Abstract
Objectives:
To assess the kinetics of procalcitonin (PCT) and C-reactive protein (CRP) in pediatric patients who required extracorporeal membrane oxygenation (ECMO) and to analyze its relationship with morbidity and mortality.
Patients and methods:
Prospective observational study including pediatric patients who required ECMO. Both PCT and CRP were sequentially drawn before ECMO (P0) and until 72 hours after ECMO.
Results:
A total of 40 patients were recruited. Two cohorts were established based on the value of the P0 PCT (>10 ng/mL). Comparing the kinetics of PCT and CRP in these cohorts, the described curves were the expected for each clinical situation. The cutoff for P0 PCT to predict multiple organ dysfunction syndrome was 2.55 ng/mL (sensibility 83%, specificity 100%). Both PCT and CRP did not predict risk of neurologic sequelae or mortality in any group.
Conclusions:
Procalcitonin does not seem to be modified by ECMO and could be a good biomarker of evolution.
Keywords: ECMO, procalcitonin, C-reactive protein, infection, multiple organ dysfunction syndrome, outcome
Introduction
Extracorporeal membrane oxygenation (ECMO) has become the greatest support in cases of refractory cardiac or respiratory failure in adults and children.1,2 The improvements in this technique in the past years, with an increasing survival rate, have motivated interest in the associated morbidity.3 Among the complications of ECMO, diagnosis of early infection has gained importance given the consequences that can emerge from a late detection of infection.4 Furthermore, overexposure to broad-spectrum antibiotics over a long period of time is becoming a problem in intensive care units due to increasing resistance.5
Similar to patients undergoing cardiopulmonary bypass, ECMO patients have a systemic inflammatory response syndrome (SIRS) that may be difficult to differentiate from sepsis.5 This SIRS seems to be secondary to exposure to strange surfaces of the circuit and conditions a massive release of pro-inflammatory cytokines which activate humoral and cellular immunity.6 The intensity of the inflammatory response seems to be related to morbidity.
In recent years, procalcitonin (PCT) has become an important biomarker in the diagnosis of bacterial infection.7 There are multiple studies about its superiority to C-reactive protein (CRP) as an infection biomarker. Its utility has been demonstrated in invasive infections,8 nosocomial infection (NI),9–12 and after cardiopulmonary bypass.13,14
There are few data on the behavior of PCT in patients in ECMO, especially in children. In 2012, a series of 20 pediatric ECMO cases in which PCT values were surprisingly low in the presence of infection was published.15 The authors also found a greater relationship between PCT and multiple organ dysfunction syndrome (MODS).
The main objective of this study was to evaluate whether ECMO modifies PCT and CRP kinetics. The secondary objective was to analyze whether the PCT at any time could predict the prognosis.
Materials and Methods
Setting and study population
This was a prospective observational single-center study. Patient recruitment was conducted from January 2007 to December 2016 at the pediatric intensive care unit (PICU) of a third-level hospital.
All pediatric patients admitted to the PICU who required ECMO by any disease were included.
All patients with rheumatologic or other forms of systemic disease and those lacking parental consent were excluded.
Characteristics of ECMO support
The ECMO circuit was MAQUET (Rastatt, Germany), which works with a ROTAFLOW centrifugal blood pump, a QUADROX oxygenator and a biocompatible circuit (MAQUET). The pump was able to guarantee a blood flow up to 100% or more of the cardiac output. Anticoagulation was managed by intravenous heparin infusion with a target-activated coagulation time of 180 to 200 seconds and cephalin depending on the clinical situation of the patient. Local ECMO protocol was based on ELSO (Extracorporeal Life Support Organization) guidelines.16
Laboratory analysis
Both PCT and CRP were sequentially analyzed in 4 periods: pre-ECMO (P0), 24 hours post-ECMO (P1), 48 hours post-ECMO (P2), and 72 hours post-ECMO (P3). The values of both biomarkers were also collected in case of NI beyond period P3.
After general analysis, 2 cohorts of patients were established according to the PCT value in P0: group 1, with PCT >10 ng/mL at P0, corresponding to severe septic patients, and group 2, with PCT <10 ng/mL at P0, in which there were patients with other causes for the refractory failure. This specific analysis was conducted because PCT levels >10 ng/mL have traditionally been described in scientific literature as a criterion for severe sepsis in patients with compatible clinical data. The values of PCT <0.5 ng/mL were considered normal.
The PCT values were determined in duplicate by means of the LUMItest PCT immunoluminometric assay (ATOM SA; Brahms Diagnostica, Middletown, VA, USA), which uses 2 monoclonal antibodies and requires 20 μL of serum or plasma; the assay sensitivity was 0.02 ng/mL. C-reactive protein was obtained using the immunoturbidimetric assay (COBAS INTEGRA; Roche ®400 plus).
Definitions
Diagnosis of infection was performed by the PICU medical team and infectious team. Sepsis diagnosis was defined according to the international definition of pediatric sepsis published in 200517 and revised in 2012.18 Nosocomial infection was diagnosed according to the criteria of the Centers for Disease Control and Prevention.19 Cultures of the different biological samples were processed in case of suspected infection.
The risk of poor prognosis before the onset of ECMO was assessed with the Sequential Organ Failure Assessment (SOFA) score. This score was recorded prior to the ECMO. We considered organ dysfunction as a SOFA score >9 as in previous studies.
Antibiotics
Cefotaxime and vancomycin were used for community-acquired infections, piperacillin-tazobactam/meropenem and vancomycin for NIs and as prophylaxis in those patients who required ECMO without sepsis. Other antibiotics (aminoglycosides, clindamycin, or metronidazole) were added according to the cause of the infection.
Variables
Baseline demographic data were recorded including underlying disease and reason for admission. Clinical data before ECMO were collected. Pediatric Risk of Mortality Score (PRISM III) and SOFA were recorded prior to ECMO. Hemodynamic instability was assessed according to the vasoactive-inotropic score20 and respiratory support according to the hours of mechanical ventilation (MV). PaO2/FiO2 ratio (PFi, ratio of partial pressure arterial oxygen and fraction of inspired oxygen) was recorded for evaluating hypoxemia degree before ECMO. All data about infection were collected, including sepsis diagnosis, NI, and changes in antibiotic treatment during ECMO. Morbidity was assessed in terms of days of MV, days in ECMO, days of PICU stay, and total hospital length of stay (LOS). Neurologic sequelae were assessed as the presence of cerebral infarction during evolution. Mortality was analyzed in the first 28 days from admission and at 3 months.
Statistical analysis
Statistical analysis was performed with SPSS 22.0 for Windows.
Qualitative variables were expressed as frequencies and percentages. Due to sample size, quantitative variables were expressed as medians and interquartile range (IQR). All data were analyzed with nonparametric tests: Mann-Whitney U test was used to compare quantitative variables, Wilcoxon rank sum test was used for paired data, and Spearman rank correlation coefficient (r) was used to analyze the independent variables associated with evolution. A P value of <.05 was considered significant. Receiver operating characteristic analysis was performed: biomarker cutoff, area under the curve (AUC), sensitivity, and specificity were analyzed using Medi-Cal Program 11.3.
The study was conducted in accordance with the Helsinki Declaration recommendations and approved by the Sant Joan de Déu Ethical Investigational Committee. Written informed consent was omitted as the ECMO database was previously approved and there was no any intervention.
Results
General results
A total of 40 patients were recruited. The median age was 1.33 years (IQR: 0.31-6.63) and 21 (52.5%) were men. Cannulation was arterial-venous in 34 (85%) and venovenous in 6. Overall demographic and pre-ECMO data, support requirement data, and outcome are reported in Table 1. We established 2 cohorts according to the PCT value in P0, as is explained in section “Materials and Methods.” The deceased patients in the first 72 hours of ECMO were excluded from the analysis of the long-term outcomes (days of MV, days of ECMO, days in PICU, and days of hospitalization). Six patients died later, between 4 and 20 days from the start of ECMO. There were statistical differences between the 2 groups in the inotropic support prior to ECMO (group 1, median of 53.7 [IQR: 23-108]; group 2, 25 [10-53.7], with P = .027) and initial lactate acid level (group 1, median of 5.9 [2.7-10]; group 2, 2.35 [1.65-4.53], with P = .016). There were also differences according to the need for hemodiafiltration (in group 1, 15 patients required it (78.9%), and in group 2, 6 patients required it (28.6%), with P = .001).
Table 1.
Overall demographic and pre-ECMO data, mechanical support data, and outcome.
| All (n = 40) | Group 1 (n = 19) | Group 2 (n = 21) | P value | |
|---|---|---|---|---|
| Demographic data | ||||
| Males | 21 (52.5%) | 11 (58.9%) | 10 (47.6%) | .516 |
| Age, y | 1.33 (0.31-6.63) | 3.29 (0.48-7.5) | 0.62 (0.075-4.51) | .136 |
| Weight, kg | 10 (4.85-20) | 15 (6.5-33) | 7 (3.65-15.25) | .095 |
| Data prior ECMO | ||||
| PRISM III | 12 (6-17) | 17 (6-24.25) | 11 (5-13) | .116 |
| SOFA | 13 (10-14) | 14 (13-16) | 11 (9-12.75) | 0 |
| Hours before ECMO | 30 (12-96) | 24 (12-96) | 32 (12-96) | .431 |
| Previous pathology | 18 (45%) | 7 (36.8%) | 11 (52.4%) | .324 |
| Inotropic score | 30.35 (15-67.75) | 53.7 (23-108) | 25 (10-53.7) | .027 |
| PFi score | 76 (49-155) | 81 (54-160) | 60.5 (37.25-114.25) | .191 |
| Oxygen index | 31.6 (12.7-51) | 33.8 (12.7-53.06) | 26.83 (11.61-47.21) | .613 |
| Lactate previous, mmol/L | 3.2 (1.8-7) | 5.9 (2.7-10) | 2.35 (1.65-4.53) | .016 |
| ECMO setup | ||||
| PICU initiation, h | 30 (12-96) | 24 (12-96) | 32 (12-96) | .413 |
| Hemodiafiltration | 21 (52.5%) | 15 (78.9%) | 6 (28.6%) | .001 |
| Cannulation AV | 34 (85%) | 17 (89.5%) | 17 (80.9%) | .451 |
| Outcome | ||||
| MV, d | 18 (12-22) | 18 (14-21) | 17.5 (12-25.75) | .824 |
| ECMO support, d | 7 (5-10) | 7 (4-10) | 7.5(5.25-10.75) | .843 |
| PICU admission, d | 27 (21-53) | 25 (21-53) | 30 (20-56.25) | .730 |
| Hospital admission, d | 52 (30-73) | 53 (31-73) | 48 (30-73.75) | .748 |
| Neurologic sequelae | 9 (22.5%) | 4 (21.1%) | 5 (23.8%) | .907 |
| Mortality | 13 (32.5%) | 8 (42.1%) | 5 (23.8%) | .173 |
| Mortality <3 d of ECMO | 7 (53.8%) | 6 (75%) | 1 (20%) | .053 |
Abbreviations: AV, arteriovenous; ECMO, extracorporeal membrane oxygenation; LOS, length of stay; MV, mechanical ventilation; PFi, PaO2/FiO2 ratio; PICU, pediatric intensive care unit; PRISM, Pediatric Risk of Mortality Score; SOFA, Sequential Organ Failure Assessment.
Values expressed as median (interquartile range). Mann-Whitney U test.
The pathology that determined the need for ECMO support was acute respiratory distress syndrome (ARDS) in 13 patients (32.5%), sepsis in 9 (22.5%), and postcardiopulmonary bypass in 4 (10%). All patients of group 1 (PCT > 10 ng/mL at P0) have a suspicion of sepsis according to previous definition. In group 2 (PCT < 10 ng/mL at P0), patients presented ARDS, pulmonary hypertension, incessant arrhythmias, and myocarditis, among others.
Kinetics of PCT
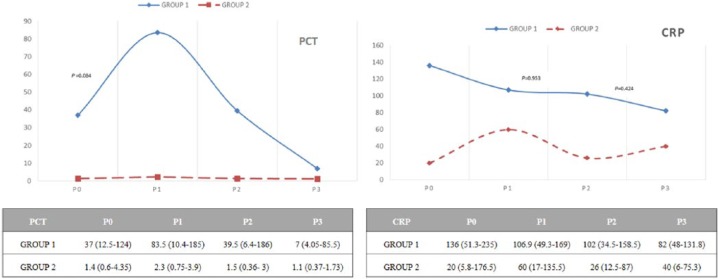
In group 1 of severe septic patients (47.5% cases), PCT increased between time P0 and P1, with subsequent decrease in P2 and P3: 37 (IQR: 12.5-124), 83.5 (IQR: 10.4-185), 39.5 (IQR: 6.4-186), and 7 ng/mL (IQR: 4.05-85.5), with P = .084, .003, and .002, respectively.
In group 2, PCT dropped significantly from P1 to P2 and P3: 1.4 (IQR: 0.6-4.35), 2.3 (IQR: 0.75-3.9), 1.5 (IQR: 0.36-3), and 1.1 ng/mL (IQR: 0.37-1.73), with P = .717, .005, and .002, respectively.
CRP did not show statically significant variations at any time or in any groups. All data are reflected in Figure 1.
Figure 1.
Differences in PCT and CRP values in the first 72 hours of extracorporeal membrane oxygenation regarding both cohorts. In group 1, PCT significantly increased between time P0 and P1, with subsequent decrease in P2 and P3. In group 2, PCT values remain similar along the determinations. CRP did not show significant changes in time. Wilcoxon rank sum test was performed. Values were expressed as median (interquartile range). CRP indicates C-reactive protein (mg/L); PCT, procalcitonin (ng/mL).
Outcomes and relationship with PCT
In group 1, PCT at P1, P2, and P3 correlated with pre-ECMO lactate (r = .687, P = .005; r = .546, P = .044 and r = .610, P = .027). There was also a correlation between PCT in P0 and PRISM III (r = .575, P = .032). In group 2, PCT in P1, P2, and P3 also correlated with pre-ECMO lactate (r = .753, P = .000; r = .676, P = .003 and r = .635, P = .008). There was also a correlation between PFi and PCT in P2 and P3 (r = −.492, P = .045 and r = −.569, P = .023).
Apart from the noted correlations, there were no statistically significant correlations between PCT at any moment, not with PRISM III, SOFA, and neither inotropic support prior to ECMO.
According to the outcomes, both groups were analyzed excluding patients who died within the first 72 hours of ECMO (6 in group 1 and 1 in group 2). In group 1, there were significant correlations between PCT values at P1, P2, and P3 and duration of ECMO and also for PCT at P1 and LOS. In group 2, there were significant positive correlations between PCT at P1, P2, and P3 and days in PICU and total LOS. In both groups, there were no correlations in terms of PCT and duration of MV. All data are reflected in Table 2.
Table 2.
Correlations between procalcitonin values and duration of ECMO support, mechanical ventilation, PICU admission, or hospital admission for each group.
| Group 1 |
||||||||
|---|---|---|---|---|---|---|---|---|
| PCT P0 |
PCT P1 |
PCT P2 |
PCT P3 |
|||||
| r | P | r | P | r | P | r | P | |
| ECMO support, d | −.435 | .18 | −.618 | .057 | −.710 | .022 | −.741 | .009 |
| PICU LOS, d | −.610 | .046 | .000 | NS | −.280 | NS | −.296 | NS |
| MV in days, d | −.534 | NS | −.468 | .172 | −.518 | NS | −.530 | NS |
| Hospital LOS, d | −.191 | NS | .733 | .016 | .188 | NS | .282 | NS |
| Group 2 |
||||||||
| PCT P0 |
PCT P1 |
PCT P2 |
PCT P3 |
|||||
| r | P | r | P | r | P | r | P | |
| ECMO support, d | .007 | NS | .087 | NS | .009 | NS | .033 | NS |
| PICU LOS, d | .044 | NS | .541 | .046 | .626 | .017 | .671 | .012 |
| MV, d | .208 | NS | .303 | NS | .25 | NS | .254 | NS |
| Hospital LOS, d | −.143 | NS | .572 | .033 | .648 | .012 | .742 | .004 |
Abbreviations: ECMO, extracorporeal membrane oxygenation; LOS, length of stay; MV, mechanical ventilation; NS, nonsignificant; PCT, procalcitonin (ng/mL); PICU, pediatric intensive care unit.
For this analysis, patients who died were excluded. Spearman correlation was performed. Values are expressed as r correlation and P value for each time.
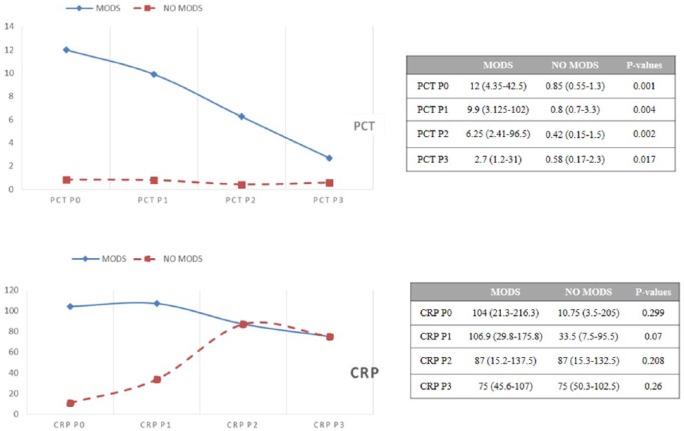
There were 31 patients (77.5%) with MODS: 19 in group 1 (100%) and 12 in group 2 (57.1%), P = 0.003. Those patients with organ failure had higher PCT values at all times compared with those without MODS: at P0, 12 ng/mL; P1, 9.9 ng/mL; P2, 6.25 ng/mL; and P3, 2.7 ng/mL, in contrast to patients without multiorgan failure: at P0, 0.85 ng/mL; P1, 0.8 ng/mL; P2, 0.42 ng/mL; and P3, 0.58 ng/mL, with P values of .001, .004, .002, and .017, respectively. C-reactive protein did not demonstrate statistically significant differences according to MODS at any time. Results are summarized in Figure 2.
Figure 2.
PCT and CRP values related to time according to the presence of MODS. Those patients with organ failure had higher PCT values at all times compared with those without multiorgan failure. CRP did not demonstrate statistically significant differences according to MODS at any time. Values are expressed as median (interquartile range). Mann-Whitney U test. CRP indicates C-reactive protein (mg/L); PCT, procalcitonin (ng/mL); MODS, multiple organ dysfunction syndrome.
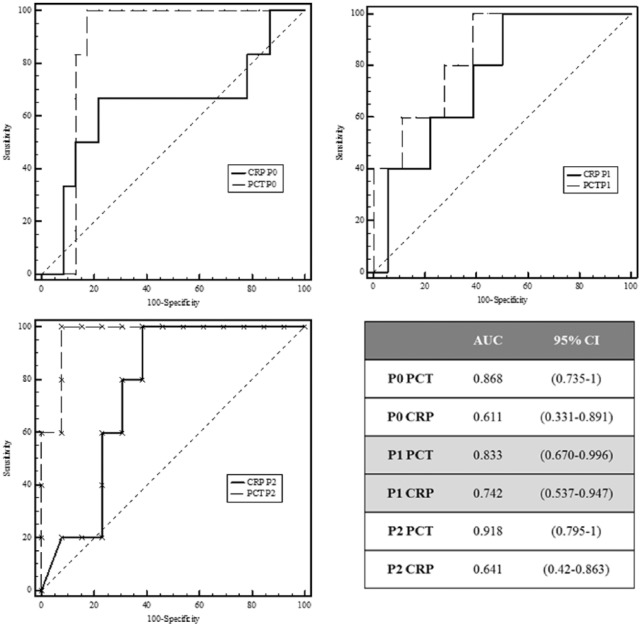
Receiver operating characteristic analysis was performed to compare organ failure with PCT and CRP for each time point P0, P1, and P2. Areas under the curve and 95% confidence interval (CI) for each time point are shown in Figure 3. Areas under the curve were better for PCT than for CRP for detecting MODS. At P0, AUC for PCT was 0.868 (95% CI: 0.69-0.963). Area under the curve was highest for PCT at P2: 0.918 (95% CI: 0.72-0.99). The best cutoff for PCT at P0 to predict evolution to MODS was 2.55 ng/mL, with a sensitivity of 83% and specificity of 100%.
Figure 3.
ROC analysis: PCT versus CRP values and MODS. AUCs were better for PCT than for CRP for detecting MODS at different times. AUCs, areas under the curve; CI, confidence interval; CRP, C-reactive protein; PCT, procalcitonin; ROC, receiver operating characteristic.
Both PCT and CRP in the first 72 hours did not seem to predict risk of neurologic sequelae or mortality in any group with P values greater than .05. All these results are summarized in Table 3.
Table 3.
Summary of P values after Mann-Whitney test performed between different PCT and CRP values related to time and the cases of exitus or neurologic sequelae.
| PCT and CRP versus exitus | ||||||
|---|---|---|---|---|---|---|
| Group 1 (n = 19) |
Group 2 (n = 21) |
|||||
| Exitus (n = 5) | No exitus (n = 14) | P | Exitus (n = 1) | No exitus (n = 5) | P | |
| PCT P0 | 125 (121-260) | 17.2 (11.7-42.5) | .54 | 1 | 1.7 (0.7-4.6) | .427 |
| PCT P1 | 185 (83-340) | 29.5 (40-155) | .76 | — | 2.3 (0.7-3.9) | — |
| PCT P2 | 246 (59-433) | 16.3 (6.4-163) | .944 | — | 1.5 (0.4-3) | — |
| PCT P3 | — | 7 (4-85) | — | — | 1.1 (0.4-1.7) | — |
| CRP P0 | 136 (67-194) | 115 (24-213) | .441 | — | 22.5 (4.9-218) | — |
| CRP P1 | 130 (27-194) | 109 (57-152) | .56 | — | 70 (17-149) | — |
| CRP P2 | — | 102 (34-136) | .796 | — | 33 (11-87) | — |
| CRP P3 | — | 95 (52-141) | — | — | 47 (5.5-75) | — |
| PCT and CRP versus neurologic sequelae | ||||||
| Group 1 (n = 19) |
Group 2 (n = 21) |
|||||
| Sequelae | No sequelae | P | Sequelae | No sequelae | P | |
| PCT P0 | 83 (39-337) | 17 (12-121) | .151 | 3.7 (0.8-6) | 1 (0.6-3.1) | .257 |
| PCT P1 | 102 (86-390) | 29.5 (10-213) | .257 | 2.3 (1.3-4.7) | 2.3 (1.3-3.9) | .752 |
| PCT P2 | 109 (59-271) | 16.3 (5.9-186) | .31 | 0.9 (0.3-3.3) | 1.8 (0.3-3) | .752 |
| PCT P3 | 73 (61-100) | 7 (3.4-86) | .39 | 0.5 (0.3-2.1) | 1.2 (0.4-1.8) | .777 |
| CRP P0 | 89 (45-210) | 140 (40-213) | .571 | 25 (94-13) | 20 (3-240) | .946 |
| CRP P1 | 104 (74-153) | 126 (32-163) | .866 | 96 (32-187) | 50 (13-145) | .428 |
| CRP P2 | 139 (110-234) | 94 (16-161) | .18 | 87 (33-135) | 26 (10-58) | .182 |
| CRP P3 | 41 (107-175) | 88 (56-141) | .182 | 75 (69-86) | 21 (5-73) | .122 |
Abbreviations: CRP, C-reactive protein (mg/L); PCT, procalcitonin (ng/mL).
Values expressed as median (interquartile range).
PCT and infection during ECMO
Eight patients (20%) had documented infection during ECMO support: 2 in group 1 and 6 in group 2. The median number of days from the onset of ECMO and a positive culture was 5 (IQR: 2.5-6.25). Fungi were isolated in 5 patients (62.5%), all Candida spp. There were 2 positive blood cultures for Candida; the other ones were respiratory cultures. There were 2 patients with positive cultures for Stenotrophomona maltophilia and 1 patient for Enterobacter cloacae, all positive in respiratory culture samples. There was no significant increase in PCT values of these patients at the moment of infection diagnosis, with P < .05 and PCT <10 ng/mL (median: 1.35, IQR).
Discussion
To our knowledge, this is the first study with a substantial number of pediatric patients in ECMO in which the behavior of the PCT is assessed and compared with the classical biomarker, CRP.
The sample was classified into 2 groups because it was considered to follow different patterns: those who started from a very high PCT value before entering ECMO and those with a lower value. The first group includes septic patients, with around 24 hours of evaluation before entering ECMO, so PCT levels were quite elevated. This was in accordance with literature, which describes the better PCT cutoff point for sepsis around 10 ng/mL. Group 2 included different pathologies in which PCT did not increase. It has to be considered that other clinical situations may stimulate PCT production (major surgery or polytraumatic patients) but with PCT lower values (under 10 ng/mL and around 2-5 ng/mL) because the inflammatory trigger was less potent.9,21–23
There is literature that supports the presence of higher PCT values in sepsis, with a decrease in PCT values when response to treatment is correct.24 Procalcitonin kinetics in group 1 describes the expected curve for the clinical situation of this group of septic cases, although patients were in ECMO. It was observed that PCT increased in the first 24 hours and then dropped, very similar to what was seen in septic patients without ECMO.24 However, in group 2, the PCT was practically normal before ECMO, remained unchanged in the first hours of ECMO support, and progressively decreased with time. Procalcitonin does not significantly elevate after exposure to exogenous ECMO material, as it does not change after cardiopulmonary bypass or other situations which may activate inflammatory response syndrome.13,25
There are few studies of the kinetics of PCT in ECMO. Data in adults suggest that PCT analyzed together with CRP may be an optimal method to detect the presence of infection in patients with arteriovenous ECMO.26 The limited data available in pediatrics suggest that PCT may be a good marker of morbidity and MODS, rather than of infection.15 Although there was a small sample of patients (20 cases) in the study performed by Rungatscher et al, the trend to higher levels of PCT was associated with MODS and suggested a PCT value >10 ng/mL after 48 hours as a predictor of adverse effects. According to this, our results show that the higher PCT levels were associated with MODS at all times of the PCT analysis, with better AUC than CRP. The cutoff which could alert to MODS complication was around 2.55 ng/mL pre-ECMO. Procalcitonin seems to correlate with classic markers of severity, such as lactate and PFi, so it could be a good prognosis biomarker. The SOFA score >15 has been associated with higher mortality rate in pediatric patients (up to 90%) so it has been considered a good marker of prognosis. However, scores seem to be more useful in the general population, whereas specific biomarkers, such as PCT, could help in the individual case.
Regarding days of ECMO support and LOS, PCT showed a positive correlation, indicating that it is useful as a prognosis factor. In adults, PCT appears to predict mortality. However, our study did not demonstrate any association. This could be explained by the small number of patients who died in our series.14
With respect to infection as an ECMO complication, data reported from 1998 to 2008 by the ELSO differ from our results.16 They reported a total of 2418 infections in 20 741 ECMO patients, corresponding to 11.7% of cases, with higher frequency in those who stayed in ECMO for more than 14 days. In our study, we found 8 infected patients (20%), all of them before 14 days of support in ECMO. The main causal agent in the ELSO database was the coagulase-negative Staphylococci, but Candida spp was the most frequent in the pediatric age, as in our study. Our results only defined PCT values at the moment of infection diagnosis. They did not show any significant increase in infected patients, probably because they were localized infections and caused by a fungus. Although there is little evidence, it appears that PCT minimally increases in the presence of fungal infection.27
The main limitations of our study are the size of the sample, the variety of diagnosis pre-ECMO, the single-center character of the design, and the few infections reported.
Regarding neurologic sequelae, we do not currently have a comprehensive follow-up of subsequent evolution so we are not aware of more subtle sequelae in our patients beyond cerebral infarctions that manifested during their PICU admission.
Conclusions
The kinetics of PCT and CRP in septic patients in ECMO seems to be quite similar to those described in patients who do not require it. Therefore, PCT could be useful in the same situations than in patients without ECMO (infection and even prognosis diagnosis). More studies are needed to establish the definitive PCT utility in ECMO patients.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: IJ, JR, SB, and AS conceived and designed the experiments. IJ, JR, and SB analyzed the data. SB and JR-F wrote the first draft of the manuscript. IJ, MB, and JM contributed to the writing of the manuscript. All authors agree with the manuscript results and conclusions, jointly developed the structure and arguments for the paper, made critical revisions and approved the final version, and reviewed and approved the final manuscript.
Disclosures and Ethics: The study was conducted in accordance with Helsinki Declaration and approved by the Sant Joan de Déu Ethical Investigational Committee.
References
- 1. Corsi F, Lebreton G, Bréchot N, et al. Life-threatening massive pulmonary embolism rescued by venoarterial-extracorporeal membrane oxygenation. Crit Care. 2017;21:76 http://ccforum.biomedcentral.com/articles/10.1186/s13054-017-1655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lin JC. Extracorporeal membrane oxygenation for severe pediatric respiratory failure. Respir Care. 2017;62:732–750. http://insights.ovid.com/crossref?an=00130478-201608000-00011. [DOI] [PubMed] [Google Scholar]
- 3. Rozencwajg S, Pilcher D, Combes A, Schmidt M. Outcomes and survival prediction models for severe adult acute respiratory distress syndrome treated with extracorporeal membrane oxygenation. Crit Care. 2016;20:392 http://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biffi S, Di Bella S, Scaravilli V, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. 2017;50:9–16. http://dx.doi.org/10.1016/j.ijantimicag.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 5. Yusuf E, Van Herendael B, Verbrugghe W, et al. Emergence of antimicrobial resistance to Pseudomonas aeruginosa in the intensive care unit: association with the duration of antibiotic exposure and mode of administration. Ann Intensive Care. 2017;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20:387 http://ccforum.biomedcentral.com/articles/10.1186/s13054-016-1570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Andriolo BN, Andriolo RB, Salomão R, Atallah ÁN. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev. 2017:CD010959 http://dx.doi.org/10.1002/14651858.CD010959.pub2. [DOI] [PMC free article] [PubMed]
- 8. Fioretto JR, Martin JG, Kurokawa CS, et al. Comparison between procalcitonin and C-reactive protein for early diagnosis of children with sepsis or septic shock. Inflamm Res. 2010;59:581–586. [DOI] [PubMed] [Google Scholar]
- 9. Pfister R, Kochanek M, Leygeber T, et al. Procalcitonin for diagnosis of bacterial pneumonia in critically ill patients during 2009 H1N1 influenza pandemic: a prospective cohort study, systematic review and individual patient data meta-analysis. Crit Care. 2014;18:R44 http://www.ncbi.nlm.nih.gov/pubmed/24612487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suberviola B, Rellan L, Riera J, et al. Role of biomarkers in early infectious complications after lung transplantation. PLoS ONE. 2017;12:e0180202 http://www.ncbi.nlm.nih.gov/pubmed/28704503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Launes C, Esteban E, Balaguer M, Alsina M, Cambra FJ, Jordan I. Procalcitonin-guidance reduces antibiotic exposure in children with nosocomial infection (PRORANI). J Infect. 2016;72:250–253. [DOI] [PubMed] [Google Scholar]
- 12. Ozsurekci Y, Oktay Arıkan K, Bayhan C, et al. Can procalcitonin be a diagnostic marker for catheter-related blood stream infection in children? J Pediatr. 2016;92:414–420. http://linkinghub.elsevier.com/retrieve/pii/S0021755716300249. [DOI] [PubMed] [Google Scholar]
- 13. Garcia IJ, Gargallo MB, Torné EE, Lasaosa FJ, Viñas AT, Tolosa CV, et al. Procalcitonin: a useful biomarker to discriminate infection after cardiopulmonary bypass in children. Pediatr Crit Care Med. 2012;13:441–445. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&;an=00130478-201207000-00011. [DOI] [PubMed] [Google Scholar]
- 14. Bobillo Pérez S, Rodríguez-Fanjul J, García IJ, Hernando JM, Sanz MI. Procalcitonin is a better biomarker than C-reactive protein in newborns undergoing cardiac surgery: the PROKINECA study. Biomark Insights. 2016;11:123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rungatscher A, Merlini A, De Rita F, et al. Diagnosis of infection in paediatric veno-arterial cardiac extracorporeal membrane oxygenation: role of procalcitonin and C-reactive protein. Eur J Cardio-Thoracic Surg. 2013;43:1043–1049. [DOI] [PubMed] [Google Scholar]
- 16. Zwischenberger J, Bartlett RH. The history of ECMO: firsthand accounts. In: Brogan TV, Lequier L, Lorusso R, Maclaren G, Peek G. eds. ELSO Red Book. 2nd ed. Ann Arbor, MI: ELSO Publications; 2000. [Google Scholar]
- 17. Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. [DOI] [PubMed] [Google Scholar]
- 18. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 20. Davidson J, Tong S, Hancock H, Hauck A, da Cruz E, Kaufman J. Prospective validation of the vasoactive-inotropic score and correlation to short term outcomes in neonates and infants after cardiothoracic surgery. Intensive Care Med. 2012;38:1184–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meisner M, Adina H, Schmidt J. Correlation of procalcitonin and C-reactive protein to inflammation, complications, and outcome during the intensive care unit course of multiple-trauma patients. Crit Care. 2006;10:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benoist JF, Edouard AR, Assicot M, Bohuon C, Roussy IG. Procalcitonin and C-reactive protein during the early posttraumatic systemic inflammatory response syndrome. 1998;24:185–188. [DOI] [PubMed] [Google Scholar]
- 23. Bölke E, Jehle PM, Trautmann M, et al. Different acute-phase response in newborns and infants undergoing surgery. Pediatr Res. 2002;51:333–338. [DOI] [PubMed] [Google Scholar]
- 24. Rey C, Los Arcos M, Concha A, et al. Procalcitonin and C-reactive protein as markers of systemic inflammatory response syndrome severity in critically ill children. Intensive Care Med. 2007;33:477–484. [DOI] [PubMed] [Google Scholar]
- 25. Davidson J, Tong S, Hauck A, Lawson DS, da Cruz E, Kaufman J. Kinetics of procalcitonin and C-reactive protein and the relationship to postoperative infection in young infants undergoing cardiovascular surgery. Pediatr Res. 2013;74:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pieri M, Greco T, De Bonis M, et al. Diagnosis of infection in patients undergoing extracorporeal membrane oxygenation: a case-control study. J Thorac Cardiovasc Surg. 2012;143:1411–1416. http://dx.doi.org/10.1016/j.jtcvs.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 27. Pieralli F, Corbo L, Torrigiani A, et al. Usefulness of procalcitonin in differentiating Candida and bacterial blood stream infections in critically ill septic patients outside the intensive care unit. Intern Emerg Med. 2017;12:629–635. http://www.ncbi.nlm.nih.gov/pubmed/28161884. [DOI] [PubMed] [Google Scholar]