Abstract
Background:
The benefit of 5 years of adjuvant endocrine therapy for women with hormone receptor-positive (HR+) breast cancer (BC) is beyond discussion. Nevertheless, the risk of recurrence of luminal BC persists for 15 years or more after diagnosis. Consequently, approaches of extended adjuvant therapy have been investigated in large clinical trials, with the ultimate aim of further reducing the risk of recurrence in patients with HR+ BC.
Methods:
A review of recently published trial data is presented to provide a solid basis for discussion. A discussion of the side effects of long-term endocrine treatment, multigenetic tests aiming to identify patients at particular risk, and an outlook for further promising targets are additional aims of this review.
Conclusion:
Extended adjuvant therapy seems beneficial in reducing distant relapse and contralateral BC for a selected group of patients with HR+ BC, particularly if aromatase inhibitors (AIs) are used after initial tamoxifen therapy. However, patients with lower risk of recurrence and initial AI therapy may suffer more from side effects than benefit from extended therapy.
Keywords: adjuvant therapy, early breast cancer, extended adjuvant therapy, hormone receptor-positive breast cancer, postmenopausal
Introduction
Estrogen receptor-positive (ER+) early breast cancer (EBC) is often considered as a chronic disease. Even if patients remain disease-free during the first 5 years of adjuvant treatment, about two-thirds of all breast cancer (BC) deaths and half of the recurrences occur up to 15 years after diagnosis,1 with a gross average annual risk of recurrence (ROR) of 2% per year.2,3 Others even assume that the long-term ROR continues steadily to at least year 20 and affects even patients with T1N0 disease.4 Thus, it stands to reason that extended adjuvant therapy might be beneficial for a patient’s long-term outcome.
Nodal positive (N+) disease, increased tumor size5 as well as ER+ BC, in comparison with ER-negative BC,6 seem to be associated with higher risk of late recurrence. Trying to characterize an adequate tool to identify patients at particular risk for recurrence is therefore more than reasonable. However, the potential reduction of late recurrences and deaths must outweigh the burden of toxic side effects as well as higher economic costs for prolonged therapy.7 Until now, no established standard regimen has been defined.
In this article, we review the most recent trial data with the aim of clarifying the following issues: whether extended endocrine treatment provides substantial beneficial value for all hormone receptor-positive (HR+) EBC patients or only for a selected group, and whether any predictive factor can be determined to identify those who benefit the most.
Methods
We performed a systematic literature search in the following electronic databases: PubMed, Cochrane Library and Embase. Unpublished data presented at the San Antonio Breast Cancer Symposium (SABCS) in 2016 were obtained from the SABCS homepage (https://www.sabcs.org/Portals/SABCS2016/). The search terms included: extended endocrine therapy, hormone receptor-positive early breast cancer and postmenopausal women. Reported data from long-term studies were also used for this review.
Findings
Aromatase inhibitors (AIs) for 5 years are superior to tamoxifen (TAM) in postmenopausal women with HR+ EBC.
A meta-analysis with 31,920 patients published in 2015 compared different adjuvant treatment regimens: 5 years AI versus 5 years TAM; 5 years AI versus 2–3 years TAM followed by AI to year 5; or 5 years of TAM versus 2–3 years TAM followed by AI to year 5.8 Significantly lower overall recurrence rates in years 0–4 could be demonstrated for 5 years of AI therapy compared with 5 years of TAM therapy [p < 0.00001; years 0–1 rate ratio (RR) 0.64 (0.52–0.78); years 2–4 RR 0.80 (0.68–0.93)]. Distant, local and contralateral recurrence rates were all significantly reduced. A significant reduction of 10-year BC mortality risk could be achieved with 5 years of AI as well [RR 0.89 (0.81–0.97), p = 0.009].8
Another essential finding of this meta-analysis is that TAM followed by AI is an advantageous alternative to TAM alone for 5 years. The ROR in years 2–4 [RR 0.56 (0.46–0.67)] and the 10-year BC mortality were significantly lower (8.7 versus 10.1%, p = 0.015) in patients switching to AI than in those remaining on TAM. However, the AI monotherapy remains superior to the sequenced regimen TAM/AI in recurrence rate [13.8 versus 14.5%, RR 0.90 (0.81–0.99), p = 0.045], although BC mortality rates were not influenced significantly [8.2 versus 9.3%, RR 0.89 (0.78–1.03), p = 0.11].8
Endocrine therapy beyond 5 years
TAM after TAM
The ATLAS trial was designed to clarify the additional benefit of extended endocrine treatment with TAM. This multicenter study included HR+ EBC patients without clinical progression after 5 years of TAM, who were randomized to a further 5 years of TAM or placebo.
Extended TAM treatment resulted in significant reduction of risk of BC recurrence (p = 0.002), BC mortality (p = 0.01) and overall mortality (p = 0.01). Furthermore, it especially lowered mortality after year 10 until year 20 after the primary diagnosis. During years 5–14 an absolute BC mortality risk reduction of 2.8% could be achieved for women undergoing extended TAM when compared with controls (BC mortality: 12.2% versus 15.0%). The BC mortality data for patients after year 15 were not mentioned separately. All in all, after reaching year 10 a significantly reduced BC mortality RR could be observed [0.71 (0.58–0.88), p = 0.0016].
Nevertheless, increased RRs for pulmonary embolism [1.87 (1.13–3.07), p = 0.01] and endometrial cancer [1.74 (1.30–2.34), p = 0.0002] must be taken into account in patients with extended TAM therapy. The cumulative risk for endometrial cancer during years 5–14 was 3.1% in patients with extended therapy versus 1.6% in the control group.9
Of the survivors, 91% were followed up 10 years after diagnosis, 77% until year 15. Nevertheless, a longer follow-up period is required to definitely prove side effects of extended TAM intake since it is known that 5 years of TAM therapy produces an absolute 15-year risk for endometrial cancer of about 2–3%, whereas 10 years of TAM intake would mean an additional risk by year 15 of approximately 2%.3,9
The aTTom trial, published in 2013 by Gray and colleagues,10 has in general the same inclusion criteria and confirms these results. Extending TAM to 10 years showed a significantly decreased risk of BC recurrence (16.7 versus 19.3%, p = 0.003). The RR was time dependent, 0.99 (0.86–1.15) during years 5–6, 0.84 (0.73–0.95) during years 7–9, and 0.75 (0.66–0.86) in later years. The overall mortality did not decrease significantly under the influence of extended therapy (24.4 versus 26.1%, p = 0.1); a RR of 1.05 (0.90–1.22) during years 5–9 and a RR of 0.86 (0.75–0.97) later was found. Endometrial cancer was more frequently diagnosed in patients with extended TAM therapy [RR 2.20 (1.31–2.34), p < 0.0001] than in those without endocrine therapy beyond 5 years. In patients with extended TAM therapy, an increased mortality of endometrial cancer could be observed as well (1.1 versus 0.6%, absolute hazard 0.5%, p = 0.02).11
However, a precise definition of ‘breast cancer recurrence’, whether locoregional, contralateral or distant recurrence was meant, is missing. A further limitation of the aTTom trial is that the ER status was untested in 4198 of 6953 patients (60%), thus the ER status was estimated to be positive in 80% of those with unknown status.
In comparison, in the ATLAS trial 37% of the women included had an unknown ER+ status. Consequently, only patients with ER+ BC were included in recurrence and BC mortality rate calculations.
To sum up, endocrine therapy with TAM beyond 5 years leads to significantly longer recurrence-free, disease-free and probably also overall survival (OS) (Table 1); however, as far as the existing data allow us to draw conclusions, an increased incidence of pulmonary embolism and endometrial cancer as well as an increase of endometrial cancer mortality must be balanced against the benefits. Further, whether the limited survival benefits substantially outweigh economic costs and the above-mentioned adverse events (AEs) must be considered.
Table 1.
Extended endocrine treatment with TAM after 5 years of TAM.
| Trial | Year | Study population | Inclusion criteria | Study arms | RFS | DFS | OS |
|---|---|---|---|---|---|---|---|
| ATLAS | 2013 | 6846 | ER+ EBC | 5a TAM versus placebo after initial 5a TAM | RR 0.84 (95% CI 0.76–0.94), p = 0.002 | 19% versus 21%, p = 0.01 |
|
| aTTom | 2013 | 6953 | ER+ EBC | 5a TAM versus no therapy after initial 5a TAM | 16.7% versus 19.3%, p = 0.003 | 24.4% versus 26.1%, p = 0.1 |
CI, confidence interval; DFS, disease-free survival; EBC, early breast cancer; ER+, estrogen receptor-positive; OS, overall survival; RFS, recurrence-free survival; RR, rate ratio; TAM, tamoxifen; 5a, 5years. Bold font for p-valures indicates statistical significance.
AI after TAM
The following three studies investigated the value of extending AI after initial TAM therapy in postmenopausal women with ER+/HR+ EBC.
MA.17 is a phase III, randomized, double-blinded, placebo-controlled study that investigated extended letrozole treatment in postmenopausal patients with ER+ BC after 5 years of initial TAM treatment. The study demonstrated beneficial effects on disease-free survival [DFS; Hazard Ratio = 0.58 (0.45–0.76), p < 0.001] and distant DFS [HR = 0.60 (0.43–0.84), p = 0.002], whereas no statistically significant difference was found for OS [HR = 0.82 (0.57–1.19)]. However, in lymph node-positive patients, a significantly improved OS was found for patients with extended letrozole treatment [HR = 0.61 (0.38–0.98), p = 0.04].12,13 A limitation of this study is a short follow-up period with a median of 30 months. Further, it must be kept in mind that all patients initially underwent 5 years of TAM, which has a long plasma and tissue half-life time.14 This might lead to a confounded toxicity assessment of AIs. Nevertheless, new diagnosed osteoporosis (8.1 versus 6.0%, p = 0.003) was significantly more frequently reported in patients taking letrozole. Whereas new bone fractures (5.3 versus 4.6%, p = 0.25), cardiovascular events (5.8 versus 5.6%, p = 0.76) and the occurrence of endometrial cancer (0.2 versus 0.4%, p = 0.12) did not differ significantly between the two groups.13 The MA.17 trial primarily included women who were premenopausal at initial diagnosis; a subgroup analysis published in 2012 showed that extended letrozole after 5 years of initial TAM therapy was effective in both pre- and postmenopausal patients (at time of diagnosis).15
The results of the NSABP B-33 study published in 2008 supported these results. The trial included 1598 postmenopausal women, who had been suffering from clinical T1-3, N0-1, M0, HR+ BC and were disease-free after 5 years of adjuvant therapy with TAM. In a 1:1 ratio patients were either assigned to 5 years of further exemestane treatment or to 5 years of placebo. A nonstatistically significant reduction in DFS event of 32%, translating to a 2% absolute improvement in 4-year DFS (89% for placebo versus 91% for exemestane, p = 0.07) and a statistically significant improvement in 4-year recurrence-free survival (RFS; 96 versus 94%, p = 0.004)16 were observed. At 2 years after study initiation, the published MA.17 trial17 showed beneficial outcomes for patients with extended AI therapy. At this time, the NSABP B-33 study was unblinded and 344 of the 779 patients of the placebo group (44%) decided to switch to the exemestane group. This might have led to confounded results due to a relatively small control group. Additionally, the lack of significance regarding OS might be explained by the small number of deaths that had occurred.16
The ABCSG trial 6a included 856 patients with HR+ BC, remaining disease-free after 5 years of TAM, who then were randomized to 3 years of AI therapy or to no further treatment. After a median follow up of 63 months a significant risk reduction of 38% for any recurrence (HR 0.62, p = 0.031) could be demonstrated for patients receiving anastrozole. The main difference could be achieved for distant metastases [HR 0.53 (0.29–0.96), p = 0.034], while locoregional [HR 0.79 (0.36–1.769), p = 0.564] or contralateral [HR 0.67 (0.25–1.80), p = 0.422] recurrences had not shown significant differences.18 In a subgroup analysis, additional benefit was also shown for patients with both ER+ and progesterone-receptor positive (PR+)tumors, who were randomized to extended therapy (HR 0.32, p < 0.001). The most common complications or AEs were hot flushes, asthenia, somnolence and allergy.19 A limitation of this trial is the nonplacebo-controlled design and the limited patient number.
Therefore, extended endocrine treatment with AI leads to prolonged RFS, DFS, but does not consistently impact OS (Table 2).
Table 2.
Extended endocrine treatment with AI after TAM.
| Trial | Year | Study population | Inclusion criteria | Study arms | RFS | DFS | Distant DFS | OS | Subgroup |
|---|---|---|---|---|---|---|---|---|---|
| MA.17 | 2003 | 5187 | ER+ EBC, postmenopausal | 5a letrozole versus placebo after initial 5a TAM | HR 0.58 (0.45–0.76),p < 0.001 | HR 0.60 (0.43–0.84) p = 0.002 | HR 0.82 (0.57–1.19) p = 0.3 | ||
| NSABP-33 | 2008 | 1598 | ER+ EBC, postmenopausal | 5a exemestane versus placebo after initial 5a TAM | 96% versus 94%, RR 0.44, p = 0.004 | 91% versus 89%, RR 0.68 p = 0.07 |
|||
| ABCSG 6a | 2007 | 856 | HR+ EBC, postmenopausal | 3a anastrozole versus no therapy after initial 5a TAM (± AI) | HR 0.62 (0.40–0.96) p = 0.031 | HR 0.89 (0.59–1.34) p = 0.57 | ER+ and PR+: RFS HR 0.32 (0.18–0.58) p > 0.001 |
AI, aromatase inhibitor; DFS, disease-free survival; EBC, early breast cancer; ER+, estrogen receptor-positive; HR, Hazard Ratio; OS, overall survival; PR+, progesterone-receptor positive; RFS, recurrence-free survival; RR, rate ratio; TAM, tamoxifen; 5a, 5years. Bold font for p-valures indicates statistical significance.
Recently published data
AI after TAM/AI
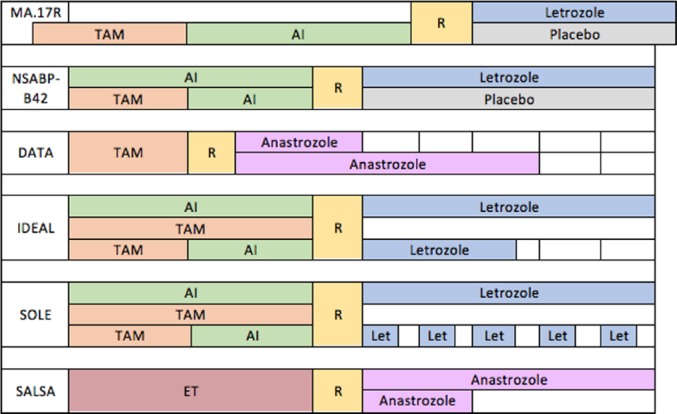
Since sequencing TAM and AI is a commonly used adjuvant therapy constellation,8 the question arises whether extending AI after initial TAM/AI therapy adds further benefit. There are five recently published studies and one ongoing study dealing with this issue. The study designs are summarized in Figure 1; the results of the already presented data are shown in Table 3.
Figure 1.
Study designs of MA.17R, NSABP-B42, DATA, IDEAL, SOLE and SALSA (ABCSG 16) trials.
AI, aromatase inhibitor; ET, endocrine therapy; Let, Letrozole; TAM, tamoxifen; R, randomization.
Table 3.
Extending AI after initial 5 years of any endocrine therapy.
| Trial | Year | Study population | Inclusion criteria | Study arms | DFS | OS | Subgroup |
|---|---|---|---|---|---|---|---|
| MA.17R | 2016 | 1918 | Postmenopausal | 5a letrozole versus placebo after initial 5a AI | 5a: 95% versus 91%, HR 0.66 (0.48–0.91),p = 0.01 | 93% versus 94%, HR 0.97 (0.73–1.28) p = 0.83 | |
| NSABP-42 | 2016 | 3923 | ER or PR+ EBC, postmenopausal | 5a exemestane versus placebo after initial 5a of ET | 7a: 84% versus 81%, HR 0.85 (0.73–0.99)p = 0.048 (NS) | 92% versus 92%, HR 1.15 (0.99–1.44) | Prior TAM: DFS HR 0.75, p = 0.04 |
| DATA | 2016 | 1660 | HR+ EBC, postmenopausal | 6a versus 3a anastrozole after initial 2–3a TAM | 5a: 83% versus 79%, HR 0.79 (0.62–1.02),p = 0.07 | 5a: 90% versus 90%, HR 0.91 (0.65–1.29), p = 0.60 | Better outcome: ER+ and PR+; N+, pT2+, prior chemotherapy or HER2-BC |
| IDEAL | 2016 | 1824 | HR+BC | 2.5a letrozole versus 5a letrozole after initial 5a ET | 5a: 88.4% versus 87.9%, HR 0.96 (0.76–1.20), p = 0.70 | 93.5% versus 92.6%, HR 1.08 (0.81–1.45), p = 0.59 | Incidence of 2nd BC: 1.9% versus 0.9% HR 0.37 (0.18–0.77), p = 0.008, better outcome: N+ |
AI, aromatase inhibitor; BC, breast cancer; DFS, disease-free survival; EBC, early breast cancer; ER+, estrogen receptor-positive; ET, endocrine therapy; HR, hazard ratio; N+, node-positive; NS, not significant; OS, overall survival; PR+, progesterone-eceptor positive; RR, risk ratio; TAM, tamoxifen; a, years. Bold font for p-valures indicates statistical significance.
The MA.17R trial, a double-blind, placebo-controlled trial, included 1918 patients who had received AI for a total of 4.5–6 years. In most cases, AI therapy was preceded by TAM treatment. Only 20.7% of the patients underwent AI as single adjuvant endocrine therapy. Patients were further randomized to either 5 years of AI or placebo. After a median follow up of 6.3 years, the 5-year DFS was significantly longer for patients receiving letrozole instead of placebo (95 versus 91%). The hazard ratio for local or contralateral recurrence under letrozole therapy was 0.66 (0.48–0.91, p = 0.01) compared with placebo. However, the 5-year OS did not differ significantly between patient groups (93 versus 94%, p = 0.83).
Bone-related side effects were associated with letrozole intake. A significant decrease in bone density could be found in patients receiving letrozole (mean loss -3.2% in letrozole group versus +1.4% in placebo group, p < 0.001).20
A very similarly designed trial is the recently presented, randomized, double-blind, placebo-controlled clinical trial NSABP-B42. Postmeno-pausal women with ER+ or PR+, stage I, II or IIIa BC were included if they had remained disease-free during adjuvant endocrine therapy with either AI for 5 years or TAM for a maximum of 3 years followed by AI to complete 5 years of therapy. A total of 1959 patients were randomized to a further 5 years of letrozole therapy whereas 1964 patients received placebo therapy. The median follow up was 6.9 years. For patients with extended letrozole therapy, a risk reduction for DFS events of 15% was achieved. In contrast with the findings of the MA.17R trial, the difference in DFS between control and placebo group did not reach statistical significance [7-year DFS 84.7 versus 81.3%, HR 0.85 (0.73–0.999), p = 0.048, statistical significance level 0.0418]. Regarding OS, a significant difference between control and placebo group was also not found [91.8 versus 92.3%, HR 1.15 (0.99–1.44), p = 0.22]. However, patients under extended endocrine therapy were significantly less frequently affected by distant recurrence [HR 0.72 (0.53–0.97), p = 0.03]; a risk reduction of 28% was observed. Further, a significantly longer BC-free interval (BCFI), defined as time to recurrence or contralateral BC as first event, could be observed in the letrozole group [incidence of BCFI events 6.7 versus 10.0%, HR 0.71 (0.56–0.89), p = 0.003].
It could be demonstrated that extended AI therapy was most beneficial for patients with prior TAM intake (HR 0.75, p = 0.04) and with a T-score ⩽-2.0 (HR 0.70, p = 0.03).
Extended letrozole intake was associated with an increased risk of arterial thrombotic events (HR 1.85, p = 0.007), and the incidence of osteoporotic fractures remained unaffected.21
The DATA trial was designed to investigate the effect of extended AI therapy after TAM. In this multicenter phase III trial, 1660 postmenopausal women with HR+ EBC, who underwent 2–3 years TAM therapy, were randomized to 6 or 3 years anastrozole daily. The primary endpoint was the adapted DFS (aDFS), defined as DFS starting 3 years after randomization. The 5-year aDFS did not differ significantly [83.1 versus 79.4%, HR 0.79 (0.62–1.02), p = 0.07].
However, a subgroup analysis showed an increased 5-year aDFS for patients with both ER+ and PR+ tumors [84.8 versus 78.8%, HR 0.70 (0.53–0.92)], as well as for patients with high-risk characteristics such as nodal positivity [82.2 versus 77.4%, HR 0.72 (0.52–1.00)], pT2 or larger tumors [81.5 versus 75.3%, HR 0.72 (0.52–0.98)], prior (neo)adjuvant chemotherapy [85.8 versus 80.1%, HR 0.68 (confidence interval (CI) 0.49–0.92)] or women who suffered from HER2-negative BC [83.2 versus 79.7%, HR 0.79 (0.61–1.03)]. In the existing data presentation, no p-values are available.
For patients with all of the above-mentioned factors [ER+ and PR+, HER2-negative, ⩾pT2, N+, prior (neo)adjuvant chemotherapy], a further increase of the 5-year aDFS was observed when randomized to 6 years of anastrozole [86.0 versus 75.9%, HR 0.58 (0.39–0.89), p = 0.01].
Thus, patients with worse tumor characteristics, such as ⩾pT2, N+ and prior chemotherapy, seem to have a greater benefit from extending adjuvant therapy.
The incidence of AEs after 3 years of randomization was higher in patients receiving AI for 6 years, although only grade 1–2 AEs were observed.
In conclusion, Tjan-Heijnen and colleagues did not recommend extended adjuvant therapy to all postmenopausal patients with HR+ BC, whereas a selected group of patients seems to benefit. Instead of extending the duration of adjuvant endocrine therapy, they suggest exploring promising new targeting therapies.22 Limitations of this study were a short follow-up period (median adapted follow up was 4.1 years) as well as the nonplacebo-controlled and nonblinded study design. The study design of the DATA trial did not include patients with initial sequenced TAM/AI or AI monotherapy.
The IDEAL trial is a multicenter phase III trial, which included 1824 women with HR+ BC randomized between 2007 and 2011 with the intention to determine the optimal duration of extended adjuvant letrozole therapy. Patients had to complete 5 years of any commonly used endocrine therapy regimens and then subsequently were randomized to extended adjuvant letrozole therapy, either 2.5 years or 5 years. The primary endpoint of this study was DFS. The median follow up was 6.5 years. No significant difference in 5-year DFS could be found between patients with either 2.5 years or 5 years extended letrozole therapy [88.4 versus 87.9%, HR 0.96 (0.76–1.20), p = 0.70]. The 5-year OS did not differ significantly between those two groups either [93.5 versus 92.6%, HR 1.08 (0.81–1.45), p = 0.59] A subgroup analysis showed that patients with N+ disease had a major advantage from longer extended adjuvant therapy than those with nodal-negative BC. Due to unpublished data, HR cannot be mentioned.
However, it could be shown that the incidence of secondary BC after 5 years of randomization was higher in women with shorter extended adjuvant therapy than in patients with 5 years of additional AI therapy [1.9 versus 0.9%, HR 0.37 (0.18–0.77), p = 0.008].
The safety assessment found a low number of additional AEs in those patients with further 5 years of letrozole therapy.23 The nonplacebo-controlled study design might be a limitation.
The SOLE study was recently presented at the ASCO annual meeting in June 2017. This phase III trial included 4884 postmenopausal women with HR+, N+ early-stage BC with the purpose to investigate the effect of a new therapy concept of letrozole. It was designed to assess the role of continuous versus intermittent letrozole intake. After 5 years of adjuvant endocrine therapy patients were either randomized to 5 years of continuous (n = 2441) or to 5 years of intermittent (n = 2443) letrozole administration whereby 3-month treatment-free intervals must be adhered to. The hypothesis is that intermittent letrozole intake will permit some estrogenic stimulation during the respective pauses. Within this hormonal withdrawal period, residual cancer might be susceptible to letrozole reintroduction, which should translate into a higher DFS, the primary endpoint of this study.24 After 60 months of follow up, similar 5 year DFS rates were observed in patients with intermittent and continuous letrozole administration [85.8 versus 87.5%, HR 1.08 (0.93–1.26), p = 0.31]. Moreover, no significant difference was observed for BC-free interval [HR 0.98 (0.81–1.19)], distant recurrence-free survival [HR 0.88 (0.71–1.09)] or OS rates [HR 0.88 (0.68–1.07)]. P-values were not presented in the available abstract.25
This trial confirmed non-inferiority of the intermittent letrozole administration. Pauses in treatment up to 3 months per year could be tolerated, given that the efficacy of the adjuvant treatment does not decrease. Although it might be assumed that a more flexible therapy administration leads to an increase of patient compliance, in both therapy arms 24% of patients discontinued letrozole earlier.
Interestingly, AEs >grade 3 were reported slightly more often in patients with intermittent letrozole intake (43.5 versus 41.6%, no p-value available).
A still ongoing study is the ABCSG 16–SALSA trial. It is designed as a prospective, randomized, multicenter phase III study aiming to assess the efficacy of secondary adjuvant endocrine anastrozole therapy for a further 2 years versus 5 years, after an initial 5 years of endocrine therapy.
The trial includes postmenopausal women with an invasive T1–3, N0 or N+, M0 and HR+ BC, with or without previous chemotherapy or radiotherapy.
The primary endpoint of this study is the effect of 2 versus 5 years of additional anastrozole intake on DFS.27
Results are expected by the end of 2017.
Recently presented data could not confirm advantageous effects of extended adjuvant therapy for all women with ER+ BC. Nevertheless, patients with ER+ and PR+ BC, or patients at higher risk like N+, pT2 or larger tumors or women after (neo)adjuvant chemotherapy, seem to benefit from extending adjuvant therapy with AI.
AI after TAM versus AI after AI
Data show that the two main therapy strategies, AI after TAM versus AI after AI, provide different beneficial impact on DFS.
There is evidence that the regime AI after TAM comes along with a lower HR for DFS events than the one with AI after AI. Placebo-controlled trials, such as MA.17,17 NSABP B-3316 and ABCSG 6a,19 that compare AI after TAM have shown a HR of 0.57–0.68 regarding DFS events.
As soon as the initial endocrine therapy contains AI, a lower benefit, referring to a lower risk reduction of DFS events, can be anticipated when AI therapy is further extended. This statement is based on results of trials investigating the effect of extended AI after initial AI or TAM/AI therapy. The MA.17R,20 NSABP B-4221 and the IDEAL trial23 observed HRs of 0.80–0.88, translating to a lower risk reduction.
A subgroup analysis within the NSABP B-42 trial showed that patients who received initial ET containing TAM had a significant lower HR regarding DFS than those without prior TAM (HR 0.75 versus 0.91).21,28
Adverse events under extended treatment
An increase in fracture rates and bone density loss in patients with BC caused by AI treatment is well known. The reason is that AI suppresses the conversion of androgens to estrogens, resulting in estrogen depletion.29 As shown in many trials, adjuvant endocrine therapy compromises bone health and causes osteopenia and even osteoporosis. The prevention of these side effects could be achieved with the additional administration of bisphosphonates or anti-RANK ligand antibodies.
The ABSCG 12 trial compared adjuvant endocrine therapy regime with tamoxifen or anastrozole ± zoledronic acid in premenopausal patients with ER+ EBC. The addition of zoledronic acid resulted in an increased DFS.30
Several studies provided evidence that zoledronic acid reduces incidence of micrometastasis in the bone marrow.31,32 To summarize, zoledronic acid does not only have antitumor effects in the bone, but also a beneficial impact on DFS.30
The ABCSG 18 trial shows in a multicenter, randomized, double-blind, placebo-controlled, phase III trial the positive effect of denosumab, an anti-RANK ligand antibody, on bone health and delayed time to the first clinically relevant fractures. The trial showed that the time to first fracture was doubled compared with placebo.33
The risk of bone loss and fractures is a relevant factor in all of the trials about extended endocrine therapy. The meta-analysis of the EBCTCG8 showed that the risk of clinically relevant fractures varies in the different age groups. In the age group >55 years, a bone fracture incidence of 7.2% was registered, and this increased with age. In the >70 years group a bone loss incidence of up to 15.9% was reported. Bone-protective drugs such as bisphosphonate or denosumab should be considered additionally to prevent bone-related (severe) AEs.
There is evidence that adjuvant bisphosphonates reduce BC recurrences in bone and improve BC survival.34–36 Patients with a low-estrogen environment at baseline of their therapy seem to benefit most.37 A standardized bone-protective therapy in patients with long-term endocrine treatment is a daily application of calcium-cholecalciferol supplementation and should be provided for all patients, but there is evidence that calcium supplementation can increase cardiovascular events in healthy older (postmenopausal) women. Therefore, it is important to evaluate the necessity of calcium supplementation individually. A higher dietary calcium intake does not cause cardiovascular events.38
Genomic risk classifiers, really helpful tools?
The question arises as to how to identify patients at particular risk. Genomic testing seems to be a reliable tool for risk stratification.27
Patients with ER+ BC seem to be at higher risk for late distant recurrence than patients with ER-negative BC, which is mostly recurring within the first 5 years.
Yu and colleagues demonstrated by analyzing data of 111,993 BC patients that the hazard of BC-specific mortality in the years 0–2 (HR 3.97; 95% CI 3.66–4.30) and 2–5 (HR 1.94; 95% CI 1.85–2.05) was higher among patients with ER-negative tumors, keeping ER+ as reference. However, 5–10 years after diagnosis, patients with ER+ BC have an increased risk of BC-specific mortality compared with those with ER-negative BC (HR 0.71; 95% CI 0.66–0.76).6
Wolmark and colleagues investigated the utility of the 21-Gene Recurrence Score (RS), designed to predict late (>5 years) distant recurrence in stage I and II BC, in combination with the quantitative ER expression level (ESR1).28 RS seems to be a strong predictor for distant recurrence after 5 years in patients with high ESR1 levels. ROR is low for women with low RS. The results suggested that patients with intermediate and high RS with higher ESR1 expression levels at initial diagnosis might profit from extended adjuvant therapy. However, there are no clinical data supporting this hypothesis.
The PAM50 is another prognostic multigene test. Based on the PAM50 gene analysis, the PAM50 ROR score can be calculated and patients can be assigned to defined ROR-based risk groups. Filipits and colleagues could demonstrate the usefulness of this test by performing the PAM50 analysis in 1246 patients of the ABCSG-8 trial.39 Depending on their ROR score, patients were assigned to a low, intermediate or high-risk group. Between years 5 and 15, an absolute risk of distant recurrence was found of 17.5% in the high-risk and 2.4% in the low-risk group [HR 6.90 (1.89–11.87), p < 0.001], respectively. Thus,40 the PAM50 ROR score and the ROR-based risk groups are a reliable tool to predict late distant recurrence.41
Although it could be demonstrated that the PAM50 ROR score accurately predicted the risk of late distant recurrence, Filipits and colleagues concluded that it is still not clear whether patients in the high-risk group will benefit from extended adjuvant therapy.42
The findings of Sestak and colleagues confirmed these results further. Follow-up data from 2137 postmenopausal women with HR+ EBC from the ABCSG 8 and the TransATAC trial were analysed. It could be shown that the PAM50 ROR score added clinically important prognostic information to the clinical treatment score, which was developed on the TransATAC data set. In the follow-up years 5–10, the ROR score was significantly prognostic by itself. Still, the question remains open if patients with higher ROR scores would benefit from extended adjuvant therapy beyond 5 years.43
Another multigene test is the EndoPredict (EP) with its EPclin score, which combines the EP with tumor size and nodal status. Dubsky and colleagues could demonstrate that the EP test provides significant prognostic information in the early (0–5 years) and the late (>5 years) time interval after diagnosis. A total of 1702 postmenopausal women with ER+/HER2-negative BC, who were treated with 5 years of ET and participated in the ABCSG 6 or the ABCSG 8 trial, were assigned to risk groups based on their EP score. A total of 49% of patients were stratified according to their EP score to the low-risk group. With an absolute of 1.8% of late distant metastasis at 10 years of follow up, patients in the low-risk group had a significantly improved outcome.44
The two-gene expression ratio, HOXB13/IL17BR (H/I) is a prognostic biomarker in ER+ EBC patients. A high H/I ratio is statistically significantly associated with a reduced ROR in patients receiving extended letrozole therapy. In patients without extended letrozole therapy, a high H/I ratio identifies a subgroup that is at higher risk for late recurrence.45
Multigene assays such as EP, PAM50 ROR score, HOXB13/IL17BR including the Breast Cancer Index seem to improve the prognostic abilities of well-established clinical predictors such as TNM stage and hormone receptor status. The identified patients at low risk for recurrence seem to have a favorable prognosis, and thus seem to have only a very little or no benefit from extended adjuvant therapy.46 However, further investigation is required to clarify if patients at higher risk substantially benefit from adjuvant therapy beyond 5 years.
The use of biomarkers to guide decisions on adjuvant endocrine therapy in patients with early-stage BC is still a matter of debate.
There is evidence that the urokinase plasminogen activator (uPA) and its inhibitor plasminogen activator inhibitor-1 (PAI-1) can be used as predictive biomarkers in patients with HR+ HER2-negative BC.47,48 They seem to play a major role in cancer invasion and metastasis. High levels of uPA and PAI-1 predict on the one hand poor prognosis but on the other hand benefit from adjuvant chemotherapy.49,50
Regarding the predictive value of microtubule associated protein (MAP)-Tau mRNA or circulating tumor cells (CTCs)51,52 as biomarkers, controversial results can be found.48 Higher levels of tumor infiltrating leukocytes (TILs), for example, are associated with an increased benefit of trastuzumab in HER2+ BC.53 Nevertheless, especially in HR+ HER2-negative BC, there seems to be not enough evidence to use TILs to guide decisions on adjuvant systemic therapy.48
What is coming next?
Currently, new therapeutic targets are under investigation. Apart from HRs and HER2neu-receptor, oncogenic tyrosine kinases and related pathways, such as the PI3K/AKT/mTOR pathway, are potential targets.
Cyclin-dependent kinases play an important role in cell cycle progression. Targeting these kinases lead to a cell cycle block and might improve the outcome in aggressive BC subtypes. Based on this, CDK4/6 inhibitors seem to be promising agents and are already part of clinical phase III trials. A well-tested substance is palbociclib, either used as monotherapy or in combination with trastuzumab or cytotoxic chemotherapy.54 Results of the currently ongoing PALLAS trial are awaited. It is a prospective, multicenter, randomized, two arm, open-label phase III study with the purpose to determine if the addition of 2 years of palbociclib to at least 5 years of standard adjuvant treatment comes along with an improved outcome in patients with HR+, HER2-negative early BC.
Another new approach is blocking the insuline-like growth factor (IGF) pathway, which is activated in 90% of BC cases and is closely related to both HER2neu-receptor and mTOR pathway. However, a single inhibition of the IGF pathway can induce a diffuse upregulation of mechanisms that potentially trigger further malignant growth progression. A promising option is a combination of IGF blockers with mTOR inhibitors, which leads to a clinical improvement and even to partial remission in metastatic BC.55,56
BC stem cells seem to be important mediators of therapy resistance. A new approach is to target the self-renewing signaling pathways. Agents like Delta-ligand 4-blockers are under investigation in xenograft models and show anti-BC stem cells effect.57
The initiation process of metastatic disease might find its origin in dormant disseminated tumor cells.58 Those cells can enter dormancy, even at a very early stage and become immune to different forms of therapy. They can develop metastatic growth59 even after long periods of dormancy and can express, by that time, a completely different tumor biology compared with the primary tumor.60 This might explain a lack of response to therapy even if the initially administered treatment was effective. New therapeutic strategies are aiming to either kill awoken dormant cells or keep them permanently dormant.61
Conclusion
Recent data demonstrate clear superiority of AI to TAM for the first 5 years as standard treatment in postmenopausal patients with HR+ BC.
Taken together, there is evidence that extended adjuvant endocrine therapy has a benefit on DFS; this has been proven in several clinical trials. The initial regime, meaning if TAM or AI was administered first, must be taken into account in the decision-making process, regarding further extended ET.
To sum up, extended ET should be favored in patients who received prior treatment with TAM, especially because of the well-known fact that TAM is associated with rare but serious risk of venous thromboembolism. The extended treatment with AI after AI should be considered carefully in relation to AEs and the tumor risk profile.
Patients at higher clinical risk, such as N+ with larger tumors, seem to have more benefit from extended endocrine treatment. Patients with low recurrence risk as defined by different multigenomic tools have a very good prognosis and seem to have only very limited or no additional improvement due to prolonged endocrine treatment. Extension of endocrine therapy beyond 5 years seems to be not reasonable for these patients.
However, feasible predictive markers to identify those who could probably benefit from prolonged endocrine treatment, are still unknown. The patients with clearly defined high clinical risk for recurrence (N+, large tumors etc.) should be carefully selected. The individual benefit and risk for AEs under prolonged therapy, as well as need for additional medication (for example, calcium-cholecalciferol supplementation, bisphosphonates, denosumab) must be clarified.
The beneficial effect of prolonged treatment for high-risk patients, identified by using multigenomic tests, remains unclear. Thus, further studies need to be performed to confirm the advantage of extended adjuvant endocrine therapy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: K.W., M.B., Y.D. and B.K. are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review. R.E. has received travel and accommodation expenses reimbursement from Roche. S.S. has received personal fees from AstraZeneca, and travel or accommodation expenses reimbursement from Roche. F.F. has received travel or accommodation expenses reimbursement from Roche. M.G. has received institutional grants from Sanofi-Aventis, Novartis, Roche, GlaxoSmithKline, Pfizer, and Smith Medical, and personal fees from Novartis, Roche, GlaxoSmithKline, AstraZeneca, Nanostring Technologies, and Accelsiors, all outside this work.
Contributor Information
Kerstin Wimmer, Department of Surgery and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Stephanie Strobl, Department of Surgery and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Michael Bolliger, Department of Surgery and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Yelena Devyatko, Department of Surgery and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Belgin Korkmaz, Department of Surgery and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Ruth Exner, Department of Surgery and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Florian Fitzal, Department of Surgery and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria.
Michael Gnant, Department of Surgery and Comprehensive Cancer Center, Medical University of Vienna, Waehringer Guertel 18–20, 1090, Vienna, Austria.
References
- 1. Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer. Cochrane Database Syst Rev 2001: CD000486. [DOI] [PubMed] [Google Scholar]
- 2. Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol 1996; 14: 2738–2746. [DOI] [PubMed] [Google Scholar]
- 3. Davies C, Godwin J, Early Breast Cancer Trialists’ Collaborative Group et al.. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomized trials. Lancet 2011; 378: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan H, Davies C, Peto R, et al. ; for the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Predictors of recurrence during years 5–14 in 46,138 women with ER+ breast cancer allocated 5 years only of endocrine therapy (ET). J Clin Oncol 2016; 34(Suppl. 15) 505–505. abstract 505. [Google Scholar]
- 5. Kennecke HF, Olivotto IA, Speers C, et al. Late risk of relapse and mortality among postmenopausal women with estrogen responsive early breast cancer after 5 years of tamoxifen. Ann Oncol 2007; 18: 45–51. [DOI] [PubMed] [Google Scholar]
- 6. Yu KD, Wu J, Shen ZZ, et al. Hazard of breast cancer-specific mortality among women with estrogen receptor-positive breast cancer after five years from diagnosis: implication for extended endocrine therapy. J Clin Endocrinol Metab 2012; 97: E2201–E2209. [DOI] [PubMed] [Google Scholar]
- 7. Cianfrocca M. Overcoming recurrence risk: extended adjuvant endocrine therapy. Clin Breast Cancer 2008; 8: 493–500. [DOI] [PubMed] [Google Scholar]
- 8. Dowsett M, Forbes JF, Early Breast Cancer Trialists’ Collaborative Group et al. Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomized trials. Lancet 2015; 386: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 9. Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomized trial. Lancet 2013; 381: 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gray RG, Rea D, Handley D, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; 31(Suppl.): abstract 5. [Google Scholar]
- 11. Gray R, Handley K, Bowden SJ, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6953 women with early breast cancer. J Clin Oncol 2013; 31(Suppl. 15): abstract 5. [Google Scholar]
- 12. Goss PE, Ingle JN, Martino S, et al. Efficacy of letrozole extended adjuvant therapy according to estrogen receptor and progesterone receptor status of the primary tumor: National Cancer Institute of Canada Clinical Trials Group MA.17. J Clin Oncol 2007; 25: 2006–2011. [DOI] [PubMed] [Google Scholar]
- 13. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005; 97: 1262–1271. [DOI] [PubMed] [Google Scholar]
- 14. Fabian C, Sternson L, Barnett M. Clinical pharmacology of tamoxifen in patients with breast cancer: comparison of traditional and loading dose schedules. Cancer Treat Rep 1980; 64: 765–773. [PubMed] [Google Scholar]
- 15. Goss PE, Ingle JN, Martino S, et al. Impact of premenopausal status at breast cancer diagnosis in women entered on the placebo-controlled NCIC CTG MA17 trial of extended adjuvant letrozole. Ann Oncol 2013; 24: 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mamounas EP, Jeong JH, Wickerham DL, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 trial. J Clinical Oncol 2008; 26: 1965–1971. [DOI] [PubMed] [Google Scholar]
- 17. Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med 2003; 349: 1793–1802. [DOI] [PubMed] [Google Scholar]
- 18. Schmid M, Jakesz R, Samonigg H, et al. Randomized trial of tamoxifen versus tamoxifen plus aminoglutethimide as adjuvant treatment in postmenopausal breast cancer patients with hormone receptor-positive disease: Austrian breast and colorectal cancer study group trial 6. J Clin Oncol 2003; 21: 984–990. [DOI] [PubMed] [Google Scholar]
- 19. Jakesz R, Greil R, Gnant M, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst 2007; 99: 1845–1853. [DOI] [PubMed] [Google Scholar]
- 20. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med 2016; 375: 209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mamounas EP, Bandos H, Lembersky BC, et al. A randomized, double-blinded, placebo-controlled clinical trial of extended adjuvant endocrine therapy (tx) with letrozole (L) in postmenopausal women with hormone-receptor (+) breast cancer (BC) who have completed previous adjuvant tx with an aromatase inhibitor (AI): Results from NRG Oncology/NSABP B-42. Presented at San Antonio Breast Cancer Symposium, December 6–10, 2016; San Antonio, TX. Abstract S1–05. [Google Scholar]
- 22. Tjan-Heijnen VC, Peer PG, Swinkels AC, et al. First results from the multicenter phase III DATA study comparing 3 versus 6 years of anastrozole after 2–3 years of tamoxifen in postmenopausal women with hormone receptor-positive early breast cancer. Presented at San Antonio Breast Cancer Symposium, December 6–10, 2016; San Antonio, TX. Abstract S1–03. [Google Scholar]
- 23. Blok EJ, Meershoek-Klein Kranenbarg EM, Putter H, et al. Optimal duration of extended letrozole treatment after 5 years of adjuvant endocrine therapy; results of the randomized phase III IDEAL trial (BOOG 2006–05). Presented at San Antonio Breast Cancer Symposium, December 6–10, 2016; San Antonio, TX. Abstract S1–04. [Google Scholar]
- 24. Colleoni M. Letrozole in preventing cancer in postmenopausal women who have received 4–6 years of hormone therapy for hormone receptor-positive, lymph node-positive, early-stage breast cancer (SOLE). Cancer Res 2011; 71(Suppl. 24): Abstract nr OT2–02–01. [Google Scholar]
- 25. Colleoni M, Luo W, Karlsson P, et al. SOLE (Study of Letrozole Extension): a phase III randomized clinical trial of continuous vs intermittent letrozole in postmenopausal women who have received 4–6 years of adjuvant endocrine therapy for lymph node-positive, early breast cancer (BC). J Clin Oncol 2017; 35(Suppl. 15): abstract 503. [Google Scholar]
- 26. Coutant C, Rouzier R, Qi Y, et al. Distinct p53 gene signatures are needed to predict prognosis and response to chemotherapy in ER-positive and ER-negative breast cancers. Clin Cancer Res 2011; 17: 2591–601. [DOI] [PubMed] [Google Scholar]
- 27. Gnant M, Steger GG, Greil R, et al. A prospective, randomized, open, multicentre phase III study to assess the efficacy of secondary adjuvant endocrine anastrozole therapy for 2 further Yrs vs 5 further Yrs in patients with HR +ve breast cancer after 5-ye primary adjuvant endocrine therapy. Unpublished data.
- 28. Wolmark N, Mamounas EP, Baehner FL, et al. Prognostic impact of the combination of recurrence score and quantitative Estrogen Receptor Expression (ESR1) on predicting late distant recurrence risk in estrogen receptor-positive breast cancer after 5 years of tamoxifen: results from NRG oncology/national surgical adjuvant breast and bowel project B-28 and B-14. J Clin Oncol 2016; 34: 2350–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Forbes JF, Cuzick J, Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ Group et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 2008; 9: 45–53. [DOI] [PubMed] [Google Scholar]
- 30. Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009; 360: 679–691. [DOI] [PubMed] [Google Scholar]
- 31. Rack B, Juckstock J, Genss EM, et al. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res 2010; 30: 1807–1813. [PubMed] [Google Scholar]
- 32. Aft R, Naughton M, Trinkaus K, et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomized, phase 2 trial. Lancet Oncol 2010; 11: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gnant M, Pfeiler G, Dubsky PC, et al. Adjuvant denosumab in breast cancer (ABCSG-18): a multicentre, randomized, double-blind, placebo-controlled trial. Lancet 2015; 386: 433–443. [DOI] [PubMed] [Google Scholar]
- 34. Strobl S, Korkmaz B, Devyatko Y, et al. Adjuvant bisphosphonates and breast cancer survival. Annu Rev Med 2016; 67: 1–10. [DOI] [PubMed] [Google Scholar]
- 35. Gnant M. Role of bisphosphonates in postmenopausal women with breast cancer. Cancer Treat Rev 2014; 40: 476–484. [DOI] [PubMed] [Google Scholar]
- 36. Coleman R, Powles T, Early Breast Cancer Trialists’ Collaborative Group et al. Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomized trials. Lancet 2015; 386: 1353–1361. [DOI] [PubMed] [Google Scholar]
- 37. Ottewell PD, Wang N, Brown HK, et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res 2014; 20: 2922–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bolland MJ, Grey A, Reid IR. Calcium supplements and cardiovascular risk: 5 years on. Ther Adv Drug Saf 2013; 4: 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jakesz R, Jonat W, Gnant M, et al. Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 2005; 366: 455–462. [DOI] [PubMed] [Google Scholar]
- 40. Gnant M, Sestak I, Filipits M, et al. Identifying clinically relevant prognostic subgroups of postmenopausal women with node-positive hormone receptor-positive early-stage breast cancer treated with endocrine therapy: a combined analysis of ABCSG-8 and ATAC using the PAM50 risk of recurrence score and intrinsic subtype. Ann Oncol 2015; 26: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 41. Filipits M, Nielsen TO, Rudas M, et al. The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 2014; 20: 1298–1305. [DOI] [PubMed] [Google Scholar]
- 42. Gnant M, Filipits M, Greil R, et al. Predicting distant recurrence in receptor-positive breast cancer patients with limited clinicopathological risk: using the PAM50 Risk of Recurrence score in 1478 postmenopausal patients of the ABCSG-8 trial treated with adjuvant endocrine therapy alone. Ann Oncol 2014; 25: 339–345. [DOI] [PubMed] [Google Scholar]
- 43. Sestak I, Cuzick J, Dowsett M, et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. J Clin Oncol 2015; 33: 916–922. [DOI] [PubMed] [Google Scholar]
- 44. Dubsky P, Brase JC, Jakesz R, et al. The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2- breast cancer patients. Br J Cancer 2013; 109: 2959–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sgroi DC, Carney E, Zarrella E, et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013; 105: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Knauer M, Filipits M, Dubsky P. Late recurrences in early breast cancer: for whom and how long is endocrine therapy beneficial? Breast Care (Basel) 2014; 9: 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duffy MJ. The urokinase plasminogen activator system: role in malignancy. Curr Pharm Des. 2004; 10: 39–49. [DOI] [PubMed] [Google Scholar]
- 48. Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline summary. J Oncol Pract 2016; 12: 384–389. [DOI] [PubMed] [Google Scholar]
- 49. Duffy MJ, McGowan PM, Harbeck N, et al. uPA and PAI-1 as biomarkers in breast cancer: validated for clinical use in level-of-evidence-1 studies. Breast Cancer Res 2014; 16: 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gouri A, Dekaken A, El Bairi K, et al. Plasminogen activator system and breast cancer: potential role in therapy decision making and precision medicine. Biomark Insights 2016; 11: 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rack B, Schindlbeck C, Juckstock J, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014; 106: pii: dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lucci A, Hall CS, Lodhi AK, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 2012; 13: 688–695. [DOI] [PubMed] [Google Scholar]
- 53. Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014; 25: 1544–1550. [DOI] [PubMed] [Google Scholar]
- 54. Xu H, Yu S, Liu Q, et al. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J Hematol Oncol 2017; 10: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zardavas D, Baselga J, Piccart M. Emerging targeted agents in metastatic breast cancer. Nat Rev Clin Oncol 2013; 10: 191–210. [DOI] [PubMed] [Google Scholar]
- 56. Di Cosimo S, Sathyanarayanan S, Bendell JC, et al. Combination of the mTOR inhibitor ridaforolimus and the anti-IGF1R monoclonal antibody dalotuzumab: preclinical characterization and phase I clinical trial. Clin Cancer Res 2015; 21: 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hoey T, Yen WC, Axelrod F, et al. DLL4 blockade inhibits tumor growth and reduces tumor-initiating cell frequency. Cell Stem Cell 2009; 5: 168–177. [DOI] [PubMed] [Google Scholar]
- 58. Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer 2009; 9: 302–312. [DOI] [PubMed] [Google Scholar]
- 59. Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev 2011; 21: 42–49. [DOI] [PubMed] [Google Scholar]
- 60. Husemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell 2008; 13: 58–68. [DOI] [PubMed] [Google Scholar]
- 61. Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014; 14: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]