major phase 3 trials of pharmacologic therapy in heart failure with preserved ejection fraction (HFPEF). I-PRESERVE indicates irbesartan in patients with heart failure and preserved systolic function; PEP-CHF indicates Perindopril in Elderly People with Chronic Heart Failure; TOPCAT, Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist; CHARM-P, Candesartan in heart failure - assessment of moRtality and Morbidity - preserved; DIG-Ancillary, digitalis investigation group trial - ancillary study; J-DHF, Japanese diastolic heart failure study.
Exercise training and rehabilitation
Exercise training is one of the few interventions in HFPEF that has shown statistically significant positive results in regard to functional capacity.38 These improvements are likely predominantly driven by peripheral mechanisms, such as improved endothelial function, oxygen extraction, and skeletal muscle function, given that no consistent effect of exercise training on systolic or diastolic function has been shown. Targeting impaired mobility and strength with exercise training as early as possible to prevent progressive frailty is important; however, data are lacking in regard to hospitalisation. A post hoc analysis of the TOPCAT trial demonstrated that patients with lower levels of activity had higher rates of adverse outcomes, including HF hospitalisation,39 and further trials are in progress to definitely answer the role of exercise training (Rehabilitation Therapy in Older Acute Heart Failure Patients [REHAB-HF]; NCT02196038).
Phenomapping
Considering the marked phenotypic heterogeneity of HFPEF, primarily contributed to by varying causes and pathophysiology, it is somewhat unsurprising that previous trials of pharmacological therapy in unselected patients with HFPEF have been negative. To discover pathophysiologically homogeneous subgroups, Shah et al40 used novel machine learning techniques, specifically unsupervised learning, to identify 3 clusters of patients based on specific phenotypic domains. Each ‘phenogroup’ demonstrated markedly different rates of hospitalisation, and studies are underway to target therapy based on this characterization.41
Specialised Clinics
In view of the complexity in the phenotype of HFPEF, specialised HFPEF clinics have been proposed to standardise the approach to diagnosis and treatment, comprehensively characterise the sub-phenotype, and offer targeted therapy based on developing evidence and physiologic mechanism for symptom limitation.42 Such standardised workup protocols can include specific lab testing, cardiac evaluation with a pre-specified focused echocardiographic protocol, alternate imaging such as cardiac magnetic resonance imaging, cardiopulmonary exercise testing, and pulmonary function testing in a detailed evaluation. Multi-disciplinary teams, including medical, nursing, and allied health staff, can target non-cardiac comorbidities as an important part of this approach, particularly with regard to objectifying frailty with scoring systems accounting for both physical and cognitive ability to more appropriately target impairments of function, as seen in the geriatric literature.43 Specialised clinically based programmes with a shared research component permit the dedicated systematic screening of hospital records to identify patients with HFPEF and then can standardise the approach to treatment in regard to lifestyle factors (diet, exercise) and pharmacologic therapy (based on phenotype and comorbid conditions) and offer centralised access to the many active research trials.
Conclusions
Heart failure with preserved EF is a common disorder with complex pathophysiology, a heterogeneous phenotype, with a profound effect on mortality and morbidity, driven predominantly by hospitalisation. Varying patient populations have been included in previous trials of pharmacologic therapy in HFPEF, contributing to neutral results. Hospitalisation occurs in more than 50% of patients with HFPEF and is a profound contributor to mortality. Around 20% of patients are admitted for HF; non-cardiovascular causes for readmission are common. Clinical trial design and registries need to include HF admission and readmission as a key metric of outcome in patients with HFPEF with specific details as to the cause and consequence. Importantly, interventions for HFPEF may demonstrate greatest benefit in patients who have had a previous hospitalisation, in view of the higher event rates (including mortality) in this population.
Hospitalisation highlights a high-risk population, and enrolling such patients in clinical trials and/or involving them in specialised HFPEF clinics provide intensive standardised follow-up with a multidisciplinary team in the early post-discharge time period for both cardiovascular and non-cardiovascular purposes. Review should occur within the first 30 days after hospitalisation as patients are at highest risk during this period. Careful attention must be provided to the management of cardiac and non-cardiac comorbidities with a particular focus on cognitive and physical function. Further research is required into the identification of high-risk patients with HFPEF to prevent hospitalisation.
Footnotes
Special Collection:Prevention of re-hospitalization in congestive heart failure
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: The paper was drafted by SN and HP, with critical appraisal from DMK. All three authors take responsibility for the intellectual content.
References
- 1. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Go AS, Mozaffarian D, Roger VL, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186–2198. doi: 10.1016/j.clinthera.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 5. Reddy YNV, Andersen MJ, Obokata M, et al. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017;70:136–148. doi: 10.1016/j.jacc.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868–1877. doi: 10.1056/NEJMcp1511175. [DOI] [PubMed] [Google Scholar]
- 7. Nanayakkara S, Marwick TH, Kaye DM. The ageing heart: the systemic and coronary circulation [published online ahead of print November 1, 2017]. Heart. doi: 10.1136/heartjnl-2017-312114. [DOI] [PubMed] [Google Scholar]
- 8. Wong CX, Ganesan AN, Selvanayagam JB. Epicardial fat and atrial fibrillation: current evidence, potential mechanisms, clinical implications, and future directions. Eur Heart J. 2016;38:1294–1302. doi: 10.1093/eurheartj/ehw045. [DOI] [PubMed] [Google Scholar]
- 9. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 10. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 11. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 12. Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 13. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA Guideline for the management of heart failure. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 14. Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 15. Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic stress echocardiography: hemodynamic validation and clinical significance of estimation of ventricular filling pressure with exercise. J Am Coll Cardiol. 2006;47:1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 16. Maeder MT, Kaye DM. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53:905–918. doi: 10.1016/j.jacc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 17. Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Hear Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sorajja P, Borlaug BA, Dimas VV, et al. SCAI/HFSA clinical expert consensus document on the use of invasive hemodynamics for the diagnosis and management of cardiovascular disease. Catheter Cardiovasc Interv. 2017;89:E233–E247. doi: 10.1002/ccd.26888. [DOI] [PubMed] [Google Scholar]
- 19. Anjan VY, Loftus TM, Burke MA, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110:870–876. doi: 10.1016/j.amjcard.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J. 2014;35:1022–1032. doi: 10.1093/eurheartj/ehu067. [DOI] [PubMed] [Google Scholar]
- 21. Solomon SD, Rizkala AR, Gong J, et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction - rationale and design of the PARAGON-HF trial. JACC Hear Fail. 2017;5:471–482. doi: 10.1016/j.jchf.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 22. Pieske B, Maggioni AP, Lam CSP, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38:1119–1127. doi: 10.1093/eurheartj/ehw593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Heerebeek L, Paulus WJ. Impact of comorbidities on myocardial remodeling and dysfunction in heart failure with preserved ejection fraction. SOJ Pharm Pharm Sci. 2014;2014:1–20. [Google Scholar]
- 24. Ziaeian B, Heidenreich PA, Xu H, et al. Race/ethnic differences in outcomes among hospitalized Medicare patients with heart failure and preserved ejection fraction. JACC Hear Fail. 2017;5:483–493. http://www.heartfailure.onlinejacc.org/content/early/2017/05/02/j.jchf.2017.02.012?_ga=2.220851162.314566952.1499985878-925717646.1498776923. Accessed July 14, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arora S, Lahewala S, Hassan Virk HU, et al. Etiologies trends: predictors of 30-day readmissions in patients with diastolic heart failure. Am J Cardiol. 2017;120:616–624. doi: 10.1016/j.amjcard.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 26. Dalos D, Mascherbauer J, Zotter-Tufaro C, et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:189–199. doi: 10.1016/j.jacc.2016.04.052. [DOI] [PubMed] [Google Scholar]
- 27. Kristensen SL, Jhund PS, Køber L, et al. Relative importance of history of heart failure hospitalization and N-terminal Pro–B-type natriuretic peptide level as predictors of outcomes in patients with heart failure and preserved ejection fraction. JACC Hear Fail. 2015;3:478–486. doi: 10.1016/j.jchf.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 28. Welles CC, Ku IA, Kwan DM, Whooley MA, Schiller NB, Turakhia MP. Left atrial function predicts heart failure hospitalization in subjects with preserved ejection fraction and coronary heart disease: longitudinal data from the heart and soul study. J Am Coll Cardiol. 2012;59:673–680. doi: 10.1016/j.jacc.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cannon JA, Shen L, Jhund PS, et al. Clinical outcomes according to QRS duration and morphology in the irbesartan in patients with heart failure and preserved systolic function (I-PRESERVE) trial. Eur J Heart Fail. 2016;18:1021–1031. doi: 10.1002/ejhf.547. [DOI] [PubMed] [Google Scholar]
- 30. Joseph J, Claggett BC, Anand IS, et al. QRS duration is a predictor of adverse outcomes in heart failure with preserved ejection fraction. JACC Heart Fail. 2016;4:477–486. doi: 10.1016/j.jchf.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 31. Nichols GA, Reynolds K, Kimes TM, Rosales AG, Chan WW. Comparison of risk of re-hospitalization, all-cause mortality, and medical care resource utilization in patients with heart failure and preserved versus reduced ejection fraction. Am J Cardiol. 2015;116:1088–1092. doi: 10.1016/j.amjcard.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 32. Carson PE, Anand IS, Win S, et al. The hospitalization burden and post-hospitalization mortality risk in heart failure with preserved ejection fraction. JACC Hear Fail. 2015;3:429–441. doi: 10.1016/j.jchf.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 33. Patel HC, Hayward C, Dungu J, et al. Assessing the eligibility criteria in phase III randomized controlled trials of drug therapy in heart failure with preserved ejection fraction: the critical play off between a ‘pure’ patient phenotype and the generalizability of trial findings. J Card Fail. 2017;23:517–524. doi: 10.1016/j.cardfail.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 34. Cleland JGF. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 35. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 36. Pitt B, Pfeffer MA, Assmann SF, et al. ; TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 37. Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 38. Pandey A, Parashar A, Kumbhani DJ, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sweitzer NK, Fang JC, Pitt B, Pfeffer MA, Solomon SD. Physical activity and prognosis in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial [published online ahead of print June 21, 2017]. Circulation. doi: 10.1161/CIRCULATIONAHA.117.028002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shah SJ, Katz DH, Selvaraj S, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2014;131:269–279. doi: 10.1161/CIRCULATIONAHA.114.010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shah SJ, Kitzman DW, Borlaug BA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction. Circulation. 2016;134:73–90. doi: 10.1161/CIRCULATIONAHA.116.021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shah SJ, Cogswell R, Ryan JJ, Sharma K. How to develop and implement a specialized heart failure with preserved ejection fraction clinical program. Curr Cardiol Rep. 2016;18:122. doi: 10.1007/s11886-016-002-1. [DOI] [PubMed] [Google Scholar]
- 43. Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: she was probably able to ambulate, but I’m not sure. JAMA. 2011;306:1782–1793. doi: 10.1001/jama.2011.1556. [DOI] [PubMed] [Google Scholar]


