Abstract
Background:
There is a paucity of stroke-specific instruments to assess health-related quality of life in the Norwegian language. The objective was to examine the validity and reliability of a Norwegian version of the 12-domain Stroke-Specific Quality of Life scale.
Methods:
A total of 125 stroke survivors were prospectively recruited. Questionnaires were administered at 3 months; 36 test–retests were performed at 12 months post stroke. The translation was conducted according to guidelines. The internal consistency was assessed with Cronbach’s alpha; convergent validity, with item-to-subscale correlations; and test–retest, with Spearman’s correlations. Scaling validity was explored by calculating both floor and ceiling effects. A priori hypotheses regarding the associations between the Stroke-Specific Quality of Life domain scores and scores of established measures were tested. Standard error of measurement was assessed.
Results:
The Norwegian version revealed no major changes in back translations. The internal consistency values of the domains were Cronbach’s alpha = 0.79–0.93. Rates of missing items were small, and the item-to-subscale correlation coefficients supported convergent validity (0.48–0.87). The observed floor effects were generally small, whereas the ceiling effects had moderate or high values (16%–63%). Test–retest reliability indicated stability in most domains, with Spearman’s rho = 0.67–0.94 (all p < 0.001), whereas the rho was 0.35 (p < 0.05) for the ‘Vision’ domain. Hypothesis testing supported the construct validity of the scale. Standard error of measurement values for each domain were generated to indicate the required magnitudes of detectable change.
Conclusions:
The Norwegian version of the Stroke-Specific Quality of Life scale is a reliable and valid instrument with good psychometric properties. It is suited for use in health research as well as in individual assessments of persons with stroke.
Keywords: Stroke-Specific Quality of Life scale, stroke, validity, reliability
Introduction
Stroke has been ranked among the most common causes of disability worldwide1,2 and is associated with a decrease in health-related quality of life (HRQOL).3–5 Traditionally, outcome assessments in stroke rehabilitation have focused on improvement in symptoms and restoration of function, whereas patient-centred assessments, such as subjective well-being and HRQOL, are a more recent initiative.6 Patient-reported outcomes (PRO) enhance our understanding of treatment outcomes by indicating the impact of disease symptoms from the individual persons’ perspective.7 Of these measures, HRQOL has been recognized as increasingly important after stroke because it improves our understanding of the impact of symptoms on persons’ lives and enables us to evaluate how treatment affects persons’ functioning and well-being.7
Numerous generic and disease-specific scales that measure HRQOL after stroke are available. Generic instruments are frequently used to compare HRQOL outcomes across populations and diseases, while disease-specific instruments assess more nuanced states or concerns of specific diagnostic groups.8 A limitation of generic instruments is the lack of specificity regarding the quality of life-related consequences of a particular condition, such as stroke.9 Furthermore, they do not detect clinically relevant changes in HRQOL for a specific condition.10 The Stroke-Specific Quality of Life (SS-QOL) scale is a HRQOL measure that is specific and clinically relevant for assessing persons with stroke. The SS-QOL scale is a multidimensional PRO measure that assesses specific aspects of functioning and HRQOL issues relevant to ischaemic stroke survivors. As part of a current multicentre stroke study in Norway and Denmark, we decided to adapt the SS-QOL. However, although it has been validated and applied in Denmark, it has not been validated for use in Norwegian stroke survivors.
The original SS-QOL questionnaire measures 12 domains with 49 items. The domains and items were derived from interviews with stroke survivors in the United States.11 The SS-QOL scale has also been validated for persons with aneurysmal subarachnoid haemorrhage.12,13 The validity of the SS-QOL scale has been examined in persons after stroke in various countries, for example, in Denmark with an ischaemic stroke population,14 Nigeria (Yoruba language),15 Mexico,16 and Germany, where a short and long version for survivors of haemorrhagic or ischaemic stroke has been validated.17 A version with 8 instead of 12 factors was proposed that replicated the eight factors well. Hsueh et al.18 compared the construct validity of the 8- and 12-domain versions but favoured the latter, as it covered additional domains, thereby enhancing participants’ perspectives on HRQOL.
The objective of this study was to (1) translate and cross-culturally adapt the 12-domain SS-QOL scale, version 2.0, into Norwegian for persons with ischaemic and haemorrhagic stroke; (2) examine the scale reliability (internal consistency) and test–retest stability, and (3) assess aspects of the construct validity of the Norwegian scale.
Methods
Participants
This validation study was part of a multicentre cohort study, including persons 18 years or older with acute ischaemic (I63) or haemorrhagic stroke (I64). Persons with stroke admitted to one of the three stroke units at the University Hospital of North Norway who were living in the region were invited to participate. Stroke survivors excluded from the Norwegian National Stroke Register were also excluded from this study, for example, those with stroke related to brain malignancy, subarachnoid haemorrhage and/or traumatic brain injury (TBI).
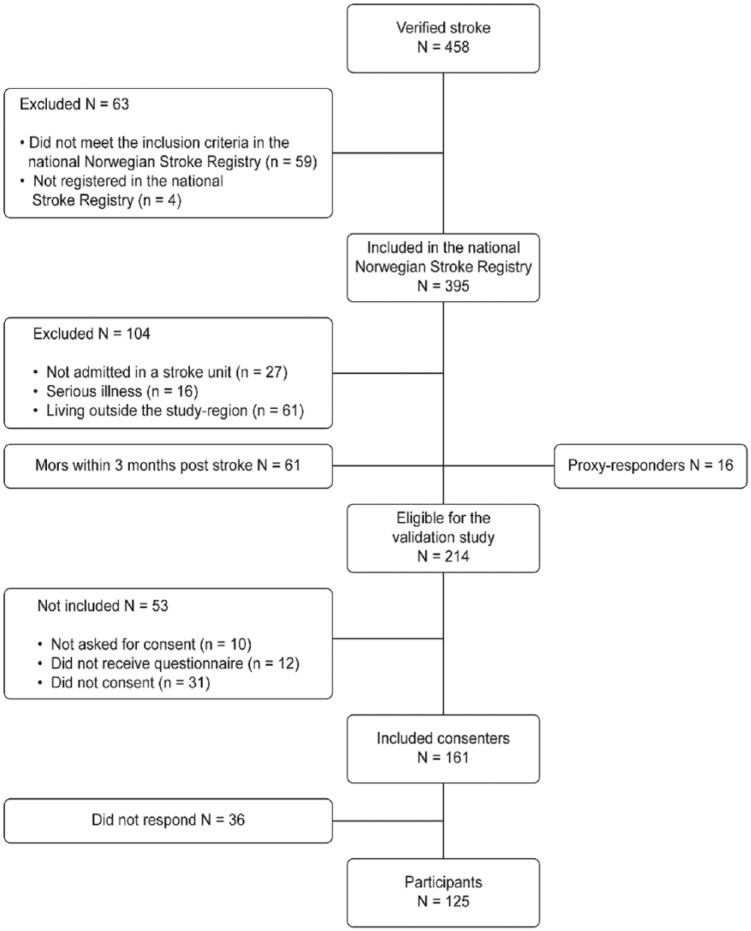
From March 2014 through December 2014, this study prospectively included 125 participants who completed or nearly completed the SS-QOL questionnaire at 3 months after stroke. These participants accounted for 58% of the 214 eligible stroke survivors during the defined recruiting period (Figure 1). The response rate was 78% (125 of 161 eligible), and among these, 41 participants (33%) received support filling out the forms. The size of the recruited sample was based on the sample size used in comparable published studies examining related research hypotheses.14
Figure 1.
Flowchart of persons with acute ischaemic or haemorrhagic stroke registred in the county of Troms in Norway during the recruiting period.
Table 1 shows the sociodemographic and stroke characteristics of the 125 participants. The median age was 72 years (range 25–96 years). Over 90% lived in their homes at 3 months, and 73% did not need any assistance.
Table 1.
Sociodemographic and stroke characteristics.
| Responders (N = 125) | |
|---|---|
| Age at the time of injury, mean (SD) | 70.5 (13.1) |
| Gender, n (%) | |
| Female | 48 (38) |
| Male | 77 (62) |
| Stroke subtype, n (%) | |
| Ischaemic | 113 (90) |
| Haemorrhagic | 12 (10) |
| Marital status, n (%) | |
| Married/cohabitant | 80 (64) |
| Widowed/single | 45 (36) |
| Education, n (%) | |
| ≤10 years | 60 (48) |
| >10 | 62 (50) |
| Unknown | 3 (2) |
| Living conditions at 3 months, n (%) | |
| Home, without assistance | 92 (73) |
| Home, with assistance | 23 (19) |
| Institution/residence for elderly | 10 (8) |
| Work status at 3 months, n (%) | |
| Student/unemployed/working fulltime or part-time | 23 (18) |
| Retired/sick-leave | 102 (82) |
| Modified Rankin Scale at 3 months, n (%) | |
| 0–1 no symptoms or no significant disability | 84 (67) |
| 2–3 slight or moderate disability | 33 (26) |
| 4–5 severe disability | 8 (7) |
SD: standard deviation.
Data collection procedures
Stroke survivors were informed about the study by nurses in the stroke units or by health professionals responsible for updating the information in the Norwegian National Stroke Register. Each hospital had one to three local health professionals who collected all the data and asked for participants’ written consent, which was obtained prior to commencing the study. A questionnaire package was mailed to the participants’ home address 3 months post stroke. Marital status, education and work status were collected from the questionnaires. Information on age, gender, living situation, stroke characteristics and modified Rankin Scale (mRS) was obtained from stroke registry data. The mRS is a scale that measures the degree of disability or dependence in activities of daily living (ADL).19 The scale ranges from 0 to 6 (‘perfect health to death’) and is widely used internationally throughout hospital services.
A sub-sample of 40 persons provided the consent to participate in a test–retest study at 12 months post-stroke onset, as stability in disease functioning and HRQOL scores may be presumed.20,21 Among the 40 recipients of the SS-QOL at 12 months, 36 responded within the desired timeframe. We re-administered the tool within 7 days from the date of the first administration. The COSMIN guidelines (consensus-based standards for the selection of health measurement instruments) were used as a checklist in the validation process.22
Predefined hypotheses
As suggested by Mokkink et al.,22,23 we predefined hypotheses for the directions and magnitude of construct relationships (i.e. correlation patterns and sizes) based on the available literature or, alternatively, a priori consensus discussions between two of the authors (S.G.P and A.A.). Construct validity was considered supported if ≥75% of the results were in correspondence with these hypotheses.24 Convergent validity was established by comparing the linear association of the individual domain score with the score of an established outcome measure for that specific domain. None of the established measures covered the Vision and Language domains, and these domains were thus tested with single items from the Norwegian National Stroke Register. In the first three columns of Table 2, the domains and corresponding outcome measures (all previously validated in Norwegian), as well as the predefined hypotheses regarding construct relationships, are presented. We expected significant positive correlations between related HRQOL constructs. We expected negative correlations between the SS-QOL domain scores (i.e. Energy, Mood and Personality and total score) and measures of global psychological distress and depression (Hospital Anxiety and Depression Scale: total and depression score). No significant correlation was expected between the SS-QOL score and participants’ gender.
Table 2.
Construct validity of the SS-QOL scale domains: Results of hypotheses testing in 125 participants 3 months post stroke.
| SS-QOL domain | Measure | Correlation hypothesis | Spearman’s ρ |
|---|---|---|---|
| Mobility | N-NSR item ‘Mobility’ | Low to moderate | 0.47 |
| EQ-5D domain ‘Gait’ | Moderate to high | 0.63 | |
| Energy | HADs total score | Moderate to high (negative) | −0.65 |
| EQ-5D: EQ VAS scale | Moderate to high | 0.48 | |
| Upper extremity function | N-NSR self-care ‘Getting dressed’ | Low to moderate | 0.47 |
| EQ-5D domain ‘Usual activities’ | Moderate | 0.62 | |
| Work/productivity | EQ-5D domain ‘Usual activities’ | Moderate to high | 0.73 |
| Mood | HADs depression | Moderate (negative) | −0.65 |
| Self-care | QOLIBRI-OS item ‘Daily activities’ | Moderate to high | 0.54 |
| EQ-5D domain ‘Personal hygiene’ | Moderate to high | 0.68 | |
| Social roles | QOLIBRI-OS item | ||
| ‘Personal and social life’ | Moderate to high | 0.56 | |
| QOLIBRI-OS total score | Moderate | 0.62 | |
| Family roles | QOLIBRI-OS item | ||
| ‘Personal and social life’ | Moderate to high | 0.58 | |
| QOLIBRI-OS total score | Moderate | 0.64 | |
| Vision | N-NSR item ‘problems with vision not present prior to stroke’ | Moderate | 0.25 |
| Language | N-NSR item ‘Problems speaking not present prior to stroke’ | Moderate | 0.42 |
| Thinking | QOLIBRI-OS item | ||
| ‘Concentrate/remember/thinking’ | High | 0.65 | |
| QOLIBRI-OS total score | Moderate | 0.64 | |
| Personality | QOLIBRI-OS item | ||
| ‘Feelings/emotional state’ | Moderate to high | 0.48 | |
| HADs Depression | Low to moderate (negative) | −0.52 | |
| SS-QOL total | QOLIBRI-OS total score | Moderate to high | 0.71 |
| EQ-5D total score | Moderate | 0.73 | |
| HADs total | Moderate to high (negative) | −0.69 | |
| Gender | No correlation | −0.17 |
N-NSR: Norwegian–National Stroke Register at 3 months. Single-item questions (n = 112–114). EQ-5D: The EuroQol Quality of Life Scale-5D; HADs: Hospital Anxiety and Depression Scale; QOLIBRI-OS: The Quality of Life After Brain Injury, Overall Scale; SS-QOL: Stroke-Specific Quality of Life scale (n = 125)
Correlation coefficients: ±0.1 small; ±0.3 medium; ±0.5 large.
Measurements
Stroke-Specific Quality of Life (SS-QOL) scale, version 2.0, by Williams et al.11 consists of 49 items covering 12 domains: Mobility, Energy, Upper Extremity Function, Work and Productivity, Mood, Self-Care, Social Roles, Family Roles, Vision, Language, Thinking and Personality. Each domain is measured by three to six items using a 5-point (1–5) Likert scale (higher scores indicate better function). An average non-weighted raw score for each domain can be generated. The overall SS-QOL summary score is most often used as the primary outcome, although the separate domain scores are helpful for identifying specific areas that are affected by stroke or that improve the most or least over time.11 The validity of the SS-QOL has been examined when administered by telephone,25,26 self-report and mail14 and with proxy responders.17,27
The Quality of Life After Brain Injury, Overall Scale (QOLIBRI-OS) is a brief HRQOL index originally constructed as a self-report scale for persons with TBI.28 The QOLIBRI-OS measures six functional areas using single-item questions assessing the following: (1) physical condition, (2) cognition, (3) emotions, (4) function in daily life, (5) personal and social life and (6) current situation and future prospects. Each item is scored from 1 (‘not at all’) to 5 (‘very’), and the sum score is arithmetically converted to a percentage score from 0 to 100 (worst–best).28 The QOLIBRI-OS has demonstrated good validity and reliability in persons with TBI29 and subarachnoid haemorrhage30 and was recently validated for use in persons with stroke.
The EuroQol Quality of Life Scale-5D (EQ-5D) is a generic HRQOL measure that evaluates five dimensions: mobility, self-care, ADL, pain, and anxiety/depression. Self-ratings were categorized into three groups in relation to possible levels of problems (1 = no, 2 = mild/moderate, and 3 = severe).31 It is possible to assign health-state utility indices based on different value sets, although according to the literature,32,33 construct validation should be performed on descriptions of the five dimensions and not the preference-based index that can be derived from the measure. In this study, we used individual dimensions to test the construct validity. The total score was used to test the convergent validity of the total SS-QOL scale. The EQ-5D has been evaluated extensively in different cultures and languages, and it was designed to be self-administered and quick enough to complement other quality of life measures.31
EuroQol Visual Analogue Scale (EQ VAS) is the second part of the EQ-5D questionnaire. The participants rate their state of health by drawing a line from a box marked ‘Your health state today’ to a point on the VAS scale, which ranges from 0 to 100 (worst to best imaginable health).31
Hospital Anxiety and Depression Scale (HADS) consists of 14 items that assess non-vegetative symptoms of depression (7 items) and anxiety (7 items). It can be used to reliably and validly detect these two mental health states.34 The HADS has been used as a screening tool in several languages and is particularly suited for hospital populations,35 including persons with stroke.36 It uses a response scale of 0–3 (higher is worse). Subscale sum scores range from 0 to 21,34 and a cut-off score ≥8 has been used to indicate a potential diagnosis of depression37 in Norwegian samples. The total HADS score (range 0–42) can additionally be considered a global measure of psychological and emotional distress.38
Stroke registry data
To test convergent validity, responses to the ADL questions ‘Mobility’ and ‘Getting dressed’ from the Norwegian National Stroke Register were used, as well as ‘Problems with vision’ and ‘Problems speaking’ (not present prior to stroke).
Translation and cross-cultural adaptation
The translation process followed standard guidelines, which included forward–backward translation, expert validation and field testing.39 Three bilingual translators conducted independent forward translations from English to Norwegian. A multidisciplinary, bilingual committee of four health professionals, all with a neurological background and special competence in stroke, prepared a reconciled Norwegian language version from the translations. The committee also reviewed the introductory statements and the instructions for the questionnaire. Three independent, bilingual health professionals performed the back translations from the Norwegian to the English version. Disagreements were resolved through discussion, and the discrepancies resulted in small changes to the Norwegian version, explained below.
In the English version, the response categories for the domains Mobility, Upper Extremity Function, Self-Care, Vision, Language and Work/Productivity were ‘Couldn’t do it at all’, ‘A lot of trouble’, ‘Some trouble’, ‘A little trouble’ and ‘No trouble at all’. In Norwegian, trouble and difficulty have the same meaning, but difficulty is more commonly used. This modification was made based on the different back translations generated. As in the Danish version,14 the response category for ‘personality’ in the additional psychometric section of the questionnaire was altered to obtain a more appropriate response in Norwegian. Explanatory examples within the items were excluded in the Norwegian version. To ensure that the translation was fully comprehensible, four participants admitted to a stroke unit were asked to complete the questionnaire and provide feedback if they found any item, response category or instruction unclear or misleading.
Statistical analyses
All analyses were conducted in SPSS 23. The distributional properties of the subscales were examined visually and parametrically (e.g. kurtosis and skewness). The descriptive data were presented as the means, standard deviations (SDs) and ranges or as proportions. Chi-square (or Fisher’s exact) tests were used to compare categorical data, whereas independent-sample t-tests were used to compare the mean differences between two groups. Non-normally distributed data were examined with non-parametric statistical analyses (e.g. Mann–Whitney test). Occasionally missing items were replaced with the mean of the subscale if less than three items were missing. The internal consistency of the SS-QOL total and domain scores were examined by calculating Cronbach’s alpha values (higher than 0.7 are preferable).40
Floor and ceiling effects were calculated as the percentage of participants with the minimum or maximum score in each domain. Floor and ceiling frequencies higher than 15% were considered substantial.24 Item-total correlations within the range of 0.4–0.9 were considered acceptable.41 Test–retest stability was examined by two means: (1) Spearman’s ρ, to quantify the magnitude of the relationship between the scores on the first and second administration, which should preferably surpass 0.7,24,40 and (2) intra-class correlation coefficients (ICCs), to assess stability in the use of the response scale by comparing the consistency with absolute agreement estimates. The ICC should also surpass 0.7.24 A distribution-based method was used to calculate the standard error of measurement (SEM). The formula was based on Cronbach’s alpha and the SD.42
Data quality
All primary missing data were recorded and summarized for each item in the SS-QOL questionnaire. Missing data were collected from participants by telephone when possible, and when not, the mean of the domain score was used as a replacement when only one or two items were missing from the total scale. SS-QOL questionnaires with more than two missing were not included in the study.
Ethics
This study was conducted according to the Helsinki Declaration regarding informed consent and confidentiality. The Regional Norwegian Ethical Committee Health Region North approved the study (2013/1461).
Results
Translation
The forward- and back-translation process confirmed a satisfactory match in semantic meaning between the original and the back-translated SS-QOL items. The few exceptions were resolved through consensus discussion, which resulted in removing explanatory examples and revising the layout, for example.
Missing data
The degree of missing data in the SS-QOL was very low (1.4%), and these data were replaced by consulting the participants or using the domain mean (Table 3). The most frequently missing item was ‘I had sex less often than I would like’, which was in the Social roles domain.
Table 3.
Reliability of the Norwegian version of the SS-QOL scale – data quality, internal consistency, floor and ceiling effects and test–retest reliability.
| SS-QOL domain (N = 125) | Numbers of items | Missing (%) | Mean (SD) | Internal consistency Cronbach’s α | Floor and ceiling effects (%) | Test–retest reliability (N = 36) |
||
|---|---|---|---|---|---|---|---|---|
| Spearman’s ρ | p | |||||||
| Mobility | 6 | 2 | 4.28 (0.92) | 0.93 | 0.8 | 36.0 | 0.84 | <0.001 |
| Energy | 3 | 2 | 3.36 (1.45) | 0.92 | 11.1 | 29.4 | 0.67 | <0.001 |
| Upper extremity function | 5 | 0.5 | 4.28 (1.04) | 0.93 | 1.6 | 43.7 | 0.94 | <0.001 |
| Work and Productivity | 3 | 1 | 4.21 (1.07) | 0.92 | 3.2 | 47.6 | 0.94 | <0.001 |
| Mood | 5 | 2 | 3.93 (1.05) | 0.84 | 1.6 | 27.8 | 0.84 | <0.001 |
| Self-care | 5 | 0 | 4.46 (0.93) | 0.92 | 2.4 | 54.8 | 0.89 | <0.001 |
| Social roles | 5 | 3 | 3.29 (1.30) | 0.91 | 7.1 | 15.9 | 0.80 | <0.001 |
| Family roles | 3 | 2 | 3.96 (1.19) | 0.83 | 2.4 | 38.9 | 0.79 | <0.001 |
| Vision | 3 | 0 | 4.58 (0.75) | 0.79 | 1.6 | 62.7 | 0.35 | <0.05 |
| Language | 5 | 0.3 | 4.59 (0.70) | 0.91 | 0.8 | 56.3 | 0.74 | <0.001 |
| Thinking | 3 | 2 | 3.79 (1.19) | 0.83 | 3.2 | 32.5 | 0.65 | <0.001 |
| Personality | 3 | 2 | 3.99 (1.18) | 0.87 | 2.4 | 41.3 | 0.83 | <0.001 |
| SS-QOL total | 49 | 1.4 | 4.09 (0.80) | 0.97 | 0.8 | 8.8 | 0.89 | <0.001 |
Ceiling effects
The SS-QOL total score had an acceptably low ceiling effect (8.8%), whereas all the domain scores had ceiling effects surpassing the 15% limit (Table 3). The domains Self-Care, Vision and Language had considerable ceiling effects (above 50%). Conversely, floor effects were predominantly absent.
Reliability
The domains of the SS-QOL scale showed acceptable and good internal consistency, with Cronbach’s alpha coefficients ranging from 0.79 to 0.93. The alpha value for the SS-QOL total score was 0.97. The item-total correlations ranged between 0.44 and 0.83 for all 49 items.
In all, 36 participants returned the retest, enabling an examination of the measurement stability. The test–retest stability was generally good, as Spearman’s correlations were all high (Table 3), except for in three domains with coefficients below 0.7 (Thinking (ρ = 0.65), Energy (ρ = 0.66) and Vision (ρ = 0.35)). The ICC values were excellent for all domains. The differences between the consistency and the absolute agreement-based ICC estimates were minor, thus indicating that participants interpreted the response scale similarly at both measurement occasions.
SEM
The SEMs are presented in Table 4 and indicate the smallest degree of change in the total or domain score that reflects a true change in the construct, that is, not confounded by measurement error. A change score of at least one SEM represents the smallest margin that can indicate a minimally clinically important difference.42 As expected, the minimally required change scores were higher for the domain scores than the SS-QOL total score. These values that can be used as guidance in studies investigate change over time.
Table 4.
Standard error of measurement (SEM).
| Mobility | 0.24 |
| Energy | 0.41 |
| Upper extremity function | 0.27 |
| Work and productivity | 0.30 |
| Mood | 0.42 |
| Self-care | 0.26 |
| Social roles | 0.39 |
| Family roles | 0.49 |
| Vision | 0.35 |
| Language | 0.21 |
| Thinking | 0.49 |
| Personality | 0.42 |
| SS-QOL total | 0.14 |
Construct validity
The correlations between the SS-QOL scale and the criterion-related measures are presented in Table 2, as are the hypothesized directions and magnitudes. As many measurements had ordinal scales and a few criterion-related variables were based on a single item, an ordinal correlation metric was preferred. All observed coefficients corresponded with the hypothesized correlations.
Discussion
This study examined the psychometric properties of the Norwegian version of the SS-QOL scale. The reliability, in terms of consistency and test–retest stability, was good. The construct validity was also supported, as the SS-QOL total and domain scores correlated as expected with the criterion-related measures.
Validity
The COSMIN panel defines validity as ‘the degree to which an instrument truly measures the construct it purports to measure’.43 Validity is a broad concept that can be distinguished into content, criterion and construct validity in the context of questionnaire validation.41 Determining the content validity of the SS-QOL involved a subjective critical evaluation of whether the SS-QOL items reflected a representative selection of indicators measuring the intended concept. The content validity of the SS-QOL has been well documented by others11 and was not re-evaluated here. Rather, we focused on the construct, or more specifically, the criterion validity of the SS-QOL by examining whether it was positively correlated with the EQ-5D sum score, specific EQ-5D domains, EQ VAS and QOLIBRI-OS as expected (convergent validity) and negatively correlated with the HADS total score and HADS depression score (divergent validity).41
Convergent and divergent validity
The SS-QOL scale is a comprehensive measurement, and as recommended in the COSMIN guidelines,22 we hypothesized the magnitudes and directions of the correlations for all the specific domains and for the total SS-QOL scale against related measurements. The Vision domain had a lower correlation than estimated, whereas all other correlations were as expected or higher than expected, supporting the construct validity of the questionnaire (Table 2).
Reliability
Internal consistency
The reliability, or internal consistency, of the domain and total SS-QOL scores were high. The degree of consistency reported in this study was comparable and slightly higher than that reported by Williams et al.,11 Muus et al.,14 and Hsueh et al.18 Of the 12 domains, 7 had alpha values >0.90. The observed differences were most likely related to variances in sample size and sampling procedures. Our study included more participants than the study by Williams et al.,11 for example, and likely a more heterogeneously composed sample than those of the abovementioned studies. Most of the participants had mild- or moderate-severity stroke, similar to corresponding studies.11,14 However, our study did not exclude persons with more severe stroke, with aphasia and/or cognitive problems or with comorbidities. Our more heterogeneous sample may partly explain the higher alpha values.41 According to de Vet et al.,41 Cronbach’s alpha values above 0.90 may indicate redundancy of items and suggest the need to shorten the scale. However, since the domains of the SS-QOL scale consist of three to six items, we would not recommend this approach.
Test–retest reliability
The test–retest reliability of the SS-QOL was satisfactory, with only three domains showing values below 0.7 (Table 3). Other SS-QOL validation studies using the same test–retest timeframe (1–2 weeks) displayed correlations from 0.71 to 0.9616 and 0.65 to 0.99.14 The Vision domain was again problematic, showing the lowest test–retest correlation with Spearman’s rho of 0.35 (p < 0.05). One of the items ‘Did you have trouble reaching for things because of poor eyesight?’ might have been ambiguous to the participants, as it may convey two meanings: physical problems with reaching for items independent or dependent of eyesight. ‘Trouble reaching for things’ after a stroke can be related to sensory motor deficits or other perceptual and cognitive impairments such as apraxia, agnosia, neglect or other visuospatial challenges. It may be difficult for persons with stroke to establish the reason why they are experiencing difficulties. As some of the included participants reported vision problems, this health problem should not be overlooked, and a future approach may be to improve the clarity of this question. The psychometric properties of the Vision domain were not satisfactory, and we suggest rephrasing at least one of the three items and then re-validating the domain.
The Energy and Thinking domains also had test–retest coefficients below 0.7, which may reflect a true day-to-day fluctuation (e.g. the need to rest, various levels of ability to concentrate) rather than an unreliable domain, as Cronbach’s alpha values were satisfactory. The high reliability and the low SEM scores in several of the domains in our study, all below 0.5 SD, indicate that the SS-QOL scale is highly suitable for assessing individual participants’ HRQOL, as well as in researching HRQOL among stroke survivors.
Quality of data
The data quality in this study was good, with a low amount of missing data. As noted by others, for example, Muus et al.,14 the item ‘I had sex less often than I would like’ (Social Roles domain) had the largest number of missing responses (15%, n = 19). This item might be considered less relevant or too sensitive or private for some of the participants. Another item, ‘change in personality’ (prior to stroke), was also often incomplete. Personality changes can be difficult to assess by the individuals themselves, or the question might be too sensitive to answer. Another possibility, also noted by Muus et al.,14 is that the layout of the questionnaire, with this particular item separated from the others, could make it easier to overlook. Overall, the high data quality indicated that the SS-QOL questionnaire was understandable and easy to complete.
Floor/ceiling effects
Less than 9% of the total SS-QOL scores exceeded the ceiling threshold, which may be considered acceptably low. The ceiling effects of the domain scores were higher but on par with previous findings,11,14,18 ranging from moderate to high. A ceiling effect was particularly present for the Vision domain (62.7%), as Muus et al.14 also reported. The variations in ceiling effects reflected areas that were more or less affected by stroke. The observed ceiling effect in the Vision, Language and Self-Care domains may indicate that these areas are less frequently affected among the responders in this stroke population.
As persons with stroke are a heterogeneous group with various and different degrees of symptoms, some degree of ceiling (or floor) effects is expected. These effects may be problematic because they weaken the ability to distinguish participants in the higher (ceiling) or lower (floor) levels of the construct, whereas the middle area is less affected. However, a high score within an SS-QOL domain simply reflects normal functioning within this area, and according to de Vet et al.,41 when a large proportion of the population has no functional problems, it should not be considered a ceiling effect. In contrast, a lower score may indicate a particular functional problem within a domain. Due to the considerable heterogeneity in the symptoms and functional consequences of stroke, ceiling (or floor) effects are normally more present in domain scores than the total score, and thus, the total score is more suitable for measuring changes in the follow-up period than the domain scores.
Limitations and strengths of the study
As reported in previous studies,11,14 most of the respondents had mild to moderate stroke. Although some eligible stroke survivors were lost due to administrative errors, we consider the study population reasonably representative for measuring HRQOL following stroke.
The convergent and discriminant validity, as indicators of construct validity, is a strength of the study, which tested predefined hypothesis and expected associations among similar and dissimilar measures as recommended in the literature.40,41 For two domains in our study, the only available option was to correlate the SS-QOL domain with a single-item question from the Norwegian National Stroke Register. It could be argued that these questions are not as valid as a validated questionnaire, though the directions of the correlations were as expected in these occasions as well. Our choice of measurements did consider respondent burden, and we thus chose measurements that were practical, not too extensive, and appropriate for HRQOL assessment post stroke in this population.
Examining the discriminative validity of the SS-QOL using factor analytic methods was not deemed appropriate in this study due to the low subject-to-item ratio. The large number of items and particularly the large number of latent factors (12 domains) would require a considerably larger sample size to achieve satisfactory statistical power.44 Due to the low power in this study to test such complex models, the risk of type II error would be unduly high. The 12-domain factor structure has previously been examined using confirmatory factor analysis in a sample of 388 stroke survivors, and the results supported the current factor model.18
Conclusion
The Norwegian version of the SS-QOL scale is an instrument with good psychometric properties. It is suited for use in health research as well as in individual assessments of persons with stroke.
Acknowledgments
We would like to acknowledge the participants, translators, and Neurology Departments in Norway and the physical therapists in the stroke unit in Tromsø for the systematic collection of data. S.G.P., A.A., C.A. and G.H. contributed in designing this study, analysed the data and had the overall decision-making in this study. S.G.P. and G.H. were responsible for producing the data. A.A., C.A. and O.F. contributed with supervision on the overall use of methods, the data analysis and interpretation of data. O.F., J.F.N. and H.H. overlooked statistical analyses and interpretation. S.G.P. drafted the manuscript and all authors contributed to critical revision of the article.
Footnotes
Clinical messages: • Our results support the Norwegian version of the SS-QOL scale as an instrument with good psychometric properties.
• Construct validity of the scale is well supported.
• The instrument is applicable, understandable and easy to complete.
• The SS-QOL scale is suited for use in research as well as in assessments of individual stroke survivors.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: This study was conducted according to the Helsinki Declaration regarding informed consent and confidentiality. The Regional Norwegian Ethical Committee Health Region North approved the study (2013/1461).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Northern Norway Regional Health Authorities funded this work (grant no. SFP1174-14).
Informed consent: Patients were informed about the study by nurses in the stroke units or by health professionals responsible for updating the information in the Norwegian National Stroke Register. Written informed consent was obtained from all the participating subjects before study initiation. Each hospital had one to three local health professionals who collected all the data.
References
- 1. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A Systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2197–1223. [DOI] [PubMed] [Google Scholar]
- 2. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. an Mierlo ML, Schröder C, van Heugten CM, et al. The influence of psychological factors on health-related quality of life after stroke: A systematic review. Int J Stroke 2014; 9: 341–348. [DOI] [PubMed] [Google Scholar]
- 4. Alguren B, Fridlund B, Cieza A, et al. Factors associated with health-related quality of life after stroke: A 1-year prospective cohort study. Neurorehabil Neural Repair 2012; 26: 266–274. [DOI] [PubMed] [Google Scholar]
- 5. Carod-Artal FJ, Egido JA. Quality of life after stroke: The importance of a good recovery. Cerebrovasc Dis 2009; 27(Suppl. 1): 204–214. [DOI] [PubMed] [Google Scholar]
- 6. Salter KL, Moses MB, Foley NC, et al. Health-related quality of life after stroke: What are we measuring? Int J Rehabil Res 2008; 31: 111–117. [DOI] [PubMed] [Google Scholar]
- 7. Wiklund I. Assessment of patient-reported outcomes in clinical trials: The example of health-related quality of life. Fundam Clin Pharmacol 2004; 18: 351–363. [DOI] [PubMed] [Google Scholar]
- 8. Patrick DL, Deyo RA. Generic and disease-specific measures in assessing health status and quality of life. Med Care 1989; 27: S217–S132. [DOI] [PubMed] [Google Scholar]
- 9. Hobart JC, Williams LS, Moran K, et al. Quality of life measurement after stroke: Uses and abuses of the SF-36. Stroke 2002; 33: 1348–1356. [DOI] [PubMed] [Google Scholar]
- 10. Williams LS. Health-related quality of life outcomes in stroke. Neuroepidemiology 1998; 17: 116–120. [DOI] [PubMed] [Google Scholar]
- 11. Williams LS, Weinberger M, Harris LE, et al. Development of a stroke-specific quality of life scale. Stroke 1999; 30: 1362–1369. [DOI] [PubMed] [Google Scholar]
- 12. Boosman H, Passier PE, Visser-Meily JM, et al. Validation of the Stroke-Specific Quality of Life scale in patients with aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 2010; 81: 485–489. [DOI] [PubMed] [Google Scholar]
- 13. Wong GK, Lam SW, Ngai K, et al. Validation of the Stroke-Specific Quality of Life for patients after aneurysmal subarachnoid hemorrhage and proposed summary subscores. J Neurol Sci 2012; 320: 97–101. [DOI] [PubMed] [Google Scholar]
- 14. Muus I, Williams LS, Ringsberg KC. Validation of the Stroke Specific Quality of Life Scale (SS-QOL): Test of reliability and validity of the Danish version (SS-QOL-DK). Clin Rehabil 2007; 21: 620–627. [DOI] [PubMed] [Google Scholar]
- 15. Akinpelu AO, Odetunde MO, Odole AC. Cross-cultural adaptation and initial validation of the Stroke-Specific Quality of Life scale into the Yoruba language. Int J Rehabil Res 2012; 35: 339–344. [DOI] [PubMed] [Google Scholar]
- 16. Cruz-Cruz C, Martinez-Nuñez JM, Perez ME, et al. Evaluation of the Stroke-Specific Quality of Life (SSQOL) Scale in Mexico: a preliminary approach. Value Health Reg Issue 2013; 2: 392–397. [DOI] [PubMed] [Google Scholar]
- 17. Ewert T, Stucki G. Validity of the SS-QOL in Germany and in survivors of hemorrhagic or ischemic stroke. Neurorehabil Neural Repair 2007; 21: 161–168. [DOI] [PubMed] [Google Scholar]
- 18. Hsueh IP, Jeng JS, Lee Y, et al. Construct validity of the stroke-specific quality of life questionnaire in ischemic stroke patients. Arch Phys Med Rehabil 2011; 92: 1113–1118. [DOI] [PubMed] [Google Scholar]
- 19. Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J 1957; 2: 200–215. [DOI] [PubMed] [Google Scholar]
- 20. Kwakkel G, Kollen B, Twisk J. Impact of time on improvement of outcome after stroke. Stroke 2006; 37: 2348–2353. [DOI] [PubMed] [Google Scholar]
- 21. Luengo-Fernandez R, Gray AM, Bull L, et al. Quality of life after TIA and stroke: Ten-year results of the Oxford vascular study. Neurology 2013; 81: 1588–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mokkink LB, Terwee CB, Knol DL, et al. The COSMIN checklist for evaluating the methodological quality of studies on measurement properties: A clarification of its content. BMC Med Res Methodol 2010; 10: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual Life Res 2010; 19: 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007; 60: 34–42. [DOI] [PubMed] [Google Scholar]
- 25. Williams LS, Redmon G, Saul DC, et al. Reliability and telephone validity of the Stroke-Specific Quality of Life (SS-QOL) scale. Stroke 2001; 32: 339. [Google Scholar]
- 26. Muus I, Ringsberg KC. Stroke Specific Quality of Life Scale: Danish adaptation and a pilot study for testing psychometric properties. Scand J Caring Sci 2005; 19: 140–147. [DOI] [PubMed] [Google Scholar]
- 27. Williams LS, Redmon G, Martinez B, et al. Proxy ratings of stroke-specific quality of life (SS-QOL) stores. Stroke 2000; 31: 301. [Google Scholar]
- 28. von Steinbuechel N, Wilson L, Gibbons H, et al. QOLIBRI overall scale: A brief index of health-related quality of life after traumatic brain injury. J Neurol Neurosurg Psychiatry 2012; 83: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 29. Polinder S, Haagsma JA, van Klaveren D, et al. Health-related quality of life after TBI: A systematic review of study design, instruments, measurement properties, and outcome. Popul Health Metr 2015; 13: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wong GK, Lam SW, Ngai K, et al. Quality of Life after Brain Injury (QOLIBRI) Overall Scale for patients after aneurysmal subarachnoid hemorrhage. J Clin Neurosci 2014; 21: 954–956. [DOI] [PubMed] [Google Scholar]
- 31. EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990; 16: 199–208. [DOI] [PubMed] [Google Scholar]
- 32. Brazier J. Measuring and valuing health benefits for economic evaluation. New York: Oxford University Press, 2007. [Google Scholar]
- 33. Brazier J, Deverill M. A checklist for judging preference-based measures of health related quality of life: Learning from psychometrics. Health Econ 1999; 8: 41–51. [DOI] [PubMed] [Google Scholar]
- 34. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 35. Soberg HL, Roe C, Anke A, et al. Health-related quality of life 12 months after severe traumatic brain injury: A prospective nationwide cohort study. J Rehabil Med 2013; 45: 785–791. [DOI] [PubMed] [Google Scholar]
- 36. Aben I, Verhey F, Lousberg R, et al. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics 2002; 43: 386–393. [DOI] [PubMed] [Google Scholar]
- 37. Oyane NM, Bjelland I, Pallesen S, et al. Seasonality is associated with anxiety and depression: The Hordaland health study. J Affect Disord 2008; 105: 147–155. [DOI] [PubMed] [Google Scholar]
- 38. Pallant JF, Tennant A. An introduction to the Rasch measurement model: An example using the Hospital Anxiety and Depression Scale (HADS). Br J Clin Psychol 2007; 46: 1–18. [DOI] [PubMed] [Google Scholar]
- 39. Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: Literature review and proposed guidelines. J Clin Epidemiol 1993; 46: 1417–1432. [DOI] [PubMed] [Google Scholar]
- 40. Reeve BB, Wyrwich KW, Wu AW, et al. ISOQOL recommends minimum standards for patient-reported outcome measures used in patient-centered outcomes and comparative effectiveness research. Qual Life Res 2013; 22: 1889–1905. [DOI] [PubMed] [Google Scholar]
- 41. de Vet HC, Terwee CB, Mokkink LB, et al. Measurement in medicine: a practical guide. New York: Cambridge University Press, 2011. [Google Scholar]
- 42. Copay AG, Subach BR, Glassman SD, et al. Understanding the minimum clinically important difference: A review of concepts and methods. Spine J 2007; 7: 541–546. [DOI] [PubMed] [Google Scholar]
- 43. Mokkink LB, Terwee CB, Patrick DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol 2010; 63: 737–745. [DOI] [PubMed] [Google Scholar]
- 44. Wolf EJ, Harrington KM, Clark SL, et al. Sample size requirements for structural equation models: An evaluation of power, bias, and solution propriety. Educ Psychol Meas 2013; 76: 913–934. [DOI] [PMC free article] [PubMed] [Google Scholar]