Highlights
-
•
Dynamic peer facial stimuli recruit key regions involved in emotion processing.
-
•
LPFC shows a nonlinear age trend across adolescence to labeling dynamic peer faces.
-
•
MOFC/vMPFC shows a linear decrease with age to viewing dynamic peer faces.
-
•
No significant age trends were observed in amygdala during viewing or labeling dynamic peer faces.
Keywords: Adolescence, Dynamic affect, Emotion labeling, Peers, Medial orbitofrontal cortex, Lateral prefrontal cortex
Abstract
Adolescence is a sensitive period of social-affective development, characterized by biological, neurological, and social changes. The field currently conceptualizes these changes in terms of an imbalance between systems supporting reactivity and regulation, specifically nonlinear changes in reactivity networks and linear changes in regulatory networks. Previous research suggests that the labeling or reappraisal of emotion increases activity in lateral prefrontal cortex (LPFC), and decreases activity in amygdala relative to passive viewing of affective stimuli. However, past work in this area has relied heavily on paradigms using static, adult faces, as well as explicit regulation. In the current study, we assessed cross-sectional trends in neural responses to viewing and labeling dynamic peer emotional expressions in adolescent girls 10–23 years old. Our dynamic adolescent stimuli set reliably and robustly recruited key brain regions involved in emotion reactivity (medial orbital frontal cortex/ventral medial prefrontal cortex; MOFC/vMPFC, bilateral amygdala) and regulation (bilateral dorsal and ventral LPFC). However, contrary to the age-trends predicted by the dominant models in studies of risk/reward, the LPFC showed a nonlinear age trend across adolescence to labeling dynamic peer faces, whereas the MOFC/vMPFC showed a linear decrease with age to viewing dynamic peer faces. There were no significant age trends observed in the amygdala.
1. Introduction
Adolescence is often perceived to be a sensitive period for social and emotional development. Indeed, compared to children, adolescents spend more time interacting with peers, and exhibit greater concern with social status, friendships, and romantic relationships (Brown and Larson, 2009, Furman et al., 2009, Suleiman and Harden, 2016). The behaviors, beliefs, or mere presence of peers have also been shown to influence adolescents at various behavioral and neurobiological levels (Doom et al., 2016, Peake et al., 2013, Shulman et al., 2016). In addition to this period of heightened sensitivity to peers, adolescents may display greater emotional variability than adults (Larson et al., 2014), heightened intensity of emotion, regardless of valence (Silk et al., 2009), and particular difficulty regulating emotions elicited by social stimuli (versus nonsocial; Silvers et al., 2012). Taken together, these concurrent changes during adolescence highlight a significant need to better understand the developmental trajectories of affective processing elicited by peer faces.
For the past decade and a half, there has been growing research interest in understanding how these social and affective changes are related to neural development, given that adolescence is also a period of remarkable neural plasticity (Kadosh et al., 2013, Zelazo and Carlson, 2012). Indeed, it is now widely accepted that key regions and networks involved in social cognition, emotional reactivity, and emotion regulation all undergo significant functional development during adolescence (Casey et al., 2008, Pfeifer and Blakemore, 2012, Somerville et al., 2011a). However, the field’s existing models of socio-affective neurodevelopment in adolescence (Nelson et al., 2016, Nelson et al., 2005) largely describe which brain regions are implicated in these tasks, rather than more precisely proposing when and how the functioning in these regions change (Pfeifer and Allen, 2016). In comparison, dual systems and imbalance models have been used extensively to explain sensitive periods in adolescent risk behavior, and propose age-based trajectories for sensitivity to rewards and regulatory capacities, as well as describe the relationships between these trajectories (Blakemore and Robbins, 2012, Casey et al., 2016, Shulman et al., 2016). These models have sometimes been applied to understanding affective changes in adolescence (Crone and Dahl, 2012, Pfeifer and Allen, 2012, Somerville et al., 2011a), but have been less extensively tested in this domain. Therefore, one aim of this study was to explore whether brain functioning elicited by socio-affective stimuli reveals a similar sensitive period of imbalance in our cross-sectional sample (following Giuliani and Pfeifer, 2015, Hare et al., 2008, Somerville et al., 2013); such that neural indices of socio-affective reactivity would exhibit non-linear developmental trajectories, with activity peaking by middle adolescence, and that regulation-related responses would display roughly linear patterns of change across the duration of adolescence. In order to acknowledge the importance of peers for affective and regulatory neurodevelopment in adolescence, the current study used a novel set of dynamic adolescent facial expressions.
1.1. Brain regions and networks implicated in the regulation of emotion processing
The adult neuroimaging literature provides a solid roadmap for beginning to understand adolescent affective reactivity and regulation. Passive viewing of facial affective stimuli by adults is associated with increased activity in key neural regions such as the amygdala (Fitzgerald et al., 2006, Fusar-Poli et al., 2009, Sabatinelli et al., 2011), medial orbitofrontal cortex (MOFC; (Liang et al., 2009, Monk et al., 2003, Ochsner et al., 2002) and ventral medial prefrontal cortex (vMPFC; (Ebner et al., 2012). When further cognitive processes are layered onto viewing affective stimuli, such as reappraisal or labeling of the emotional content of the stimulus, additional regions are recruited by adults to modulate the response to the affective stimuli, particularly the lateral prefrontal cortex (LPFC; (Buhle et al., 2014, Lieberman et al., 2007, Wager et al., 2008).
Across adolescence, these key regions from networks supporting reactivity and regulation show varied nonlinear and linear age-related patterns of functional activity, respectively, to affective stimuli. For example, some studies show an adolescent peak in amygdala activity to affective facial stimuli (Guyer et al., 2008, Hare et al., 2008, Monk et al., 2003), although others have not observed this non-linear pattern (Gee et al., 2012, Pfeifer et al., 2011); a similar finding with non-facial affective stimuli has been observed (Vink et al., 2014). Meanwhile, functional recruitment of the LPFC often shows a linear pattern across adolescence, although the direction of change can vary. In affect regulation tasks using facial stimuli, increases have been observed (Gee et al., 2012), while regulation of non-facial affect has shown both increases (McRae et al., 2012) and decreases (Vink et al., 2014). In broader assessments of LPFC engagement across a variety of regulatory and executive function tasks, findings have likewise been mixed (for review, see Crone and Dahl, 2012). Finally, contrary to adult studies using affective paradigms, adolescent studies do not show the same MOFC activity to facial affective stimuli; however, MOFC is frequently observed in studies of adolescent reward sensitivity and non-facial emotion processing (Galvan et al., 2006, Rothkirch et al., 2012, Vink et al., 2014). One possibility for the absence of MOFC activity in adolescent studies of facial affective processing is that adult faces are less rewarding or motivationally salient to adolescents. This suggests that aspects of task design including stimuli selection may critically influence the degree or kind of neurodevelopmental changes in cortical and subcortical recruitment that are observed in adolescence, and thus our inferences about whether adolescence is a sensitive period with respect to particular processes (Blakemore and Robbins, 2012, Crone and Dahl, 2012, Pfeifer and Allen, 2012).
1.2. Developmentally salient stimuli
As adolescents increasingly build autonomy from their parents, more time is spent with peers and a greater value is placed on peer and romantic relationships (Brown and Larson, 2009, Furman et al., 2009, Suleiman and Harden, 2016), making the ability to identify peers’ emotions particularly important for successful social functioning. However, prior research examining changes in reactivity and regulatory regions across adolescence has been limited by primarily relying on adult facial expressions to probe emotion processing (Ekman faces; Ekman and Friesen, 1976, Tottenham et al., 2009), or other adult-oriented affective stimuli, such as the International Affective Picture System (IAPS; Britton et al., 2006, Vink et al., 2014), with a few notable exceptions (child stimuli sets: CAFE; LoBue and Thrasher, 2015; NIMH-chEFS; Egger et al., 2011).
There is evidence to suggest that child and adult faces may elicit different patterns of brain activity (Leibenluft et al., 2004). In fact, studies that have directly compared peer and adult facial expressions found both children and adolescents recruit largely similar brain regions to peer and adult faces, but exhibit increased amygdala activity to positive peer faces and angry adult faces, which may represent an age-appropriate response to the stimuli (8–16 year olds: Marusak et al., 2013; 5–6 year olds: Hoehl et al., 2010). In addition, when adolescents were asked to rate and receive feedback from peers (based on viewing peer faces), 8–17 year old females showed increased nucleus accumbens activity across age (Guyer et al., 2009). These data suggest that peer faces, particularly in adolescence, may modulate the affective response elicited and/or the observed neural correlates. Despite the importance of peers during this sensitive period of facial affective processing, however, there is still a relative dearth of studies examining adolescent development of neural responses elicited by peer facial expressions or other adolescent-oriented affective stimuli.
Additionally, the stimuli used in the extant literature are largely static images, and are of relatively more extreme expressions or situations than the emotional content individuals experience on a daily basis. Neuroimaging studies in adults suggest that relative to static emotional expressions, neural responses to dynamic emotional expressions are more robust (Pitcher et al., 2011, Sato et al., 2004). Taken together, this research suggests that dynamic emotional expressions made by peers, rather than adults, may be particularly salient to adolescents and an important option to pursue in the attempt to enhance the ecological validity of fMRI tasks.
1.3. Implicit regulation
In addition to the use of adult, static faces, previous paradigms used to assess adolescent brain function associated with emotion processing have ranged from implicit, non-instructed passive viewing (Pfeifer et al., 2011) to explicit, instructed regulation or reappraisal tasks (Crowley et al., 2014, McRae et al., 2012, Pitskel et al., 2014, Silvers et al., 2012), with some paradigms falling in between, such as behavioral inhibition tasks that utilize affective stimuli (Somerville et al., 2011b). The literature on emotion regulation in adolescence has predominantly focused on explicit (e.g., instructed to regulate) or partially explicit emotion regulation strategies (e.g, instructed to inhibit response, such as go/no-go tasks; Mauss et al., 2007). However, implicit emotion regulation, or regulation without intention, awareness, or insight, is an equally important strategy that is not mutually exclusive from explicit emotion regulation (Gyurak et al., 2011). Research suggests that these strategies recruit the same key reactivity and regulatory brain regions as explicit emotion regulation paradigms in adults (Gyurak et al., 2011, Lieberman et al., 2007), but may additionally serve as a more ecologically valid assessment of real-world tendencies to regulate emotions (Berkman and Lieberman, 2009, Mauss et al., 2007).
One experimental paradigm that assesses implicit emotion regulation is affect labeling, which asks participants to label the emotion expressed by a facial stimulus (Lieberman et al., 2007, Torrisi et al., 2013). Affect labeling has been shown to recruit regulatory regions associated with explicit regulation (e.g., LPFC), as well as down-regulate reactivity regions (e.g., the amygdala; (Lieberman et al., 2007). This assessment of implicit regulation may be particularly valuable for real world translation of automatic, or implicit regulation that occurs on a daily basis (Berkman and Lieberman, 2009). However, to our knowledge, only a few studies have assessed affect labeling in some fashion in adolescents (9–17 years old; Monk et al., 2003), including assessing impairments in facial processing for male children and adolescents (8–23 years old) with autism spectrum disorder (Wang et al., 2004); impairments in adolescents (15–23 years old) at risk for psychosis (Gee et al., 2012); and parental contributions to 17 year old adolescents’ emotional processing (Telzer et al., 2014). These studies suggest that labeling static adult faces engages similar brain regions during adolescence as in adulthood, although Telzer et al. (2014) additionally showed adolescents displayed increased right vLPFC and right amygdala during affect labeling (compared to patterns displayed by their parents). Therefore, affect labeling may similarly function as a proxy for implicit regulation in adolescence, although there may be some notable differences, and normative developmental trajectories would benefit from further exploration and characterization.
1.4. Current study
In the current study, we assessed cross-sectional trends in neural responses to viewing and labeling dynamic emotional expressions in adolescent girls across a broad age range (10–23 years old). To decrease heterogeneity due to the influence of biological sex on pubertal onset, emotion processing, and brain structural and functional development, we used an all female sample (Lenroot and Giedd, 2010, McClure, 2000). We implemented an affect labeling paradigm that reliably recruits key brain regions in emotion reactivity and regulation (amygdala, MOFC/vMPFC, and LPFC), and can be easily performed across a wide age range. Importantly, we used new stimuli characterized by dynamic adolescent affective expressions, to reflect the importance of peers during adolescence. Dominant neurobiological models of adolescent brain maturation applied to the affective domain would predict nonlinear developmental trends in amygdala activity when viewing emotional faces (with responses peaking by middle adolescence), and a linear developmental trend in LPFC when labeling emotional faces. However, due to increased peer influence during this sensitive period of development, it is particularly important to understand how adolescents process affect expressed by peers. Based on the literature review above, we hypothesized that utilizing socially relevant (peer) faces and dynamic emotional expressions would maximize our ecological validity and ability to demonstrate the neurodevelopmental trends predicted by application of imbalance models to the affective domain: nonlinear age trends in amygdala and MOFC/vMPFC activity (with an adolescent peak), and linear LPFC activity across age. However, if the data demonstrated different developmental trends, this would provide evidence that the models’ utility may be restricted to a more limited set of stimuli, paradigms, and constructs. The stimulus set included positive, neutral, and negative expressions, but we did not have specific a priori hypotheses about whether the affective valence would interact with the developmental patterns of brain activity during viewing or labeling of dynamic adolescent affective stimuli.
2. Methods
2.1. Participants
Sixty-one female adolescents between the ages of 10 and 23 years (M = 16.66, SD = 3.68, range 10.16-22.89 years) were recruited from the Eugene/Springfield, OR metropolitan area. Two participants were excluded prior to completing the task due to recognition of peers used in stimulus set, and one participant was excluded from analyses due to movement resulting in incomplete images, resulting in a total of 58 participants (M = 16.70, SD = 3.77, range 10.16–22.89 years). Participants were evenly distributed across the age range (M = 4.62, SD = 0.77, range 3–6 participants per age-year). Participants were predominantly middle to upper class (median income = $40,000-$75,000, median maternal education = bachelor’s degree), and self-identified as 84.5% non-Hispanic/White, 10% mixed race, 1.72% Hispanic/Latino, 1.72% Asian, 1.72% not listed. Individuals were excluded if they were left-handed, non-native English speakers, possessed MRI contraindications (e.g., orthodontia), or reported a history of neurological or psychological disorders, head trauma, or current psychoactive medication. Parents and participants over 18 years of age provided informed consent, and minors under 18 years of age provided informed assent in accordance with the University of Oregon Institutional Review Board.
2.2. Task description
The stimuli used in the dynamic affect labeling task consisted of 60 videos of children and adolescents, ages 8–18 years old, making a range of emotional expressions. Videos were created by utilizing adolescents enrolled in a local theatrical performance camp, so demographics were representative of the Eugene area (approximately 85% Caucasian). Videos were externally validated using the same procedure as other widely used facial stimuli sets (e.g., those with >70% agreement with intended expression were retained, as in Tottenham et al., 2009). Complete details on validation are outlined in another manuscript (Giuliani et al., in press). The videos selected from the stimulus set consisted of an equal number of males and females (N = 20).
From the participants’ perspective, there were only two types of instructions: Label or View. Participants saw each video for 1.1 s and then it froze on the last frame of the video, which displayed the prototypic emotion, for 3.4 s (viewing the stimuli for a total of 4.5 s). During Label trials (N = 60 across two runs, indicated by the presence of two emotion words above the video), participants were instructed to watch the video and press the button corresponding with the label that best matched the expression displayed in the video. During View trials (N = 60 across two runs, indicated by the presence of two nonsense words consisting of punctuation symbols above the video), participants were instructed to watch the video and press a button if the video got blurry at any point. Most videos in the View condition did not blur, but 25% of the time the videos were blurry, either from the beginning of the stimulus presentation period or at the very end of the stimulus presentation period. These blurred trials were used as our control condition (N = 20 across two runs) to account for the visual features represented in both Label and View conditions besides facial expressions and to ensure sustained attention during the View condition, but participants were unaware of the role of control trials. Finally, there were fixation trials (N = 20 across two runs) consisting of rest with fixation, as required for an event-related design. Facial expressions were equally distributed between valence and task across two runs: 40 positive (20 View, 20 Label), 40 negative (20 View, 20 Label), and 40 neutral (20 View, 20 Label). A genetic algorithm (GA; Wager and Nichols, 2003) was used to determine stimulus order, with four conditions (Fixation, Blur, View, Label), optimized for the following contrasts: Label > Blur, View > Blur, and View > Label. Valence was randomized within condition (View, Label).
2.3. Functional MRI data acquisition
Data were acquired using a 3.0 T Siemens Skyra scanner at the University of Oregon’s Robert and Beverly Lewis Center for Neuroimaging. Blood oxygen-level dependent echo-planar images (BOLD-EPI) were acquired with a T2*-weighted gradient echo sequence (TR = 2000 ms, TE = 30 ms, flip angle = 90, matrix size = 64 × 64, 33 contiguous axial slices with interleaved acquisition, field of view = 200 mm, slice thickness = 4 mm; total time = 7 min 50 s per run × 2 runs). For each participant, a high-resolution structural T1-weighted 3D MPRAGE pulse sequence (TR = 2300 ms, TE = 2.1 ms, matrix size = 192 × 192, 160 contiguous axial slices, voxel size = 1 mm, slice thickness = 1 mm; total time = 5 min 59 s) was acquired coplanar with the functional images.
2.4. Functional MRI data analysis
DICOM images were converted to NIfTI format via MRIConvert (http://lcni.uoregon.edu/∼jolinda/MRIConvert/). Before preprocessing, non-brain tissue was removed from the brain images using robust skull stripping with the Brain Extraction Tool (BET) in FMRIB’s Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl/). Image preprocessing was implemented in SPM12 (Welcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/), which included realignment and co-registration of each subject’s own high-resolution structural image to a mean of the functional images using a six-parameter rigid body transformation model, reorientation of all images to the plane containing the anterior and posterior commissures, segmentation of the structural image into three tissue priors, spatial normalization of all images into Montreal Neurological Institute (MNI) template space using the deformations resulting from segmentation, and smoothing using an 8 mm full-width at half-maximum Gaussian kernel.
Statistical analyses were implemented in SPM12. For each subject, event-related condition effects were estimated according to the general linear model, using a canonical hemodynamic response function, high-pass filtering (200 s) to fit the task design and a first-order autoregressive error structure. At the subject level, BOLD signal was modeled in a fixed effects analysis with separate regressors modeling each condition of interest during the picture presentation period (4.5 s); because response times were only available for the label condition, all durations were set to 4.5 s (onset of video to end of freeze frame). Six-parameter motion regressors were calculated as deviations from the origin, and entered into single-subject models as covariates of no-interest. The average mean functional optimal threshold explicit mask was used for both fixed and random effects models. No voxels were excluded at the subject level to allow all individuals to contribute to the random effects level mask. No implicit mask or global normalization was applied. Relative differences in BOLD signal between contrasts were measured as an approximation of neural activity; for simplicity, BOLD signal will be referred to as neural activity.
Primary contrasts, collapsed across valence included: Label > Blur, View > Blur, and Label > View. Individual contrasts were entered into subsequent group level random effects analyses. We ran one-sample t-tests for the main effects of task (Label > Blur, View > Blur, and Label > View). A combined voxel-height and cluster-extent correction was applied for multiple comparisons to control type 1 error at alpha = 0.05, calculated in AFNI (Cox, 1996) by 3dClustSim software version AFNI_16.1.06 (Mar 6 2016). This version is updated to account for recently identified software bugs (Eklund et al., 2016). Smoothness estimates entered into 3dClustSim were an average of subject level spatial autocorrelation function (acf) parameters based on individual subjects’ residuals from each group level model, as calculated by 3dFWHMxyz using the −acf flag. 3dClustSim takes into account the size of the search space and the estimated smoothness of the data to generate probability estimates (using Monte-Carlo simulations) of a random field of noise producing a cluster of voxels of a given size for a set of voxels passing a given voxel-wise p-value threshold. In our data set, these simulations determined that a voxel-wise threshold of p < 0.0001 combined with a spatial extent threshold of 14 voxels corresponded to a p < 0.05 family wise error (FWE) correction. The results observed at this threshold were consistent with results observed at whole-brain voxel-wise FWE correction (t = 5.11). In addition, confirmatory permutation thresholding using Statistical Non-Parametric Mapping (SnPM) toolbox (http://warwick.ac.uk/snpm) revealed substantively identical results. Due to the robustness of results even at voxel-level FWE correction in the whole-brain Label > View contrast, we were unable to fully break down expansive (15,000+ voxel) clusters in SPM, precluding a comprehensive table of results for this contrast. Instead, un-thresholded group level statistical parameter maps for this contrast and all other contrasts reported on in the manuscript can be accessed at http://neurovault.org/collections/1302, http://neurovault.org/collections/1303, http://neurovault.org/collections/1304, http://neurovault.org/collections/1305, http://neurovault.org/collections/2093, and http://neurovault.org/collections/2094.
2.5. ROI analyses and correlations with age and performance
To decompose the direction of effects and interactions, we extracted parameter estimates of activity in a priori regions of interest (ROIs; amygdala, MOFC, dLPFC, and vLPFC) for every subject using MarsBaR (http://marsbar.sourceforge.net/) for each condition of interest (i.e., Label > Blur, View > Blur, Label > View, Label Positive (LPos) > Blur, Label Negative (LNeg) > Blur, Label Neutral (LNeut)> Blur, View Positive (VPos) > Blur, View Negative (VNeg) > Blur, View Neutral (VNeut) > Blur). This allowed us to statistically compare brain activity across various contrasts and correlate activation with age or behavioral performance (accuracy and reaction time) in SPSS. ROIs were defined as spheres with a radius of 8 mm, centered at regional peak voxels in clusters resulting from the whole-brain analysis at the group level. We chose this method in order to examine more ROIs (e.g., by allowing separation of dorsal and ventral LPFC, as well as identification of bilateral amygdala, all of which were activated as part of expansive clusters). The contrast of Label > Blur was used to define bilateral dorsal and ventral LPFC. The contrast of View > Blur was used to define MOFC (which in the present study, extended slightly into vMPFC) and bilateral amygdala (which was robustly engaged in both task conditions). However, for confirmation, we also compared these sphere-defined ROIs results with results from ROIs defined anatomically where possible (i.e., bilateral amygdala), as well as from ROIs defined as the entire cluster resulting from the whole-brain analysis at the group level (i.e., both MOFC and bilateral amygdala from the View > Blur contrast). Post-hoc paired-sample t-tests were performed to statistically compare differential activation in these ROIs. Developmental trends in significant clusters of activity observed in these ROIs were investigated using hierarchical linear regressions with linear age (step 1) and quadratic age (step 2). Age was mean-centered in all analyses, and mean-centered linear age was included in all mean-centered quadratic age analyses. To further explore developmental trends in neural correlates of viewing and labeling dynamic emotional expressions, we also entered mean-centered linear age, and mean-centered quadratic age (respectively), as whole-brain regressors to predict our main effect contrasts. Finally, Pearson’s correlations were run to investigate whether accuracy and/or reaction times were associated with age.
2.6. Functional connectivity analyses
To further interrogate our amygdala activation during affective viewing and labeling dynamic peer faces, we conducted task-dependent psychophysiological interaction (PPI; e.g., context-dependent connectivity) analyses with our left amygdala seed (a sphere defined as equivalent to the left amygdala ROI described above) using the generalized PPI toolbox in SPM (gPPI: McLaren et al., 2012). Subject level gPPI models included physiological and psychological regressors, as well as the PPI interaction terms, for each condition: Lpos, Lneg, Lneut, Vpos, Vneg, Vneut, and Blur. At the group level, we ran one-sample t-tests to characterize left amygdala functional connectivity during our two tasks independently (Label > Blur, View > Blur) and for our task by valence interaction for left amygdala (Label Emotions (LEmo) > Labeling Neutral (LNeut). For PPI analyses, 3dClustSim determined that a voxel-wise threshold of p < 0.001 combined with a spatial extent threshold of 40 voxels corresponded to a p < 0.05 family wise error (FWE) correction. Developmental trends in left amygdala during LEmo > LNeut were assessed by enterings mean-centered linear age, and mean-centered quadratic age, as whole-brain regressors.
3. Results
3.1. Task behavior
To confirm task comprehension, accuracy and reaction times in the Label condition were assessed across the sample. Total accuracy was high (M = 96%), and neither it nor reaction time (M = 1.66s) significantly correlated with age, r(57) = −0.07, p = 0.62 and, r(57) = −0.15, p = 0.25, respectively). Total accuracy by valence was not significantly correlated with age for positive (M = 94%), negative (M = 96%), or neutral (M = 98%) expressions (r(57) = −0.14, p = 0.31; r(57) = 0.01, p = 0.92; and r(57) = −0.03, p = 0.84, respectively). Total reaction time by valence was not significantly correlated with age for positive (M = 1.63s), negative (M = 1.71s) or neutral (M = 1.64s) expressions, (r(57) = −0.18, p = 0.17; r(57) = −0.77, p = 0.57, r(57) = −0.15, p = 0.25, respectively), but there was a significant decrease in mean reaction time with age to neutral expressions (r(57) = −0.30, p = 0.02). However, there were no significant correlations between activity in our ROIs and neutral valence mean reaction time (ps > 0.05); therefore, it was not controlled for in subsequent analyses.
3.2. fMRI: main effects of task (view and label)
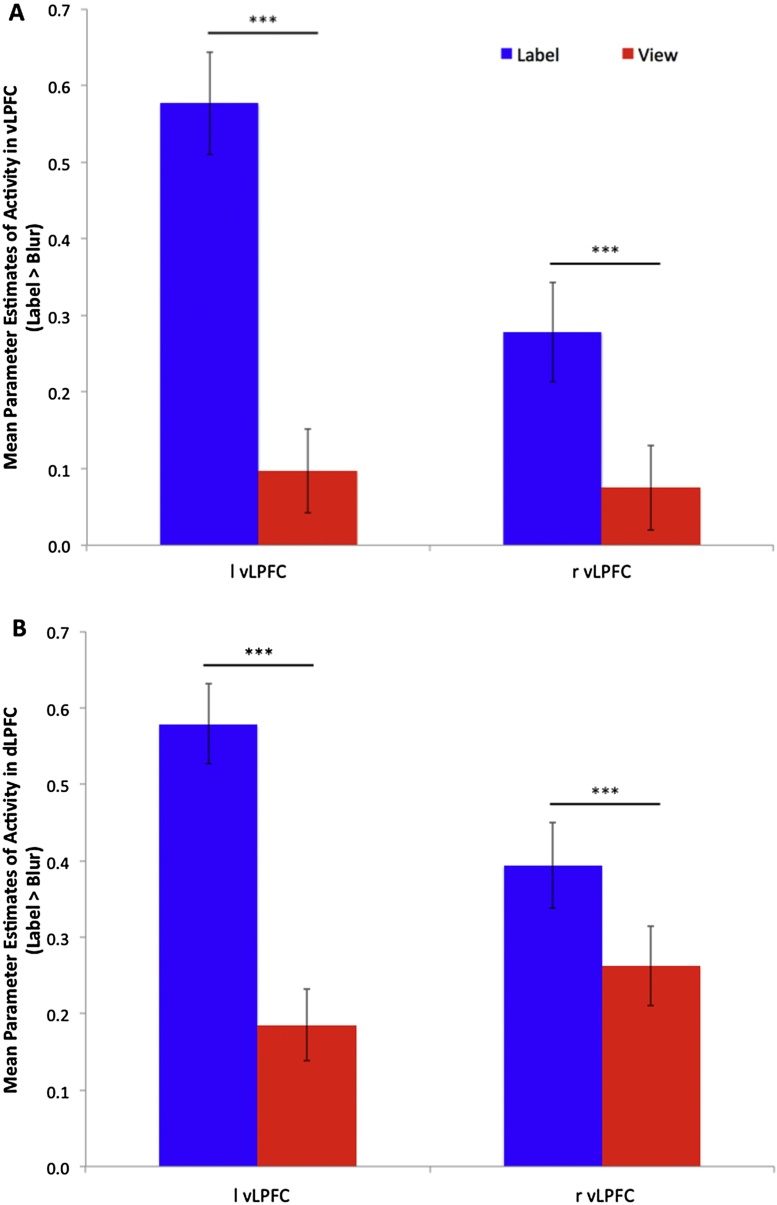
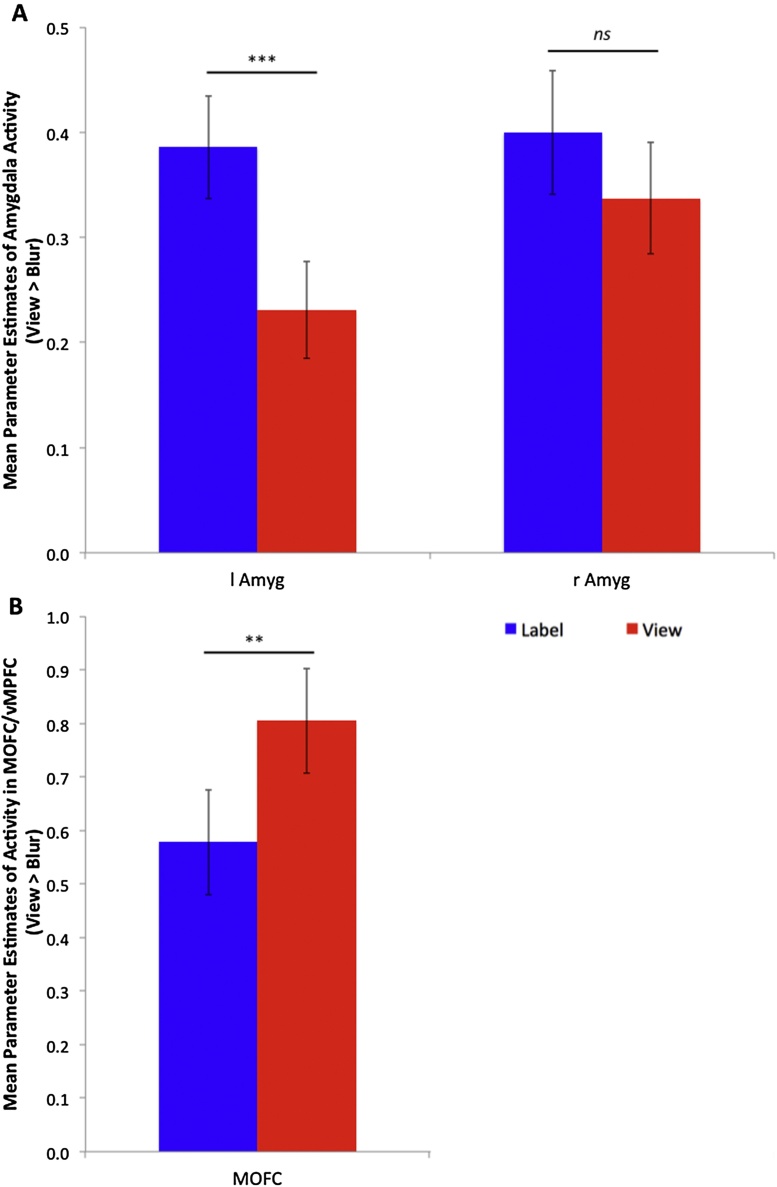
In support of our hypotheses, the View > Blur whole-brain one-sample t-test revealed robust activity in the MOFC (extending slightly into vMPFC) and bilateral amygdala, along with significant activity in the right posterior superior temporal sulcus (pSTS), bilateral anterior inferior temporal sulcus, and bilateral dorsal LPFC (see Fig. 1, Table 1, and http://neurovault.org/collections/1304/). In the Label > Blur whole-brain one-sample t-test, we found significant activity in the MOFC/vMPFC, bilateral amygdala, bilateral dorsal LPFC, as well as robust activation in the bilateral ventral LPFC, dMPFC, bilateral pSTS, dorsal anterior cingulate cortex (dACC), and primary motor cortex (see Fig. 1, Table 2, and http://neurovault.org/collections/1302/). Whole-brain analyses of the Label > View one-sample t-test revealed significant activity in the bilateral inferior frontal cortex, including pars opercularis, triangularis, and orbitalis extending into premotor cortex and insular cortex; cerebellum; large swaths of visual cortex including cuneus, lingual, and fusiform gyri; bilateral rostal inferior parietal lobule; bilateral thalamus; dorsal and rostral anterior cingulate cortex; bilateral caudate extending into bilateral amygdala; bilateral hippocampus and parahippocampal gyri; dorsal medial prefrontal cortex; midbrain (see Table 3 and http://neurovault.org/collections/1303 for un-thresholded whole-brain group level statistical parameter maps). Whole-brain analyses of the View > Label one-sample t-test revealed significant activity extending from subgenual ACC into MOFC/vMPFC, as well as in the posterior cingulate cortex, posterior precuneus, and middle temporal gyrus (see Table 3 for full list and http://neurovault.org/collections/1303). In a direct comparison of parameter estimates extracted from sphere-defined ROIs, Label > View revealed greater bilateral dorsal and ventral LPFC activity (see Fig. 2A and B), while View > Label revealed greater MOFC/vMPFC activity (see Fig. 3A). However, a direct comparison of parameter estimates extracted from sphere-defined ROIs revealed greater left amygdala activation during Label > View, and no difference in right amygdala activation between Label and View (see Fig. 3B and Table 4).
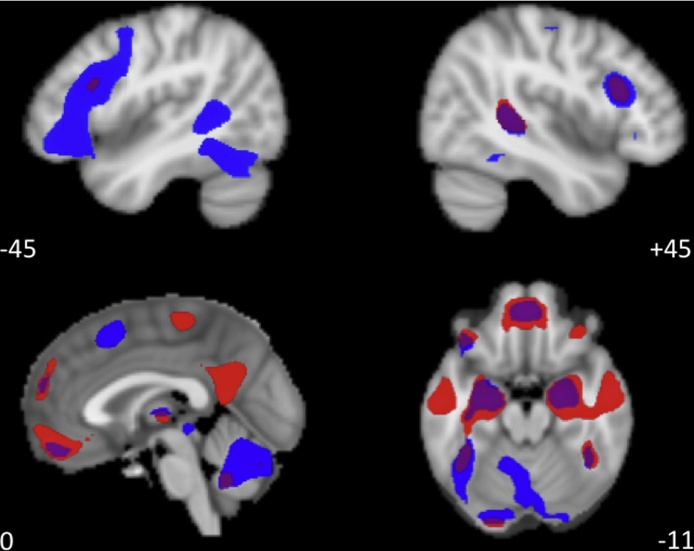
Fig. 1.
Label > Blur and View > Blur Overlay of Neural Activity. Label > Blur contrast activity is depicted in blue. View > Blur contrast activity is depicted in red. Overlap is depicted in purple. A combined voxel-height and cluster-extent correction was applied as calculated by 3dClustSim (p < 0.0001, k ≥ 14). View > Blur revealed activity in medial orbital frontal cortex (extending slightly into ventral medial prefrontal cortex) and bilateral amygdala, along with significant activity in the right posterior superior temporal sulcus, bilateral anterior inferior temporal sulcus, and bilateral dorsal LPFC. Label > Blur also revealed medial orbital frontal cortex (extending into ventral medial prefrontal cortex), bilateral amygdala, bilateral dorsal LPFC, right posterior superior temporal sulcus, as well as robust activation in the bilateral ventral LPFC, dMPFC, dorsal anterior cingulate cortex (dACC), and primary motor cortex. Data Availability: Data are available via NeuroVault, a public repository of unthresholded brain activation maps. Complete group-level statistics for View > Blur and Label > Blur are available at http://neurovault.org/collections/1302/andhttp://neurovault.org/collections/1304/, respectively.
Table 1.
View > Blur. Whole brain parameter estimates of activity in contrast View > Blur. A combined voxel-height and cluster-extent correction was applied as calculated by 3dClustSim (p < 0.0001, k ≥ 14). MOFC = medial orbital frontal cortex; dLPFC = dorsal lateral prefrontal cortex.
| Region | x | y | z | t | k | |
|---|---|---|---|---|---|---|
| Occipital Cortex | R | 27 | −94 | −7 | 11.76 | 107 |
| Occipital Cortex | L | −21 | −100 | −7 | 11.15 | 127 |
| MOFC (Orbital Gyrus/Medial Frontal Gyrus) | 0 | 50 | −19 | 8.94 | 165 | |
| Amygdala/Hippocampus/Parahippocampal Gyrus | R | 21 | −7 | −19 | 7.64 | 120 |
| Posterior Superior Temporal Sulcus | R | 48 | −37 | 5 | 7.59 | 88 |
| Amygdala/Hippocampus/Parahippocampal Gyrus | L | −24 | −10 | −19 | 6.91 | 72 |
| Anterior Temporal Cortex (Inferior/Middle Temporal Gyrus) | R | 54 | −7 | −19 | 6.62 | 96 |
| dLPFC (Middle/Inferior Frontal Gyrus) | R | 45 | 20 | 20 | 6.54 | 70 |
| Fusiform Gyrus | R | 42 | −43 | −16 | 5.89 | 14 |
| Anterior Temporal Cortex (Inferior/Middle Temporal Gyrus) | L | −57 | −10 | −19 | 5.78 | 55 |
| Posterior Cingulate Cortex/Precuneus | 6 | −52 | 20 | 5.17 | 44 | |
| Paracentral Gyrus/Supplementary Motor Area | 9 | −25 | 65 | 4.86 | 19 | |
| dLPFC (Inferior Frontal Gyrus) | L | −42 | 17 | 23 | 4.64 | 20 |
Table 2.
Label > Blur. Whole brain parameter estimates of activity in contrast Label > Blur. A combined voxel-height and cluster-extent correction was applied as calculated by 3dClustSim (p < 0.0001, k ≥ 14). dLPFC = dorsal lateral prefrontal cortex; vLPFC = ventral lateral prefrontal cortex; dMPFC = dorsal medial prefrontal cortex; MOFC = medial orbital frontal cortex.
| Region | x | y | z | t | k | |
|---|---|---|---|---|---|---|
| Occipital Cortex | R | 27 | −94 | −7 | 13.47 | 2301 |
| Occipital Cortex | L | −21 | −100 | −7 | 11.65 | |
| Fusiform Gyrus | L | −42 | −49 | −19 | 10.79 | |
| Amygdala/Hippocampus/Parahippocampal Gyrus | L | −30 | −4 | −22 | 10.42 | |
| Amygdala/Hippocampus/Parahippocampal Gyrus | R | 21 | −7 | −19 | 9.99 | |
| Posterior Superior Temporal Sulcus | L | −51 | −43 | 5 | 9.30 | |
| Hippocampus | L | −21 | −31 | −4 | 8.61 | |
| Vermis | 0 | −58 | −34 | 7.62 | ||
| Midbrain | R | 12 | −31 | −4 | 6.35 | |
| dLPFC (Middle/Inferior Frontal Gyrus) | L | −42 | 17 | 26 | 12.40 | 980 |
| vLPFC (Inferior Frontal Gyrus; Pars Orbitalis) | L | −48 | 26 | −10 | 9.38 | |
| vLPFC (Inferior Frontal Gyrus; Pars Triangularis) | L | −51 | 29 | 2 | 7.82 | |
| Precentral Gyrus | L | −45 | −1 | 53 | 5.65 | |
| Anterior Insula | L | −27 | 23 | −1 | 4.86 | |
| dLPFC (Middle/Inferior Frontal Gyrus) | R | 45 | 20 | 20 | 10.22 | 252 |
| vLPFC (Inferior Frontal Gyrus; Pars Triangularis) | R | 57 | 29 | 2 | 5.65 | |
| Supplementary Motor Area (Medial Frontal Gyrus) | −6 | 17 | 47 | 8.07 | 258 | |
| dMPFC (Medial Frontal Gyrus) | −6 | 59 | 23 | 7.34 | ||
| MOFC (Orbital Gyrus/Medial Frontal Gyrus) | 6 | 50 | −19 | 7.84 | 71 | |
| Posterior Superior Temporal Sulcus | R | 48 | −37 | 2 | 6.77 | 86 |
| Precentral Gyrus | R | 36 | −7 | 62 | 6.45 | 136 |
| Fusiform Gyrus | R | 42 | −43 | −16 | 6.11 | 39 |
| Putamen/Caudate | R | 24 | 2 | 11 | 5.77 | 238 |
| Superior Parietal Lobule/Precuneus | L | −27 | −61 | 53 | 5.13 | 21 |
Table 3.
Whole brain parameter estimates of activity in contrast View > Label and Label > View. A combined voxel-height and cluster-extent correction was applied as calculated by 3dClustSim (p < 0.0001, k ≥ 14). MOFC = medial orbital frontal cortex; LPFC = lateral prefrontal cortex; dMPFC = dorsal medial prefrontal cortex.
| Region | x | y | z | t | k | |
|---|---|---|---|---|---|---|
| View > Label | ||||||
| Angular Gyrus | L | −39 | −76 | 41 | 7.82 | 55 |
| Angular Gyrus. | R | 45 | −70 | 41 | 7.51 | 77 |
| Posterior Cingulate Cortex/Precuneus | 3 | −37 | 41 | 7.45 | 233 | |
| Frontal Pole (Superior Frontal Gyrus) | L | −24 | 62 | 2 | 6.84 | 71 |
| Superior Frontal Sulcus | R | 24 | 29 | 35 | 6.74 | 173 |
| mOFC (Orbital Gyrus/Medial Frontal Gyrus) extending into Subgenual Anterior Cingulate Cortex | −9 | 32 | −10 | 6.02 | 247 | |
| Middle/Superior Temporal Gyrus | R | 63 | −22 | −7 | 5.68 | 92 |
| Supramarginal Gyrus | R | 48 | −46 | 35 | 5.41 | 56 |
| Superior Frontal Sulcus | L | −27 | 41 | 38 | 5.25 | 104 |
| Superior/Middle Temporal Gyrus | L | −48 | −19 | −1 | 5.06 | 36 |
| Frontal Pole (Superior Frontal Gyrus) | R | 27 | 62 | 2 | 4.89 | 28 |
| Paracentral Lobule | −6 | −28 | 59 | 4.71 | 21 | |
| Label > View | ||||||
| LPFC/Insula/Cerebellum/Occipital Cortex/Fusiform/Thalamus/Caudate/Putamen/Amygdala/Hippocampus/Anterior Cingulate Cortex/Supplementary Motor Area | −45 | 2 | 8 | 13.88 | 15137 | |
| Postcentral Gyrus/Somatosensory Cortex | L | −57 | −22 | 20 | 11.31 | 1566 |
| Postcentral Gyrus/Somatosensory Cortex | R | 54 | −19 | 20 | 6.89 | 584 |
| Brainstem | 3 | −34 | −46 | 6.83 | 59 | |
| dMPFC (Medial Frontal Gyrus) | 3 | 59 | 23 | 5.74 | 24 | |
Fig. 2.
Neural activity in lateral prefrontal cortex by task. Panel A depicts mean parameter estimates of activity in bilateral ventral lateral prefrontal cortex (vLPFC) by task. Panel B depicts mean parameter estimates of activity in bilateral dorsal lateral prefrontal cortex (dLPFC) by task. Bilateral ventral and dorsal lateral prefrontal cortex regions of interest defined by 8 mm sphere around the peak within that region from the Label > Blur contrast (at p < 0.0001, k ≥ 14).
Fig. 3.
Neural activity in medial orbital frontal cortex and amygdala by task. Panel A depicts mean parameter estimates of activity in bilateral amygdala (Amyg) across task. Panel B depicts mean parameter estimates of activity in medial orbital frontal cortex/ventral medial prefrontal cortex (MOFC/vMPFC) across task. Medial orbital frontal cortex and bilateral amygdala regions of interest defined by 8 mm sphere around the peak within that region from the View > Blur contrast (at p < 0.0001, k ≥ 14).
Table 4.
Paired Sample T-tests of mean parameter estimates of activity between tasks. Bilateral dorsal and ventral lateral prefrontal cortex regions of interest defined by 8 mm sphere around the peak within that region from the Label > Blur contrast (at p < 0.0001, k ≥ 14). Medial orbital frontal cortex and bilateral amygdala regions of interest defined by 8 mm sphere around the peak within that region from the View > Blur contrast (at p < 0.0001, k ≥ 14).
| Paired Sample T-test | M | SE | CI | t | df | p |
|---|---|---|---|---|---|---|
| (Label > Blur) > (View > Blur) | ||||||
| Left Dorsal Lateral Prefrontal Cortex. | 0.39 | 0.04 | (0.30, 0.48) | 8.88 | 57 | <0.001 |
| Right Dorsal Lateral Prefrontal Cortex | 0.13 | 0.03 | (0.06, 0.20) | 3.86 | 57 | <0.001 |
| Left Ventral Lateral Prefrontal Cortex | 0.48 | 0.05 | (0.38, 0.58) | 9.63 | 57 | <0.001 |
| Right Ventral Lateral Prefrontal Cortex | 0.2 | 0.04 | (0.12, 0.28) | 4.9 | 57 | <0.001 |
| (View > Blur) > (Label > Blur) | ||||||
| Medial Orbital Frontal Cortex | 0.23 | 0.07 | (.08, 0.37) | 3.16 | 57 | <0.01 |
| Left Amygdala | −0.15 | 0.04 | (−0.08, −0.23) | −4.3 | 57 | <0.001 |
| Right Amygdala | −0.06 | 0.04 | (−0.14, 0.01) | −1.72 | 57 | 0.09 |
3.3. fMRI: moderation of task effects by valence
Next, we assessed how the valence of stimulus expression moderated the main task level effects, via parameter estimates of activity from sphere-defined ROIs in a 2 × 3 repeated measures ANOVA. Significant task by valence interactions revealed increased bilateral ventral LPFC and left amygdala activity to labeling both positive and negative expressions, relative to labeling neutral expressions (i.e., LPos > LNeut, LNeg > LNeut; see Table 5), and significantly increased bilateral dorsal LPFC activity to labeling negative expressions, relative to positive or neutral expressions (i.e., LNeg > LPos, LNeg > LNeut; see Table 5). Significant task by valence interactions also revealed increased MOFC/vMPFC activity to viewing positive and neutral expressions relative to viewing negative expressions (i.e., VPos > VNeg, VNeut > VNeg; see Table 5). For interested readers, we note that whole-brain main effects of valence were observed in pSTS, left lateral orbitofrontal cortex, and fusiform gyrus (see http://neurovault.org/collections/1305).
Table 5.
Paired Sample T-tests of mean parameter estimates of activity within tasks. Bilateral dorsal and ventral lateral prefrontal cortex regions of interest defined by 8 mm sphere around the peak within that region from the Label > Blur contrast (at p < 0.0001, k ≥ 14). Medial orbital frontal cortex and bilateral amygdala regions of interest defined by 8 mm sphere around the peak within that region from the View > Blur contrast (at p < 0.0001, k ≥ 14). Pos = Positive; Neg = Negative; Neut = Neutral.
| Paired Sample T-test | M | SE | CI | t | df | p |
|---|---|---|---|---|---|---|
| Left Dorsal Lateral Prefrontal Cortex | ||||||
| Label Neg > Label Pos. | 0.16 | 0.07 | (0.30, 0.02) | 2.36 | 57 | 0.02 |
| Label Neg > Label Neut | 0.23 | 0.05 | (0.05, 0.13) | 4.4 | 57 | <0.001 |
| Right Dorsal Lateral Prefrontal Cortex | ||||||
| Label Neg > Label Pos | 0.18 | 0.07 | (0.31, 0.04) | 2.69 | 57 | <0.01 |
| Label Neg > Label Neut | 0.2 | 0.43 | (0.06, 0.09) | 3.61 | 57 | <0.001 |
| Left Ventral Lateral Prefrontal Cortex | ||||||
| Label Pos > Label Neut | 0.21 | 0.08 | (0.06, 0.36) | 2.76 | 57 | <0.01 |
| Label Neg > Label Neut | 0.29 | 0.07 | (0.14, 0.43) | 3.9 | 57 | <0.001 |
| Right Ventral Lateral Prefrontal Cortex | ||||||
| Label Pos > Label Neut | 0.23 | 0.07 | (0.10, 0.37) | 3.45 | 57 | <0.01 |
| Label Neg > Label Neut | 0.3 | 0.05 | (0.20, 0.40) | 5.85 | 57 | <0.001 |
| Left Amygdala | ||||||
| Label Pos > Label Neut | 0.17 | 0.06 | (0.04, 0.30) | 2.69 | 57 | <0.01 |
| Label Neg > Label Neut | 0.26 | 0.06 | (0.15, 0.37) | 4.61 | 57 | <0.001 |
| Medial Orbital Frontal Cortex | ||||||
| View Pos > View Neg | 0.26 | 0.1 | (0.05, 0.46) | 2.6 | 57 | 0.01 |
| View Neut > View Neg | 0.33 | 0.09 | (0.51, 0.15) | 3.73 | 57 | <0.001 |
3.4. fMRI: developmental trends
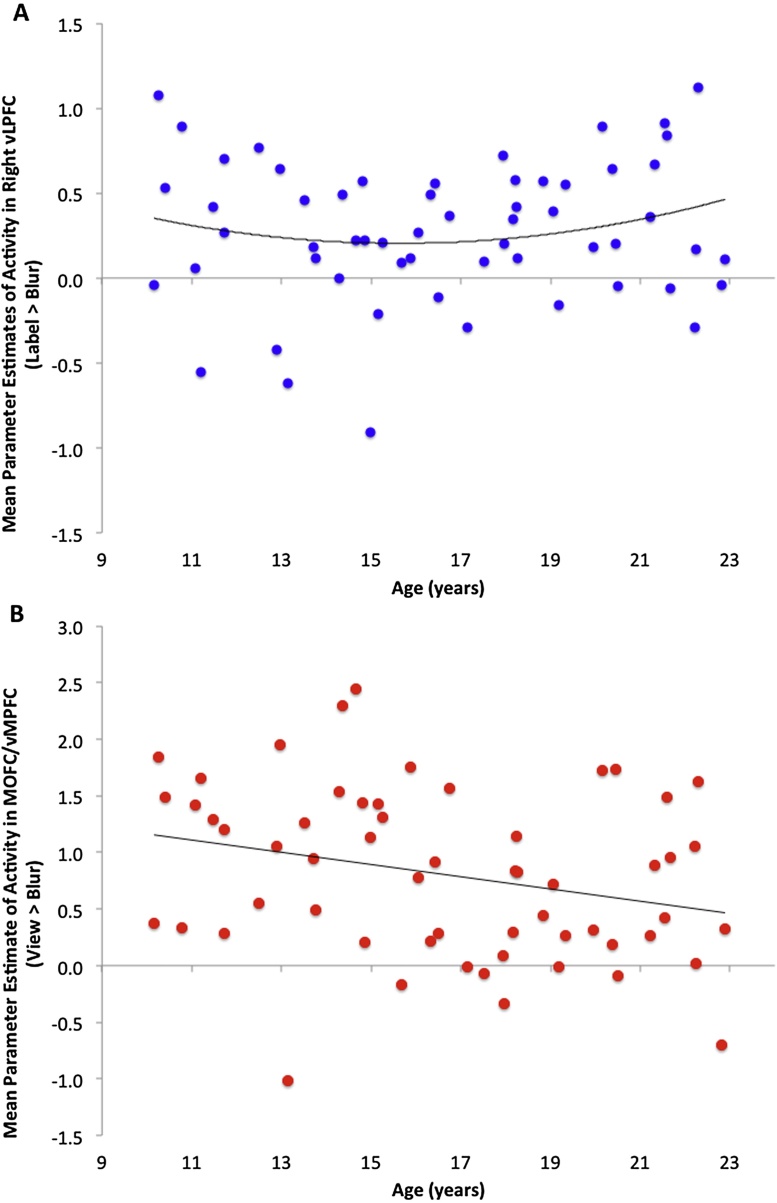
Next, to investigate developmental trends in our a priori ROIs, we ran hierarchical linear regressions (step 1: linear age, step 2: quadratic age) for the sphere-defined ventral and dorsal LPFC (Label > Blur), bilateral amygdala (Label > Blur), and MOFC/vMPFC (View > Blur). Responses in all ROIs were relative to our high level control (Blur) to assess age trends in both processes (i.e., labeling and viewing) independently. These analyses revealed a significant positive quadratic age trend in right ventral LPFC activity while labeling peer expressions, such that right ventral LPFC activity to labeling peer expressions was lowest in mid-adolescence (see Fig. 4A and Table 6). Left ventral LPFC and bilateral dorsal LPFC showed no significant linear or quadratic age trends (see Table 5). There were no significant developmental trends in the amygdala, such that left and right amygdala activity while labeling peer expressions remained consistently high across all ages studied (see Table 6). Sensitivity analyses using bilateral amygdala ROIs defined by the entire cluster resulting from the whole-brain analysis at the group level View > Blur contrast, as well as using the Harvard-Oxford probabilistic atlas (set at 75% probability of being in the amygdala) likewise revealed no significant developmental trend in left or right amygdala to viewing peer expressions. Finally, MOFC/vMPFC revealed a significant linear decrease in activity across age to viewing peer expressions (see Fig. 4B and Table 6). At our conservative threshold, no age trends were observed in the whole-brain regression; however, when we relaxed the threshold to p < 0.005, k = 20, right dorsal LPFC (Label > Blur) showed a nonlinear trend, MOFC (View > Blur) showed a linear trend, and no age trends (linear or quadratic) with amygdala activity were observed in either contrast. Importantly, opposite trends were not observed in either region in these contrasts at this relaxed threshold, providing consistent evidence for the direction of the age trends.
Fig. 4.
Neural Activity Across Age. Panel A depicts mean parameter estimates of activity in right ventral lateral prefrontal cortex (vLPFC) by age (10–23 years) across participants. Panel B depicts mean parameter estimates of activity in medial orbital frontal cortex/ventral medial prefrontal cortex (MOFC/vMPFC) by age (10–23 years) across participants. Right ventral lateral prefrontal cortex region of interest defined by 8 mm sphere around the peak within that region from the Label > Blur contrast (at p < 0.0001, k ≥ 14). Medial orbital frontal cortex region of interest defined by 8 mm sphere around the peak within that region from the View > Blur contrast (at p < 0.0001, k ≥ 14).
Table 6.
Hierarchal Linear Regressions in a priori regions of interest using linear and quadratic age. Bilateral dorsal and ventral lateral prefrontal cortex regions of interest defined by 8 mm sphere around the peak within that region from the Label > Blur contrast (at p < 0.0001, k ≥ 14). Medial orbital frontal cortex and bilateral amygdala regions of interest defined by 8 mm sphere around the peak within that region from the View > Blur contrast (at p < 0.0001, k ≥ 14).
| Hierarchical Linear Regression | R2 | Δ in R2 | B | β | t | p |
|---|---|---|---|---|---|---|
| Medial Orbital Frontal Cortex | ||||||
| age linear. | 0.09 | 0.09 | −0.06 | −0.29 | 3.27 | 0.03 |
| age quadratic | 0.09 | 0.00 | 0.00 | 0.05 | −2.23 | 0.70 |
| Right Ventral Lateral Prefrontal Cortex | ||||||
| age linear | 0.01 | 0.01 | 0.01 | 0.10 | 0.76 | 0.45 |
| age quadratic | 0.08 | 0.07 | 0.008 | 0.27 | 2.05 | 0.05 |
| Right Dorsal Lateral Prefrontal Cortex | ||||||
| age linear | 0.03 | 0.03 | 0.02 | 0.19 | 1.48 | 0.14 |
| age quadratic | 0.08 | 0.05 | <0.01 | 0.22 | 1.73 | 0.08 |
| Left Dorsal Lateral Prefrontal Cortex | ||||||
| age linear | <0.01 | <0.01 | <0.01 | 0.09 | 0.66 | 0.51 |
| age quadratic | 0.02 | 0.02 | 0.004 | 0.13 | 0.99 | 0.33 |
| Left Ventral Lateral Prefrontal Cortex | ||||||
| age linear | 0.01 | 0.01 | −0.01 | −0.08 | −0.63 | 0.53 |
| age quadratic | 0.07 | 0.06 | 0.01 | 0.24 | 1.82 | 0.08 |
| Left Amygdala | ||||||
| age linear | 0.03 | 0.03 | −0.01 | −0.16 | −1.18 | 0.24 |
| age quadratic | 0.03 | 0.001 | 0.001 | 0.03 | 0.24 | 0.81 |
| Right Amygdala | ||||||
| age linear | 0.01 | 0.01 | 0.01 | 0.12 | 0.86 | 0.40 |
| age quadratic | 0.01 | <0.01 | <0.01 | 0.03 | 0.23 | 0.82 |
We reran 2 × 3 repeated measures ANOVAs with age as a covariate to assess if there was a significant difference in the age effect depending on factor level (task or valence). There was not a significant interaction between age and task or valence factor levels (label/view or positive/negative/neutral). For completeness, however, we provide the estimated age effects across all factor levels. There was a significant positive quadratic age trend in right ventral LPFC activity to labeling positive emotions (R = 0.30, t(55) = 2.29, p = 0.03). There were no significant age related changes in left amygdala activity to labeling faces between expressions (ps > 0.05). The linear age trend in MOFC/vMPFC activity during view, however, was significant across all valence conditions (R = −0.32, t(56) = −2.51, p = 0.02; R = −0.31, t(55) = −2.46, p = 0.02; and R = −0.30, t(56) = −2.31, p = 0.03, for positive, negative, and neutral expressions, respectively). Sensitivity analyses using bilateral amygdala ROIs defined by the entire cluster resulting from the whole-brain analysis at the group level View > Blur contrast, as well as using the Harvard-Oxford probabilistic atlas (set at 75% probability of being in the amygdala) likewise revealed no significant developmental trend in left or right amygdala to labeling positive, negative, or neutral expressions.
Differences in the effect of age on brain activity between our regions of interest (right vLPFC (Label > Blur), and MOFC (View > Blur)) were tested using a linear model (specifically, the “lm” function) in R 3.3.0 (R Core Team, 2016). The average BOLD response within each ROI was regressed on age, age2, and an ROI factor (with a level for each ROI: right vLPFC, sphere extracted from Label > Blur, and MOFC, sphere extracted from View > Blur), and the interaction of ROI with age and age2. The presence of a significant interaction would indicate that the effect of age or age2 differs between the ROIs. The best fitting model included an interaction between ROI and the linear effect of age. Although only right vLPFC had a quadratic effect significantly different from 0, we cannot reject the null that there is no difference in their quadratic effects. However, at mean centered age, the linear slopes significantly differ (b = −2.54, t (54) = −2.46, p = 0.017).
3.5. fMRI: functional connectivity with left amygdala
We explored patterns of functional connectivity by focusing on the left amygdala, a region of great interest in prior neurodevelopmental studies of connectivity during affective processing (Gee et al., 2013), which was robustly activated during both Label and View. Relative to our high-level visual control (Blur), during View, the left amygdala showed positive functional connectivity with posterior cingulate cortex, extending into precuneus; however, it showed negative functional connectivity (i.e., decoupling) with dorsal anterior cingulate cortex, premotor cortex, bilateral inferior parietal lobule and secondary somatosensory cortex, posterior insula, middle frontal gyrus, and precuneus (see Supplementary Table 1 and http://neurovault.org/collections/2093/). Relative to our high-level visual control (Blur), during Label the left amygdala exhibited no significant positive functional connectivity, but revealed similar patterns of negative functional connectivity with dACC, bilateral inferior parietal lobule, secondary somatosensory cortex, posterior insula, middle frontal gyrus, and precuneus, as well as portions of occipital cortex (see Supplementary Table 2 and http://neurovault.org/collections/2094/). Direct contrasts between Label and View produced no significant differences in functional connectivity at our statistical thresholds, and whole-brain regressions with linear and quadratic age likewise produced no significant developmental effects at our statistical thresholds.
4. Discussion
The aim of this study was to examine the neural correlates of facial affect processing across this sensitive period of adolescent socioemotional development by using a highly ecologically valid stimulus set and paradigm (assessing implicit regulation of dynamic adolescent expressions) in a healthy sample of 10–23 year-old females. Consistent with prior studies that employed various emotion regulation paradigms (Lieberman et al., 2007, McRae et al., 2012, Ochsner et al., 2009, Pitskel et al., 2011, Silvers et al., 2015), our novel peer dynamic stimulus set reliably and robustly recruited key neural regions involved in the network of emotion reactivity (MOFC/vMPFC, bilateral amygdala) and regulation (bilateral dorsal and ventral LPFC). These data suggest that viewing peer faces (compared to labeling) was associated with heightened MOFC/vMPFC activity, while labeling peer faces (compared to viewing) was associated with heightened activity in bilateral ventral LPFC and bilateral dorsal LPFC as well as left amygdala. However, in our cross-sectional study spanning most of adolescence, none of our a priori regions of interest (MOFC/vMPFC, amygdala, dLPFC, and vLPFC) demonstrated the age-related trends in activity to dynamic peer faces that could be expected by extending imbalance models to the affective domain. These findings suggest that the field’s characterization of sensitive periods in socio-affective neurodevelopment may be highly influenced by the particular stimuli and paradigms used. In particular, when using stimuli and paradigms that may be more socially salient (peer faces) and ecologically valid (dynamic expressions and implicit regulation), the neurodevelopmental trends in emotional reactivity and regulation may vary from commonly assumed patterns derived from applying dual systems or imbalance models to the affective domain.
4.1. Neural correlates of affective reactivity and implicit regulation elicited by dynamic peer expressions
While viewing dynamic emotional expressions made by other adolescents, participants engaged the MOFC/vMPFC and amygdala as predicted: key regions associated with encoding emotional information, emotional memory formation, and mentalization (Davis and Whalen, 2001, Krueger et al., 2009, Viviani, 2014). These regions involved in emotion processing help encode both the positive and negative affective salience of the environment (Davis and Whalen, 2001, Gallagher and Chiba, 1996) to modulate implicit and explicit memory, heighten attention and awareness of the affective stimuli, aid in mentalizing, and facilitate encoding the rewarding and social value of the affective stimuli (Krueger et al., 2009, Viviani, 2014).
Labeling these expressions continued to engage the reactivity regions, but additionally recruited bilateral LPFC (both dorsal and ventral aspects), which is involved in linguistic and regulatory processes (Buhle et al., 2014, Silvers et al., 2015, Somerville et al., 2011b, Wager et al., 2008). Direct comparison between conditions confirmed greater MOFC/vMPFC activity during viewing compared to labeling as well as greater bilateral dorsal and ventral LPFC activity during labeling compared to viewing. The dorsal LPFC (dLPFC) and ventral LPFC (vLPFC) differentially modulate affective reactivity. The dLPFC, which has strong connections with the dorsal medial prefrontal cortex (dMPFC), is centrally involved in selective attention, working memory, and affective regulation during emotion processing (Morawetz et al., 2016, Petrides, 2005). The vLPFC, however, has stronger connections with the vMPFC and amygdala, and is more centrally involved in response inhibition as well as modification of emotional arousal intensity (Morawetz et al., 2016, Petrides, 2005).
Unexpectedly, there was greater left amygdala during labeling than during viewing, and no significant difference between conditions for right amygdala. These results are different than adult studies of affect labeling (Lieberman et al., 2007), suggesting that labeling dynamic emotional expressions made by other adolescents may not decrease affective reactivity during this stage. Instead, these data may be seen as consistent with Telzer et al. (2014), in which 17 year olds exhibited greater amygdala and right vLPFC than adults during affect labeling. However, a pattern of increased amygdala activity to peer faces is also consistent with prior studies directly comparing peer and adult faces, particularly to positive peer faces (Hoehl et al., 2010, Marusak et al., 2013). Exploratory analyses suggested that while viewing dynamic peer expressions, left amygdala showed positive connectivity with the posterior cingulate cortex, a region associated with emotional stimuli and self-referential processing (Bzdok et al., 2015, Maddock, 1999). While both labeling and viewing, the left amygdala also showed negative connectivity with regions associated with regulation (e.g., dACC, and lateral prefrontal cortex) and social cognition (e.g., inferior parietal lobule, and precuneus). The amygdala activity may thus have remained relatively similar between labeling and viewing due to unexpected implicit efforts to decode and regulate affect during viewing (for a similar effect, see Pfeifer et al., 2011). These data provide further evidence of the complexity of patterns and contextual influences, particularly socially salient influences, on amygdala activation across adolescence (Crone and Dahl, 2012, Pfeifer and Allen, 2012, Silvers et al., 2012).
Finally, task by valence interactions revealed greater MOFC/vMPFC activity during the viewing of positive and neutral emotional expressions (versus negative); greater left amygdala and bilateral vLPFC activity during the labeling of both positive and negative emotional expressions (versus labeling neutral); and greater bilateral dLPFC during the labeling of negative emotional expressions (versus labeling positive and neutral). As noted above, these valence specific findings are consistent with the functions typically associated with these brain regions, namely the central involvement of MOFC in valuation and reward processes (Rothkirch et al., 2012); the robust engagement of amygdala during emotion processing (Davis and Whalen, 2001); and vLPFC activity during emotion recognition and modification of emotional arousal as well as dLPFC activity during affective reappraisal (Morawetz et al., 2016).
4.2. Age-related changes in neural patterns of affective reactivity and implicit regulation
Dominant models of adolescent neurodevelopment predict non-linear trends in reactivity regions associated with socioemotional, motivational, and affective processing and linear trends in lateral frontal and parietal regions associated with cognitive control (Shulman et al., 2016), creating a sensitive period of imbalance between systems in adolescence. However, due to significantly less evidence for this in the affective domain, we sought to evaluate these predictions using a highly ecologically valid stimulus set and paradigm, to maximize the opportunity to observe a sensitive period in adolescence. In contrast to the expected nonlinear adolescent-specific increase in reactivity, we observed that MOFC/vMPFC activity (regardless of valence) showed a significant linear trend, as its activity during viewing was highest among the youngest participants (pre/early adolescence) but steadily declined as the age of the participant increased. Additionally, despite robust amygdala activity across the full sample, there were no significant age-related changes in its response during either viewing or labeling. Although the linear decrease with age in MOFC/vMPFC activity may represent some degree of diminished valuation or motivational significance of the peer expressions to the older adolescents, the sustained engagement of amygdala across age suggests the affective stimuli remained highly salient for participants of all ages included in the sample; together, these results provide mixed evidence about an adolescent-specific sensitive period for socioemotional reactivity. Finally, in contrast to the expected linear trend during implicit regulation, the right vLPFC showed a positive non-linear (quadratic) trend across age. Although there were no task by valence interactions with age, analysis of simple effects suggest this non-linear pattern was driven primarily by changes in right vLPFC activity during the labeling of positive emotional expressions, compared to negative or neutral expressions. It may be worth noting that there was no age trend in dLPFC during labeling of negative emotional expressions, suggesting both that emotional valence may moderate neurodevelopmental trends and that the mid-adolescent participants did not simply disengage from the task. Though these findings are consistent with the hypothesis that adolescents demonstrate different patterns of affective reactivity and implicit regulatory responses to peer faces, the specific age-related trajectories observed were unexpected based on prior literature suggesting adolescence is a sensitive period of heightened socioemotional reactivity and diminished regulatory capacity.
4.3. Implications of diversifying the stimuli and tasks
The current study implemented several modifications to paradigms commonly used to assess adolescent emotion processing that may account for the results described above. First, using adolescent faces may have increased the salience of the stimuli, which is consistent with prior studies indicating heightened impulsivity and reactivity in the presence of peers (Chein et al., 2011). Additionally, the familiarity of an emotional expression may produce differences in amygdala activity (Somerville and Whalen, 2006), therefore it is possible that these stimuli recruited unexpected age-related pattern of activation because they depicted more familiar expressions and targets for adolescents (i.e., both non-exaggerated facial expressions as well as adolescent faces). Second, the dynamic nature of the stimuli may elicit different patterns of activity across age, as well as account for the robust amygdala activation across our participants. This explanation would suggest a need to prioritize dynamic stimuli presentation in future studies of emotion processing for enhanced ecological validity. Third, despite recruiting the same key regions implicated in explicit or partially explicit emotion regulation, it is possible that labeling serves a different implicit regulatory function in adolescence, particularly in response to peer faces. This explanation would also suggest the need to incorporate implicit regulatory tasks for greater understanding of real-world tendencies to use regulatory strategies. These considerations may provide further evidence of the need to diversify our stimuli and tasks used in testing hypotheses derived from our current neurobiological models, to identify when effects are robust or sensitive to such paradigm modifications (Pfeifer and Allen, 2016).
4.4. Limitations and future directions
There were several limitations to the present study that lend themselves directly to suggestions for future research. First, due to sex differences in emotion regulation, pubertal onset and brain development, we limited our sample to females. Future work should investigate whether and how labeling dynamic emotional expressions made by peers differs between males and females across adolescence. Second, similar to all previous cross-sectional developmental studies utilizing adult faces across all ages, our participants were not individually age- or sex- matched with the stimulus set. Therefore all participants saw all stimuli, consisting of male and female adolescents ranging in age from 8 to 18. This was done to ensure all participants saw the same stimuli, while preventing participant fatigue (i.e., increased task length). While the stimuli used in the present study were closer in age to the age of the participants than previous child and adolescent studies using adult face stimuli, future studies should investigate differences in emotion processing using stimuli individually-matched to the participant, in order to better understand how neurodevelopmental trends may vary in response to affect displayed by age-matched peers versus other kinds of social partners. These data did not directly compare adult and peer stimuli, but strongly suggest that diversifying our stimuli constructs to assess these processes is important when trying to characterize developmental patterns of neural activity during affective reactivity and implicit regulation. Future studies should seek to directly compare neural responses to affective displays by younger, older, and age-matched adolescents within subjects.
An important possibility to consider is that some developmental changes in affective processing may be better characterized by relative changes in connectivity, representing varied contributions of neural activity across regions within a network (Casey et al., 2016, Gabard-Durnam et al., 2014, Gee et al., 2013, Perlman and Pelphrey, 2011, Todd et al., 2011, Wu et al., 2016). Future work should therefore assess connectivity more comprehensively to better characterize the relative activation of brain regions implicated in emotion processing, as well as identify longitudinal patterns in associated neural activity to identify changes in intensity of neural responses across development.
4.5. Conclusions
Taken together, these data suggest that when using dynamic peer facial expressions, there may still be an age-related imbalance in cortical and subcortical regions involved in emotion processing. However, the patterns of activity and the observed developmental trends are different than the dominant models would predict in key regions associated with affective reactivity and regulation. Studies on this topic are particularly relevant due to the increased influence of peers on adolescent behavior, particularly given that peer presence is shown to heighten impulsivity and reactivity (Albert et al., 2013, Blakemore and Robbins, 2012, Chein et al., 2011). More broadly, this study suggests that we should consider how experimental design choices may constrain our assessments of whether adolescence is a period of increased sensitivity to emotions and peers, and the underlying associated neurodevelopmental processes. Although adolescence is typically emphasized as a sensitive period in socioemotional development, these data suggest neural sensitivity to affective stimuli that are highly socially relevant may already be pronounced by late childhood in some regions (e.g., MOFC/vMPFC), and may not decline in late adolescence in other regions (e.g., amygdala). These findings reveal important new directions for future research to help researchers understand how developing brains process and are influenced by peer affect, dynamic expressions, and implicit regulation during adolescence.
Acknowledgments
Funding for this project was provided by the Oregon Medical Research Foundation (New Investigator Grant to J. H. P.). J. E. F. was supported by the National Science Foundation under Graduate Research Fellowship 2015172132. J.H.P. was also supported by P50 DA034763, NIDA, U.S. PHS (PIs: Chamberlain & Fisher), and R01 MH107418, NIMH, U.S. PHS (PI: Pfeifer). This work was made possible through generous support from the Lewis Family Endowment to the UO which supports the Robert and Beverly Lewis Center for Neuroimaging. We would like to thank the staff of the Lewis Center for Neuroimaging at the University of Oregon, the members of the Developmental Social Neuroscience Laboratory, as well as all of our participants and their families.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.dcn.2017.02.003.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Albert D., Chein J., Steinberg L. The teenage brain: peer influences on adolescent decision making. Curr. Dir. Psychol. Sci. 2013;22(2):114–120. doi: 10.1177/0963721412471347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E.T., Lieberman M.D. Using neuroscience to broaden emotion regulation: theoretical and methodological considerations: emotion regulation. Soc. Person. Psychol. Compass. 2009;3(4):475–493. doi: 10.1111/j.1751-9004.2009.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S.-J., Robbins T.W. Decision-making in the adolescent brain. Nat. Neurosci. 2012;15(9):1184–1191. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Britton J.C., Taylor S.F., Sudheimer K.D., Liberzon I. Facial expressions and complex IAPS pictures: common and differential networks. Neuroimage. 2006;31(2):906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Brown B.B., Larson J. Peer relationships in adolescence. In: Lerner R.M., Steinberg L., editors. Handbook of Adolescent Psychology. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2009. Retrieved from. [Google Scholar]
- Buhle J.T., Silvers J.A., Wager T.D., Lopez R., Onyemekwu C., Kober H. Cognitive reappraisal of emotion: a meta-analysis of human neuroimaging studies. Cereb. Cortex. 2014;24(11):2981–2990. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D., Heeger A., Langner R., Laird A.R., Fox P.T., Palomero-Gallagher N. Subspecialization in the human posterior medial cortex. Neuroimage. 2015;106:55–71. doi: 10.1016/j.neuroimage.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B., Galván A., Somerville L.H. Beyond simple models of adolescence to an integrated circuit-based account: a commentary. Dev. Cogn. Neurosci. 2016;17:128–130. doi: 10.1016/j.dcn.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry: peer influence on risk taking. Dev. Sci. 2011;14(2):F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Crowley D.M., Hill L.G., Kuklinski M.R., Jones D.E. Research priorities for economic analyses of prevention: current issues and future directions. Prev. Sci. 2014;15(6):789–798. doi: 10.1007/s11121-013-0429-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M., Whalen P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Doom J.R., Doyle C.M., Gunnar M.R. Social stress buffering by friends in childhood and adolescence: effects on HPA and oxytocin activity. Soc. Neurosci. 2016;0:1–14. doi: 10.1080/17470919.2016.1149095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner N.C., Johnson M.K., Fischer H. Neural mechanisms of reading facial emotions in young and older adults. Front. Psychol. 2012;3 doi: 10.3389/fpsyg.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger H.L., Pine D.S., Nelson E., Leibenluft E., Ernst M., Towbin K.E., Angold A. The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): a new set of children’s facial emotion stimuli: child emotional faces stimuli. Int. J. Methods Psychiatr. Res. 2011;20(3):145–156. doi: 10.1002/mpr.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A., Nichols T.E., Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc. Natl. Acad. Sci. U. S. A. 2016;113(28):7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. Measuring facial movement. Environ. Psychol. Nonverbal Behav. 1976;1(1):56–75. [Google Scholar]
- Fitzgerald D.A., Angstadt M., Jelsone L.M., Nathan P.J., Phan K.L. Beyond threat: amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30(4):1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Furman W., Low S., Ho M.J. Romantic experience and psychosocial adjustment in middle adolescence. J. Clin. Child Adolesc. Psychol. 2009;38(1):75–90. doi: 10.1080/15374410802575347. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Placentino A., Carletti F., Landi P., Allen P., Surguladze S. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J. Psychiatry Neurosci.: JPN. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E. The development of human amygdala functional connectivity at rest from 4 to 23years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M., Chiba A.A. The amygdala and emotion. Curr. Opin. Neurobiol. 1996;6(2):221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G., Casey B.J. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J. Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Karlsgodt K.H., van Erp T.G.M., Bearden C.E., Lieberman M.D., Belger A. Altered age-related trajectories of amygdala-prefrontal circuitry in adolescents at clinical high risk for psychosis: a preliminary study. Schizophr. Res. 2012;134(1):1–9. doi: 10.1016/j.schres.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., Goff B., Telzer E.H., Shapiro M. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J. Neurosci. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani, N. R., Flournoy, J. C., Ivie, E., Von Hippel, A., Pfeifer, J. H. (in press). Presentation and validation of the DuckEES child and adolescent dynamic facial expressions stimulus set. International Journal of Methods in Psychiatric Research. doi: 10.1002/mpr.1553. [DOI] [PMC free article] [PubMed]
- Giuliani N.R., Pfeifer J.H. Age-related changes in reappraisal of appetitive cravings during adolescence. Neuroimage. 2015;108:173–181. doi: 10.1016/j.neuroimage.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., Nelson E.E., Roberson-Nay R., Adler A.D. A developmental examination of amygdala response to facial expressions. J. Cogn. Neurosci. 2008;20(9):1565–1582. doi: 10.1162/jocn.2008.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., McClure-Tone E.B., Shiffrin N.D., Pine D.S., Nelson E.E. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyurak A., Gross J.J., Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn. Emot. 2011;25(3):400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. Biological substrates of emotional reactivity and regulation in adolescence during an emotional Go-Nogo task. Biol. Psychiatry. 2008;63(10):927–934. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehl S., Brauer J., Brasse G., Striano T., Friederici A.D. Children’s processing of emotions expressed by peers and adults: an fMRI study. Soc. Neurosci. 2010;5(5–6):543–559. doi: 10.1080/17470911003708206. [DOI] [PubMed] [Google Scholar]
- Kadosh K.C., Linden D.E.J., Lau J.Y.F. Plasticity during childhood and adolescence: innovative approaches to investigating neurocognitive development. Dev. Sci. 2013;16(4):574–583. doi: 10.1111/desc.12054. [DOI] [PubMed] [Google Scholar]
- Krueger F., Barbey A.K., Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends Cogn. Sci. 2009;13(3):103–109. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Larson R., Csikszentmihalyi M., Graef R. Mood variability and the psycho-social adjustment of adolescents. In: Csikszentmihalyi M., editor. Applications of Flow in Human Development and Education. Springer Netherlands; Dordrecht: 2014. pp. 285–304.http://link.springer.com/10.1007/978-94-017-9094-9_15 Retrieved from. [Google Scholar]
- Leibenluft E., Gobbini M.I., Harrison T., Haxby J.V. Mothers’ neural activation in response to pictures of their children and other children. Biol. Psychiatry. 2004;56(4):225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):46. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Zebrowitz L.A., Aharon I. Effective connectivity between amygdala and orbitofrontal cortex differentiates the perception of facial expressions. Soc. Neurosci. 2009;4(2):185–196. doi: 10.1080/17470910802453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Eisenberger N.I., Crockett M.J., Tom S.M., Pfeifer J.H., Way B.M. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol. Sci. 2007;18(5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- LoBue V., Thrasher C. The Child Affective Facial Expression (CAFE) set: validity and reliability from untrained adults. Front. Psychol. 2015;5 doi: 10.3389/fpsyg.2014.01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock R.J. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Carré J.M., Thomason M.E. The stimuli drive the response: an fMRI study of youth processing adult or child emotional face stimuli. Neuroimage. 2013;83:679–689. doi: 10.1016/j.neuroimage.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Mauss I.B., Bunge S.A., Gross J.J. Automatic emotion regulation. Soc. Person. Psychol. Compass. 2007;1(1):146–167. [Google Scholar]
- McClure E.B. A meta-analytic review of sex differences in facial expression processing and their development in infants, children, and adolescents. Psychol. Bull. 2000;126(3):424–453. doi: 10.1037/0033-2909.126.3.424. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., Robertson E.R., Sokol-Hessner P., Ray R.D. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., Zarahn E., Bilder R.M., Leibenluft E. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20(1):420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Baudewig J., Kirilina E., Heekeren H.R. Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cereb. Cortex. 2016;26(5):1923–1937. doi: 10.1093/cercor/bhv005. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Leibenluft E., McClure E.B., Pine D.S. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol. Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Jarcho J.M., Guyer A.E. Social re-orientation and brain development: an expanded and updated view. Dev. Cogn. Neurosci. 2016;17:118–127. doi: 10.1016/j.dcn.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D.E. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Hughes B., Robertson E.R., Cooper J.C., Gabrieli J.D.E. Neural systems supporting the control of affective and cognitive conflicts. J. Cogn. Neurosci. 2009;21(9):1841–1854. doi: 10.1162/jocn.2009.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peake S.J., Dishion T.J., Stormshak E.A., Moore W.E., Pfeifer J.H. Risk-taking and social exclusion in adolescence: neural mechanisms underlying peer influences on decision-making. Neuroimage. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 2011;108(3):607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. B: Biol. Sci. 2005;360(1456):781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn. Sci. 2012;16(6):322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. The audacity of specificity: moving adolescent developmental neuroscience towards more powerful scientific paradigms and translatable models. Deve. Cogn. Neurosci. 2016;17:131–137. doi: 10.1016/j.dcn.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Blakemore S.-J. Adolescent social cognitive and affective neuroscience: past, present, and future. Soc. Cogn. Affect. Neurosci. 2012;7(1):1–10. doi: 10.1093/scan/nsr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Moore W.E., Oswald T.M., Mazziotta J.C., Iacoboni M., Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69(5):1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D., Dilks D.D., Saxe R.R., Triantafyllou C., Kanwisher N. Differential selectivity for dynamic versus static information in face-selective cortical regions. Neuroimage. 2011;56(4):2356–2363. doi: 10.1016/j.neuroimage.2011.03.067. [DOI] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Crowley M.J., Pelphrey K.A. How grossed out are you? The neural bases of emotion regulation from childhood to adolescence. Dev. Cogn. Neurosci. 2011;1(3):324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitskel N.B., Bolling D.Z., Kaiser M.D., Pelphrey K.A., Crowley M.J. Neural systems for cognitive reappraisal in children and adolescents with autism spectrum disorder. Dev. Cogn. Neurosci. 2014;10:117–128. doi: 10.1016/j.dcn.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkirch M., Schmack K., Schlagenhauf F., Sterzer P. Implicit motivational value and salience are processed in distinct areas of orbitofrontal cortex. Neuroimage. 2012;62(3):1717–1725. doi: 10.1016/j.neuroimage.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E.E., Li Q., Siddiqui A., Krafft C., Oliver W.T. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54(3):2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Sato W., Kochiyama T., Yoshikawa S., Naito E., Matsumura M. Enhanced neural activity in response to dynamic facial expressions of emotion: an fMRI study. Cogn. Brain Res. 2004;20(1):81–91. doi: 10.1016/j.cogbrainres.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Shulman E.P., Smith A.R., Silva K., Icenogle G., Duell N., Chein J., Steinberg L. The dual systems model: review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J.S., Siegle G.J., Whalen D.J., Ostapenko L.J., Ladouceur C.D., Dahl R.E. Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Dev. Psychopathol. 2009;21(01):7–26. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., McRae K., Gabrieli J.D.E., Gross J.J., Remy K.A., Ochsner K.N. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12(6):1235–1247. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers J.A., Weber J., Wager T.D., Ochsner K.N. Bad and worse: neural systems underlying reappraisal of high- and low-intensity negative emotions. Soc. Cogn. Affect. Neurosci. 2015;10(2):172–179. doi: 10.1093/scan/nsu043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Whalen P.J. Prior experience as a stimulus category confound: an example using facial expressions of emotion. Soc. Cogn. Affect. Neurosci. 2006;1(3):271–274. doi: 10.1093/scan/nsl040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Fani N., McClure-Tone E.B. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev. Neuropsychol. 2011;36(4):408–428. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J. Cogn. Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Ruberry E.J., Dyke J.P., Glover G., Casey B.J. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychol. Sci. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman A.B., Harden K.P. The importance of sexual and romantic development in understanding the developmental neuroscience of adolescence. Dev. Cogn. Neurosci. 2016;17:145–147. doi: 10.1016/j.dcn.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Qu Y., Goldenberg D., Fuligni A.J., Galván A., Lieberman M.D. Adolescents’ emotional competence is associated with parents’ neural sensitivity to emotions. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R.M., Evans J.W., Morris D., Lewis M.D., Taylor M.J. The changing face of emotion: age-related patterns of amygdala activation to salient faces. Soc. Cogn. Affect. Neurosci. 2011;6(1):12–23. doi: 10.1093/scan/nsq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi S.J., Lieberman M.D., Bookheimer S.Y., Altshuler L.L. Advancing understanding of affect labeling with dynamic causal modeling. Neuroimage. 2013;0:481–488. doi: 10.1016/j.neuroimage.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M., Derks J.M., Hoogendam J.M., Hillegers M., Kahn R.S. Functional differences in emotion processing during adolescence and early adulthood. Neuroimage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- Viviani R. Neural correlates of emotion regulation in the ventral prefrontal cortex and the encoding of subjective value and economic utility. Front. Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Nichols T.E. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18(2):293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Neural mechanisms of emotion regulation: evidence for two independent prefrontal-subcortical pathways. Neuron. 2008;59(6):1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Dapretto M., Hariri A.R., Sigman M., Bookheimer S.Y. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(4):481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wu M., Kujawa A., Lu L.H., Fitzgerald D.A., Klumpp H., Fitzgerald K.D. Age-related changes in amygdala-frontal connectivity during emotional face processing from childhood into young adulthood: amygdala-frontal connectivity development. Hum. Brain Mapp. 2016;37(5):1684–1695. doi: 10.1002/hbm.23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo P.D., Carlson S.M. Hot and cool executive function in childhood and adolescence: development and plasticity. Child Dev. Perspect. 2012 n/a–n/a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.